Abstract
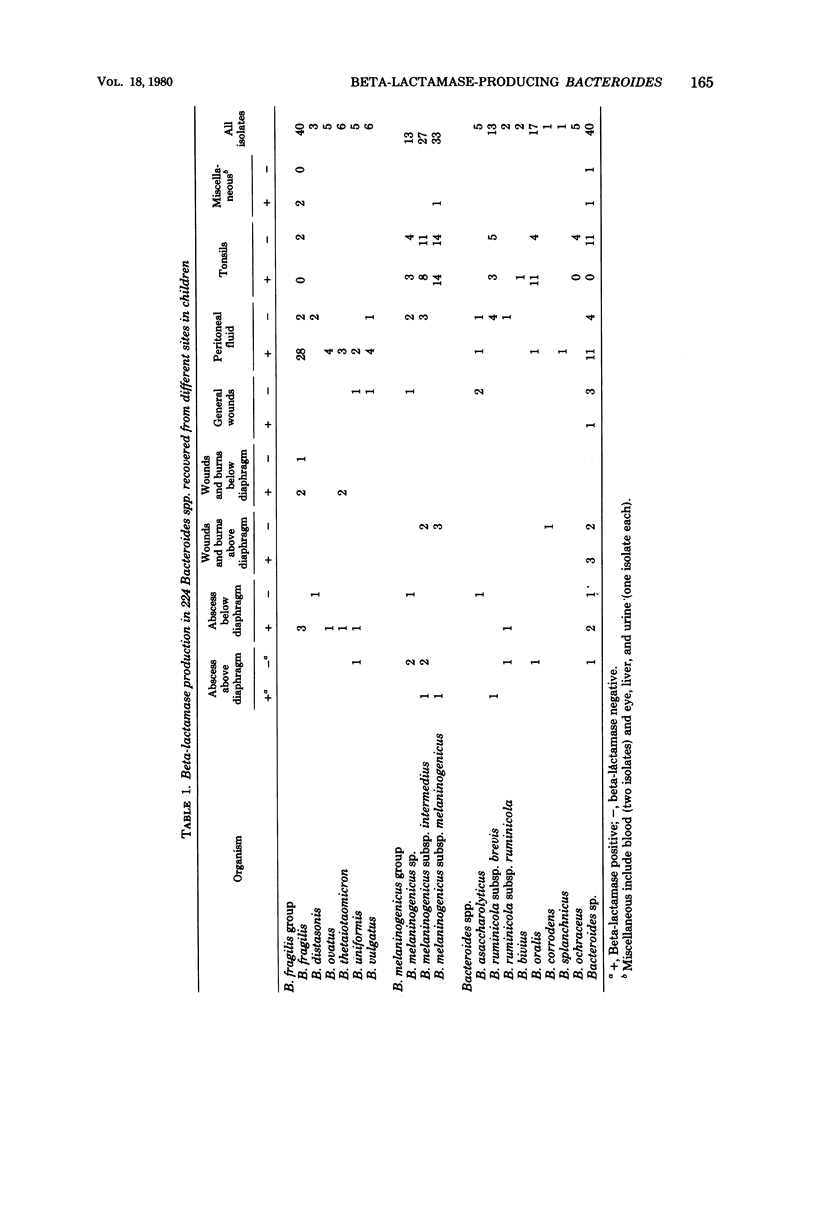
Two hundred twenty-four isolates of Bacteroides sp. were recovered from recurrently inflamed tonsils, infected peritoneal fluid, abscesses, wounds, and burns of hospitalized children. Isolates were examined for beta-lactamase production by the chromogenic cephalosporin analog 87/312 methodology. Altogether, 119 isolates were beta-lactamase producers. Of these, 53 were in the B. fragilis group, 28 were in the B. melaninogenicus groups, 12 were B. oralis, 4 were B. ruminicola subsp. brevis, and 22 were Bacteroides sp. Of 28 beta-lactamase-producing strains of B. melaninogenicus, 25 were recovered from tonsils. These observations indicate that in pediatric patients there is a significant incidence of beta-lactamase producers among anaerobes other than B. fragilis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourgault A. M., Rosenblatt J. E. Characterization of anaerobic gram-negative bacilli by using rapid slide tests for beta-lactamase production. J Clin Microbiol. 1979 Jun;9(6):654–656. doi: 10.1128/jcm.9.6.654-656.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook I., Martin W. J., Cherry J. D., Sumaya C. V. Recovery of anaerobic bacteria from pediatric patients. A one-year experience. Am J Dis Child. 1979 Oct;133(10):1020–1024. doi: 10.1001/archpedi.1979.02130100044009. [DOI] [PubMed] [Google Scholar]
- Hackman A. S., Wilkins T. D. Influence of pencillinase production by strains of Bacteroides melaninogenicus and Bacteriodes oralis on pencillin therapy of an experimental mixed anaerobic infection in mice. Arch Oral Biol. 1976;21(6):385–389. doi: 10.1016/s0003-9969(76)80007-6. [DOI] [PubMed] [Google Scholar]
- Murray P. R., Rosenblatt J. E. Penicillin resistance and penicillinase production in clinical isolates of Bacteroides melaninogenicus. Antimicrob Agents Chemother. 1977 Apr;11(4):605–608. doi: 10.1128/aac.11.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkus G., Veo G., Braude A. I. Bacteroides penicillinase. J Bacteriol. 1968 Oct;96(4):1437–1438. doi: 10.1128/jb.96.4.1437-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P. W. Bacteriological monitoring in penicillin treatment of streptococcal sore throat. J Hyg (Lond) 1971 Sep;69(3):355–360. doi: 10.1017/s0022172400021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMON H. J., SAKAI W. Staphylococcal antagonism to penicillin-G therapy of hemolytic streptococcal pharyngeal infection. Effect of oxacillin. Pediatrics. 1963 Mar;31:463–469. [PubMed] [Google Scholar]
- Sutter V. L., Finegold S. M. Susceptibility of anaerobic bacteria to 23 antimicrobial agents. Antimicrob Agents Chemother. 1976 Oct;10(4):736–752. doi: 10.1128/aac.10.4.736. [DOI] [PMC free article] [PubMed] [Google Scholar]


