Abstract
It can be useful to present a different image to each of the two eyes while they cooperatively view the world. Such dichoptic presentation can occur in investigations of stereoscopic and binocular vision (e.g., strabismus, amblyopia) and vision rehabilitation in clinical and research settings. Various techniques have been used to construct dichoptic displays. The most common and most flexible modern technique uses liquid-crystal (LC) shutters. When used in combination with cathode ray tube (CRT) displays, there is often leakage of light from the image intended for one eye into the view of the other eye. Such interocular crosstalk is 14% even in our state of the art CRT-based dichoptic system. While such crosstalk may have minimal impact on stereo movie or video game experiences, it can defeat clinical and research investigations. We use micromirror digital light processing (DLP™) technology to create a novel dichoptic visual display system with substantially lower interocular crosstalk (0.3%; remaining crosstalk comes from the LC shutters). The DLP system normally uses a color wheel to display color images. Our approach is to disable the color wheel, synchronize the display directly to the computer’s sync signal, allocate each of the three (former) color presentations to one or both eyes, and open and close the LC shutters in synchrony with those color events.
Keywords: dichoptic, stereoscopic, binocular vision, digital light processing, medical device development
Introduction
In studies of visual perception, often it is useful to present a different image to each of the two eyes (dichoptic presentation). Two relatively common uses are investigations of stereoscopic vision (the ability to see depth only through disparities between retinal images1) and binocular visual function (the relative alignment of the eyes and the tolerance to accommodative and prismatic stress: fixation disparity2, 3, 4). Another use has been investigations that probe (using interocular transfer) the visual pathway location at which a certain visual process such as the motion after-effect might occur, at least to distinguish between pre- and postchiasm.5, 6, 7, 8, 9 Dichoptic stimulation is a particularly valuable tool for this when coupled with functional MRI.10 We are interested in the function and alignment of both eyes in patients with bilateral central scotomas (blind regions) that occur in macular degeneration and many other diseases.11 Available systems did not meet our needs, so we developed a system capable of mapping the visual field of each eye separately while both eyes are open. The system is also used to evaluate the use of monocular vision aids, allowing us to determine whether a stimulus is detected via the aided or fellow eye.
Most people with central scotomas that include both the foveas use a location in their residual retina to fixate: the preferred retinal locus.12 Traditionally, the location of the preferred retinal locus is found by measurement of a monocular visual field (i.e., with the other eye covered). For about a third of people with bilateral fovea-loss scotomas, the preferred retinal locus differs between the two eyes, i.e., noncorresponding retinal locations.13, 14 The preferred retinal locus may be characterized directly on an image of the retina, or indirectly using perimetry (visual field measurement). Since most people use both eyes, the preferred retinal locus used for binocular viewing is of functional importance. Currently it is not possible to determine the binocular preferred retinal locus using retinal images. When visual fields are measured binocularly (and all stimuli could be seen by each eye), it is not clear which eye controls fixation and whether or not the preferred retinal locus remains the same. Knowing separately what each eye is seeing when viewing with both eyes provides valuable information. This can be accomplished by dichoptic perimetry, which presents a perimetry stimulus to one eye while a fixation target (and the rest of the test environment) is visible to both eyes.
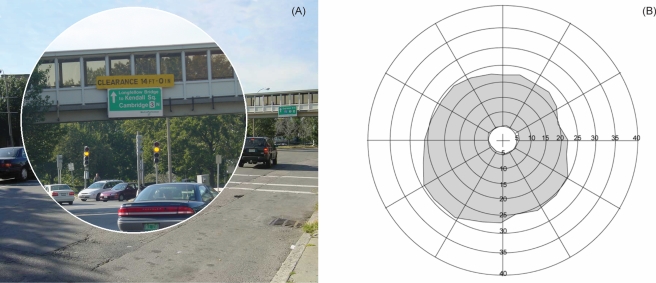
Viewing monocularly through a spectacle-mounted bioptic telescope (of the sort used as a magnification aid for reduced visual acuity) creates a “ring scotoma,” an area of the natural scene that is invisible to that eye due to the area of the retina occupied by the magnified image15, 16 (Fig. 1). Driving with bioptic telescopes is now permitted in 39 states in the United States and in the Netherlands. When using the telescope, the ring scotoma may block important areas of the scene, and thus could be hazardous. There has been controversy over whether or not the fellow eye, that views without the telescope, detects targets that are blocked by the ring scotoma apparent to the telescope-wearing eye. (See Lippman, Corn, and Lewis17 for a review of the issues.)
Figure 1.
Ring scotoma with a bioptic telescope. (a) The enlarged image on the retina through a spectacle-mounted telescope necessarily blocks a portion of the see-through view around the telescope’s field. (b) A visual field plot shows that only the portions of the scene not blocked by the magnified image or actually seen through the telescope are detectable, creating a blind area known as a ring scotoma (gray area in the figure).
Targets in the ring scotoma are generally detected when testing with conventional binocular perimetry,15, 17, 18 but that may be an artifact of the test conditions: high-contrast stimuli and a lack of competing images between the eyes. With binocular perimetry, it is difficult to determine which stimuli are presented within the ring scotoma, as the position of the scotoma is dependent on the alignment of the eye and telescope. With dichoptic perimetry, the stimulus can be presented to just the fellow eye, in the area of the ring scotoma of the telescope eye, while a more-natural background is presented to both eyes. There is no need to patch the fellow eye when determining the location of the scotoma, thus avoiding manipulations that may alter the scotoma location.19
When fitting prisms as an aid to people with hemianopia (loss of vision on the same side in both eyes) by shifting the image presented to one or both eyes,20, 21 it is necessary to understand the effect of the inherent prism scotomas, as minor position shifts can have a significant effect on the utility of the prisms.19 While it is possible to make these determinations without dichoptic presentation, dichoptic perimetry is considerably easier and less error-prone than binocular perimetry, and it eliminates the need for patching (covering) one eye, and the potential perturbations from patching.
The applications described before place a number of significant constraints on a dichoptic perimetry test system. While existing systems meet some of these requirements, no previous system we are aware of meets all of them simultaneously. The most important issue is interocular crosstalk, defined as the degree to which images intended for just one eye are visible to the fellow eye (known as leakage or ghosting). While some interocular crosstalk can be tolerated in some applications, such as stereo displays (where visual masking may hide the crosstalk) and some clinical applications (like the Hess screen for strabismus22), interocular crosstalk can be a critical problem in other applications. In vision-science studies using static stimuli, interocular crosstalk can be a problem requiring control conditions to test that the crosstalk has not influenced results.5, 6, 7, 8, 9 For the applications described before, perimetry is usually performed with moving (kinetic) stimuli of high contrast. Using cathode-ray tube (CRT)-based dichoptic systems, we found that dichoptic stimuli that were not detected when presented in a fixed location (static) were detected easily when kinetic.23
In applications other than perimetry, pair-wise comparisons of images may be made with each image (or stimulus) viewed by just one eye. For example, Peli and Lang24 evaluated the characteristics of a multifocal intraocular lens (IOL) by showing a clear image to the patient’s eye with the IOL and a simulated image, transformed by the calculated optical characteristics of the IOL, to the other eye. The parameters of the simulation were modified to determine the point of subjective equality, where the patient perceived that the images in both eyes appeared minimally different. A component of that study24 was limited by the interocular crosstalk in their CRT-based dichoptic system. The dim but sharp residual image visible to the eye without the multifocal IOL changed the degree of blur perceived. They masked the effect by replacing the sharp image with a bright screen during the interval in which it was to be hidden, but that reduced the resulting contrast and limited the accuracy of the results.
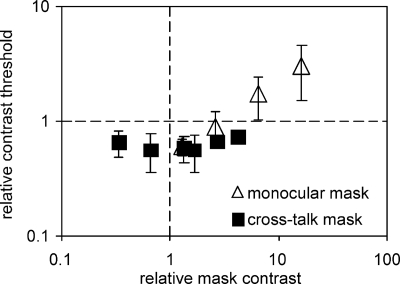
Interocular crosstalk can affect results even when the crosstalk image itself is subthreshold. For instance, the detection threshold for a monocular stimulus changes if the other eye is stimulated also. A stimulus that occupies the same location in the visual field (colocal) and is presented simultaneously to the other eye is known as a dichoptic mask. Of particular interest is Legge’s demonstration that dichoptic and monocular masks can facilitate detection at low mask contrasts and inhibit detection at higher contrasts.25 If there is interocular crosstalk, an object intended for the other (i.e., nontested) eye may act as a mask. If so, there would be difference between the contrast detection threshold measured with and without the presentation of an object to the “closed” eye, and it would depend on the contrast of that object, as is demonstrated in Fig. 11. Thus, care must be taken when conducting control experiments that test the impact of interocular crosstalk on results.
Figure 11.
Threshold facilitation. The effect of interocular crosstalk when using our EIZO CRT-based dichoptic display and FE-1 (ferroelectric LC) shutter goggles. Contrast thresholds were determined with the left eye covered. When a mask was presented to the right (open) eye, there was facilitation of contrast detection for relative mask contrasts less than about three times the contrast threshold with no mask, and inhibition for higher contrast monocular mask conditions (open symbols). When a mask was presented to the closed (left) eye, it should not have been visible to the open (right) eye, and there should have been no effect on contrast thresholds (i.e., filled symbols should all be along the horizontal dashed line). crosstalk caused facilitation of contrast thresholds at all measured closed-eye mask contrasts, including contrasts that could not be detected (i.e., to the left of the vertical dashed line). Stimulus and mask were 1- cycle∕deg, 1 octave Gabor patches. Error bars are 95% confidence limits.
There are some physical constraints imposed by our needs. While perimetry is conducted, the subject often wears corrective lenses (e.g., spectacles). For the evaluation of vision aids, including the bioptic telescopes and prisms described earlier, those aids must be worn during perimetry. Thus, a suitable dichoptic system needs to have its display or display optics sufficiently far from the eyes, to not interfere with corrective lenses or visual aids. Also, when evaluating visual aids, the dichoptic system should allow natural use of the vision aid, allowing the subject to adopt head and eye postures appropriate for the device. Thus, the system should not depend on head restraints such as brow and bite bars. For some studies, we are interested in tracking gaze location during testing, so an ideal system would allow, and not interfere with, head and eye tracking systems.
To avoid unnatural eye alignment (binocular posture) and disparities of binocular and accommodative cues, the system must not introduce a binocular disparity. The apparent distance of the images should be at least a meter from the subject’s eyes,20 and a horizontal field of view of 60 deg or more may be needed.
Dichoptic systems can be broadly categorized as dual or single channel, according to whether separate sources and optical paths are used for each eye, or a single (multiplexed) source is somehow separated into the images for each eye. Interocular crosstalk is a significant issue (addressed with varying degrees of success) in the design of single-channel systems. Inherently, dual-channel systems do not suffer from interocular crosstalk, but they do fail to meet our needs in other ways.
Head-mounted displays (HMDs)
The growing popularity of stereovision applications for consumers means that devices for that market have become considerably less expensive. They would be attractive for research and clinical devices if they worked well enough. However, HMDs may limit the room for study-oriented optics, they may interfere with eye tracking, and they operate at a fixed focal distance, so that the natural relationship between binocular vergence and accommodative focus is not guaranteed.26, 27 Unless very carefully aligned, HMDs induce unnatural ocular posture, which the subject must overcome. This may cause eye strain, discomfort, and in some cases double vision, all of which may affect the experimental result.
Haploscopes, amblyoscopes, and synoptophores
Haploscopes, originally described by Hering,28 are broadly defined but commonly taken to include any dual-channel device used to investigate, diagnose, or treat issues of cooperation between eyes, such as strabismus, amblyopia, and the ability to perceive stereoscopic depth. Purpose-built research devices, often on optical benches,29, 30 tend to be called haploscopes and are too varied to be characterized here. Commercial devices are generally called amblyoscopes or synoptophores. Examples include the hand-held Worth’s amblyoscope31 and the highly-adjustable Clement Clarke synoptophore (Harlowe, Essex, United Kingdom). (Note that the phase difference haploscope,32 although it uses two projectors, is best understood as a single-channel system, in that the projected images are viewed on a single screen.) Haploscopes demand precise alignment of the optical paths and eyes, and thus require that the head be restrained. The optical tubes of synoptophores limit the field of view to about 15 deg, making them suitable for foveal and parafoveal applications, but not investigation of wider visual fields. Unless very carefully adjusted, they induce unnatural binocular posture, and it is difficult to maintain natural accommodation and convergence relationships. In addition, they generally do not allow for study-oriented optics. These devices typically operate with images on slides, and thus do not provide the flexible stimuli needed for perimetry.
Single-channel systems can allow for free head movement with respect to the display, which is important when evaluating visual aids. Single-channel systems can be characterized by the way in which they achieve multiplexing of the images to share the channel, and each type of multiplexing carries advantages and disadvantages. Multiplexing in single-channel systems can be classified as spatial, temporal, spectral, or polarized, depending on the way in which images for a designated eye are passed and images intended for the fellow eye are blocked, and combinations of these techniques can be employed. For spatial multiplexing, the images for each eye appear in spatially distinct areas of the display, and the optics or barriers are expected to convey just the proper images to each eye. Spectral multiplexing distinguishes the images by color (anaglyphic system), and tuned filters are expected to pass or block the images for each eye. Polarized multiplexing uses polarizing filters to distinguish and transmit or block the images. Temporal multiplexing presents the images at distinct instances, and shutters are expected to pass or block the images at the proper times. The type of multiplexing bears directly on sensitivity to head positioning and the potential sources of interocular crosstalk.
Spatial multiplexing
Autostereoscopic displays implement spatial multiplexing typically by dividing the displayed image into thin vertical stripes, with alternating stripes destined for each eye. Cylindrical lenses in front of each stripe, or mechanical barriers (septa) between the stripes, allow each eye to see just the intended half of the stripes, if the head is positioned properly. Positioning is critical, as any head shift can destroy or even reverse the stereo effect. The displays can be quite large, but the sensitivity to head position and interpupillary distance (IPD) limits autostereoscopic displays to applications such as 3-D visualization that can tolerate significant interocular crosstalk.
The Turville infinity balance method is a clinical binocular vision test that uses a septum to block half of the view to each eye.33 In a similar manner, dichoptic presentation for research can be achieved by using a single septum close to the subject to block half of the display for each eye, which also results in only the temporal half of each eye receiving an image.34 While this approach was simple, it is not useful for many other purposes, wherein images need to appear in the same retinal area of each eye. By using base-in prisms with a similar septum, the subject can fuse the images,25 allowing testing of the same retinal area in both eyes. The limitation that the images can be only a half-screen wide remains, and the high-power prisms needed have their own side effects.
Spectral multiplexing
Anaglyphic systems display one color, typically red, for one eye and another color, typically green or blue, for the other. Corresponding filters worn over the eyes provide the needed blocking and image separation. Aside from the obvious disadvantage that these systems cannot represent full-color images (a shortcoming shared by the system we developed), they also suffer in that each eye sees a colored image, and the color is different for each eye (which, as explained later, is not the case for our system).
In spectrally multiplexed systems, the light (and hence information) available to each eye depends on the combination of spectral transmission of the filter over that eye and the spectral luminance of the display. If there is an overlap of the spectral transmission of the filter of that eye with the spectral luminance of the information intended for the other eye, interocular crosstalk will occur. To avoid interocular crosstalk, typically the source for each eye must have sufficiently narrow bandwidth (such as a laser or LED), or each filter must have sufficiently narrow bandwidth. Sufficiently eliminating interocular crosstalk has historically been a problem for spectrally multiplexed systems, due to the imperfect nature of the interposed color filters as well as the spectral width and overlap of some illumination sources, such as CRT phosphors. As a result, their use has typically been restricted to applications such as binocular visual training33, 35, 36 that can tolerate the interocular crosstalk and put a premium on relatively inexpensive implementations.
Polarized multiplexing
Typically, a pair of cross-polarized filters is used at the display source, one for each of the two image streams. Each of a corresponding pair of filters, one per eye, passes the images intended for that eye and block images for the fellow eye. In its simplest configuration, two projectors with cross-polarized filters could project images simultaneously on a screen, and filters with the corresponding polarization would be placed in front of each eye. Circular (clockwise and anticlockwise) polarization is preferable, as linear polarization (e.g., vertical and horizontal) is sensitive to head orientation.37 A liquid-crystal display (LCD) with alternatingly polarized stripes would be an example of combined spatial and polarizing multiplexing, while a single display with a switchable polarizer that alternates orientation between frames (mechanically or electronically) would combine temporal and polarized multiplexing. Polarized multiplexing depends on the quality of the filters to avoid interocular crosstalk and reflectance properties of the screen if projected. As it is relatively inexpensive, polarized multiplexing is used in ophthalmic clinical settings for binocular vision assessment33, 37, 38, 39 and for stereo entertainment, but it has not found wide applicability in research settings due to interocular crosstalk.
Temporal multiplexing
Temporal multiplexing is commonly accomplished by presenting alternating video frames for each eye and using synchronized shutters to block frames intended for the fellow eye. The video can be shown directly via CRT or LCD, or via a projector. To avoid flicker, video is typically presented at a 120-Hz frame rate, yielding 60 images per eye per second.
Shutters can be mechanical (usually rotating) or electronic. Mechanical shutters are not conducive to headgear and free head movement, so electronic shutters in goggles are generally used when freedom of motion is a requirement. Electronic shutters generally use liquid crystals (LC) that can be switched from high to low transmittance at a rapid rate. The primary concern for LC shutters is interocular crosstalk, due either to slow switching times or to an insufficiently low extinction ratio (“OFF” transmittance divided by “ON” transmittance). High-quality ferroelectric LC shutter goggles, such as the Cambridge Research Systems (Rochester, Kent, United Kingdom) FE-1 shutter goggles that we use, are fast enough, with switching times on the order of 10 μs. Their extinction ratio of ∼0.25% is suitable for some, but not all, of our purposes, as discussed later. No better shutter technology was available to us.
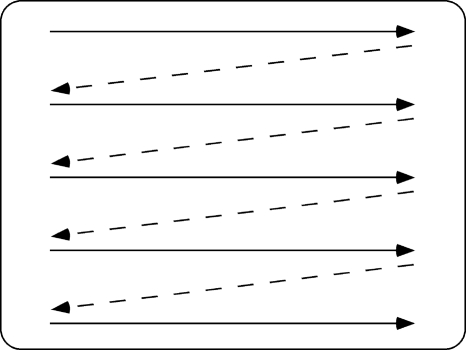
Temporally multiplexed CRTs have found common use in psychophysical studies that require dichoptic presentation. However, phosphor decay time can result in considerable interocular crosstalk.24, 40 A CRT image is drawn sequentially, but shutters expose and block essentially the entire display at once. The lines at the bottom of the screen are drawn almost instantaneously before the lines for the next frame begin to draw at the top of the screen (Fig. 2). Each line of the display must reach its intended luminance, hold it for a sufficient time, and decay to near zero before the shutters open for the alternate frame. This means that the last line drawn must disappear in the fraction of time represented by the vertical blanking interval. Since this interval is typically less than 10% of the frame time, for a 120-Hz display it represents less than 0.83 ms. Even “fast” phosphors, typically with decay times of ∼2.5–8 ms, cannot meet this requirement. With our fast EIZO (Ishikawa, Japan) monitor, we have measured about 14% ghosting at the center of the monitor (Fig. 3). If measured at the bottom of the raster, this would have been still worse.
Figure 2.
Schematic diagram of a raster scan. The electron beam scans later pixels in the lower lines not long before the next frame is initiated. Hence a “late” pixel, even with a fast phosphor, may not have decayed completely before the next frame sync (vsync signal).
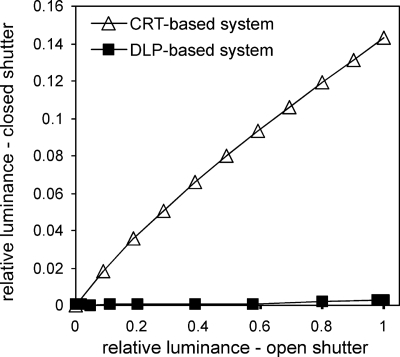
Figure 3.
Luminance measured (at display center) through the closed FE-1 (ferroelectric LC) shutter increased as luminance was increased. Interocular crosstalk, the slope of these functions, was substantially less for our DLP-based dichoptic visual test system (<0.3%, black squares) than the EIZO CRT-based dichoptic system (14%, open diamonds). The luminance data were normalized to a maximum value of one for a digital video input value of (255, 255, 255) for each system.
LCD-based approaches, popular in relatively inexpensive dual-channel systems, have not proven suitable in single-channel systems. They do not suffer the constraints of sequential illumination that limit CRTs, but their slow switching times result in considerable interocular crosstalk, even for the fastest models commonly available, currently advertising switching times as low as 2 ms.
Projector-based systems vary according to the technologies used to modulate the light beam, and whether color is produced sequentially or on parallel color channels. CRT- and LCD-based projectors suffer the same interocular crosstalk problems as their direct-display counterparts. Liquid crystal on silicon (LCOS) chips used in some high-end projectors are potentially fast enough, but we have found none with appropriate properties for our application. Systems using digital light processing (DLP™) chips have the potential to avoid interocular crosstalk, and that is the technology used in our system. (An explanation of DLP technology, with its millions of fast-switching, hinged microscopic mirrors on a chip, each corresponding to a single pixel, is available at http://www.dlp.com/tech/what.aspx.)
While DLP-based displays are not susceptible to the sustained stimulation problem of CRT- and LCD-based displays, framing errors are a significant concern. Framing error refers to the presentation of an image to an eye when it is supposed to be blocked; that is, an imperfect synchronization of shutter and display. Framing errors can be introduced several ways. Most obviously, if the computer provides video at a rate different from that used by the display, the goggle synchronization signals from the computer’s video card will bear no useful relationship to the timing of the images. Even when the computer is set to the same frame rate used by the display, there can be variability in the phasing of the frames. This is often the case for displays that use color wheels to separate a single image source into sequential color (red, green, and blue) subframes. Those projectors typically use the color wheel position for synchronization, and that mechanical system can vary slightly in phasing with respect to the computer’s signal. More insidiously, the processor in the display itself may blend the information from successive frames in complex ways to improve brightness or the motion compensation involved when deinterlacing is needed.
The monochromatic (grayscale) dichoptic solution we report here is DLP-based. As described in the Methods section (Sec. 2), much of our solution involved turning the challenges associated with a color-wheel-based projector into an advantage. The Results section (Sec. 3) characterizes the dichoptic system, based on direct measurement and illustrative applications. In the Discussion section (Sec. 4), we identify some residual problems, as well as new opportunities made possible by technological advances that were unavailable to us when we implemented this solution.
Methods
Our monochromic dichoptic video display system incorporates a modified Davis Powerbeam VI DLP color projector (Davis A∕S, Drammen, Norway), FE-1 shutter goggles, and shutter control electronics that we developed. The Powerbeam is a single optical channel projector that uses a color wheel to filter color sequentially within a video frame.
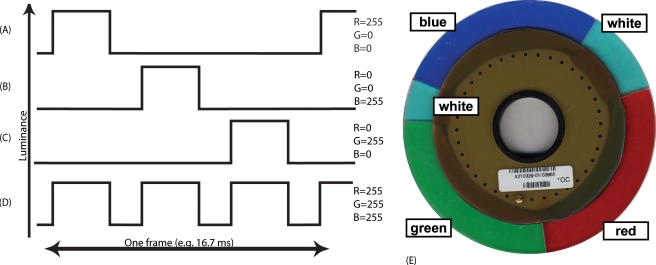
Color-wheel-based projectors buffer a frame of video information so that they can present the red, blue, and green channels of the image sequentially. The color wheel has red, green, and blue sectors. As the wheel turns, the red, green, or blue channel data are used to activate the micromirrors needed to display pixels with a color component of the color wheel sector currently in the projector beam. Figures 4a, 4b, 4c, 4d shows the temporal-luminance sequence of the simplest color-wheel-based display. In practice, many systems also include one or more white sectors, and show data from all three channels when a white sector is current, thus providing a brighter overall display. Figure 4e shows a color wheel (from the Davis Powerbeam VI projector) with two white sectors. More recent DLP displays often have more than one sector for each color in the color wheel. In DLP-based systems, light intensity modulation is accomplished by rapidly switching the mirrors between the on and off positions, with an “on” time proportional to intensity. In the on position, a mirror directs the light source to the mirror’s corresponding pixel position on the screen. In the off position, that portion of the beam is deflected to a light trap. The longer a mirror is in the on position, the brighter its pixel will be on the screen.
Figure 4.
Temporal-luminance sequence during one frame of a single-chip DLP display. (a) to (d) are schematic illustrations of the luminance for (a) red only, (b) blue only, (c) green only, and (d) white. (e) Color wheel from a Davis Powerbeam VI. The temporal windows of each color are used in combination (temporal multiplexed) to provide the gamut of colors. As shown in Fig. 5, the luminance profile of a Davis Powerbeam VI is more complicated, with variations in luminance within each color window, and it includes two short white windows.
By removing the color wheel from the projector (or the light path), we are able to use the red, green, and blue data channels to temporally multiplex grayscale information. The projector continues to use the color information sequentially to modulate the beam intensity during the intervals that the color wheel sectors would be in the beam, but no color filtering occurs, resulting in a grayscale-only display. The shutter control electronics we developed synchronize the shutter goggles with the projector, though the color wheel is not longer in use, opening the shutter for one eye during the red interval and the shutter for the other eye during the blue interval. Thus, information coded in red in the video frame and the information coded in blue are directed to the eyes separately, thereby achieving grayscale dichoptic presentation.
Since the red, blue, and green video color channels are used to designate eye destination, not color, we refer to the channels as R, G, and B to maintain their correspondence with color channels in the computer data while avoiding using confusing color designations for data that are, in fact, presented in grayscale.
We selected the Powerbeam VI projector because it includes a knob that removes the color wheel from the beam path and was amenable to our modifications. In particular, we were able to intercept the color wheel sync pulse in the projector and replace it with frame sync information from the input video, to provide the “color wheel” synchronization to the projector. The shutter control electronics implement switch-settable delays relative to the video sync pulse to open and close the shutters at the times that the projector electronics would be sending the corresponding channel information to its DLP chip, as described next. The resulting effect is that information coded in red in the source video is directed via the R channel to one eye (in grayscale), while information coded in blue is directed via the B channel to the other eye. It is also possible to direct information encoded in green on the G channel to both eyes, which could be convenient for applications needing to provide binocular fusion cues. It would also be possible to provide settings that showed the G channel to just one eye for increased brightness, but we have not done that, nor do we open the shutters during the times corresponding to the white portions of the color wheel.
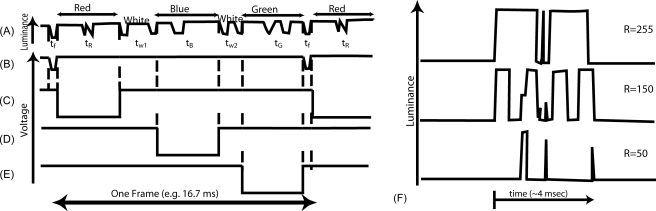
In actuality, the relationship between input channel level and mirror activity in a DLP projector can be quite complex (and proprietary). The microscopic mirrors have switching times of about 2 μs and are switched on and off for periods of about 15 μs. The projector’s processor uses various algorithms to improve the projected image. Figure 5 illustrates the relationship between channel input and projected output of the Powerbeam VI when displaying fully white frames (R=255, G=255, and B=255) with its color wheel removed. Figure 5b shows the two consecutive sync pulses that bound one frame of video. Figure 5a shows the measured beam luminance during that interval. Figures 5c, 5d, 5e show the periods that a goggle shutter should be open to display the R, G, and B channels, respectively. When displaying gray, many more interruptions of the beam occur, as illustrated for the R segment in Fig. 5f.41
Figure 5.
Temporal sequence of (a) the luminance output of the Davis Powerbeam VI DLP display showing bright white; (b) the frame sync; and the shutter controller (c) “red” circuit; (d) “blue” circuit; and (e) “green” circuit. The three “color” circuits control the shutters. Negative voltage opens the shutter (high light transmission) for the eye specified for that circuit. For example, the right eye might be driven by the “red” and “green” circuits, therefore opening twice per frame. Meanwhile the “blue” and “green” circuits might drive the left eye. “Green” elements of the image could be seen binocularly (e.g., fusion lock). The temporal-luminance sequence of the Powerbeam VI display is more complex than the schematic in Fig. 4, and actually even more complex at other luminances, as shown in (f) for various values of just the red channel. This is a function of the binary pulse-width modulation technique used to create gray levels41 and the addition of “white” to enhance perceived brightness and contrast. Measurements were made with the color wheel removed, so the appearance was always grayscale. The time of each segment of the frame sequence was measured empirically and used to establish the shutter control switch settings.
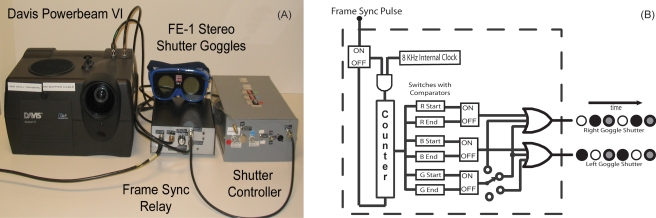
Figure 6a is a photograph of the components of our monochromatic dichoptic vision test system, including the projector, FE-1 shutter goggles, frame sync relay, and shutter controller. The frame sync relay allows us to choose whether the computer, a signal generator, or the position of the projector’s color wheel is used as the sync source sent to the projector’s electronics. The signal generator option is provided for debugging and maintenance operations, and the projector source is included so that the projector can be used in its standard mode for other applications. The shutter controller generates the control signals for the left and right goggle shutters, which it derives based on timing relative to the sync pulse.
Figure 6.
Our DLP-based dichoptic visual test system. (a) The frame sync relay allows us to switch between the color wheel, the computer, or a 60-Hz signal generator. (b) Conceptual diagram of the shutter controller. Delay after the onset of the frame sync pulse is used to time the shutter events (i.e., open and close) in each frame (Fig. 5). The rows of circles on the right represent two cycles (frames) of shutter events. Each cycle has three periods that are established by the on periods of three flip-flops, which, in turn, are set and reset at the times the counter reaches the values set in the switches of their associated comparators. First the “red” flip-flop opens the right goggle lens (open circle) while the left goggle lens remains closed (filled), then the “blue” flip-flop opens the left goggle lens while the right goggle lens is closed. The gray circles in the shutter event cycle (third and sixth events as shown) represent the state of the “green” counter, if connected. The four-way switch allows monocular or binocular or no shutter opening. At the switch location shown, the gray circles would represent both goggle lenses being open (a binocular view), although we have not used that setting in our applications.
Figure 6b is a conceptual diagram of the shutter controller we built. An 8-KHz clock is gated by the incoming 60-Hz sync pulse and counted by a 7-bit counter. This provides 1∕8-ms resolution over a 0- to 16-ms range. Six sets of dual inline package (DIP) switches and comparators select the start and end counts for R, B, and G intervals that lie within the R, B, and G periods, respectively, of the projector’s 16.7-ms frame cycle. A switch selects whether the “on” time signal of the G channel is merged with the “on” time of the R and B channels to produce the left and right goggle shutter control signals. After 16 ms, the clock is gated off to await the next sync pulse. (In actuality, due to the way the circuitry evolved, we have separate R, B, and G counters, each of which is stopped at the end of its on cycle.) Four-bit DIP switches provide selection variability over a range of 0 to 2 ms relative to hardwired starting counts for each switch bank. If we were to do it again, we would use a microprocessor-based controller rather than counters, comparators, and switches.
We used a high-speed silicon photodiode (model PDA55, DC-10MHz, Thorlabs, Newton, New Jersey) to detect the light output of the projector as one color (R, G, or B) was sent, and determined, via a two-channel oscilloscope, the timing of each color window relative to the frame sync pulse. We then set the shutter controller switches so that the left and right goggle shutters would open shortly after the start of their corresponding color interval, and close shortly before the end of the interval. The settings were verified by viewing the signal to the goggles with one trace of the oscilloscope and the projector output with the other, while just a single “color” was displayed,
A custom software application allows an operator to select a fixation target, a stimulus, and a background. Each can be selected to be shown to the right eye, left eye, or both, and the intensity level of each can be selected on a 0 to 255 scale. The program generates the appropriate image components and colors them the corresponding level of red (R), blue (B), or purple (R+B) for left, right, or both-eye presentation, respectively. The monochrome dichoptic DLP-based projection system just described then directs the images to the correct eyes. The operator console displays a polar visual field plot diagram, and by moving the mouse cursor the operator controls the position of the stimulus on the rear projection screen viewed by the subject. The mouse buttons control whether the stimulus is on or off, and the plotting of points as seen or unseen as the subject’s visual fields are probed. Thus we have a monochromatic dichoptic vision test system, with interocular crosstalk from framing errors eliminated by ensuring that the counter settings of the shutter controller correspond to on times that are in sync with the behavior of the projector electronics. The Results section (Sec. 3) characterizes the performance of this system.
Results
Interocular Crosstalk
Using a Minolta LS-100 luminance meter (Minolta Camera Company, Limited, Japan), we measured the ratio of light transmission through an FE-1 shutter in steady off and on states for a range of digital input values to our DLP-based system and our EIZO CRT-based system (same shutters for each). In an ideal system there should be no measurable luminance through the closed shutter. When the luminance through the closed shutter is plotted against the luminance through the open shutter, the slope of the function is the interocular crosstalk. The interocular crosstalk for the DLP-based system was less than 0.3%, while it was 14% for the CRT-based system [Fig. 3a].
Framing Error and Gamma Function
The procedure for setting the shutter controller switches, as described before, ensured that there could be no framing errors. This was tested using the high-speed Thorlabs photodiode, and no conditions were found that produced a framing error. While our approach ensured that light intended for the eye was visible, that was achieved by trimming the color event at each end. Thus, it is possible that the gamma function would be affected. For example, if the duration of mirror-on events increased in a simple monotonic manner as with increasing digital input value, trimming early and late signal could produce a nonmonotonic gamma function (e.g., Fig. 5). The Minolta luminance meter was used to measure luminance through the open FE-1 shutter separately for each R, B, and G interval. The resulting gamma functions did not show nonmonotonic behavior (within measurement noise).
Temporal Modulation Transfer Function
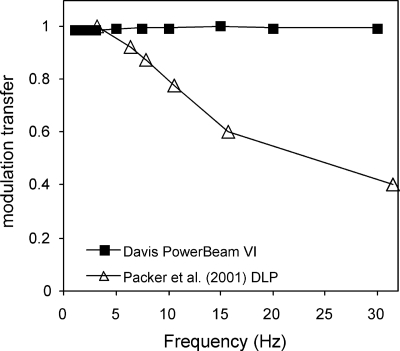
The temporal modulation transfer function (MTF) plots the relative luminance against stimulus temporal frequency. Curiously, Packer et al.42 reported a roll-off with frequency of the temporal MTF in their three-DLP-based stimulator, but did not identify the cause. Therefore, we checked the temporal MTF of our system using the Thorlabs photodiode to measure the luminance output for sinusoidal variations (1 to 30 Hz) in digital input value. So that the digital input values would range from 0 to 255, the tested temporal frequencies were integral multiples of the frame rate, and the short test sequences were initiated at a frame sync. The temporal MTF of our system showed no loss up to the maximum possible 30 Hz (Fig. 7).
Figure 7.
Temporal modulation transfer function of the single-chip DLP used in our dichoptic system (Davis PowerBeam VI: solid squares) showed no loss up to the maximum possible 30 Hz, unlike the three-chip DLP (open triangles) reported by Packer et al.42
Actual Use for Visual Field Evaluation
Our main motivation in developing this dichoptic vision test system was to be able to measure monocular visual fields (perimetry) when both eyes are open and viewing naturally. This is of particular interest in the evaluation visual field loss and the impact of visual aids for rehabilitation of vision impairments. With conventional perimetry and binocular viewing, it is not possible to locate the physiological blind spot where the optic nerve exits the retina, as the other eye can detect stimuli in that location. Our DLP-based dichoptic vision test system measured monocular visual fields during binocular viewing so the physiological blind spots of a normally sighted subject were located [Fig. 8a]. This is of particular value for patients with central scotomas, as it was possible to distinguish regions of monocular (hatched fill) and binocular (cross-hatched fill) regions of visual field loss, and to investigate changes in the fixation pattern as the fixating eye was manipulated [Fig. 8b]. Our inability to perform dichoptic perimetry with our CRT-based system for such evaluations was the initial impetus for development of the DLP-based system.
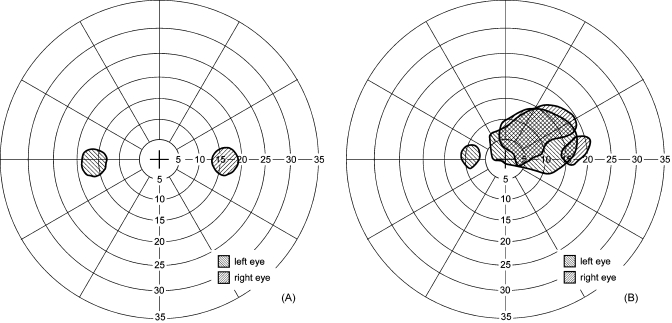
Figure 8.
Dichoptic visual fields. Monocular, right (right-tilted stripes) and left (left-tilted stripes) scotomas found in the visual fields when measured with our dichoptic visual test system while viewing binocularly for (a) a normally sighted subject, and (b) a patient with bilateral central scotomas. For the normally sighted subject, both physiological blind spots (optic nerve heads) are measured separately while the observer was fixating on a binocularly visible target and was seeing the physical screen binocularly. The patient used the same preferred retinal locus (PRL) for fixation here as when viewing monocularly with the right eye (i.e., not the left PRL). Note how the left eye central scotoma includes the fixation target. These visual fields illustrate the ability to measure each eye separately under binocular conditions.
In another usage, we used dichoptic perimetry to determine if a stimulus falling within the ring scotoma of a (monocular) bioptic telescope would be detected by the fellow (nontelescope) eye. The ability to detect stimuli within the ring scotoma has been controversial but not tested,17 and used as an argument against bioptic telescopes as a safe visual aid for driving.43, 44 With our dichoptic system, we were able to measure the ring scotoma of the telescope eye while simultaneously probing the nontelescope eye within that ring scotoma (Fig. 9).15 Subjects detected most of those stimuli presented to the fellow eye, indicating that the information was not suppressed.
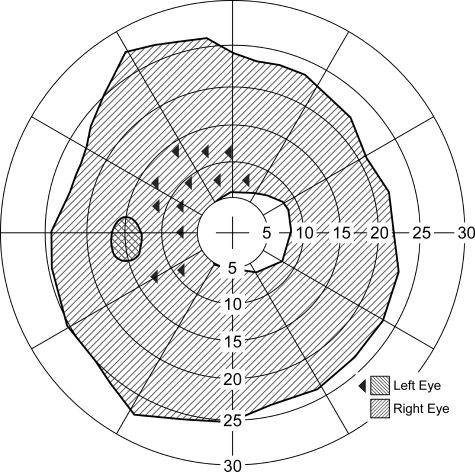
Figure 9.
Ring scotoma probe. Dichoptic visual field plot of a normally sighted subject fixating through a 3× bioptic telescope mounted on the right spectacle lens while the left eye was open. The clear central area represents the visual field visible to the right eye through the telescope, and the large hatched area is the ring scotoma caused by the 3× magnification of the telescope. The small cross-hatched area is not seen by either eye, as it is in the physiological blind spot of the left eye. The boundaries of those scotomas were found using kinetic stimuli. The left-pointing triangles represent locations at which static stimuli were presented only to the left eye. All of these static stimuli, presented within the ring scotoma of the right eye, were detected by the left eye. Under these conditions, vision in the left (nontelescope) eye was not suppressed, and therefore, objects appearing within the ring scotoma would be detected when viewing binocularly. The exception is the area of overlap of the ring scotoma with the blind spot, which is a (small) binocular scotoma.
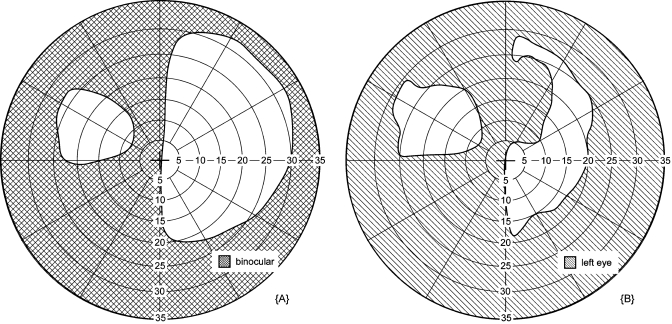
Figure 10 shows the effect of the apical scotoma of a prism fitted to provide visual field expansion for people with hemianopia. This prism was of a higher power than has been fitted in the past, and caused a gap between the patient’s visual field (to the right of the vertical midline) and the expansion region formed by the prism that is worn over one eye (here the left).19 This gap is not found with lower power prisms (e.g., 40 prism diopters).20, 21 The expansion region is expected to be less effective when there is a gap. Dichoptic visual field measurement identified that the right edge of the expansion region was being limited by the right edge of the prism rather than the visual field of the left eye [Fig. 10b]. With this knowledge, better fitting of the prism was achieved by relocating the prism on the left spectacle lens.
Figure 10.
Prism scotoma fitting. A peripheral prism for hemianopia creates visual field expansion (patient without the peripheral prism would only see to the right of the vertical midline). (a) Binocular visual field with an upper peripheral prism worn over the left eye. When probed binocularly, the fellow eye compensates (on the seeing side, here the right side) for the prism’s apical scotoma, so there is no reduction in the right portion of the visual field. (b) Monocular visual field of the left eye when stimuli are presented dichoptically so they were seen only by the prism eye: the effect of the apical scotoma is evident. A plot of this nature helps identify proper fitting of the peripheral prism. In this case, the peripheral prism could be moved to the right to reduce or eliminate the gap between the normal and expanded visual fields in binocular viewing. The fixation mark and background were presented binocularly in both plots. The difference in the extent of the visual field on the right side [ending at 30 deg in (a) and 20 deg in (b)] is due to the greater limitation from the frame of the goggles on the left eye than the right eye (available in the binocular test).
Discussion
We developed and characterized a grayscale single-channel dichoptic digital video display system capable of showing common or different images to each eye. The system largely eliminates interocular crosstalk—the visibility of the images intended for one eye in the fellow eye. Extremely low interocular crosstalk is a requirement for applications of interest to us, including measuring the visual fields to locate the preferred retinal locus used when viewing binocularly, and the determination of which eye detects targets at various locations when a monocular bioptic telescope is being used. The system allows for free head movements, does not adversely prevent the use of optical aids and tracking devices, provides a wide field of view, and preserves the normal relationship between accommodation and convergence.45
The system’s reduction of crosstalk was limited by the imperfect extinction ratio of even the high-quality ferroelectric LC shutter goggles used. Sometimes it was possible to detect the imperfectly extinguished moving stimuli used in kinetic perimetry, and thus possibly compromised the results. Interocular crosstalk of a white stimulus against a black background could readily be detected by some observers, although interocular crosstalk against a gray background was not (but may nonetheless be a source of subthreshold interocular facilitation, as shown in the Appendix).
Interocular crosstalk through ferroelectric LC shutter goggles is also somewhat angle dependent, and this can be a concern in applications that require both a wide field of view and fixed head position. Our applications, however, do not have those requirements simultaneously, so off-axis viewing is not of particular concern to us. Viewers of early DLP-based projection systems like the Davis PowerBeam often reported picture breakup (“rainbow artifacts”) associated with saccadic eye movements.46 This is most noticeable as flashes of rainbow-like color when a color wheel is used to sequentially filter white light into its component primary colors, but has also been reported in monochrome systems, although it is much less distracting there.26, 47, 48 In many applications, including those of primary interest to us, the subject is told to maintain fixation on a stationary target, and trials in which fixation is lost (i.e., an eye movement is made) are to be rejected, so picture breakup is not an issue.
Due to the reports of picture breakup, most manufacturers now produce so-called two-times and four-times DLP displays. Such devices display each frame two or four times. Effectively, these DLP displays are running at 120 and 240 Hz, respectively, with colors changing 360 and 720 times per second, respectively. While four-times displays are too fast for current LC shutter goggles, the color event timings of a two-times DLP display that we examined appeared amenable to our modifications with the ferroelectric LC shutters. It had a simpler (cleaner) color event sequence than the Powerbeam VI (close to the idealized color sequence shown in Fig. 4), and we could remove the colors simply by disabling the motor and positioning the color wheel to be always in white. (There were three color segments: red, blue, and green, and one white segment.)
The conversion of computer display rate to DLP display rate can lead to an asynchrony due to deletion or insertion of frames. Using the frame sync from the computer provides a good solution, since then the video signal rate and the frame rate to the DLP are the same. Despite this, as noted by Brainard, Pelli, and Robson,46 with our DLP-based system we determined that there is still one frame delay between video input and image display due to video processing. That asynchrony was stable under a variety of conditions. For investigators who have an interest in precise control of display timing, this may be advantageous (even if dichoptic display is not of interest).
To improve on the system further by reducing the current 0.3% interocular crosstalk, we have considered the possibility of using polymer dispersed liquid crystals.49 They block the image transmission by diffusing rather than absorbing the light, and given the amount of light scatter in the “closed” state, should be able to more effectively mask interocular crosstalk. Currently available filters (Translucent Technologies, Toronto, Ontario, Canada) do not yet have the fast switching times (about 0.5 ms) required for our application, but could be made.50
In the years that have elapsed since we first implemented our system, DLP projector technology has improved. The 120-Hz video frame rate of the InFocus DepthQ projector from Lightspeed Design Group (Bellevue, Washington, see http://www.depthq.com) has recently enabled us to produce a full-color dichoptic vision test system. When we purchased it, the DepthQ projector was the only model we found that guaranteed synchronization of the color wheel with the input frames. Even when sync is maintained, the projector control electronics can intentionally blend data across frames to enhance the image. Only the “Film” preset mode of the DepthQ projector avoids that sort of blending, and it does it at a considerable loss in brightness. A similar conclusion was recently reached in excellent survey articles.51, 52, 53, 54, 55, 56
In Film mode, the DepthQ projector maintains displayed frame synchrony with the input video, with a constant one-frame delay between input and presentation (like our system). When a suitable video card is used in OpenGL stereo mode, a square wave is produced that alternates between 1 and 0 levels in synchrony with alternate video frames, and is used to control shutter goggles. Thus odd-numbered video frames can be directed to one eye, and even frames to the other. The resulting system implements frame-sequential temporal multiplexing. Since the DepthQ system still depends on LC shutter goggles, it is still limited by their interocular crosstalk.
In his acceptance speech for the 2008 Bressler Award,57 Horton described an anaglyphic system he was using to evaluate strabismic suppression. As with our systems, he used a DLP projector, but used red and blue filters to isolate the images intended for each eye. He reported interocular crosstalk of less than 0.01%. That was probably achieved by using high-Q interference filters of the type developed by Infitec (Infitec GmbH, Ulm, Germany). The extremely narrowband interference filters currently available from Infitec make it possible to create a dichoptic system using spectral multiplexing,58 potentially combined with temporal multiplexing. The filters are narrow enough that two sets of three color filters, each containing filters that have small spectral offsets relative to the corresponding filter in the other set, can be employed with little interocular crosstalk. The filters of each set act as the three standard trichromatic primaries (red, green, and blue) for additive color mixing. A display system that can produce two images with sets of primaries that match the spectral transmissions of the filter sets is required. A dichoptic image encoded with the colors of one filter set can be viewed through the narrow filters with one eye, while the other eye views approximately metameric images created with the colors of the other filter set. An anaglyphic system of that sort can thus produce displays perceived as full color.58 Barco (Kortrijk, Belgium, see http://www.barco.com) currently offers three-chip DLP projectors that employ temporal multiplexing to display alternate frames with the alternating pairs of trichromatic primaries, at up to a 110-Hz frame rate. They also offer two-projector configurations with one set of trichromatic primaries per projector, and thus do not need temporal multiplexing. Dolby 3D Digital Cinema59 is just such a projection system for commercial theaters. These systems are not affected by viewing angle, nor do they require special screens. They are, however, still very expensive, with entry systems currently costing more than $100,000.60
Conclusions
The grayscale dichoptic video display system we developed is proven effective in minimizing interocular crosstalk, making it useful for a flexible array of static perimetry applications and some kinetic perimetry. The system permits more-natural viewing than is possible with conventional haploscopes of the type generally used in clinical and laboratory applications. We continue to seek technologies with sufficient extinction ratios and switching speeds for the most demanding kinetic perimetry tasks.
Acknowledgments
Supported in part by NIH grants EY12890 and EY07957. Frank Rogers fabricated the electronic circuitry, Robert Giorgi established and verified the timing settings, Amy Doherty collected the data presented in Fig. 9 and Nicole Ross collected the data presented in Fig. 10.
Appendix: Significance of Subthreshold Interocular Crosstalk
Interocular crosstalk, even that which in itself is undetectable by the subject (subthreshold), can alter the outcome of some experiments. We demonstrated this in an experiment measuring contrast sensitivity. Data were collected using a high-quality CRT-based dichoptic system, the Nanao™ EIZO® FlexScan FX-E7 color monitor (120-Hz progressive-scan frame rate, 1200×600 pixels, 40×23.4 cm) driven by a VisionWorks™ system (Vision Research Graphics, Durham, New Hampshire),61 which could switch the FE-1 shutter goggles in synchrony with the frame alternations.
Contrast detection threshold (and its inverse, contrast sensitivity) is important for the detection and diagnosis of eye conditions62, 63 and is of frequent interest in vision science.64 Classically, it is tested by presenting a sinusoidal grating at a variety of contrasts to determine the contrast at which the subject can just barely detect the patch (i.e., at the contrast threshold). In a variation of the experiment, there are two gratings: one, the “mask,” is presented alone in one interval, and the other is a combination of the mask and a test grating (the test and mask do not have to be in perfect phase or orientation correspondence). For a given mask level, the test contrast is varied to determine the smallest contrast difference detectable in the presence of the mask.25 The open triangles in Fig. 11 show a typical masking curve derived that way (“monocular mask,” in that the mask and test grating are presented to the same eye), using our CRT-based system. The horizontal axis represents relative mask contrast, and the vertical axis represents relative test contrast, both normalized to the contrast detection threshold obtained with no mask. As can be seen in Fig. 11, a mask affects the stimulus detection threshold, lowering the threshold at low mask levels and then raising it at higher mask levels. The effect is the same whether the mask plus stimulus pairing is presented by summing the two, or by rapidly alternating between them (above the flicker fusion rate).
If instead of combining the mask with the stimulus in the conventional way, the mask is presented in a dichoptic system during the off interval for the seeing eye (and the fellow eye patched), the mask should have no effect, and the relative contrast threshold function shown with the filled squares in Fig. 11 would be flat. If there was interocular crosstalk, however, the mask would have an effect, and the familiar contrast sensitivity “dipper” curve would result. That is in fact what we measured with our CRT-based display and the FE-1 shutter goggles, as is shown by the solid symbols in Fig. 11. Most significantly, interocular crosstalk that in itself was undetectable (filled symbols left of the vertical dashed line) nonetheless affected contrast sensitivity. This underscores the need to understand the way small amounts of interocular crosstalk can affect experimental results.
References
- Howard I. P. and Rogers B. J., Binocular Vision and Stereopsis, Oxford University Press, Oxford, UK: (1995). [Google Scholar]
- Sheedy J. E., “Actual measurement of fixation disparity and its use in diagnosis and treatment,” J. Am. Optom. Assoc. 51(12), 1079–1084 (1980). [PubMed] [Google Scholar]
- Sheedy J. E. and Saladin J. J., “Validity of diagnostic criteria and case analysis in binocular vision disorders,” in Vergence Eye Movements: Basic and Clinical Aspects, Schor C. M. and Ciuffreda K. J., Eds., Butterworths, Boston: (1983). [Google Scholar]
- Waltuck M., McKnight R., and Peli E., “Visual function tester with binocular vision testing,” US patent no. 5,026,151, Mentor O & O, Norwell, MA (1997).
- Levi D. M., Harwerth R. S., and Smith E. L., “Binocular interactions in normal and anomalous binocular vision,” Doc. Ophthalmol. 49(2), 303–324 (1980). 10.1007/BF01886623 [DOI] [PubMed] [Google Scholar]
- Schieting S. and Spillmann L., “Flicker adaptation in the peripheral retina,” Vision Res. 27(2), 277–284 (1987). 10.1016/0042-6989(87)90190-8 [DOI] [PubMed] [Google Scholar]
- Smith A. T., “Interocular transfer of colour-contingent threshold elevation,” Vision Res. 23(7), 729–734 (1983). 10.1016/0042-6989(83)90215-8 [DOI] [PubMed] [Google Scholar]
- Chaudhuri A., “Modulation of the motion aftereffect by selective attention,” Nature (London) 344, 60–62 (1990). 10.1038/344060a0 [DOI] [PubMed] [Google Scholar]
- Wade N. J., Swanston M. T., and de Weert C. M. M., “On interocular transfer of motion aftereffects,” Perception 22(11), 1365–1380 (1993). 10.1068/p221365 [DOI] [PubMed] [Google Scholar]
- Choubey B., Jurcoane A., Muckli L., and Sireteanu R., “Methods for dichoptic stimulus presentation in functional magnetic resonance imaging—a review,” Open Neuroimag. J. 3, 17–25 (2009). 10.2174/1874440000903010017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson C., Low Vision: Principles and Practice, Butterworth, Oxford: (1998). [Google Scholar]
- Timberlake G. T., Mainster M. A., Peli E., Augliere R. A., Essock E. A., and Arend L. E., “Reading with a macular scotoma I. Retinal location of scotoma and fixation area,” Invest. Ophthalmol. Visual Sci. 27(7), 1137–1147 (1986). [PubMed] [Google Scholar]
- Labianca A. T. and Peli E., “Monocular and binocular PRL are inconsistent,” in Proc. Intl. Conf. Low Vision, Vision ’96 1, 381–387 (1997).
- Kabanarou S. A., Crossland M. D., Bellmann C., Rees A., Culham L. E., and Rubin G. S., “Gaze changes with binocular versus monocular viewing in age-related macular degeneration,” Ophthalmology 113(12), 2251–2258 (2006). 10.1016/j.ophtha.2006.06.028 [DOI] [PubMed] [Google Scholar]
- Doherty A., Bowers A. R., Woods R. L., and Peli E., “Is the ring scotoma of a monocular telescope present when viewing binocularly?,” in Proc. 9th Intl. Conf. Low Vision—Vision 2008, (2008).
- Peli E. and Vargas Martin F., “In-the-spectacle-lens telescopic device,” J. Biomed. Opt. 13(3), 0324027 (2008). 10.1117/1.2940360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann O., Corn A. L., and Lewis M. C., “Bioptic telescopic spectacles and driving performance: a study in Texas,” J. Vis. Impair. Blin. 82(5), 182–187 (1988). [Google Scholar]
- Fetchenheuer I., Peli E., and Woods R. L., “Functional visual fields of monocular bioptic telescopes (abstract),” 7th Intl. Conf. Low Vision: Activity Particip., 81 (2002).
- Ross N. C., Bowers A. R., and Peli E., “Consideration of optical scotomas in designing visual field expansion devices (abstract),” Invest. Ophthalmol. Visual Sci. 50(5), E-4734 (2009). [Google Scholar]
- Peli E., “Field expansion for homonymous hemianopia by optically-induced peripheral exotropia,” Optom. Vision Sci. 77(9), 453–464 (2000). 10.1097/00006324-200009000-00006 [DOI] [PubMed] [Google Scholar]
- Giorgi R. G., Woods R. L., and Peli E., “Clinical and laboratory evaluation of peripheral prism glasses for hemianopia,” Optom. Vision Sci. 86(5), 492–502 (2009). 10.1097/OPX.0b013e31819f9e4d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt-Johnson J. A. and Tillson G., Management of Strabismus and Amblyopia: A Practical Guide, Thieme Publishing, New York: (2001). [Google Scholar]
- Watson A. B. and Turano K., “The optimal motion stimulus,” Vision Res. 35(3), 325–336 (1995). 10.1016/0042-6989(94)00182-L [DOI] [PubMed] [Google Scholar]
- Peli E. and Lang A., “Appearance of images through a multifocal intra ocular lens,” J. Opt. Soc. Am. A Opt. Image Sci. Vis 18(2), 302–309 (2001). 10.1364/JOSAA.18.000302 [DOI] [PubMed] [Google Scholar]
- Legge G. E., “Spatial frequency masking in human vision: binocular interactions,” J. Opt. Soc. Am. 69(6), 838–847 (1979). 10.1364/JOSA.69.000838 [DOI] [PubMed] [Google Scholar]
- Peli E., “Optometric and perceptual issues with head-mounted display (HMD),” in Visual Instrumentation: Optical Design and Engineering Principles, Mouroulis P., Ed., pp. 205–276, McGraw-Hill, New-York: (1999). [Google Scholar]
- Peli E., Hedges T. R., Tang J., and Landmann D., “A binocular stereoscopic display system with coupled convergence and accommodation demands,” in SID Intl. Symp. Digest Tech. Papers 32, [53.2], 1296–1299 (2001).
- Hering E., Die Lehre vom Binocularen Sehen, Wilhelm Engelmann, Leipzig, (1868). [Google Scholar]
- Brown J. P., Ogle K. N., and Reiher L., “Stereoscopic acuity and observation distance,” Invest. Ophthalmol. Visual Sci. 4(5), 894–900 (1965). [PubMed] [Google Scholar]
- Simmons D., “Contrast threshholds for stereoscopic depth identification with isoluminant and isochromatic stimuli,” Vision Res. 34(22), 2971–2982 (1994). 10.1016/0042-6989(94)90269-0 [DOI] [PubMed] [Google Scholar]
- Lyle T. K. and Wybar K. C., Practical Orthoptics in the Treatment of Squint, H. K. Lewis and Co. Ltd, London: (1967). [Google Scholar]
- von Noorden G. K. and Campos E. C., Binocular Vision and Ocular Motility. Theory and Management of Strabismus, Mosby Inc., St. Louis, MO: (2002). [Google Scholar]
- Pickwell D., Binocular Vision Anomalies. Investigation and Treatment, Butterworths, London: (1989). [Google Scholar]
- Peli E., “Suprathreshold contrast perception across differences in mean luminance: effects of stimulus size, dichoptic presentation, and length of adaptation,” J. Opt. Soc. Am. A Opt. Image Sci. Vis 12(5), 817–823 (1995). 10.1364/JOSAA.12.000817 [DOI] [PubMed] [Google Scholar]
- Daum K. M., Rutstein R. P., and Eskridge J. B., “Efficacy of computerized vergence therapy,” Am. J. Optom. Physiol. Opt. 64, 83–89 (1987). [DOI] [PubMed] [Google Scholar]
- Groffman S., “Motivational factors in vision therapy: comparison of computerized vs. manipulative techniques,” J. Am. Optom. Assoc. 67,(6), 344–349 (1996). [PubMed] [Google Scholar]
- Peli E., “Ophthalmic applications of circular polarizers,” J. Am. Optom. Assoc. 57(4), 298–302 (1986). [PubMed] [Google Scholar]
- Borish I. M., “Subjective testing,” in Clinical Refraction, pp. 715–804, Professional Press, Chicago: (1970). [Google Scholar]
- Reading R. W., Binocular Vision. Foundations and Applications, Butterworths, Boston: (1983). [Google Scholar]
- Yeh Y. Y. and Silverstein L. D., “Spatial judgments with monoscopic and stereoscopic presentation of perspective displays,” Hum. Factors 34, 683–600 (1982). [DOI] [PubMed] [Google Scholar]
- Hornbeck L. J., “Digital light processing for high-brightness, high-resolution applications,” see http://www.vxm.com/TIDLP.html and http://www.dlp.com/dlp_technology/images/dynamic/white_papers/141_hornbeck.pdf, Texas Instruments, Austin, TX (1997).
- Packer O., Diller L. C., Verweij J., Lee B. B., Pokorny J., Williams D. R., Dacey D. M., and Brainard D. H., “Characterization and use of a digital light projector for vision research,” Vision Res. 41(4), 427–439 (2001). 10.1016/S0042-6989(00)00271-6 [DOI] [PubMed] [Google Scholar]
- Keeney A. H., Weiss S., and Silva D., “Functional problems of telescopic spectacle in the driving task,” Trans. Am. Ophthalmol. Soc. 72, 132–138 (1974). [PMC free article] [PubMed] [Google Scholar]
- Fonda G., “Bioptic telescopic spectacle is a hazard for operating a motor vehicle,” Arch. Ophthalmol. 101(12), 1907–1908 (1983). [DOI] [PubMed] [Google Scholar]
- Peli E., “The visual effects of head-mounted display (HMD) are not distinguishable from those of desk-top computer display,” Vision Res. 38(13), 2053–2066 (1998). 10.1016/S0042-6989(97)00397-0 [DOI] [PubMed] [Google Scholar]
- Brainard D. H., Pelli D. G., and Robson T., “Display characterization,” in Encyclopedia of Imaging Science and Technology, Hornak J., Ed., pp. 172–188, Wiley, Hoboken, NY: (2002). [Google Scholar]
- Peli E., “Visual issues in the use of a head mounted monocular display,” Opt. Eng. 29(8), 883–892 (1990). 10.1117/12.55674 [DOI] [Google Scholar]
- Peli E., “Real vision and virtual reality,” Opt. Photonics News 6, 28–34 (1995). 10.1364/OPN.6.7.000028 [DOI] [Google Scholar]
- Spruce G. and Pringle R. D., “Polymer dispersed liquid crystal (PDLC) films,” Electron. Commun. Eng. J. 2(2), 91–100 (1992). 10.1049/ecej:19920017 [DOI] [Google Scholar]
- Crawford G. P., Division of Engineering, Brown University, Providence, RI, Personal Communication (2006).
- Woods A. and Tan S. S. L., “Characterizing sources of ghosting in time-sequential stereoscopic video displays,” Proc. SPIE 4660, 66–77 (2002). 10.1117/12.468076 [DOI] [Google Scholar]
- Woods A. J., “Compatibility of display products with stereoscopic display methods,” in IDMC ’05 Proc. 2, 1–4 (2005).
- Stereoscopic Displays and Virtual Reality Systems XIV, Woods A. J., Dodgson N. A., Merritt J. O., Bolas M. T., and McDowall I. E., Eds., Proc. SPIE 6490, entire volume (2007).
- Woods A. J. and Rourke T., “The compatibility of consumer DLP projectors with time-sequential stereoscopic 3D visualisation,” Proc. SPIE 6490, 64900V (2007). 10.1117/12.706243 [DOI] [Google Scholar]
- Woods A. J., Rourke T., and Yuen K. L., “The compatibility of consumer displays with time-sequential stereoscopic 3D visualisation (plenary paper),” in Proc. K-IDS 3-D Display Workshop 2006, pp. 7–10 (2006).
- Woods A. J. and Yuen K. L., “Compatibility of LCD monitors with frame-sequential stereoscopic 3D visualisation (invited paper),” in IMID∕IDMC ’06 Digest [5–4] pp. 98–102 (2006).
- “Jonathan C. Horton, MD, PhD, wins 2008 Bressler Prize in vision science,” in The Guild Newsletter, The Jewish Guild for the Blind, New York, (2008).
- Jorke H. and Fritz M., “Infitec—a new stereoscopic visualisation tool by wavelength multiplexing,” see http://www.infitec.net/infitec_english.pdf, Ulm, Germany (2003).
- Shankland S., “Dolby stakes its claim in 3D movie tech,” see http://news.zdnet.com/2100-9588_22-168848.html, ZDNet, San Francsico (2007).
- Rowe D., at N. V. Barco, Dayton, OH, Personal Communication (Jul. 2009).
- Swift D., Panish S., and Hippensteel B., “The use of the VisionWorks™ in visual psychophysical research,” Spatial Vis. 10, 471–477 (1997). 10.1163/156856897X00401 [DOI] [PubMed] [Google Scholar]
- Woods R. L., Tregear S. J., and Mitchell R. A., “Screening for ophthalmic disease in older subjects using visual acuity and contrast sensitivity,” Ophthalmology 105(12), 2318–2326 (1998). 10.1016/S0161-6420(98)91235-0 [DOI] [PubMed] [Google Scholar]
- Owsley C., “Contrast sensitivity,” Ophthalmol. Clin. North Am. 16, 171–177 (2003). 10.1016/S0896-1549(03)00003-8 [DOI] [PubMed] [Google Scholar]
- Shapley R. and Lam D. M. K., Contrast Sensitivity, A Bradford Book, MIT Press, Cambridge, MA, (1993). [Google Scholar]