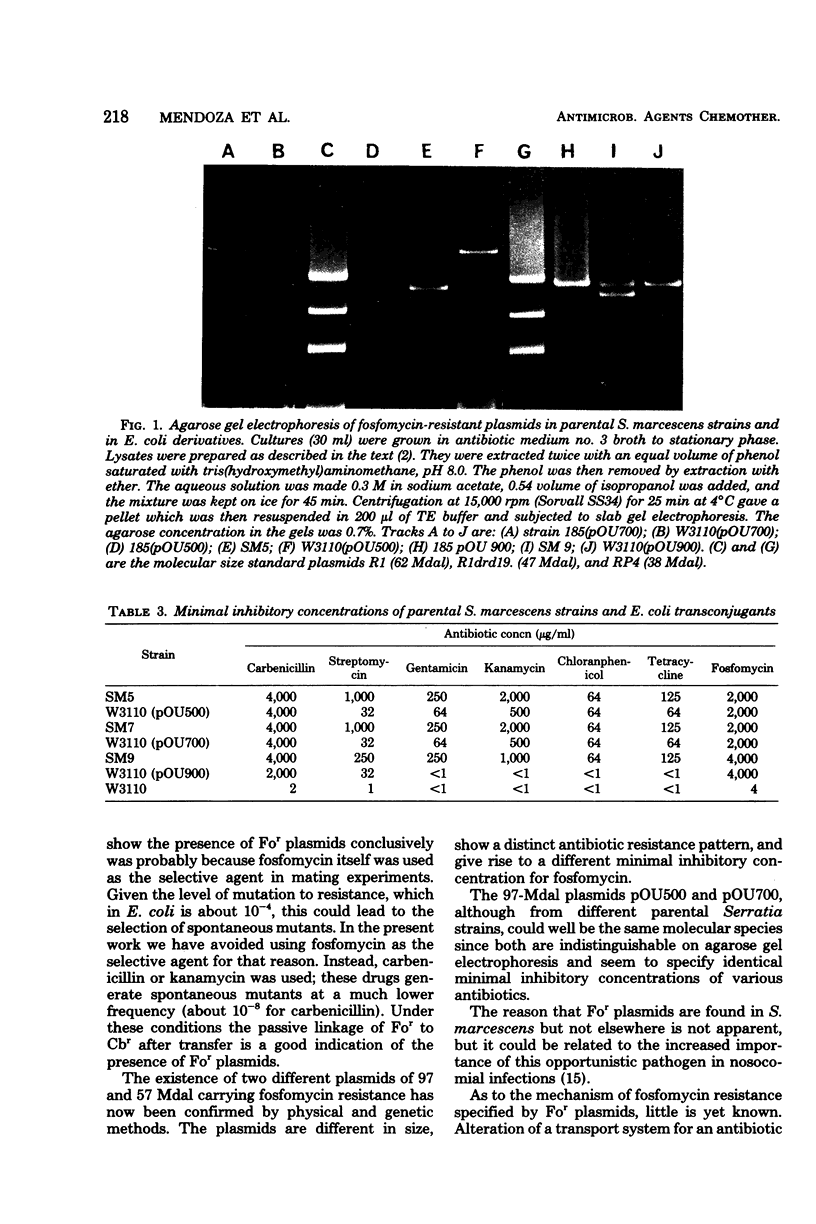
Abstract
Multiple-antibiotic-resistant strains of Serratia marcescens isolated from hospitalized patients were examined for their ability to transfer antibiotic resistance to Escherichia coli by conjugation. Two different patterns of linked transferable resistance were found among the transconjugants. The first comprised resistance to carbenicillin, streptomycin, and fosfomycin; the second, and more common, pattern included resistance to carbenicillin, streptomycin, kanamycin, gentamicin, tetracycline, chloramphenicol, sulfonamide, and fosfomycin. The two types of transconjugant strains carried a single plasmid of either 57 or 97 megadaltons in size. Both of these plasmids are present in parental S. marcescens strains resistant to fosfomycin. The 57-megadalton plasmid was transformed into E. coli.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alés J. M. Evolution of sensitivity to fosfomycin at Jimenez Diaz Foundation. Chemotherapy. 1977;23 (Suppl 1):94–98. doi: 10.1159/000222033. [DOI] [PubMed] [Google Scholar]
- Arthur A., Sherratt D. Dissection of the transposition process: a transposon-encoded site-specific recombination system. Mol Gen Genet. 1979 Oct 1;175(3):267–274. doi: 10.1007/BF00397226. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., O'Connor S. Enzymatic modification of aminoglycoside antibiotics: 3-N-acetyltransferase with broad specificity that determines resistance to the novel aminoglycoside apramycin. Antimicrob Agents Chemother. 1978 Jul;14(1):69–72. doi: 10.1128/aac.14.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulaney E. L., Ruby C. L. In vitro development of resistance to fosfomycin. J Antibiot (Tokyo) 1977 Mar;30(3):252–261. doi: 10.7164/antibiotics.30.252. [DOI] [PubMed] [Google Scholar]
- Dámaso D., Moreno-López M., Martínez-Beltrán J. Evolution of sensitivity to fosfomycin in bacteria isolated in 1973, 1974 and 1975 in the Servicio de Microbiologia y Epidemiologia of the 'Clinica Puerta de Hierro', Madrid. Chemotherapy. 1977;Suppl 1:104–111. doi: 10.1159/000222035. [DOI] [PubMed] [Google Scholar]
- Falkinham J. O., 3rd, Curtiss R., 3rd Isolation and characterization of conjugation-deficient mutants of Escherichia coli K-12. J Bacteriol. 1976 Jun;126(3):1194–1206. doi: 10.1128/jb.126.3.1194-1206.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobernado M., Santos M., Diosdado N., Pérez F. The evolution of the sensitivity to fosfomycin over the past two years. Chemotherapy. 1977;23 (Suppl 1):99–103. doi: 10.1159/000222034. [DOI] [PubMed] [Google Scholar]
- MacArthur B. S., Ackerman N. B. The significance of serratia as an infectious organism. Surg Gynecol Obstet. 1978 Jan;146(1):49–53. [PubMed] [Google Scholar]
- Martin C. M., Ikari N. S., Zimmerman J., Waitz J. A. A virulent nosocomial Klebsiella with a transferable R factor for gentamicin: emergence and suppression. J Infect Dis. 1971 Dec;124 (Suppl):S24–S29. doi: 10.1093/infdis/124.supplement_1.s24. [DOI] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruoka T., Yamada Y. Charactertization of spontaneous fosfomycin (phosphonomycin)-resistant cells of Escherichia coli B in vitro. J Antibiot (Tokyo) 1975 Nov;28(11):906–911. doi: 10.7164/antibiotics.28.906. [DOI] [PubMed] [Google Scholar]
- Venkateswaran P. S., Wu H. C. Isolation and characterization of a phosphonomycin-resistant mutant of Escherichia coli K-12. J Bacteriol. 1972 Jun;110(3):935–944. doi: 10.1128/jb.110.3.935-944.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Distribution of an inducible hexose-phosphate transport system among various bacteria. J Bacteriol. 1973 Nov;116(2):1079–1081. doi: 10.1128/jb.116.2.1079-1081.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]