Abstract
Dendritic cells (DC) represent a heterogeneous cell family of major importance for innate immune responses against pathogens and antigen presentation during infection, cancer, allergy and autoimmunity. The aim of the present study was to characterize canine DC generated in vitro with respect to their phenotype, responsiveness to toll-like receptor (TLR) ligands and T-cell stimulatory capacity. DC were derived from monocytes (MoDC) and from bone marrow hematopoietic cells cultured with either Flt3-ligand (FL-BMDC) or with GM-CSF (GM-BMDC). All three methods generated cells with typical DC morphology that expressed CD1c, CD11c and CD14, similar to macrophages. However, CD40 was only found on DC, CD206 on MΦ and BMDC, but not on monocytes and MoDC. CD1c was not found on monocytes but on all in vitro differentiated cells. FL-BMDC and GM-BMDC were partially positive for CD4 and CD8. CD45RA was expressed on a subset of FL-BMDC but not on MoDC and GM-BMDC. MoDC and FL-DC responded well to TLR ligands including poly-IC (TLR2), Pam3Cys (TLR3), LPS (TLR4) and imiquimod (TLR7) by up-regulating MHC II and CD86. The generated DC and MΦ showed a stimulatory capacity for lymphocytes, which increased upon maturation with LPS. Taken together, our results are the basis for further characterization of canine DC subsets with respect to their role in inflammation and immune responses.
Keywords: canine, dendritic cell, phenotype, TLR
1. INTRODUCTION
Dendritic cells (DC) represent a complex family of cells comprised of multiple subsets with different origin, anatomical localization and function [20]. Besides their particular ability to act as sentinels of the immune system and sensing invading pathogens and danger, they are most effective in antigen presentation and able to prime naive T cells [32], as well as regulating the orientation of distinct T-cell responses [20]. Their functioning is most critical for resistance to pathogens and tumors. DC express different pattern recognition receptors like toll-like receptors (TLR) for responding to danger and pathogen associated molecular patterns and internalizing of pathogens, and C-type lectin receptors for the internalizing of pathogens [19, 36]. To fulfil their role as sentinels, DC are not only present in the blood and lymphoid tissue [23] but also throughout the skin [25] and mucosa [28] under steady state conditions. Recent evidence from the mouse has demonstrated that DC have two major origins. In general, steady state DC present in lymphoid tissue and blood originate from an early DC progenitor population and depend on the haematopoietic cytokine Fms-like tyrosine kinase 3 ligand (Flt3L), whereas under inflammatory conditions DC can also originate directly from monocytes in a granulocyte-macrophage colony-stimulating factor (GM-CSF)-dependent manner [2, 23]. In addition, it appears that the origin of DC is organ-dependent. Particular subsets of DC in mucosal tissue including the gut and lung are partially of monocyte origin [37].
In order to translate this knowledge into the field of canine immunology, this study was aimed at phenotypically characterizing and comparing canine DC generated from bone marrow haematopoietic cells under the influence of Flt3L or GM-CSF with DC and macrophages derived from blood monocytes (MoDC and MΦ, respectively), and identifying discriminatory markers for each cell population. In order to characterize how canine DC would respond to pathogens, we analyzed their responses to different TLR ligands. To this end we established culture systems for canine DC and MΦ based on protocols published for mice, humans and pigs to generate MoDC [3, 7–9, 17, 32, 39, 43] and bone marrow haematopoietic cell-derived DC (BMDC) as documented for humans [29], mice [22], rats [35], bovines [15] and pigs [8].
2. MATERIALS AND METHODS
2.1. Animals
Blood was drawn from 25 healthy privately owned dogs with the owner’s written consent. Five laboratory Beagles used as control dogs in toxicological studies (Novartis Animal Health, Basel, Switzerland) were used as a source of bone marrow. All procedures were approved by the local animal welfare authorities.
2.2. Isolation of PBMC, monocytes and bone marrow hematopoietic cells
PBMC were isolated from freshly drawn blood anticoagulated with EDTA by histopaque (1.077 g/mL; Sigma-Aldrich, Buchs, Switzerland) gradient centrifugation as described previously [18]. Monocytes, defined as CD14+ cells, were isolated from freshly prepared PBMC with the MACS system (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. Briefly, after PBMC isolation, the cells were counted and incubated with anti-CD14 mAB CAM36A (see Tab. I). After washing with buffer, secondary goat anti-mouse microbeads were applied and the cells were placed onto an MS column. After several washing steps, CD14+ monocytes were obtained with a purity of 92–98%, controlled by flow cytometry (FCM).
Table I.
Monoclonal antibodies used for surface molecule labeling.
| Clone | Antigen | Target species | Source |
|---|---|---|---|
| CA13.1E4 | CD4 | Canine | Seroteca |
| YKIX322.3 | CD5 | Canine | Serotec |
| CA9.JD3 | CD8 | Canine | Serotec |
| Ca13.9H11 | CD1c | Canine | P. Mooreb |
| CA16.3E10 | CD11b | Canine | P. Moore |
| CA11.6A1 | CD11c | Canine | P. Moore |
| CAM36A | CD14 | Bovine | VMRDc |
| Tük4-RPE-Cy5 | CD14 | Human | Serotec |
| CA4.1D3 | CD45RA | Canine | P. Moore |
| CA24.5D4 | CD80 | Canine | P. Moore |
| CA24.3E4 | CD86 | Canine | P. Moore |
| CA2.1C12 | MHC II | Canine | P. Moore |
| 3.29B1.10 | CD206 | Human | Beckman Coulterd |
| LOB7/6 | CD40 | Human | Serotec |
Serotec, Oxford, UK.
Peter Moore, University of Davis, CA, USA.
VMRD, Pulman, USA.
Beckman Coulter, Roissy, France.
Bone marrow hematopoietic cells were obtained from the humerus of freshly euthanized dogs. The marrow was flushed with phosphate buffered saline/0.03% EDTA (PBS-EDTA) at room temperature and overlaid on histopaque for gradient centrifugation to deplete granulocytes and erythrocytes [8]. The cells were washed three times with PBS-EDTA at 4 °C.
2.3. Cell culture
For macrophage differentiation, isolated CD14+ monocytes were resuspended at 1 × 106 cells/mL in phenolred-free DMEM high in glucose (PAA Laboratories GmbH, Chemie Brunschwig AG, Basel, Switzerland) supplemented with 20% autologous serum. Recombinant human GM-CSF (rhuGM-CSF; 15 ng/mL; kindly donated by Novartis Pharma, Vienna, Austria) was added and the cells were incubated at 37 °C in a humified atmosphere with 5% CO2 for 6 days. Half of the medium was replaced on days 2 and 4.
To generate MoDC, purified monocytes were resuspended at 1 × 106 cells/mL in serum free AIM V MED medium (Invitrogen, Lubio Science, Lucerne, Switzerland) and cultured at 37 °C for 6 days in the presence of 15 U/mL recombinant canine IL-4 prepared in our laboratory and 15 ng/mL rhuGM-CSF. Half of the medium was replaced on days 2 and 4.
Isolated bone marrow haematopoietic cells were cultured at 5 × 105/mL for 12 days in IMDM (PAA) with 10% v/v FBS, 100 U/mL penicillin (Sigma), 100 μg/mL streptomycin (Sigma), 2 mM L-glutamine (Sigma) and 50 ng/mL of either feline Flt3-ligand (Flt3L) (R&D Systems), porcine recombinant Flt3L (in house), human Flt3L (PeproTech EC Ltd., London, UK) or 50 ng/mL rhuGM-CSF. Half of the medium was replaced twice per week. For each dog, the cells were cultured with both feline Flt3L and rhuGM-CSF, respectively.
All DC cultures were stimulated with different TLR ligands including Pam3Cys-SK4 at 10 μg/mL (Pam3C, TLR2 ligand; EMC microcollections, Tübingen, Germany), polyinosinic-polycytidylic acid potassium salt (PIC, TLR3 ligand; Sigma), Imiquimod at 5 μg/mL (Imi, TLR7 ligand; Invivogen, San Diego, USA), CpG oligonucleotide ODN 2395 at 5 μg/mL (TLR9 ligand; Invivogen), and LPS at 1 μg/mL (from E. coli 0128:B12, TLR4 ligand; Sigma). CpG ODN 2395 was chosen on the basis of its capacity to induce IFN-type I in canine PBMC. The responsiveness to these TLR ligands was analyzed after 24 h of culture in terms of their impact on MHC class II (MHC II) and the costimulatory molecule CD86.
2.4. Cloning of recombinant canine IL-4
We cloned canine IL-4 as described previously [33]. Briefly, PBMC were stimulated for 18 h with concanavalin A (10 μg/mL), total RNA was isolated using the RNAeasy-kit (Qiagen, Basel, Switzerland). After reverse transcription with oligo-dT primary and Omniscript RT (Qiagen), the canine IL-4 gene was amplified by standard polymerase chain reaction (PCR) using primers designed based on the published sequence of IL-4 (GenBank: AF187322.1). The PCR product was subsequently cloned into the pEAK-HIS expression vector. IL-4 was produced in HEK-293 cells using calcium-phosphate transfection. The biological activity was quantified using TF1 cells.
2.5. Phenotyping
All monoclonal antibodies (mAB) used are listed in Table I. To determine the phenotype of monocytes, PBMC were double stained with αCD14 (TÜK4 or CAM36A) in combination with all other mAB followed by anti-mouse or anti-rat isotype specific fluorescein isothiocyanate (FITC) or phycoerythrin (PE) conjugated goat F(ab’)2 Igs (Southern Biotech, Birmingham, USA). Macrophages and DC (only harvested non-adherent cells) were phenotyped by single labeling. Fluorescence intensities were quantified using a LSRII flow cytometer (BD Biosciences, San Jose, CA, USA) and data were processed with FlowJo software (Tree Star, Ashland, OR, USA).
2.6. T-cell stimulation assays
For mixed lymphocyte reactions (MLR), lymphocytes (CD14-depleted PBMC) were stained with a final concentration of 0.5 μM CFSE (Vybrant1 CFDA SE Cell Tracer Kit, Invitrogen, OR, USA). The lymphocytes were washed 4 times with DMEM 10% v/v FBS. Half of the cultured DC and macrophages were stimulated for 24 h with 1 μg/mL LPS. The other half was kept under the same culture conditions but without stimulants. CFSE-labeled lymphocytes were resuspended in RPMI (Gibco, Invitrogen) supplemented with 10% v/v FBS, 100 U/mL penicillin (Sigma) and 2 mM L-glutamine (Sigma) and seeded in triplicates of 2 × 105 cells in 96-well U-bottom plates (Corning, Vitaris, Baar, Switzerland) together with titrated numbers of DC or macrophages. After 5 days, the cells were stained with CD5 and anti-rat IgG PE-conjugated goat F(ab’)2 Ig (Jackson Immuno Research, West Grove, USA). Proliferation of CD5 positive cells, based on the CFSE profile, was assessed by FCM as described before [18].
2.7. Statistical analysis
The relative increase of the mean fluorescent intensity was calculated by dividing the stimulated by the unstimulated value. Normal distribution was assumed and a one-sample t-test was used to test for significant difference to 1. Statistical significance was considered for p ≤ 0.05. The difference of the stimulatory capacity between unstimulated versus stimulated cells in an MLR was also assumed to be normally distributed and was tested with a one-sample t-test using NCSS 2004 software (NCSS, Kaysville, Utah, USA) as well.
3. RESULTS
3.1. Generation of MoDC
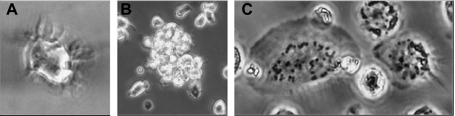
In our hands, published cell isolation protocols and corresponding culture conditions for canine MoDC differentiation [7, 39] did not consistently generate cells with dendritic morphology and some cultures had a low viability or a high proportion of granular cells (data not shown). In contrast, using modified conditions as described in Materials and Methods section, non-granulated cells with high viability and dendritic morphology were consistently generated. Within 24 h, monocytes adhered well, started to spread and appeared larger. After 3 days of culture, non-adherent cells accumulated possessing dendritic extensions protruding from the surface (Fig. 1A). These cells typically formed small clusters (Fig. 1B). Cells with this morphology further increased up to 6 days of culture. In contrast, in the absence of IL-4 strongly adhering cells with typical macrophage morphology with a large, granular cytoplasm, and a ruffled surface, could be observed (Fig. 1C).
Figure 1.
Cell morphology of canine MΦ and MoDC. (A, B) MoDC generated from monocytes cultured with human GM-CSF and canine IL-4. (A) Cells with dendritic extensions; (B) cluster of MoDC. (C) MΦ obtained from CD14+ monocytes after 6 days of culture in serum-free medium supplemented with human GM-CSF.
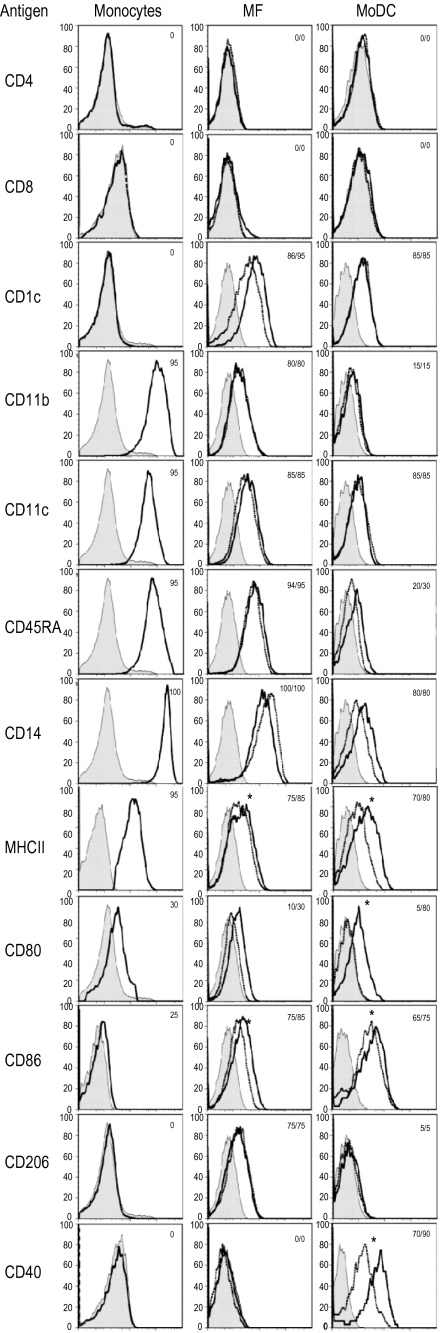
3.2. Comparative phenotype of monocytes, MΦ and MoDC
As shown in Figure 2, monocytic cells expressed the lineage markers CD11c and CD14 but were negative for CD4, CD5 (data not shown) and CD8. In contrast to differentiated cells, monocytes differed from MΦ and MoDC only by the lack of CD1c, CD206 (mannose receptor) and CD40 expression. MΦ had a similar phenotype to MoDC, but higher expression of CD11b, CD11c, CD14 and CD45RA. CD206 was only found on MΦ, whereas CD40 was restricted to MoDC. Upon LPS stimulation of MΦ, the expression of costimulatory molecules such as CD80 and CD86 but not of MHC II increased. In contrast, LPS-stimulated MoDC displayed significantly higher MHC II, CD40, CD86 and CD80 expression (Fig. 2).
Figure 2.
Phenotype of monocytes, monocyte-derived MΦ (MF) and MoDC. The filled histogram represents the conjugated controls, the overlaid lines the expression of the markers of unstimulated (dashed line) or LPS (10 μg/mL) stimulated cells (solid line) (only for MΦ and MoDC). The numbers give the percentages of positive cells calculated with the Overtone algorithm of the Flowjo software. Data are representative of 5 independent experiments. A statistical significance for the LPS stimulation is indicated by a * (p < 0.05, n = 5, assessed for CD11c, CD45RA, CD14, MHC II, CD80, CD86, CD206 and CD40).
3.3. Generation of BMDC
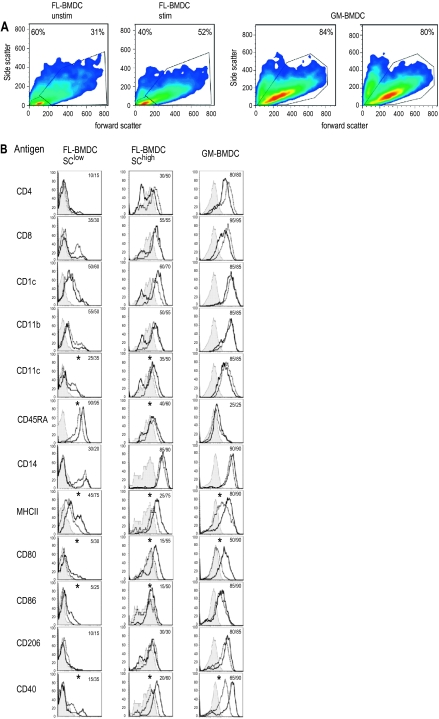
Since no canine Flt3L was commercially available, we tested the cross-reactivity of several species. In contrast to feline Flt3L, the human cytokine did not support any growth and differentiation of DC from bone marrow haematopoietic cells (data not shown). The bone marrow haematopoietic cell cultures supplemented with either GM-CSF or Flt3L differentiated into large cells with dendritic morphology within 10 to 12 days (termed GM-BMDC and FL-BMDC, respectively). Besides DC, a population of non-adherent smaller and round cells, which were presumably immature precursor cells, was visible. In addition, adherent cells with macrophage- as well as stroma-cell morphology developed. These cells were not harvested and further investigated in the present study. Based on the forward/side scatter characteristics, we found two populations of non-adherent cells: small cells with low forward and side scatter and larger cells with high scatter values. The latter population increased upon stimulation with LPS (Fig. 3A). When compared to FL-BMDC, the majority of GM-BMDC had a high scatter (Fig. 3A), relating to their bigger size and higher degree of differentiation as observed by light microscopy (data not shown).
Figure 3.
Phenotype of BMHC-derived DC. (A) Forward-side scatter plots of FL-BMDC and GM-BMDC. In FL-BMDC a region for cells with low scatter (SClow) and with high scatter (SChigh) was created. (B) Histogram plots for the markers indicated for cells in the gate defined in (A). The filled histograms represent the conjugate controls, the overlaid lines the expression of the markers of unstimulated (dashed line) or LPS (10 μg/mL) stimulated cells (solid line). The numbers are percentages of positive cells calculated with the Overtone algorithm with the Flowjo software. Data are representative of 5 independent experiments. The * indicates a statistically significant LPS-induced increase of the MFI (p < 0.05; calculated for CD11c, CD45RA, CD14, MHC II, CD80, CD86, CD206 and CD40). (A color version of this figure is available online at www.vetres.org.)
3.4. Comparative phenotype of BMDC
In contrast to MoDC, GM-BMDC and FL-BMDC with high forward and side scatter (SChigh) expressed CD4 and CD8 (Fig. 3B). Similar to monocyte-derived cells, CD1c were expressed on the majority of GM-BMDC and all FL-BMDC (Fig. 3B). CD11b showed a higher expression on the BMDC when compared to MoDC. Similar to MoDC, CD11c and CD14 were expressed on GM-BMDC and on SChigh FL-BMDC, although the presumably less differentiated SClow FL-BMDC were mostly negative for these markers. In contrast to MoDC, CD11b was expressed at high levels on BMDC. CD45RA was only expressed at high levels on SClow FL-BMDC. CD206 was highly expressed on GM-BMDC but weakly on FL-BMDC. For the maturation markers, it was interesting to note that in contrast to FL-BMDC, GM-BMDC possessed a more mature phenotype with high levels of MHC II, CD86 and CD40, with little increase after LPS stimulation. Only the expression of CD80 was highly enhanced by LPS. In contrast, LPS treatment of FL-BMDC potently enhanced the expression of all four maturation markers in the SChigh, but not in the less differentiated, SClow cells. LPS stimulation also had a positive effect on CD1c expression.
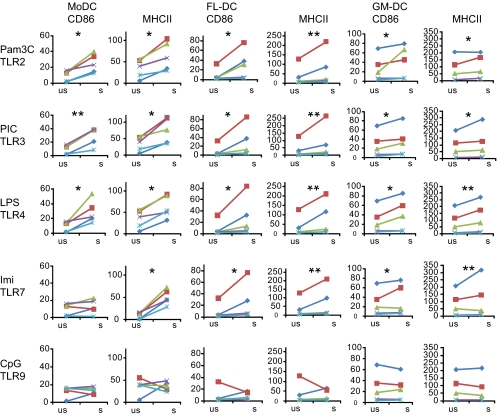
3.5. Response of canine DC to various TLR ligands
As shown in Figures 2 and 3B, dependent on the type of culture, canine DC responded to LPS (TLR4 ligand) by up regulation of MHC II and costimulatory molecules. We further investigated this function of DC using additional ligands for TLR2, TLR3, TLR7 and TLR9. MoDC from all tested dogs reacted to stimulation of TLR2, -3 and -4, with a statistically significant up regulation of CD86 and MHC II (Fig. 4 and Tab. II). The MHC II up regulation after stimulation through TLR7 was lower, but also significant (p = 0.029). In contrast, stimulation through TLR9 was inconsistent with up- and also down-regulation of both CD86 and MHC II, dependent on the experiment. With the BMDC, more variation with cells obtained from different dogs was observed (Fig. 4). Nevertheless, with FL-BMDC we observed a consistent increase of CD86 and MHC II expression upon stimulation with ligands to TLR2, -3, -4 and -7 (Fig. 4, Tab. II), although also in this cell population CpG stimulation was not consistent in its influence on MHC II and costimulatory molecules. As already described for Figure 3B, GM-BMDC reacted less well to stimulation by ligands for TLR2, TLR3, TLR4 and TLR7. Here, the increase of MHC II and CD86 expression was only significant for cells stimulated with PIC, LPS and imiquimod (Fig. 4, Tab. II). Stimulation with Pam3C induced a statistically significant increase only of MHC II expression.
Figure 4.
Phenotypic maturation of MoDC, FL-DC and GM-DC upon stimulation with different TLR ligands in terms of up regulation of MHC II and CD86. The different DC cultures were stimulated for 24 h with Pam3Cys-SK4 (Pam3C, 10 μg/mL), polyinosinic-polycytidylic acid potassium salt (PIC, 10 μg/mL), LPS (1 μg/mL), Imiquimod (Imi, 5 μg/mL) or CpG 2395 (5 μg/mL). The ratios of the MFI of stimulated and unstimulated cells were calculated and tested for significance (n = 5; *p value of 0.01–0.05; **p > 0.01). (A color version of this figure is available online at www.vetres.org.)
Table II.
Up regulation of MHC II and CD86 upon TLR ligation.
| P3Cc |
PICc |
LPSc |
Imic |
CpG odnc |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| fia | pb | fi | p | fi | p | fi | p | fi | p | ||
| MoDC | CD86 | 3.6 | 0.048 | 3.9 | 0.003 | 3.2 | 0.038 | 1.1 | 0.3 | 2.6 | 0.14 |
| MoDC | MHC II | 2.0 | 0.019 | 2.4 | 0.012 | 2.1 | 0.018 | 1.4 | 0.029 | 1.6 | 0.2 |
| FL-BMDC | CD86 | 4.7 | 0.046 | 3.9 | 0.047 | 3.6 | 0.037 | 3.2 | 0.039 | 2.1 | 0.11 |
| FL-BMDC | MHC II | 2.1 | 0.008 | 2.0 | 0.001 | 2.5 | 0.008 | 2.2 | 0.009 | 1.2 | 0.2 |
| GM-BMDC | CD86 | 1.8 | 0.08 | 1.5 | 0.04 | 1.5 | 0.02 | 1.3 | 0.07 | 0.9 | 0.28 |
| GM-BMDC | MHC II | 1.8 | 0.049 | 1.6 | 0.039 | 1.6 | 0.001 | 1.4 | 0.046 | 0.9 | 0.81 |
Average fold increase of mean fluorescent intensity (MFI) calculated as MFI of stimulated cells/MFI of unstimulated cells (n = 5).
p value determined by one-sample t-test.
P3C = Pam3Cys; PIC = Polyinosinic-Polycytidylic; Imi = Imiquimod; and CpG odn = CpG oligonucleotide ODN 2395.
3.6. T-cell stimulatory capacity
With respect to the capacity to stimulate allogeneic T lymphocytes, MΦ showed a comparable activity to MoDC (p > 0.05), but the monocyte-derived cells were less potent than BMDC (p < 0.01). Within BMDC, the GM-BMDC were more potent in this function than FL-BMDC (p < 0.02). With all four cell types, the stimulated cells showed a significantly higher T-cell stimulatory capacity than their unstimulated counterparts (p < 0.002; Fig. 5).
Figure 5.
Comparative T-cell stimulatory capacity of the different MΦ and differentially generated DC. Unstimulated and LPS-stimulated MΦ/DC were tested in mixed lymphocyte reactions. MΦ and DC were cultured at ratios of 1:10 (blue), 1:30 (red); 1:90 (green) with lymphocytes. The * indicates a statistically significant higher activity of LPS-stimulated cells. The p value for MΦ was 0.04, for MoDC 0.02, FL-BMDC 0.05 and for GM-BMDC 0.006. Standard deviations of triplicates are shown and the data are representative of three independent experiments. (A color version of this figure is available online at www.vetres.org.)
4. DISCUSSION
The dog has an increasing importance as an alternative non-murine animal model for several immunological disorders, such as atopic dermatitis [30], asthma [5], hypothyroidism [13], diabetes mellitus [31], autoimmune haemolytic anaemia [11], myopathy [44] and infectious diseases [12]. Considering the importance of DC in these diseases, the present study characterized the canine DC system in more detail. Our aim was not only to establish and optimize culture systems which allow the efficient generation of large numbers of DC but also to obtain information on the phenotype and function of canine DC, and to find discriminatory DC markers. Our study confirms previous studies that have classified canine DC as CD1c+, CD11c+, MHC II+, CD80+, CD86+ and CD40+ cells [1, 7, 9, 17, 38, 39]. However, our comparative analysis to monocytes and MΦ, which has not been systematically performed before, demonstrated that none of the markers typically used to differentiate DC from monocytes/MΦ including CD1c, CD11b, CD11c and CD14 permits to identify canine DC when used alone. This broad expression of CD11c and its unsuitability as a DC marker was also found with porcine DC and contrasts with the mouse DC system in which CD11c represents a marker frequently used to differentiate DC from monocytes, granulocytes and macrophages. Canine monocyte-derived MΦ can be phenotypically distinguished from monocytes by their expression of CD1c and the mannose receptor CD206, representing a C-type lectin receptor. However, the specificity of the αCD1c antibody used is currently under debate, since it might bind to CD1a instead of CD1c [21]. CD206 was also able to discriminate MoDC and MΦ, this time with expression restricted to the MΦ. However, BMDC also expressed CD206 which is in agreement with observations done in other species where this c-type lectin can also be found on both MΦ and DC [10, 34, 41]. Based on our data demonstrating CD40 expression even on unstimulated MoDC and BMDC, but not on MΦ and monocytes, we propose that this surface molecule is a potential discriminatory marker for DC.
In humans, CD14 is considered to be a typical monocyte/MΦ rather than a DC marker [3]. Therefore, it was unexpected to find CD14 expressed on all DC cultures, although at lower levels when compared to MΦ. Nevertheless, this finding is consistent with previous observations for dogs [7, 17], pigs [8], cattle [16], chimpanzees [4] and cats [6]. In contrast, CD14 was described to be absent from canine blood DC [24], again being comparable to the porcine DC system [34].
An interesting observation was the expression of CD4 and CD8 found on BMDC but not on blood-derived cells. CD4 and CD8 is also found on particular murine DC subsets but the function of these receptors for DC is unknown [14]. Also the differential expression of CD45RA only found on SClow FL-BMDC deserves further investigation focusing on a differential expression of these markers on DC in vivo.
An important functional criterion of DC related to their important role in innate immunity is the response to many TLR ligands in terms of maturation and cytokine production. Using this criterion, we demonstrate that LPS-stimulated MoDC in contrast to MΦ up-regulated MHC II, CD80 and CD40, similar to observations found with other species [3, 8, 33] upon stimulation with ligands to TLR2, -3 and -7 MoDC reacted with a significant up regulation of MHC II and CD86 as seen in other species [27, 34, 42]. The lack of responsiveness to TLR9 ligand CpG could be related to observations also found for humans and pigs, which describe that only plasmacytoid DC express this receptor [34, 40]. Future studies are required with more CpG sequences and the canine plasmacytoid DC need to be identified before any conclusion can be drawn. GM-BMDC expressed the highest levels of CD86 and MHC II, which did not often further increase upon stimulation indicating a spontaneous maturation in this cell culture system. This contrasted with FL-BMDC which remained in an immature phenotype and showed the most potent up regulation of MHC II and CD86 after stimulation with TLR2, -3, -4 and -7 ligands. This may relate to the central role of Flt3L in the generation of steady state DC from a clonogenic bone marrow DC precursor, whereas GM-CSF is important for the generation of inflammatory monocyte-derived DC [23, 26].
In the MLR, all DC stimulated lymphocytes. As expected, activated DC showed a significantly higher stimulatory capacity compared to their unstimulated counterparts. All together the relative efficiency of the different cells analyzed to induce T cell proliferation correlated with their expression of MHC II.
Taken together, the present study provides a detailed characterization of the phenotypic and functional properties of canine DC generated in vitro, which represent a valuable system for studying the interaction of DC with pathogens and their role in tumor immunology, autoimmunity and allergy. We also propose that these cultures contain various DC subsets with potential equivalents in vivo with distinct functions in steady state conditions and inflammation, as well as with different anatomical localizations. Future studies are required to identify these subsets in vivo, and to study their contribution to immune response against infectious agents as well as during allergies and autoimmune diseases.
Acknowledgments
This study was generously sponsored by Novartis Animal Health, St. Aubin, Switzerland. We are grateful to Peter Moore for providing the CD1c antibody, to Marcus Doherr for statistical advice and to Vivianne Molitor and Viviane Neuhaus for technical help.
References
- 1.Affolter V.K., Moore P.F., Canine cutaneous and systemic histiocytosis: reactive histiocytosis of dermal dendritic cells, Am. J. Dermatopathol. (2000) 22:40–48 [DOI] [PubMed] [Google Scholar]
- 2.Auffray C., Sieweke M.H., Geissmann F., Blood monocytes: development, heterogeneity, and relationship with dendritic cells, Annu. Rev. Immunol. (2009) 27:669–692 [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y.J., et al. , Immunobiology of dendritic cells, Annu. Rev. Immunol. (2000) 18:767–811 [DOI] [PubMed] [Google Scholar]
- 4.Barratt-Boyes S.M., Henderson R.A., Finn O.J., Chimpanzee dendritic cells with potent immunostimulatory function can be propagated from peripheral blood, Immunology (1996) 87:528–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bice D.E., Seagrave J., Green F.H., Animal models of asthma: potential usefulness for studying health effects of inhaled particles, Inhal. Toxicol. (2000) 12:829–862 [DOI] [PubMed] [Google Scholar]
- 6.Bienzle D., Reggeti F., Clark M.E., Chow C., Immunophenotype and functional properties of feline dendritic cells derived from blood and bone marrow, Vet. Immunol. Immunopathol. (2003) 96:19–30 [DOI] [PubMed] [Google Scholar]
- 7.Bonnefont-Rebeix C., de Carvalho C.M., Bernaud J., Chabanne L., Marchal T., Rigal D., CD86 molecule is a specific marker for canine monocyte-derived dendritic cells, Vet. Immunol. Immunopathol. (2006) 109:167–176 [DOI] [PubMed] [Google Scholar]
- 8.Carrasco C.P., Rigden R.C., Schaffner R., Gerber H., Neuhaus V., Inumaru S., et al. , Porcine dendritic cells generated in vitro: morphological, phenotypic and functional properties, Immunology (2001) 104:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catchpole B., Stell A.J., Dobson J.M., Generation of blood-derived dendritic cells in dogs with oral malignant melanoma, J. Comp. Pathol. (2002) 126:238–241 [DOI] [PubMed] [Google Scholar]
- 10.Cavatorta D.J., Erb H.N., Flaminio M.J., Ex vivo generation of mature equine monocyte-derived dendritic cells, Vet. Immunol. Immunopathol. (2009) 131:259–267 [DOI] [PubMed] [Google Scholar]
- 11.Day M.J., Antigen specificity in canine autoimmune haemolytic anaemia, Vet. Immunol. Immunopathol. (1999) 69:215–224 [DOI] [PubMed] [Google Scholar]
- 12.Garg R., Dube A., Animal models for vaccine studies for visceral leishmaniasis, Indian J. Med. Res. (2006) 123:439–454 [PubMed] [Google Scholar]
- 13.Happ G.M., Thyroiditis – a model canine autoimmune disease, Adv. Vet. Sci. Comp. Med. (1995) 39:97–139 [DOI] [PubMed] [Google Scholar]
- 14.Heath W.R., Carbone F.R., Dendritic cell subsets in primary and secondary T cell responses at body surfaces, Nat. Immunol. (2009) 10:1237–1244 [DOI] [PubMed] [Google Scholar]
- 15.Hope J.C., Werling D., Collins R.A., Mertens B., Howard C.J., Flt-3 ligand, in combination with bovine granulocyte-macrophage colony-stimulating factor and interleukin-4, promotes the growth of bovine bone marrow derived dendritic cells, Scand. J. Immunol. (2000) 51:60–66 [DOI] [PubMed] [Google Scholar]
- 16.Howard C.J., Brooke G.P., Werling D., Sopp P., Hope J.C., Parsons K.R., Collins R.A., Dendritic cells in cattle: phenotype and function, Vet. Immunol. Immunopathol. (1999) 72:119–124 [DOI] [PubMed] [Google Scholar]
- 17.Ibisch C., Pradal G., Bach J.M., Lieubeau B., Functional canine dendritic cells can be generated in vitro from peripheral blood mononuclear cells and contain a cytoplasmic ultrastructural marker, J. Immunol. Methods (2005) 298:175–182 [DOI] [PubMed] [Google Scholar]
- 18.Im Hof M., Williamson L., Summerfield A., Balmer V., Dutoit V., Kandimalla E.R., et al. , Effect of synthetic agonists of toll-like receptor 9 on canine lymphocyte proliferation and cytokine production in vitro, Vet. Immunol. Immunopathol. (2008) 124:120–131 [DOI] [PubMed] [Google Scholar]
- 19.Kumar H., Kawai T., Akira S., Toll-like receptors and innate immunity, Biochem. Biophys. Res. Commun. (2009) 388:621–625 [DOI] [PubMed] [Google Scholar]
- 20.Liu Y.J., Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity, Cell (2001) 106:259–262 [DOI] [PubMed] [Google Scholar]
- 21.Looringh van Beeck F.A., Zajonc D.M., Moore P.F., Schlotter Y.M., Broere F., Rutten V.P., et al. , Two canine CD1a proteins are differentially expressed in skin, Immunogenetics (2008) 60:315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lutz M.B., Kukutsch N., Ogilvie A.L., Rossner S., Koch F., Romani N., Schuler G., An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow, J. Immunol. Methods (1999) 223:77–92 [DOI] [PubMed] [Google Scholar]
- 23.Merad M., Manz M.G., Dendritic cell homeostasis, Blood (2009) 113:3418–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mielcarek M., Kucera K.A., Nash R., Torok-Storb B., McKenna H.J., Identification and characterization of canine dendritic cells generated in vivo, Biol. Blood Marrow Transplant. (2007) 13:1286–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nestle F.O., Di Meglio P., Qin J.Z., Nickoloff B.J., Skin immune sentinels in health and disease, Nat. Rev. Immunol. (2009) 9:679–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onai N., Obata-Onai A., Schmid M.A., Ohteki T., Jarrossay D., Manz M.G., Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow, Nat. Immunol. (2007) 8:1207–1216 [DOI] [PubMed] [Google Scholar]
- 27.Reis e Sousa C., Toll-like receptors and dendritic cells: for whom the bug tolls, Semin. Immunol. (2004) 16:27–34 [DOI] [PubMed] [Google Scholar]
- 28.Rescigno M., Di Sabatino A., Dendritic cells in intestinal homeostasis and disease, J. Clin. Invest. (2009) 119:2441–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siena S., Di Nicola M., Bregni M., Mortarini R., Anichini A., Lombardi L., et al. , Massive ex vivo generation of functional dendritic cells from mobilized CD34+ blood progenitors for anticancer therapy, Exp. Hematol. (1995) 23:1463–1471 [PubMed] [Google Scholar]
- 30.Sinke J.D., Rutten V.P., Willemse T., Immune dysregulation in atopic dermatitis, Vet. Immunol. Immunopathol. (2002) 87:351–356 [DOI] [PubMed] [Google Scholar]
- 31.Soon-Shiong P., Feldman E., Nelson R., Heintz R., Merideth N., Sandford P., et al. , Long-term reversal of diabetes in the large animal model by encapsulated islet transplantation, Transplant. Proc. (1992) 24:2946–2947 [PubMed] [Google Scholar]
- 32.Steinman R.M., Inaba K., Turley S., Pierre P., Mellman I., Antigen capture, processing, and presentation by dendritic cells: recent cell biological studies, Hum. Immunol. (1999) 60:562–567 [DOI] [PubMed] [Google Scholar]
- 33.Summerfield A., Guzylack-Piriou L., Schaub A., Carrasco C.P., Tache V., Charley B., McCullough K.C., Porcine peripheral blood dendritic cells and natural interferon-producing cells, Immunology (2003) 110:440–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Summerfield A., McCullough K.C., The porcine dendritic cell family, Dev. Comp. Immunol. (2009) 33:299–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talmor M., Mirza A., Turley S., Mellman I., Hoffman L.A., Steinman R.M., Generation or large numbers of immature and mature dendritic cells from rat bone marrow cultures, Eur. J. Immunol. (1998) 28:811–817 [DOI] [PubMed] [Google Scholar]
- 36.Van Kooyk Y., C-type lectins on dendritic cells: key modulators for the induction of immune responses, Biochem. Soc. Trans. (2008) 36:1478–1481 [DOI] [PubMed] [Google Scholar]
- 37.Varol C., Yona S., Jung S., Origins and tissue-context-dependent fates of blood monocytes, Immunol. Cell Biol. (2009) 87:30–38 [DOI] [PubMed] [Google Scholar]
- 38.Wang Y.S., Chi K.H., Chu R.M., Cytokine profiles of canine monocyte-derived dendritic cells as a function of lipopolysaccharide- or tumor necrosis factor-alpha-induced maturation, Vet. Immunol. Immunopathol. (2007) 118:186–198 [DOI] [PubMed] [Google Scholar]
- 39.Wang Y.S., Chi K.H., Liao K.W., Liu C.C., Cheng C.L., Lin Y.C., et al. , Characterization of canine monocyte-derived dendritic cells with phenotypic and functional differentiation, Can. J. Vet. Res. (2007) 71:165–174 [PMC free article] [PubMed] [Google Scholar]
- 40.Weber M., Lange C., Gunther W., Franz M., Kremmer E., Kolb H.J., Minor histocompatibility antigens on canine hemopoietic progenitor cells, J. Immunol. (2003) 170:5861–5868 [DOI] [PubMed] [Google Scholar]
- 41.Wollenberg A., Mommaas M., Oppel T., Schottdorf E.M., Gunther S., Moderer M., Expression and function of the mannose receptor CD206 on epidermal dendritic cells in inflammatory skin diseases, J. Invest. Dermatol. (2002) 118:327–334 [DOI] [PubMed] [Google Scholar]
- 42.Yao V., Platell C., Hall J.C., Dendritic cells, ANZ J. Surg. (2002) 72:501–506 [DOI] [PubMed] [Google Scholar]
- 43.Yoshida H., Momoi Y., Taga N., Ide K., Yamazoe K., Iwasaki T., Kudo T., Generation of canine dendritic cells from peripheral blood mononuclear cells, J. Vet. Med. Sci. (2003) 65:663–669 [DOI] [PubMed] [Google Scholar]
- 44.Ytterberg S.R., Animal models of myopathy, Curr. Opin. Rheumatol. (1991) 3:934–940 [DOI] [PubMed] [Google Scholar]