Abstract
Objective:
To investigate whether baseline CSF biomarkers are associated with hippocampal atrophy rate as a measure of disease progression in patients with Alzheimer disease (AD), patients with mild cognitive impairment (MCI), and controls, controlling for baseline neuropsychological and MRI findings.
Methods:
We assessed data from 31 patients with AD, 25 patients with MCI, and 19 controls (mean age 68 ± 8 years; 39 [52%] female) who visited our memory clinic and had received serial MRI scanning (scan interval 1.7 ± 0.7 years). At baseline, CSF biomarkers (amyloid β 1-42, tau, and tau phosphorylated at threonine 181 [p-tau]) were obtained, as well as neuropsychological data. Baseline MRI scans were assessed using visual rating scales for medial temporal lobe atrophy (MTA), global cortical atrophy, and white matter hyperintensities. Hippocampal atrophy rates were estimated using regional nonlinear “fluid” registration of follow-up scan to baseline scan.
Results:
Stepwise multiple linear regression, adjusted for age and sex, showed that increased CSF p-tau levels (β [standard error]: −0.79 [0.35]) at baseline was independently associated with higher subsequent hippocampal atrophy rates (p < 0.05), together with poorer memory performance (0.09 [0.04]) and more severe MTA (−0.60 [0.21]). The association of memory function with hippocampal atrophy rate was explained by the link with diagnosis, because it disappeared from the model after we additionally corrected for diagnosis.
Conclusions:
Baseline CSF levels of tau phosphorylated at threonine 181 are independently associated with subsequent disease progression, as reflected by hippocampal atrophy rate. This effect is independent of baseline neuropsychological and MRI predictors. Our results imply that predicting disease progression can best be achieved by combining information from different modalities.
GLOSSARY
- Aβ1-42
= amyloid β 1-42;
- AD
= Alzheimer disease;
- FOV
= field of view;
- GCA
= global cortical atrophy;
- LP
= lumbar puncture;
- MCI
= mild cognitive impairment;
- MMSE
= Mini-Mental State Examination;
- MTA
= medial temporal lobe atrophy;
- p-tau
= tau phosphorylated at threonine 181;
- TE
= echo time;
- TI
= inversion time;
- TMT
= Trail Making Test;
- TR
= repetition time;
- VAT
= Visual Association Test;
- WMH
= white matter hyperintensities.
In the diagnostic process of Alzheimer disease (AD), information from different modalities, including clinical information and information from neuropsychological assessment, neuroimaging, and CSF biomarkers, is used to increase diagnostic specificity.1,2 Loss of memory function and other cognitive deficits still form the core of the clinical diagnosis of AD,3 and severity of cognitive dysfunction is associated with the extent of neuropathological changes.4,5 Atrophy of the medial temporal lobe on MRI is associated both with a clinical diagnosis of AD6 and with neuropathological findings.7–10 Finally, CSF levels of amyloid β 1-42 (Aβ1-42), tau, and tau phosphorylated at threonine 181 (p-tau) proteins are associated with the clinical diagnosis of AD. These 3 CSF biomarkers probably reflect different aspects of the neuropathologic findings in AD, such as amyloid plaque formation and presence of neurofibrillary tangles.5,11 Evidence of an association of CSF biomarkers and progression of hippocampal atrophy is scarce,36,37 and it is unclear whether associations are independent of information from other modalities.
Especially in light of the current search for disease-modifying therapies, early prediction of disease progression has become an important research goal. A marker that could be used to monitor disease progression is hippocampal atrophy rate measured by MRI. This method is well validated, showing a positive correlation with pathologically measured hippocampal volume and with the number of neurons within the hippocampus7,12 as well as with severity of other pathologic findings related to AD.5,8–10 Additionally, the relationship of hippocampal atrophy rate and cognitive decline has been well established.13,14 Hippocampal volume measurement seems to correlate stronger with neuropathological severity than cognitive measures.5 Advances in image analysis techniques allow for a precise detection of hippocampal atrophy rate, using regional nonlinear registration techniques,15 rendering this outcome attractive to determine progression in AD.
In a population of patients with AD, patients with MCI, and controls, we investigated whether baseline CSF biomarkers were associated with subsequent atrophy rate of the hippocampus, controlling for baseline neuropsychological and MRI findings.
METHODS
Patients.
We included patients from our memory clinic with a baseline diagnosis of probable AD or MCI and controls for whom complete neuropsychological data, CSF biomarkers, and baseline MRI variables were available. Subjects underwent a repeat MRI scan and clinical assessment as described below. Data were obtained from 31 patients with AD, 25 patients with MCI, and 19 controls. The control group consisted of patients who presented with subjective symptoms at our memory clinic but were found to be normal on neuropsychological examination.
Standardized clinical assessment included medical history taking, physical examination, neuropsychological test battery, lumbar puncture (LP), and MRI scan acquired with a standardized protocol. Diagnoses at baseline visit were made in a multidisciplinary consensus meeting, using the Petersen criteria16 for the diagnosis of MCI, and the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association criteria3 for the diagnosis of probable AD. At follow-up visit, diagnoses were reevaluated according to the consensus criteria. Patients with a diagnosis other than MCI or AD at follow-up were not included in the study. Neuropsychological, EEG, routine laboratory, and MRI findings were taken into account in the diagnostic considerations.
Neuropsychology.
The Mini-Mental State Examination (MMSE) was used as a measure of global cognitive function.17 Episodic memory function was tested with the Visual Association Test (VAT; range 0–12).18 The Trail Making Test (TMT),19 was used to assess mental speed (TMT-A) and executive function (TMT-B). For TMT-A and TMT-B, time to completion was used as outcome. Finally, category fluency was used as a measure of language and executive function. In short, patients were asked to produce the name of as many animals as possible in 1 minute.
CSF biomarkers.
CSF was obtained by an LP between the L3/L4 or L4/L5 intervertebral space. A 25-gauge needle was used for the LP, and the CSF was collected in 12-mL polypropylene tubes. CSF samples were centrifuged for 10 minutes at 1,800 g at 4°C within 2 hours of collection. CSF samples were divided into aliquots in polypropylene tubes of 0.5 or 1 mL at −80°C until analysis. Aβ1-42, tau, and p-tau were measured as described previously.20 Because the manufacturer does not supply controls, the performance of the assays was monitored with pools of surplus CSF specimens. In the study period, multiple specimens with various concentrations, included in 7 to 18 runs, were used for this purpose. The coefficients of variation were (mean ± SD) 11.3 ± 4.9% for Aβ42, 9.3 ± 1.5% for tau, and 9.4 ± 2.5% for p-tau.
MRI acquisition and analysis.
Baseline and repeat MRI scans (mean scan interval 1.7 ± 0.7 years) were obtained at 1.0 T (Siemens Magnetom Impact Expert System, Siemens AG, Erlangen, Germany). Subjects were actively invited to undergo repeat MRI, obtained at the same scanner with the same scan protocol. The scan protocol included 1) a coronal, heavily T1-weighted 3-dimensional single slab volume sequence (magnetization-prepared, rapid acquisition gradient echo sequence); rectangular 250 mm field of view (FOV) with a 256 × 256 matrix; 1.5-mm slice thickness; 168 slices; 1 × 1-mm in-plane resolution; repetition time (TR) = 15 msec; echo time (TE) = 7 msec; inversion time (TI) = 300 msec; flip angle 15° and 2) a transverse fluid-attenuated inversion recovery sequence; FOV 25 mm, 256 × 256 matrix; 17 slices; 5-mm slice thickness; 1.5- to 2.0-mm slice gap; TR = 9,000 msec; TE = 101 to 105 msec; TI = 2,200 msec.
The outcome measure in this study was annualized hippocampal atrophy rate, calculated using regional, nonlinear “fluid” registration21–24 of follow-up MRI scan to the baseline scan, quantified within the manually segmented region of the hippocampus. For the segmentation of the hippocampi, the baseline 3-dimensional T1-weighted volume sequence was reformatted in 2-mm slices (in-plane resolution: 1 × 1 mm), oriented perpendicular to the long axis of the left hippocampus. Regions of interest (ROIs) of the hippocampi were constructed by manual delineation of hippocampi on both sides on the reformatted slices, using the in-house–developed software package Show_ Images 3.7.0 (VU University Medical Center, Amsterdam, The Netherlands, 2003). Delineation of the hippocampus was performed using previously described criteria,25,26 by 3 trained technicians (coefficients of variation: interrater <8%, intrarater <5%) blinded to diagnosis. Calculation of hippocampal volume change over time was performed using 3 consecutive registration steps of repeat scan onto the baseline scan. The repeat scan was linearly (6 degrees of freedom) registered to the reformatted baseline scan using the in-house–developed registration tool Visual Register. The second registration step was a regional linear registration (6 degrees of freedom), using the hippocampal ROI to optimize cost-function, to further align baseline and repeat hippocampi, using the software package MIDAS. 27 Finally, the same software package was used to perform nonlinear “fluid” registration within a cuboid, constructed by extending 16 voxels in 3 perpendicular directions from the outer margins of the hippocampal ROIs. The voxelwise jacobian determinants, derived from the nonlinear registration matrix, were used to calculate volume change. This quantification was restricted to voxels within the baseline hippocampal ROI that showed contraction from baseline to follow-up, as described previously.15 Atrophy rate, expressed as percentage change from baseline volume, was divided by scan interval to obtain an annualized atrophy rate. More negative atrophy rates represent faster decline in volume over time.
Other MRI measures consist of semiquantitative visual rating scales assessed on baseline MRI scans. Medial temporal lobe atrophy (MTA) was assessed using a 5-point rating scale (range 0–4) on both sides.28 Global cortical atrophy (GCA) was assessed using a 4-point rating scale (range 0–3).29 White matter hyperintensities (WMH) were assessed using a 4-point rating scale (range 0–3).30 Visual rating was performed by 2 raters blinded to clinical data. The raters were trained to meet consistency according to our standard operating procedure. Interrater weighted Cohen κ values were >0.8 for MTA and WMH and >0.6 for GCA. Intrarater weighted Cohen κ values were >0.8 for MTA and >0.7 for GCA and WMH.
APOE genotyping.
DNA was isolated from 10 mL ethylenediaminetetraacetic acid blood by the QIAamp DNA blood isolation kit from Qiagen (Santa Clarita, CA). APOE genotype was determined with the Light Cycler APOE mutation detection kit (Roche Diagnostics GmbH, Mannheim, Germany). We divided the subjects into 2 groups: subjects with 1 or 2 ɛ4 alleles and subjects without an APOE ɛ4 allele. APOE was available for 69 subjects.
Statistical methods.
For statistical analyses, we used SPSS version 14.0 for Windows (SPSS Inc., Chicago, IL). Because they were not normally distributed, CSF biomarker levels were log transformed (natural logarithm). Differences between groups in age, neuropsychological data, and CSF data were assessed with analysis of variance, corrected for age and sex, with post hoc Bonferroni tests. Differences in frequency distribution of sex were assessed with χ2, and differences in baseline MRI variables were assessed with Kruskal–Wallis tests. To assess whether there were associations between baseline variables and hippocampal atrophy rate, we performed linear regression analysis in the overall population, with hippocampal atrophy rate as the dependent variable and neuropsychological tests, CSF biomarkers, and baseline MRI variables as independent variables. First, we performed separate linear regression models, adjusted for age and sex, for each variable. Subsequently, looking for a model that best predicted hippocampal atrophy rate, we performed stepwise multiple regression analyses. In these analyses, all baseline variables (with the exclusion of APOE, which was not available for all subjects) were entered stepwise, after correction for age and sex in the first model and for age, sex, and diagnosis (entered as dummy variables) in the second model. Finally, we repeated the stepwise analyses, adding APOE ɛ4 status, in those patients for whom APOE ɛ4 status was available (n = 69).
Standard protocol approvals, registrations, and patient consents.
The study was approved by the institutional medical ethical committee, and all subjects or their caregivers gave written informed consent.
RESULTS
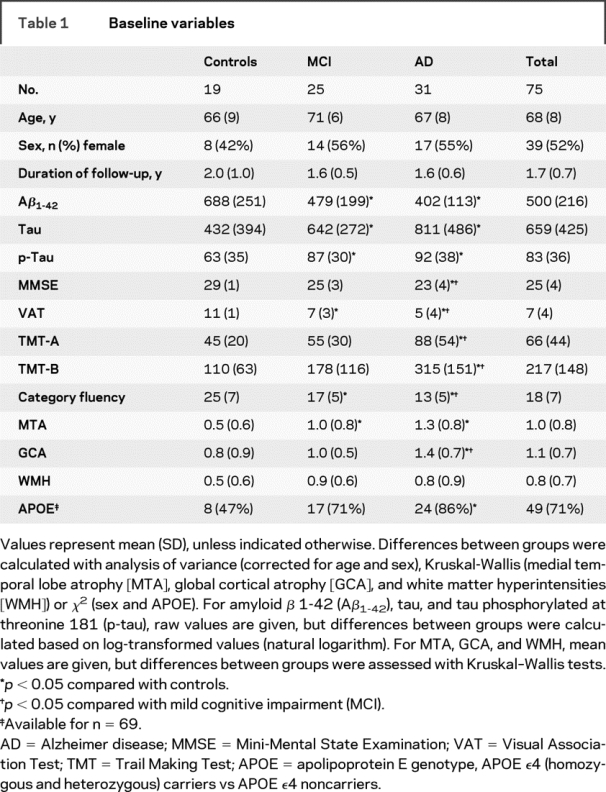
Distribution of baseline patient characteristics, with annotation for results of post hoc analysis, is given in table 1. The groups did not differ in age or distribution of sex. We found group differences for all neuropsychological tests (p < 0.001), with poorer performance of patients with MCI compared with controls and poorer performance of patients with AD compared with patients with MCI. CSF biomarker levels also differed between groups (p < 0.01 for all 3 markers), with lower Aβ1-42 levels and higher tau and p-tau levels in MCI and AD, compared with controls. Visual rating scales of MTA and GCA showed more severe atrophy in patients with MCI compared with controls and more severe atrophy in patients with AD than in patients with MCI (MTA: p < 0.01; GCA: p < 0.05). There was no significant difference in WMH score among the 3 groups. Hippocampal atrophy rates (annual percentage volume change) were −2.0% (±1.5) for controls, −3.7% (±1.2) for patients with MCI, and −3.7% (±1.1) for patients with AD (p < 0.01).
Table 1 Baseline variables
The results of linear regression analysis are shown in table 2. The separate models, corrected for age and sex, showed that CSF p-tau levels were associated with hippocampal atrophy rate. Furthermore, variables from other modalities (neuropsychology, baseline MRI markers, and APOE ɛ4 status) also showed associations with hippocampal atrophy rate. Subsequently, we constructed a stepwise multiple linear regression model, looking for the model that best predicted hippocampal atrophy rate. First, we adjusted for age and sex by entering them into the model before we stepwise entered all other variables. The model that most strongly predicted hippocampal atrophy rate included baseline CSF p-tau level, together with baseline VAT and MTA score. The explained variance (R2) of the model was 0.36. The second stepwise model (R2 = 0.35), in which we adjusted for age, sex, and diagnosis, included p-tau together with MTA as predictors of hippocampal atrophy rate. The figure illustrates the association between p-tau levels and hippocampal atrophy rate. In additional analyses, we performed the stepwise models, including APOE ɛ4 genotype (available for n = 69). These models included the same variables we show in our overall population, with the exception that tau was included in both models instead of p-tau. Regression coefficients (standard error) in the model corrected for age, sex, and diagnosis were −0.66 (0.24) for tau and −0.44 (0.19) for MTA. APOE was not included in the final model of the stepwise analyses.
Table 2 Associations of baseline variables with hippocampal atrophy rate
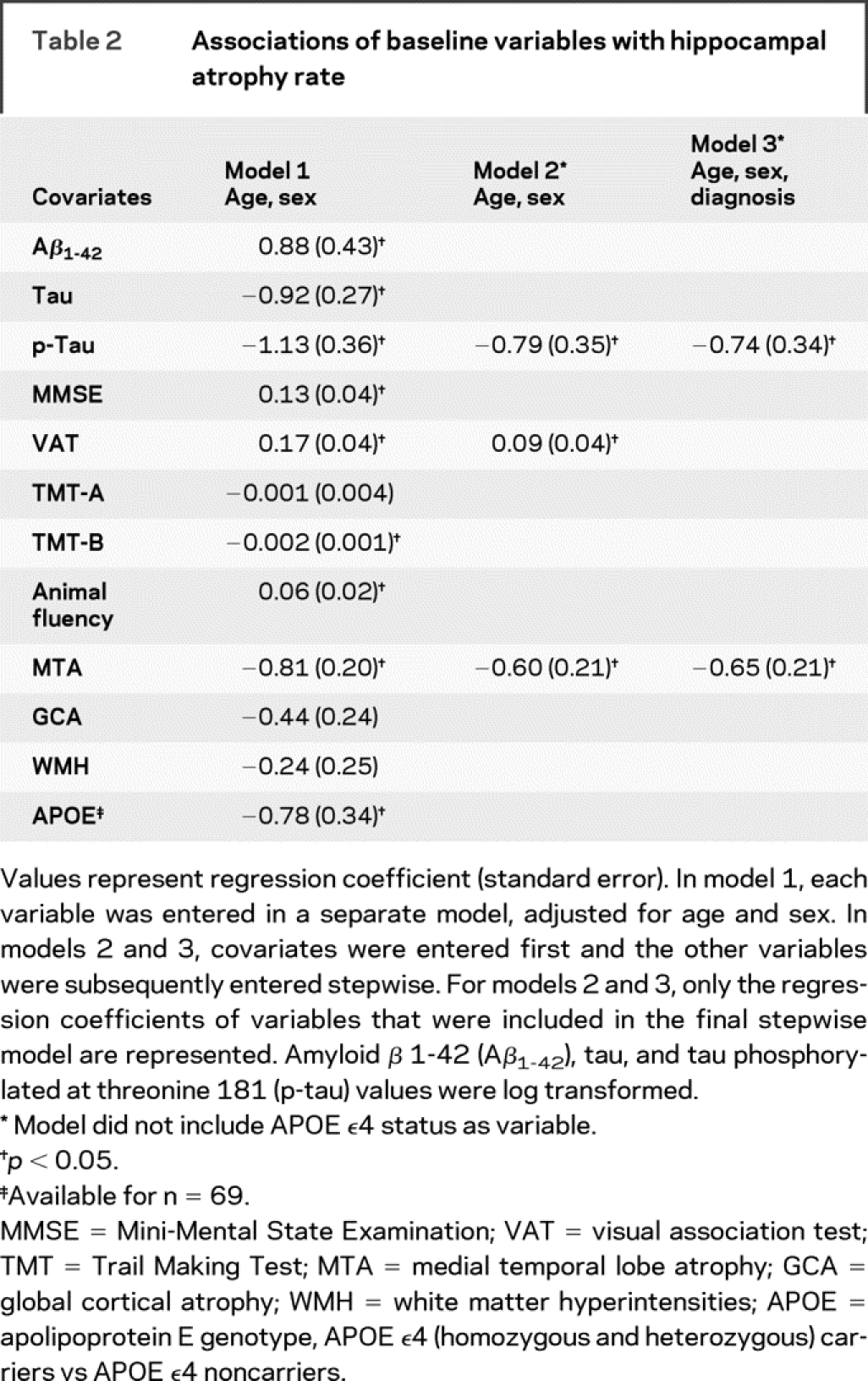
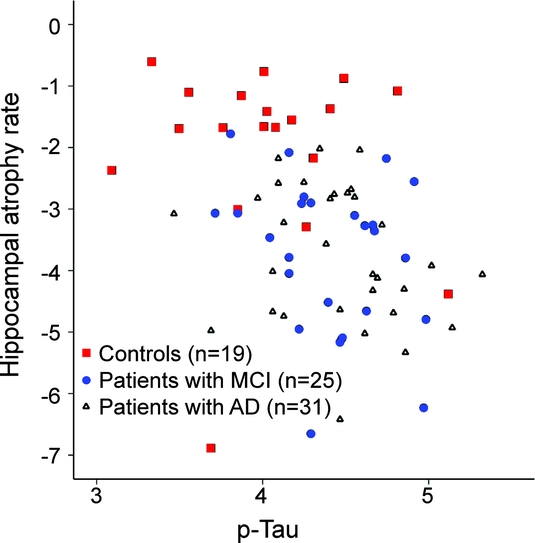
Figure p-Tau vs hippocampal atrophy rate
Scatterplot of hippocampal atrophy rate (percentage/year) by log-transformed (natural logarithm) levels of tau phosphorylated at threonine 181 (p-tau). MCI = mild cognitive impairment; AD = Alzheimer disease.
Finally, we explored the associations with hippocampal atrophy rate per diagnostic group by calculating correlation coefficients. Pearson r [in total population: −0.36 (p = 0.001)] for the correlation between p-tau and hippocampal atrophy rate was −0.25 (p = 0.18) in patients with AD, −0.29 (p = 0.16) in patients with MCI, and −0.08 (p = 0.76) in controls. When we combined only the patients with AD and MCI, excluding controls, stepwise linear regression analysis rendered p-tau as the only variable that showed an independent association (p < 0.05) with hippocampal atrophy rate (β [standard error]: −0.86 [0.40]).
DISCUSSION
We found that CSF p-tau levels, together with baseline memory function and MTA on MRI, were independently associated with hippocampal atrophy rate when corrected for age and sex in a memory clinic sample. These data indicate that different modalities give independent and complementary information about future disease progression.
The associations of memory function and baseline MTA with hippocampal atrophy rate are in concordance with previous longitudinal studies.14,26,31 Former studies addressing the association of CSF biomarkers with hippocampal atrophy on MRI are less easy to interpret. Most studies use cross-sectional hippocampal volume as outcome measure. Associations of lower Aβ1-42 and higher levels of tau and p-tau with lower hippocampal volumes have been reported by some authors,32–34 whereas others did not find an association of tau with hippocampal volume.35 One previous longitudinal study found an association of higher p-tau levels with faster hippocampal atrophy rate in patients with AD,36 and another study showed that longitudinal decrease of Aβ1-42 and increase of tau and p-tau levels over time were associated with higher hippocampal atrophy rates.37 Although we found associations of all 3 CSF biomarkers with hippocampal atrophy rate in the univariate analysis, p-tau remained the only independent predictor of the 3 CSF markers in the stepwise model. It has been suggested that p-tau reflects the formation of tangles,11 and atrophy of medial temporal lobe structures has also been related to neurofibrillary tangle burden.9 Furthermore, it has been suggested by others that tau and p-tau are correlated with rate of progression, whereas Aβ1-42 is correlated with the stage of the disease.34 We confirm previous findings, and extend on earlier studies by showing that neuropsychological, CSF, and MRI variables give additional and independent information about the prediction of disease progression over the complete cognitive continuum of AD and its preceding stages.
We previously investigated the correlation of CSF markers and whole brain atrophy rate in isolation38 and found hardly any correlations. The study showed a weak correlation of p-tau with whole brain atrophy rate in patients with AD, albeit in the other direction than the association we found in this study with hippocampal atrophy rate. These contrasting findings illustrate the difference between hippocampal atrophy rate and whole brain atrophy rate as markers in AD. We hypothesize that whole brain atrophy rate is a general marker of (advanced) neurodegeneration, whereas hippocampal atrophy rate is more specifically associated with AD-related neuropathological changes, such as the presence of neurofibrillary tangles.
Although the diagnostic groups were not large enough to perform separate analyses in, we calculated correlations within the 3 groups. This analysis showed that the association of p-tau with hippocampal atrophy rate was present in both MCI and AD groups, but not in the controls.
The fact that we used visual rating scales as baseline MRI measures, instead of more sophisticated methods such as volumetric measures, might be seen as a limitation of the study. However, we deliberately chose to use these simple methods because the more sophisticated methods are not (yet) being applied in the everyday clinical practice of a memory clinic.
The prospect of therapies that target disease-specific mechanisms and possibly stop disease progression brings forth 2 important goals that should be addressed by clinical research. The first is the ability to predict the presence of specific neuropathological changes within a patient, thus increasing the specificity of selecting patients that might benefit from such a treatment. The second is early diagnosis, enabling early start of a treatment. Our results indicate that the use of information from different modalities, including measurement of CSF biomarkers, might be a good approach to achieve these goals, because they give independent and complementary information about disease progression, as measured with hippocampal atrophy rate.
AUTHOR CONTRIBUTIONS
Statistical analyses were conducted by W.J.P. Henneman.
DISCLOSURE
Dr. Henneman, Dr. Sluimer, Dr. Verwey, Dr. Klein, and Dr. van der Flier report no disclosures. Dr. Vrenken has received non–industry-sponsored funding for travel; and receives research support from the Dutch Multiple Sclerosis Research Foundation [05-566MS, 08-633MS, and 05-358c] and the Alzheimer’s Association [EADNI-06-10004]. Dr. Barnes receives an Alzheimer’s Research Trust Fellowship partly supported by the Kirby Laing Foundation. Prof. Blankenstein serves as Associate Editor of the Annals of Clinical Biochemistry. Prof. Fox has served on scientific advisory boards of the Alzheimer’s Research Forum, GE Healthcare, and Eisai; holds an international patent for a QA Box [2007]; has served as consultant to Eli Lilly, Abbott Laboratories, and Lundbeck; has received research support (to the Dementia Research Centre) from Elan/Wyeth, Lundbeck, Sanofi, IXICO, Pfizer, and Neurochem; and receives research support from the Medical Research Council [G0801306 (PI) and G0601846 (PI)], the NIH [U01 AG024904 (Coinvestigator)], and the Alzheimer Research Trust [ART/RF/2007/1 (PI)]. Prof. Scheltens serves as an Associate Editor of the Journal of Neurology, Neurosurgery and Psychiatry, as Book Review Editor of the Alzheimer’s Disease and Associated Disorders, on the editorial board of Dementia and Geriatric Cognitive Disorders, and as Chief Editor of Tijdschrift Neurologie en neurochirugie; and receives research support from Alzheimer Nederland and the Alzheimer Center. Prof. Barkhof receives honoraria as a scientific consultant to Lundbeck, Johnson, and Roche; and serves on the editorial boards of Brain, Journal of Neurology, Neurosurgery and Psychiatry, European Radiology, the Journal of Neurology, and Neuroradiology.
Address correspondence and reprint requests to Dr. W.J.P. Henneman, Department of Radiology and Alzheimer Center, VU University Medical Center, PO Box 7057, 1007 MB Amsterdam, The Netherlands w.henneman@vumc.nl
W.J.P. Henneman is supported by the Image Analysis Center and Alzheimer Center, Amsterdam, The Netherlands. J. Barnes is supported by an Alzheimer’s Research Trust (UK) Research Fellowship.
Disclosure: Author disclosures are provided at the end of the article.
Received March 16, 2009. Accepted in final form June 25, 2009.
REFERENCES
- 1.Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 2007;6:734–746. [DOI] [PubMed] [Google Scholar]
- 2.Waldemar G, Dubois B, Emre M, et al. Recommendations for the diagnosis and management of Alzheimer’s disease and other disorders associated with dementia: EFNS guideline. Eur J Neurol 2007;14:e1–e26. [DOI] [PubMed] [Google Scholar]
- 3.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 4.Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol 2003;60:729–736. [DOI] [PubMed] [Google Scholar]
- 5.Mortimer JA, Gosche KM, Riley KP, Markesbery WR, Snowdon DA. Delayed recall, hippocampal volume and Alzheimer neuropathology: findings from the Nun Study. Neurology 2004;62:428–432. [DOI] [PubMed] [Google Scholar]
- 6.Jack CR Jr, Petersen RC, Xu Y, et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology 2000;55:484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bobinski M, de Leon MJ, Wegiel J, et al. The histological validation of post mortem magnetic resonance imaging-determined hippocampal volume in Alzheimer’s disease. Neuroscience 2000;95:721–725. [DOI] [PubMed] [Google Scholar]
- 8.Jack CR Jr, Dickson DW, Parisi JE, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology 2002;58:750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitwell JL, Josephs KA, Murray ME, et al. MRI correlates of neurofibrillary tangle pathology at autopsy: a voxel-based morphometry study. Neurology 2008;71:743–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gosche KM, Mortimer JA, Smith CD, Markesbery WR, Snowdon DA. Hippocampal volume as an index of Alzheimer neuropathology: findings from the Nun Study. Neurology 2002;58:1476–1482. [DOI] [PubMed] [Google Scholar]
- 11.Blennow K, Hampel H. CSF markers for incipient Alzheimer’s disease. Lancet Neurol 2003;2:605–613. [DOI] [PubMed] [Google Scholar]
- 12.Barkhof F, Polvikoski TM, van Straaten ECW, et al. The significance of medial temporal lobe atrophy. Neurology 2007;69:1521–1527. [DOI] [PubMed] [Google Scholar]
- 13.Petersen RC, Jack CR Jr, Xu YC, et al. Memory and MRI-based hippocampal volumes in aging and AD. Neurology 2000;54:581–587. [DOI] [PubMed] [Google Scholar]
- 14.Rusinek H, De Santi S, Frid D, et al. Regional brain atrophy rate predicts future cognitive decline: 6-year longitudinal MR imaging study of normal aging. Radiology 2003;229:691–696. [DOI] [PubMed] [Google Scholar]
- 15.van de Pol LA, Scahill RI, Frost C, et al. Improved reliability of hippocampal atrophy rate measurement in mild cognitive impairment using fluid registration. Neuroimage 2007;34:1036–1041. [DOI] [PubMed] [Google Scholar]
- 16.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol 2001;58:1985–1992. [DOI] [PubMed] [Google Scholar]
- 17.Folstein OMF, Folstein SE, Mchugh PR. Practical method for grading cognitive state of patients for clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 18.Lindeboom J, Schmand B, Tulner L, Walstra G, Jonker C. Visual association test to detect early dementia of the Alzheimer type. J Neurol Neurosurg Psychiatry 2002;73:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills 1958;8:271–276. [Google Scholar]
- 20.Bouwman FH, van der Flier WM, Schoonenboom NS, et al. Longitudinal changes of CSF biomarkers in memory clinic patients. Neurology 2007;69:1006–1011. [DOI] [PubMed] [Google Scholar]
- 21.Barnes J, Lewis EB, Scahill RI, et al. Automated measurement of hippocampal atrophy using fluid-registered serial MRI in AD and controls. J Comput Assist Tomogr 2007;31:581–587. [DOI] [PubMed] [Google Scholar]
- 22.Crum WR, Scahill RI, Fox NC. Automated hippocampal segmentation by regional fluid registration of serial MRI: validation and application in Alzheimer’s disease. Neuroimage 2001;13:847–855. [DOI] [PubMed] [Google Scholar]
- 23.Freeborough PA, Fox NC. Modeling brain deformations in Alzheimer disease by fluid registration of serial 3D MR images. J Comput Assist Tomogr 1998;22:838–843. [DOI] [PubMed] [Google Scholar]
- 24.Henneman WJP, Sluimer J, Barnes J, et al. Hippocampal atrophy rates in Alzheimer disease: added value over whole brain volume measures. Neurology 2009;72:999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jack CR Jr. MRI-based hippocampal volume measurements in epilepsy. Epilepsia 1994;35(suppl 6):S21–S29. [DOI] [PubMed] [Google Scholar]
- 26.van de Pol LA, van der Flier WM, Korf ES, et al. Baseline predictors of rates of hippocampal atrophy in mild cognitive impairment. Neurology 2007;69:1491–1497. [DOI] [PubMed] [Google Scholar]
- 27.Freeborough PA, Fox NC, Kitney RI. Interactive algorithms for the segmentation and quantitation of 3-D MRI brain scans. Comput Methods Programs Biomed 1997;53:15–25. [DOI] [PubMed] [Google Scholar]
- 28.Scheltens P, Launer LJ, Barkhof F, Weinstein HC, van Gool WA. Visual assessment of medial temporal lobe atrophy on magnetic resonance imaging: interobserver reliability. J Neurol 1995;242:557–560. [DOI] [PubMed] [Google Scholar]
- 29.Pasquier F, Leys D, Weerts JG, et al. Inter- and intraobserver reproducibility of cerebral atrophy assessment on MRI scans with hemispheric infarcts. Eur Neurol 1996;36:268–272. [DOI] [PubMed] [Google Scholar]
- 30.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 1987;149:351–356. [DOI] [PubMed] [Google Scholar]
- 31.Mungas D, Harvey D, Reed BR, et al. Longitudinal volumetric MRI change and rate of cognitive decline. Neurology 2005;65:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herukka SK, Pennanen C, Soininen H, Pirttila T. CSF A beta 42, tau and phosphorylated tau correlate with medial temporal lobe atrophy. J Alzheimers Dis 2008;14:51–57. [DOI] [PubMed] [Google Scholar]
- 33.Schroder J, Pantel J, Ida N, et al. Cerebral changes and cerebrospinal fluid beta-amyloid in Alzheimer’s disease: a study with quantitative magnetic resonance imaging. Mol Psychiatry 1997;2:505–507. [DOI] [PubMed] [Google Scholar]
- 34.Wahlund LO, Blennow K. Cerebrospinal fluid biomarkers for disease stage and intensity in cognitively impaired patients. Neurosci Lett 2003;339:99–102. [DOI] [PubMed] [Google Scholar]
- 35.Schonknecht P, Pantel J, Hartmann T, et al. Cerebrospinal fluid tau levels in Alzheimer’s disease are elevated when compared with vascular dementia but do not correlate with measures of cerebral atrophy. Psychiatry Res 2003;120:231–238. [DOI] [PubMed] [Google Scholar]
- 36.Hampel H, Burger K, Pruessner JC, et al. Correlation of cerebrospinal fluid levels of tau protein phosphorylated at threonine 231 with rates of hippocampal atrophy in Alzheimer disease. Arch Neurol 2005;62:770–773. [DOI] [PubMed] [Google Scholar]
- 37.de Leon MJ, DeSanti S, Zinkowski R, et al. Longitudinal CSF and MRI biomarkers improve the diagnosis of mild cognitive impairment. Neurobiol Aging 2006;27:394–401. [DOI] [PubMed] [Google Scholar]
- 38.Sluimer JD, Bouwman FH, Vrenken H, et al. Whole-brain atrophy rate and CSF biomarker levels in MCI and AD: a longitudinal study. Neurobiol Aging Epub 2008 Aug 7. [DOI] [PubMed]


