Abstract
Quantum physics and biology have long been regarded as unrelated disciplines, describing nature at the inanimate microlevel on the one hand and living species on the other hand. Over the past decades the life sciences have succeeded in providing ever more and refined explanations of macroscopic phenomena that were based on an improved understanding of molecular structures and mechanisms. Simultaneously, quantum physics, originally rooted in a world-view of quantum coherences, entanglement, and other nonclassical effects, has been heading toward systems of increasing complexity. The present perspective article shall serve as a “pedestrian guide” to the growing interconnections between the two fields. We recapitulate the generic and sometimes unintuitive characteristics of quantum physics and point to a number of applications in the life sciences. We discuss our criteria for a future “quantum biology,” its current status, recent experimental progress, and also the restrictions that nature imposes on bold extrapolations of quantum theory to macroscopic phenomena.
While in the days of Darwin and Mendel the life sciences were mainly focusing on botany or zoology, modern biology, pharmacology, and medicine are deeply rooted in a growing understanding of molecular interactions and organic information processing.
Quantum physics, on the other hand, was initially centered on microscopic phenomena with photons, electrons, and atoms. But objects of increasing complexity have attracted a growing scientific interest, and since the size scales of both physics and the life sciences have approached each other, it is now very natural to ask: what is the role of quantum physics in and for biology?
Erwin Schrödinger, most famous for his wave equation for nonrelativistic quantum mechanics, already ventured across the disciplines in his lecture series “What is life?” (Schrödinger, 1944). He anticipated a molecular basis for human heredity, which was later confirmed to be the DNA molecule (Watson and Crick, 1953). Since the early days of quantum physics, its influence on biology has always been present in a reductionist sense: quantum physics and electrodynamics shape all molecules and thus determine molecular recognition, the workings of proteins, and DNA. Also van der Waals forces, discrete molecular orbitals, and the stability of matter: all this is quantum physics and a natural basis for life and everything we see.
But even 100 years after its development, quantum physics is still a conceptually challenging model of nature: it is often acclaimed to be the most precisely verified theory of nature and yet its common interpretation stands in discrepancy to our classical, i.e., prequantum, world-view, and our natural ideas about reality or space-time. Is there a transition between quantum physics and our everyday world? And how will the life sciences then fit into the picture—with objects covering anything from molecules up to elephants, mammoth trees, or the human brain?
Still half a century ago, the topic had some rather skeptical reviews (Longuet-Higgins, 1962). But experimental advances have raised a new awareness and several recent reviews (e.g., Abbot et al., 2008) sketch a more optimistic picture that may be overoptimistic in some aspects.
The number of proven facts is still rather small. Many hypotheses that are formulated today may be found to have lacked either visionary power or truth by tomorrow. We will therefore start on well-established physical grounds and recapitulate some typical quantum phenomena. We will then elucidate the issue of decoherence and dephasing, which are believed to be central in the transition between the quantum and the classical world. They are often regarded to be the limiting factors if we want to observe quantum effects on the macroscopic scale of life. Next, we give an overview over modern theories and experiments at the interface between quantum physics and biology. The final section will be devoted to open speculations, some of which still face less supporting experimental evidence than theoretical counterarguments.
Experimental studies at the interface between quantum physics and the life sciences have so far been focused on two different questions: (1) can genuine quantum phenomena be realized with biomolecules?
Photon antibunching in proteins (Sanchez-Mosteiro et al., 2004), the quantum delocalization of biodyes in matter-wave interferometry (Hackermüller et al., 2003) and the implementation of elementary quantum algorithms in nucleotides (Jones and Mosca, 1999) are some recent examples.
These experiments are optimized for revealing fundamental physics, such as quantum statistics, delocalization, and entanglement. But they all also show that quantum phenomena are best observed in near-perfect isolation from the environment or at ultralow temperatures, in order to avoid the detrimental influence of decoherence and dephasing. They are thus not representative for life as such. (2) Are nontrivial quantum phenomena relevant for life?
Nontrivial quantum phenomena are here defined by the presence of long-ranged, long-lived, or multiparticle quantum coherences, the explicit use of quantum entanglement, the relevance of single photons, or single spins triggering macroscopic phenomena.
Photosynthesis, the process of vision, the sense of smell, or the magnetic orientation of migrant birds are currently hot topics in this context. In many of these cases the discussion still circles around the best interpretation of recent experimental and theoretical findings.
A BRIEF REVIEW OF ELEMENTARY QUANTUM PHENOMENA
Quantum physics includes a wide variety of phenomena. Most of them are regarded as unusual because they violate our everyday expectations of how nature should behave.
Quantum discreteness
Quantum physics derives its very name from the discreteness of nature. The latin “quantum?” asks the question “how much?” and even colloquially a quantum is nowadays a “small portion.” Several physical properties only assume a countable number of values. This is for instance true for the electronic energy in atoms or the vibrational energy in molecules. The quantized set of spectral lines, Fig. 1a, is often used in the life sciences as a finger print of chemical substances, since it relates to the discrete set of energies in all nanomatter, from atoms to large biomolecules. And yet, this aspect is regarded as atomic and chemical physics rather than a part of “quantum biology.” If, however, single photons or single spins can trigger a chain of macroscopic phenomena in organic systems, the assignment will be justified.
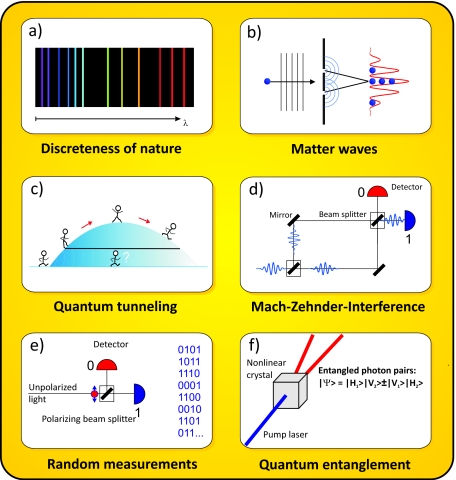
Figure 1. Review of some prominent quantum phenomena.
(a) Quantum physics emphasizes that our world is built on discrete particles that are bound in finite systems of discontinuous energies. This becomes evident in the finite number of wavelengths λ, respectively, colors that an atom emits. (b) The quantum delocalization and wave-particle duality of light and matter can be demonstrated using a double-slit experiment (see text). (c) Quantum wave effects allow tunneling through an energy barrier which would classically be insurmountable. (d) A Mach–Zehnder interferometer allows to split a wave into two widely separated paths. (e) A quantum measurement generates objective randomness. A photon beam that is divided by a 50/50 beam splitter will hit either detector randomly and yield an absolutely random sequence of zeros and ones. (f) Entanglement is the inseparable quantum correlation of two or more particles or degrees of freedom. Here, we sketch the creation of a polarization-entangled pair of red photons when a single UV pump photon interacts with a nonlinear crystal (Kwiat et al., 1995). A measurement of both photons shows perfectly anticorrelated polarizations although the result on each side individually appears to be absolutely random.
Quantum superposition
One of the intriguing aspects of physics is related to the fact that quantum states are well-defined even when they refer to situations, which we would describe as a “coexistence of mutually excluding possibilities” in classical physics. We usually adhere to Aristotle’s sentence of noncontradiction that “the same attribute cannot at the same time belong and not belong to the same subject.” Quantum physics teaches us that we either have to give up this wisdom or otherwise renounce on other well-established intellectual concepts, such as our understanding of reality, information, or space-time.
Wave-particle duality of light: A paradigmatic example for this fact is the dual nature of light that manifests itself in Young’s famous double-slit diffraction experiment [Fig. 1b]: when a light beam is sent onto a small opening in a wall, it will spread out behind the slit. If we open a neighboring slit in the screen, we will observe a fringe pattern [Fig. 1b] that exhibits dark minima at several screen positions that are still bright when only a single slit is open. This observation is explained by the superposition and interference of classical electromagnetic waves of well-defined relative phases, i.e., sufficient mutual coherence. The same wave interference experiment turns into a quantum puzzle when we dilute the optical field to the level of individual quanta of light, photons, which are always detected as localized packets of energy E=hν. The delocalized nature of photons in free flight and their localized character in the detection process are at the heart of their wave-particle duality and very incompatible with classical reasoning. Here, ν relates the frequency of the electromagnetic wave to the energy E of each individual photon through Planck’s constant of action h.
The superposition principle is also associated with the dualism between determinism and quantum randomness: The shape and location of the envelope of the interference pattern are strictly determined by a wave equation. The square of the wave amplitude represents the probability to detect a single event. Each individual realization is, however, objectively random within the predetermined probabilities.
Quantum theory describes the evolution of delocalized objects perfectly well, but a measurement signifies a break in this evolution and introduces a random element. Since it terminates the wavelike propagation of the quantum state, it is often described as a “collapse of the wave function.” It is still an open debate whether measurements have an “ontological” or rather an “epistemological” meaning, i.e., whether they describe an outside reality or our knowledge about the world. But for many practical purposes the “act of measurement” is a valid and useful concept.
It is important to note that interference always occurs, when the wave associated to a quantum object may reach the detector along at least two different but intrinsically indistinguishable paths—either in real-space or in some configuration space (e.g., potential curves). The Mach–Zehnder interferometer, sketched in Fig. 1d, is a simple textbook arrangement, which is often used to demonstrate delocalization and interference for photons over macroscopic distances. It also visualizes an idea that has been invoked in the description of coherent energy transport in organic molecules.
Quantum delocalization of matter: Diffraction and interference deviate even more from our classical expectations when we observe them with massive particles, such as electrons, neutrons, or atoms (Cronin et al. (2009), and citations therein). But chemistry teaches us that the delocalization of electrons over nanometers is rather ubiquitous, for instance across the bonds of aromatic molecules.
The wave-nature of matter is also of relevance for life science in a different context: the short de Broglie wavelength of fast electrons allows one to obtain high resolution images in electron microscopy, and neutron waves are useful in analyzing crystallized protein structures.
While electrons and neutrons are still rather small and elementary particles, recent experiments were able to demonstrate the delocalization of entire molecules, such as C60, using far-field diffraction (Arndt et al., 1999).
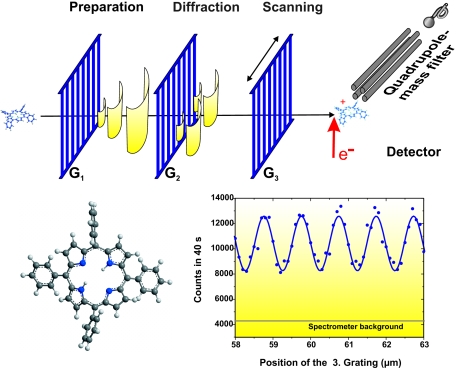
And even biodyes, such as porphyrin derivatives, revealed their quantum wave properties in a near-field interferometer (Hackermüller et al., 2003), as shown in Fig. 2, and briefly outlined below: the molecular powder was evaporated to form a beam, which was velocity selected to Δv∕v≃15–20%. The stream was directed onto a gold grating, i.e., an array of slits (450 nm wide) with a period of 990 nm. The molecules are only nanometers large, and their center-of-mass is still localized to within a few picometers inside the oven. But once ejected into the vacuum, the free quantum propagation stretches their coherence function by more than a factor of 10000, i.e., to 50 nm before they interact with the first grating. Diffraction within the slits of this grating leads to a further broadening of the molecular coherence function, up to several micrometers before the porphyrins encounters the second grating. The simultaneous and indistinguishable passage of the molecular wave amplitude through several slits leads to quantum interference, which manifests itself as a sinusoidal molecular density pattern at the location of the third grating (Fig. 2).
Figure 2. The wave-particle duality of the biodye tetraphenylporphyrin can be revealed by diffracting the molecules in a near-field interferometer of the Talbot–Lau type ( Hackermüller et al., 2003 ).
. Molecules passing the first grating are diffracted and delocalized over several micrometers at the second grating. Diffraction there leads to interference fringes, i.e., a molecular density pattern, at the position of the third mask. This is imaged by scanning grating G3 and by recording all transmitted molecules in a mass spectrometer.
These experiments show that large scale coherence of biomolecules is obviously observable. These molecules can be delocalized over many micrometers, a thousand times their physical size and a million times their de Broglie wavelength, and over time scales of a few milliseconds. But it is also clear that this demonstration required laboratory conditions, i.e., high-vacuum, to avoid observing interactions with the environment. It is interesting to note that all porphyrin molecules were heated to 690 K, and thermal excitations could drive internal state changes even during the molecular propagation through the interferometer. The reason for de Broglie interference to survive lies in the fact that at this temperature the internal states still remain effectively decoupled from the center-of-mass motion (Hackermüller et al., 2003).
Several groups have formulated serious research proposals that ask to which extent quantum superpositions might still be observable on the mass scale, not only of a single organic molecule, but possibly even of a supermassive cluster or a virus (Clauser, 1997; Reiger et al., 2006; Romero-Isart et al., 2009). Chances are high that the idea behind these proposals can actually be successfully implemented within the next decade.
Quantum tunneling: When a particle encounters a potential barrier that is higher than its kinetic energy, it will not be able to pass by any classical means. Quantum mechanics, however, allows it to tunnel through the barrier [see Fig. 1c]. In order to understand the relevance of this phenomenon for biology, it is important to see that the tunneling probability depends exponentially on the height, width, and shape of the potential barrier. The particle’s mass and kinetic energy enter in a similar way. This explains why tunneling of electrons and protons is rather ubiquitous in biology, whereas it is unobserved for entire amino acids or proteins: their mass and their interaction potentials grow with the number of molecular constituents and therefore dramatically decrease the probability for this quantum effect.
Spin: a quantum way to turn around: Angular momentum L is a well-established quantity in classical physics, but quantum physics requires that it appears only with a discrete set of values and orientations. Most particles also possess a spin, i.e., an angular momentum that is not related to any mechanical motion and therefore without any classical analog.
Spins may also be brought into superposition of two or more mutually exclusive states, comparable to a single compass needle pointing both north and south at the same time. In spite of being a pure quantum property, spin is also often responsible for magnetism in biological systems on the molecular level. The spin of protons is also exploited to derive structural and functional properties of organisms (Lauterbur, 1973). Magnetic resonance imaging of human tissue relies on the simultaneous response of many trillion spins in a macroscopic biological volume. It is, however, still an open question whether magnetization in biology can be quantized on a larger scale, whether spin entanglement or mesoscopic coherent spin transport can be found under ambient conditions and with a consequence for biological functionality—a question that is resumed below in the context of the magnetic senses in animals.
Quantum superposition of energy states: If a molecule is excited by a femtosecond optical pulse, the energy-time uncertainty relation ΔE×Δt⩾ℏ∕2 ensures that the photon energy is sufficiently undetermined to be resonant with more than a single excited state. Femtosecond spectroscopy has thus become a regular tool for characterizing biomolecular systems (Felker et al., 1982), and the coherent superposition of electronic and vibrational energy states are regularly observed in such experiments. But even when biological systems are exposed to incoherent daylight, excitonic coherences may form between electronically coupled neighboring molecules. In photosynthetic complexes excitonic coupling across several pigment molecules has been reported (Dahlbom et al., 2001), and coherence in photosynthesis is a major field of current research (see further below).
Quantum statistics
The spin of identical particles also determines their statistical behavior within an ensemble. Bosons, particles with an integer value of the spin quantum number s, tend to occupy the same quantum state when they are prepared to be indistinguishable. Fermions with half-integer quantum numbers, such as electrons, avoid each other under otherwise identical circumstances. This has important consequences: Pauli’s exclusion principle, the stability of matter, and the existence of neutron stars are important results for fermions. On the other hand, the existence of lasers and Bose–Einstein condensates are macroscopic effects of boson statistics. The observation of quantum statistics with atoms requires highly specialized environments, such as μK temperatures and ultrahigh vacuum. Interestingly, quantum degeneracy of exciton-polaritons has already been observed in a condensed matter system at T=19 K (Kasprzak et al., 2006). This is much closer to the conditions of life, but at present there is still no experimental evidence for quantum statistics on a macroscopic biological scale.
On the other hand, it is worth noting that photons are also bosons with a spin quantum number of s=1, and nonclassical states of light may well be generated in organic systems. Although we do not know of any biological laser, random lasing of artificially dye-infiltrated human tissue has been demonstrated recently (Polson and Vardeny, 2004).
Quantum entanglement and quantum information
When we extend the superposition principle to more than a single object or property, we arrive at the notion of quantum entanglement. By this we mean a nonclassical correlation, an unseparable connection between two objects or properties. Amazingly, once established, this quantum connection may theoretically persist over long distances and times, unless it is perturbed by external interactions and measurements.
The example of polarization-entangled photon-pairs shall illustrate the idea [Fig. 1f]: when photons are sent onto a properly chosen nonlinear crystal, each of them can be converted with a certain probability into two photons of lower energy. The polarizations of the two photons is then quantum correlated, i.e., entangled, in the following sense: none of the two emerging photons has a well-defined polarization before it is measured, but when the two quanta of light are polarization-entangled, we can predict in advance and with certainty that the polarization of both will always be orthogonal to each other. If the first photon is horizontally polarized, denoted as |H⟩, the partner quantum will be detected in a vertical orientation, denoted as |V⟩, and vice versa. This is why quantum physicists write this particular entangled state as ψ∝|H1⟩|V2⟩±|V1⟩|H2⟩. The sum or difference of the two products describes a quantum superposition of the two possibilities. The essence of quantum entanglement lies in the fact that nature no longer allows us to describe one particle without the other. Their individual polarization is not defined until we do a definite measurement. Each individual recording will find a random orientation for each individual photon, but if we compare the properties of paired quanta, they will always be perfectly correlated.
There are several formal definitions and measures of entanglement (Vedral and Plenio, 1998). Generally the following conditions are required to test for it: first, we have to identify at least two physical subsystems or modes. They must be clearly distinguishable and independently addressable for instance through different frequencies, locations, or detector orientations. Second, we have to be able to identify at least two complementary measurements that can be performed on each of these two modes, and it should be possible to gradually vary between the two kinds of measurements. In the photon example, these two criteria are easily fulfilled as the two polarizations are orthogonal and optical elements, such as λ∕2-plates, can transform one into the other. The two twin-photons can be emitted in spatially separated arms, too.
Quantum entanglement has been demonstrated for photons, but also for superconducting circuits, nuclear spins in small molecules, spin noise in atomic ensembles, trapped ions, and other systems [see references in Nielsen and Chuang (2000)]. All these proof-of-principle demonstration experiments were, again, performed under strictly controlled environmental conditions, often including ultrahigh vacuum and ultralow temperatures, i.e., conditions that are incompatible with living organisms.
Interestingly, entanglement may also be mediated by the exchange of light. And the transmission can be amazingly robust, as shown by the successful quantum teleportation of atomic spin properties from one room temperature atomic ensemble to another, over macroscopic distances through air (Sherson et al., 2006). Electromagnetic radiation might thus be an important coherence mediator, if entanglement should be relevant in life.
Quantum correlations are of particular interest for new information processing schemes [see references inBouwmeester et al. (2000); Nielsen and Chuang (2000)]: in contrast to classical physics, where a computing bit can only assume the values “0” or “1,” quantum systems can coexist in a superposition of states and form quantum-bits or “qubits” of the form ψ∝|0⟩+eiϕ|1⟩, where ϕ is an angle that can take a continuous, i.e., infinite, range of values. This continuity opens, in principle, a way to massively parallel computation.
As of today, several algorithms have been suggested, which would actually provide a significant advantage over all classical schemes, when implemented with many qubits [see references in Nielsen and Chuang (2000)]. Quantum computers are expected to provide faster prime number factoring for crypto-analysis using Shor’s algorithm, speed-up in database search using Grover’s algorithm, new insights into quantum games (Eisert et al., 1999) or an exponential speed-up in solving systems of linear equations (Harrow et al., 2009).
These developments in quantum information science lead to the question whether quantum methods may also be relevant for living organisms, which are stunning information processing devices. Yet, the implementation of quantum circuits in biology, as anywhere else, would require a configuration space that increases exponentially with the number of qubits involved. And given the enormous sensitivity of quantum states to most external perturbations, it is widely believed that the probability of a successful coherent computation will also be exponentially suppressed under the conditions of life as they are known to us.
Until today, first quantum computing circuits and elementary quantum algorithms could be demonstrated using nuclear magnetic resonance (NMR) on a few nuclear spins within an individual biomolecule [Jones et al., 2000, seeFig. 3a]. These experiments approach a natural setting since they were embedded in a condensed matter environment, even though at a temperature of a few Kelvin only. NMR quantum computing in a dense ensemble is mostly hampered by the difficulty to initialize all molecules to the same ground state. And also the rapid randomization of quantum phases by interactions with the host matrix is a further limiting factor.
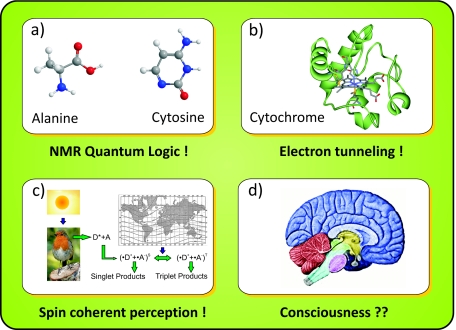
Figure 3. Some recent explorations of quantum aspects in the life sciences.
(a) The nuclear spins of amino acids have been used as qubits in quantum computing demonstrations (Jones et al., 2000). (b) Electron tunneling on nanometer scales has been established as a common phenomenon in life, for instance, in reactions with cytochrome (De Vault and Chance, 1966). (c) Electron spin entanglement and coherent spin transport are part of a possible explanation for the magnetic orientation of migratory birds (Ritz et al., 2000). (d) Speculations about the influence of quantum physics on human consciousness are often regarded as inspiring but as of today they are not substantiated by any experiment [robin picture: David Jordan, CC-BY-SA (http://commons.wikimedia.org/wiki/Commons:Reusing_content_outside_Wikimedia)].
One may also speculate whether naturally occurring quantum correlations can be used in biology. Being embedded in a macroscopic system, any initial coherent superposition of a molecule—be it in position, electronic, or vibrational excitations—will rapidly be transformed into entanglement with the environment. One could imagine that this yields a functional benefit. But again, experimental evidence for this and other meaningful quantum information processing in nature still has to be found.
THE QUANTUM-TO-CLASSICAL TRANSITION
Some general insights
In order to understand the overwhelming success of our classical world-view in the description of biological phenomena, we have to understand why quantum effects are usually hard to observe.
The kinematic argument is based on the insight that Planck’s constant of action ℏ is extremely small. For instance, the de Broglie wavelength λdB=h∕mv≃10−35 m of an adult man walking at a speed of 1 m∕s is way too small to ever be observed. Here, it is rather the value of the fundamental constants than the intrinsic structure of quantum mechanics, which forbid the observation of macroscopic quantum delocalization.
The phase averaging argument adds the insight that quantum interference relies on phase coherence. But small wavelengths are easily dephased in changing environments. Fluctuations of the geometry, electromagnetic fields, or chemical environments will easily alter the conditions for constructive and destructive interference. In most cases thermal fluctuations will render interference phenomena unobservable, when we look at macroscopic molecular ensembles. In fact, this does not necessarily mean that quantum mechanics does not play a role in biology: the stochastic dephasing of destructive quantum interference has recently been even invoked as being responsible for the fast energy transport in the photosynthetic complex (Caruso et al., 2009), as further described below.
In contrast to the notion of phase averaging, we here reserve the term decoherence for processes, which actually exchange information between the quantum system and its environment (Zurek, 1991; Joos et al., 2003). Decoherence is closely related to the act of measurement. By definition, a quantum measurement extracts information about a quantum system and correlates it to the pointer of a macroscopic “meter.” But in contrast to the prescriptions of unitary quantum physics, we never see any macroscopic meter pointing up and down at the same time.
A valid explanation for that is given by decoherence theory (Joos and Zeh, 1985): the act of measurement on a quantum superposition state will create entanglement between the system and the meter. The “quantumness” of the original system is thus diluted to a larger composite system (object and meter) and, in most cases, the quantumness of the meter diffuses over the entire detector, which may contain some 1020…1025 atoms. This is such an incredible amount of particles that we will never be able to retrieve the original quantum coherence from the enlarged system.
In contrast to decoherence theory, for which the unitary evolution of quantum physics always persists—even during a measurement—the model of an objective collapse of the quantum wave function assumes that coherences spontaneously disappear in the act of measurement. Spontaneous collapse models assume that this intrinsic loss of coherence is bound to a certain internal complexity and number of participating particles (Ghirardi et al., 1986). Recently, it has also been suggested that the inevitable coupling between mass and deformations of spacetime might cause an objective collapse of the wave function (Diosi, 1989; Penrose, 1996).
The kinematic argument, phase averaging, and decoherence are all consistent with the unitary evolution of quantum physics. They are experimentally accessible and proven to be relevant in well-controlled experiments. But none of them includes a qualitative transition between quantum and classical phenomena—only a gradual reduction of the observability of quantum effects. In contrast to that, collapse models, as taught in many modern physics textbooks, postulate a factual and abrupt change between quantum and classical dynamics. But also this assumption leaves many experimental and philosophical questions unanswered. Also from this fundamental point of view, it is therefore still open to which degree quantum physics can prevail on the macroscopic scale of living species.
Can quantumness survive in biological environments?
The observation of quantum phenomena often requires low temperatures and a high degree of isolation. And yet, quantum coherence could be observed in matter-wave experiments with biodyes even at internal temperatures exceeding 690 K (Hackermüller et al., 2003). Related experiments, however, also quantified the high sensitivity of quantum coherence to the emission or scattering of even single photons (Hackermüller et al., 2004) or molecules (Hornberger et al., 2003). A simple and general rule reads as follows: if an individual state of a quantum superposition can be resolved and detected by any interaction with its environment, the superposition will be destroyed. A practical example: A molecule that coherently traverses several slits of a diffraction grating will maintain its quantum superposition until the environment gets the capability of reading which slit the particle takes. Even if the openings are separated by only 250 nm, the scattering of a single visible photon per molecule may destroy the observation of quantum interference.
Under biological conditions, in an aqueous and warm environment, position superpositions of massive particles cannot survive more than a few collisions with electrons, atoms, photons, or phonons. In the case of strong interactions, a single collision will destroy the coherence. And even for weak perturbations, the visibility of quantum phenomena will vanish exponentially with the separation of the position states. Any delocalization in biomaterials will therefore be limited to extensions of a few nanometers and time scales typically shorter than nanoseconds, in most cases even a few hundred femtoseconds only. But a few backdoors still exists for coherence and entanglement to persist.
First, it is conceivable that special molecular architectures may shield some parts of a system from some interactions with their environment. Electrostatic forces may for instance create hydrophobic pockets from which the solvent could be excluded. But the existence and survival of decoherence free subspaces (Lidar et al., 1998) in organic matter and over the time scales of milliseconds still has to be shown experimentally.
Second, quantum error correction (Shor, 1995) is often being discussed as a loophole for the implementation of quantum information processing in living systems. The idea is based on the fact that a certain class of computational errors may even be corrected if no one knows the details of the state or the errors that occurred. This is particularly important, as any attempt to read a quantum superposition would result in its loss, i.e., its reduction to a classical state. A system with quantum error correction requires, however, even more qubits than the uncoded counterpart. The idea of the scheme has been demonstrated with a 3 bit code in NMR quantum computing with alanine (Cory et al., 1998); but, as of today, extensions to more than a handful of spins at low temperatures are not known. We thus face the following dilemma: What are the odds that quantum error correction was developed by natural selection from unprotected quantum computing if this faulty precursor process would provide no evolutionary advantage?
Finally, it is worth noting that biology operates in open systems, i.e., far from thermal equilibrium. It has been hypothesized that living systems might act as heat engines, providing local cooling in an otherwise thermal environment (Matsuno, 1999). Another perspective was opened (Cai et al., 2008) with the hypothesis that entanglement might be regularly refreshed in a thermally driven repetitive contact between neighboring molecular subsystems. If that happens on a time scale shorter than the one of decoherence, net entanglement may possibly persist. Clearly, all of these ideas are presently of an exploratory nature.
How can quantum entanglement be revealed in mesoscopic systems?
We introduced entanglement as the inseparability of quantum states. But how could we possibly detect this intricate quantumness in a complex condensed matter environment, where there is no hope of getting access to all underlying details? Interestingly, it has been found that even macroscopic thermodynamical variables may serve as entanglement witnesses (Vedral, 2003; Amico et al., 2008). And to a certain degree, entanglement may even survive in a macroscopic thermal state (Markham et al., 2008).
Since the Hamiltonian, i.e., the operator of the system’s total energy that is used for deriving all thermodynamic functions, is influenced by the entanglement of the underlying wave functions, the energy terms and therefore also all thermodynamic variables are affected by these quantum correlations. A single macroscopic observable can thus suffice to hint to the presence of quantum entanglement, and the important trick is to find an observable that can be reliably read in experiments. In practice, the magnetic susceptibility (Brukner et al., 2006) or the heat capacity might provide good indicators for entanglement (Wieśniak et al., 2008), and it will be interesting to search for further witnesses in biological systems too.
There is an enormous reduction in information when we derive a single thermodynamical variable from thousands of quantum states. Entanglement witnesses therefore cannot tell us all the details about a system. They rather indicate under which conditions a state is definitely entangled, but they neither quantify it nor do they exclude with certainty the presence of entanglement if the witness conditions are violated.
LIFE SCIENCE RESEARCH WITH AN INTERFACE TO QUANTUM PHYSICS
Single-photon phenomena in the life sciences
Single quanta of light have been relevant for illustrating fundamental quantum principles but they are also ubiquitous in the life sciences: the most efficient detection techniques for fluorescent biomolecules are sensitive on the single-photon level. Individual particles of light are also of direct relevance in biological processes as they may affect the structure of individual molecules, which, in turn, can transduce signals in living organisms. The retinal molecule can switch its conformation after absorption of very few photons and thus turns the human eye into one of the most sensitive light detecting devices that exist. Between two and seven photons are usually sufficient to be perceived by a dark-adapted human observer (Hecht et al., 1942). Various studies indicated that test persons could even count the number of photons with a reliability that was only limited by quantum shot noise (Rieke and Baylor, 1998; and citations therein).
Single-photon detectors are of great interest for quantum communication, and it has recently been suggested that octopus rhodopsin, chosen by evolution because it is well-adapted to the dark of the deep oceans, may be a useful component in such applications (Sivozhelezov and Nicolini, 2007). But also single-photon sources are gaining increasing importance in quantum communication or computation protocols, and single molecules are considered to be relevant emitters (Lounis and Orrit, 2005).
When we talk about the quantum properties of light we usually refer to its wave-particle duality, the graininess, and quantum statistical properties, such as photon bunching, antibunching, or squeezing (Glauber, 2006). Fluorescence correlation experiments with proteins (Sanchez-Mosteiro et al., 2004) have revealed both the discrete quantum nature of molecular energy states and the nonthermal quantum statistics of light: an excited single molecule may usually not absorb a second photon of identical wavelength—unless the excited state has decayed. The emitted photons are therefore released with a time structure, which differs from that of thermal light sources. Photons emitted by a single molecule come in an “antibunched” rather than a bunched time series. It remains, however, still open whether single-photon emission is explicitly used by living systems.
In contrast to that, artificially grown quantum emitters have found many applications in the life sciences. The characteristic energy of a quantum system is connected with its spatial dimensions. This is, in particular, also true for semiconductor nanocrystals, which measure only a few nanometers in diameter and whose color can be changed from blue to red by growing them to larger sizes. Fluorescent quantum dots are used as highly efficient labels for biomolecular imaging, and they allow one to follow the dynamics of marked receptors in the neural membrane of living cells (Dahan et al., 2003). Similar results have recently also been achieved with nanodiamonds. Their nitrogen vacancy centers exhibit strong and stable fluorescence, they are biocompatible, and they have also been proven to be highly sensitive quantum probes for magnetic fields on the nanoscale [e.g., Balasubramanian et al. (2008)].
Quantum tunneling in biomolecules: from enzymatic reactions to the olfactory sense?
Living organisms are enormous biochemical reactors making and breaking zillions of chemical bonds every day. To a large extent the reaction rates are controlled both by thermal activation and enzymatic catalysis. It has been a long-standing question whether quantum tunneling is also involved and whether its presence provides an evolutionary advantage. This concerns the tunneling of electrons, protons, and even entire small molecules.
The theory of electron transfer has a long history (Marcus, 1956). First evidence for electron tunneling was derived from the oxidation rate of cytochrome [see Fig. 3b] in the bacterium chromatium vinosum. Since the reaction speeds were both large and temperature independent at low temperatures (<100 K), it was concluded that they are incompatible with a thermal activation model alone (De Vault and Chance, 1966). Electron tunneling has actually been identified as a widespread process found in photosynthesis (Blankenship, 1989), cellular respiration (Gray and Winkler, 2003), and electron transport along DNA (Winkler et al., 2005).
While speculations about proton tunneling had also been around for long (Löwdin, 1963), first experimental evidence was only given in 1989 (Cha et al., 1989) for the enzyme alcohol dehydrogenase, which transfers a proton from alcohol to nicotinamide adenine dinucleotide. Since tunneling depends on the mass of the object, the tunneling rates must change when hydrogen is replaced by the chemically equivalent deuterium, which doubles the atomic mass. This kinetic isotope effect was confirmed and gives good evidence for the presence of proton tunneling. Since then, many other enzymatic reactions were ascribed to proton tunneling (Glickman et al., 1994). It has to be noted, however, that the tunneling distances involved in all these reactions are typically shorter than 0.1 nm, and the protons traverse the barrier at energies around 10 kcal∕mol (0.4 eV) below the potential maximum (Masgrau et al., 2006).
The simultaneous tunneling of several particles has also been discussed, including double, triple, and even quadruple proton exchange in cyclic molecular networks (Brougham et al., 1999). The transition rates in these experiments were measured using NMR spectroscopy, and the temperature dependence of the reaction rate and the kinetic isotope effect were taken as witnesses for the presence of hydrogen tunneling. Even the tunneling of entire small molecules, i.e., formaldehyde (CH2O), was proposed based on the temperature dependence of its photo-induced polymerization rate (Goldanskii, 1979).
Turin (1996) also opened a public debate by suggesting that we are even able to “smell” quantum tunneling. Most aspects of our sense of smell are very well understood without it. Linda Buck and Richard Axel received the Nobel prize for their description of the mammalian olfactory system. They identified transmembrane proteins that encode for odor receptors in the olfactory epithelium (Buck and Axel, 1991). Each of them can sense multiple odorants. And each odorant can be detected by different sensors. Most smells can be perfectly explained (Zarzo, 2007) by assuming a “lock and key” mechanism, where an odor molecule binds to a specific receptor combination depending on its size, shape and chemical groups.
Based on much earlier hypotheses (Dyson, 1938), Turin suggested that smell is, at least additionally, correlated with the vibrational spectrum of molecules and that the receptors perform phonon-assisted inelastic electron tunneling spectroscopy to identify the odorant. This idea should explain why our nose is able to distinguish molecular groups of similar geometry but different vibrational spectra, such as for example OH and SH or ferrocene and nickelocene (Turin, 2002). However, recent experiments (Keller and Vosshall, 2004) rejected this theory, while newer theoretical work conceded a conceptual viability of the idea—even though without being quantitatively decisive (Brookes et al., 2007).
Concluding, we see that quantum tunneling is certainly present in a large number of biological processes, but experimentally proven only on the level of small-scale chemical reactions.
Coherent excitation transfer in photosynthesis
Photosynthesis is a key process for life and often considered as a role model for future light-harvesting technologies (Blankenship, 2002). It is differently realized in plants, algae, or bacteria. But they all convert light to chemical energy. A closer look reveals that photosynthesis involves a plethora of highly complex processes such as long-ranged excitation transfer, redox-reactions, hydrolysis, proton transport, or phosphorylation. In many parts of the system—including the wet-chemical material transport—we do not expect to find significant quantum coherence or entanglement, but others may actually require the notion of quantum tunneling, coherent excitonic transfer, or matter-wave interference.
The photosynthetic complex is a membrane-bound system with many embedded functional subunits. The energy conversion starts with the absorption of an incident photon by a pigment molecule, e.g., a chlorophyll, porphyrin, or a carotenoid molecule embedded in a protein structure, the antenna complex. The large number of these dye units ensures a high photoabsorption probability, and their arrangement enables an efficient excitation transfer from the primary absorber to the reaction center.
The reaction center is a pigment-protein complex, which contains a dimer, called the “special pair.” When it is excited, it donates an electron to a neighboring acceptor molecule. Fast secondary processes prevent the recombination of the ion pair and trigger the release of protons that are first transferred across the membrane and later used to fuel, for instance, the synthesis of adenosine triphosphate (ATP) from adenosine diphosphate (ADP).
Several recent studies (van Grondelle and Novoderezhkin, 2006; Cheng and Fleming, 2009) emphasized how well the excitation transfer from the antenna pigments to the reaction center is optimized. A fast conversion is important since any delay would increase the chances of relaxation mechanisms to channel energy into heating instead of chemical potentials.
Early explanations of the energy transport based on incoherent and dipole-dipole-mediated excitation hopping between molecular sites (Förster, 1948) failed to explain the observed transfer rates. Delocalization and coherent exciton coupling between the closely packed antenna pigments were therefore suggested as the most likely explanation, with experimental support rapidly growing throughout recent years.
Modern two-dimensional Fourier transform spectroscopy allowed one to probe the various excitation transfer pathways between the molecules on a femtosecond time scale. In particular, experiments performed on a 77 K cold bacteriochlorophyll Fenna–Matthews–Olsen (FMO) (Fig. 4) antenna complex were able to reveal exciton delocalization (Brixner et al., 2005) and long-lasting coherence in the excitonic energy transfer (Engel et al., 2007).
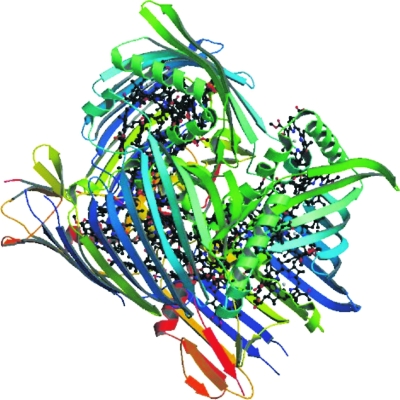
Figure 4. The FMO complex is composed of three protein-pigment structures.
. Each of them contains seven bacteriochlorophyll-a molecules (Blankenship, 2002). Electronic excitation transfer from the FMO complex to the reaction center is a key process in the light-harvesting of green photosynthetic bacteria. Two-dimensional Fourier transform spectroscopy (Engel et al., 2007) was able to document long-lived excitonic coherences across neighboring molecules in this structure (picture credits: Tronrud et al., 2009).
The observation of a spatially and temporally extended coherence, covering several nanometers and time-spans as long as a few hundred femtoseconds, is highly remarkable, and it has triggered a growing number of scientific groups to focus their theoretical and experimental work on that question. As of today, a rich set of detailed data has already been collected to characterize the energy levels, transfer rates, and intramolecular and intermolecular coherences. In particular, the latter raised the question how to connect these findings to related fields in quantum physics.
When there is coherence, what is the role of constructive or destructive interference? And are we allowed to use the language of quantum information processing to describe the highly efficient natural transfer of information and energy in light-harvesting complexes? It has been suggested that a “wavelike” sampling of the energy landscape or even a quantum search algorithm might permit to find the fastest route from the antenna to the reaction center (Engel et al., 2007). The excitation transport has also been associated with quantum random walks (Mohseni et al., 2008). In contrast to classical random walks, which we also know from the Brownian motion, the position of the quantum “walker” would not be a single random position but rather a superposition of positions.
The incorporation of interference effects in the theoretical reasoning led to further considerations concerning the possible role of the protein environment (Rebentrost et al., 2009; Olaya-Castro et al., 2008), since a close look at wave physics reveals that coherence can be both beneficial and a hindrance if the aim is to optimize the speed of transport. On the one hand, the simultaneous wavelike sampling of many parallel paths could possibly result in finding a faster way to the final goal. But on the other hand the presence of an irregular lattice of scattering centers (static disorder) may actually suppress wave transport because of destructive interference. This phenomenon, well known in solid state physics, is called Anderson localization (Anderson, 1958). In that case, thermal fluctuations of the protein environment might therefore be crucial and help to avoid localization and thus assist in the excitation transfer (Caruso et al., 2009). The importance of protein dynamics in eliminating Anderson localization was actually already discussed in an earlier paper by Balabin and Onuchic (2000), where multiple quantum pathways and interference were proposed for the electron transfer after the reduction in the special pair—instead of the excitation transfer towards the special pair that is discussed here.
The role of interference in transport phenomena can also be visualized by recalling the analogy to an optical Mach–Zehnder interferometer [as shown in Fig. 1d]: depending on the setting of phases, wave interference can guide all excitations to either one of the two exits. Quantum coherence may then be the best way to channel the interfering quanta to the desired output. But if the wave phases happened to be initially set to destructive interference, quantum coherence would be a severe handicap. In this case, even random dephasing processes would help optimize the transport efficiency.
External perturbations may also be important for energetic reasons: the electronic excitations have to be transferred between complexes of different energies. If the molecular states were too well-defined, the lacking energy overlap would reduce the transfer rate. External perturbations may broaden the transition bands and thus increase the coupling between neighboring molecules.
Recent experiments by Collini and Scholes (2009), however, hint also at another possible role of the protein environment. In their experiments they could show that coherent electronic excitation transfer along conjugated polymer chains occurs even at room temperature. These long-lasting coherences (200 fs) could only be observed in intrachain but not in interchain electronic excitation transfers.
All of the models described above bear in common that they rely on quantum coherence and decoherence and that they may be robust even under ambient environmental conditions over short time scales. It is thus the fine interplay of coherent exciton transfer, decoherence, and dephasing that yields the best results and which seems to reign one of the most important reactions in nature.
Conformational quantum superpositions in biomolecules
Since atoms can exist in a superposition of position states, this may also lead to a superposition of conformational states in molecules. A tunneling-induced superposition of conformation states is conceivable. It becomes, however, highly improbable when many atoms have to be shifted over large distances and across high potential wells during the state change.
Photoisomerization is another way of inducing structural state changes in molecules—now using photon exchange, instead of tunneling. This opens the possibility to connect even energetically separated states. The photo-induced all-trans-13-cis transition of retinal is a famous example where a single photon can cause a sizeable conformation change. But much of the subsequent atom rearrangement occurs in interactions with the thermal environment (Gai et al., 1998). In spite of that, it was possible to gain coherent quantum control in this process. Applying pulse-shaped femtosecond laser excitation to retinal in a native protein environment Prokhorenko et al. (2006) achieved a modulation of the isomerization yield by ±20%. The detected dependence on the laser phase is a good indication for the relevance of quantum interference among vibrational states. But a coherent superposition of functionally different configuration states, instead of electronic or vibrational states, has not been achieved for any large biomolecule, so far.
Decoherence has often been named to explain the prevalence of chirality in biomolecules. If a molecule may exist in two enantiomers, quantum mechanics allows, in principle, also for a coherent superposition of the left-handed and the right-handed state. In practice, however, this is not observed for larger particles. An intuitive argument is based on the fact that various scattering processes between a molecule and its environment depend on its chirality. This may include the scattering of polar light and elementary particles or the interaction through higher-order London dispersive forces between polarizable bodies. Such events may act as quantum measurements and projections onto a chirality state. And in many cases, the energy barrier between the symmetric ground states will then be too high to allow for their spontaneous mixing on a time scale comparable to the scattering events (Trost and Hornberger, 2009).
The generation and controlled decoherence of chirality superposition states in biological molecules thus still remains an open challenge. The lack of any experimental evidence for coherent conformation superpositions in large molecules also seriously questions a recent model by Hameroff and Penrose (1996) who suggested that the collapse of such superpositions in microtubuli may be the cause for the emergence of human consciousness.
Spin and the magnetic orientation of migratory birds
It is well-established that various animals are able to derive direction information from the geomagnetic field (Wiltschko and Wiltschko, 1995; Ritz et al., 2000; Johnsen and Lohmann, 2008). Some mammals perceive the Earth’s field as a polarity compass, distinguishing “north” and “south,” while birds and reptiles rely on an inclination compass that discriminates between “polewards” and “equatorwards” and which exploits both the intensity and the gradient of the field. Interestingly, it could be shown (Wiltschko and Wiltschko, 2006; and references therein) that the orientation in the magnetic field requires the presence of visible light beyond a certain photon energy and that an oscillating magnetic field (0.1–10 MHz) can disturb the bird’s senses.
It has therefore been argued that vision-based magnetosensing might be rooted in the light-induced formation of a radical pair (Schulten et al., 1978), a mechanism originally invoked to explain the photochemically induced dynamic polarization in nuclei (Closs, 1969; Kaptein and Oosterhoff, 1969): when light falls onto a donor molecule in the bird’s eye, it may excite it into a singlet state [Fig. 3c]. The molecule may then transfer an electron to a neighboring acceptor molecule. The freshly formed pair of radical molecules usually starts in a singlet state (total spin quantum number: s=0), but in the presence of hyperfine couplings with the molecular nuclei, it will undergo an interconversion between the singlet and the triplet state (s=1). Since spin is otherwise rather well protected from environmental influences on a short time scale, it is assumed that the spin pair remains quantum correlated, i.e., entangled in this process. This is also supported by a recent calculation (Rieper et al., 2009), where even a weak external oscillatory magnetic field noise was admitted and not able to fully destroy entanglement. The evolution of the electron spins both in the presence of the nuclei, and the earth’s magnetic field will vary the ratio between singlet and triplet states. Since many chemical reactions are spin-dependent—in particular, also the back-transfer of the electron from the acceptor to the donor—the spin evolution should also influence the ratio of molecular products that are finally formed in the bird’s eye. A model for the transduction from the radical pair to the neuronal correlates was proposed by Weaver et al. (2000) who also estimated the requirements on the size and the temperature dependence of the system in order to yield a certain sensitivity.
The radical pair mechanism was ascribed to the signaling protein cryptochrome that can be found in the bird’s retina (Wiltschko and Wiltschko, 2006). Both the electron transfer from a photo-excited flavin adenine dinucleotide along a chain of tryptophan molecules and the reverse recombination reaction are supposed to be sensitive to the geomagnetic field (Solov’yov and Schulten, 2009).
The idea is further supported by recent experiments of Maeda et al. (2008), who showed that the radical pair mechanism in the earth’s field is actually sufficiently strong to alter the chemical end products in a custom-designed complex that was built from a carotenoid, a porphyrin, and a fullerene C60.
In order to further corroborate that magnetosensing is related to quantum-correlated (entangled) electrons, Cai et al. (2009) suggested to use a sequence of short radio-frequency pulses to obtain active quantum control over the radical pair spins, immediately after their creation. Such and related experiments are still required to further elucidate this intriguing phenomenon.
SPECULATIONS ON QUANTUM INFORMATION AND BIOLOGY ON THE LARGE SCALE
Most puzzles of quantum physics are related to the way information is encoded and processed. Some researchers would therefore demand that “quantum biology” should be defined by its use of quantum information. The present section recapitulates two recent speculations, which aim at much larger scales than that of a few molecules. We clearly state that, as of today, these hypotheses are without any experimental justification and even disputed on theoretical grounds. But as some of them have gained rather high popularity in discussions, they merit mentioning and brief comments.
Quantum physics and the human mind
About two decades ago, Roger Penrose raised the question whether classical physics alone could suffice to explain the enormous problem solving capabilities of the human brain (Penrose, 1989). And he speculated that a combination of currently irreconcilable pieces of physics, namely, quantum theory and general relativity, might open a new window to our understanding of human consciousness, i.e., another phenomenon, which is hardly understood.
Together with the consciousness scientist Stuart Hameroff, he proposed a model that assumes that the human mind may exploit at least two conformations of microtubuli as values of a quantum bit. The quantumness of the proteins was suggested to solve complex computational problems in the brain while the act of consciousness would be linked to a gravity-induced objective collapse of the quantum wave function (Hameroff and Penrose, 1996).
Intriguing as the idea of macroscopic quantum coherence may be, the proposed model hits several hard bounds and controversies: as of today, no one has ever been able to prepare and characterize a useful coherent macroscopic quantum superposition of two conformations in a macromolecule, not even in the laboratory. And even if it existed in nature, decoherence is believed to be orders of magnitude too fast to make it relevant on physiological time scales (Tegmark, 2000; Eisert and Wiseman, 2007).
An objective collapse of the wave function is currently also only one of many models to explain the emergence of classicality from quantum physics. The dynamics of the proposed gravitational collapse is neither theoretically understood nor experimentally observed. It may also surprise that microtubuli were chosen as the decisive agents in quantum consciousness. They are by no means special to the human brain but rather ubiquitous cell support structures.
In spite of its potential deficiencies, the model serves a purpose in that it stretches the scientific fantasy to its very limits. And even though it is unlikely that all details of the proposal will survive future scientific explorations, experimental efforts in proving or disproving these details will lead to new insights into the relevance of quantum phenomena within the life sciences.
May quantum physics speed up biological evolution?
The idea starts from the question how a complex protein or strand of DNA could possibly have formed by random trials and mere chance from primordial amino acids or a series of nucleotides up to the high degree of complexity that is required to drive self-replication and evolution.
It has therefore been asked whether a faster macroscopic quantum sorting mechanism might have been involved in finding the first successfully self-replicating molecule on Earth (McFadden, 2000). However, its realization on our early Earth must have involved thousands of atoms and molecules in a warm and wet environment under the additional precondition that all sorts of molecules were available that the formation of the sample molecular structures wasenergetically accessible and that the molecules were delocalized over large areas in the given environment.
In particular the latter requirement is in variance with the findings of molecule decoherence experiments (Hornberger et al., 2003), which confirm that any measurement—be it collision with other molecules, phonons, or photons—is capable of destroying the quantum delocalization, if the interaction retrieves position information. But even if we hypothesize that large molecules could be delocalized in a primordial soup, the fastest speed-up in Grover’s quantum search has still only a “square root” advantage and the number of combinations is still stupendous. One might argue that the initial replicators were extremely tiny and that the first useful molecules for life were only influenced or catalyzed by the replication of the tinier structures. But even then a feedback loop between biological evolution and the suggested quantum coherence remains highly speculative in the light of present knowledge.
CONCLUSIONS
Quantum physics and the life sciences are both attracting increasing interest, and research at the interface between both fields has been growing rapidly. As of today, experimental demonstrations of quantum coherence in biology are still limited to the level of a few molecules. This includes for instance all quantum chemistry, tunneling processes, coherent excitation transport, and local spin effects.
In recent years quantum biology has stimulated the scientific reasoning and fantasies and has triggered hypotheses ranging from “exploratory” and “visionary” over “speculative” to “very likely to be simply wrong.” The current status of research does not always allow one to draw a precise borderline between these classifications. Experimental facts are largely missing, theoretical understanding is still an enormous challenge, and scientists are arguing both in favor and against a variety of these ideas.
Fascinating combinations of physics and biology can be understood already now. We have identified a large number of interconnects between quantum physics and the life sciences and the status of present experimental skills is great. But the complexity of living systems and high-dimensional Hilbert spaces is even greater.
When we talk about quantum information, the discussion always circles around exponential speed-up. But in living systems any improvement by a few percent might already make the difference in the survival of the fittest. Therefore, even if coherence or entanglement in living systems were limited to very short time intervals and very small regions in space—and all physics experiments up to now confirm this view—simple quantum phenomena might possibly result in a benefit and give life the edge to survive.
We still have to learn about the relevance and evolutionary advantage of quantum physics in photosynthesis, the sense of smell, or the magnetic orientation of bird. We still do not know whether quantum entanglement is useful on the molecular level under ambient conditions, whether quantum information processing could possibly be implemented in organic systems. We still do not fully understand and appreciate the philosophical implications of the quantum-to-classical transition even under laboratory conditions.
We thus conclude that the investigation of quantum coherence and entanglement in biological systems is timely and important. And it will need even more visions, further refined theories, and above all—a significantly broadened basis in carefully worked out and interdisciplinary experiments.
ACKNOWLEDGMENTS
M.A. would like to thank the FWF for support in the Wittgenstein Program Contract No. Z149-N16. V.V. would like to thank EPSRC, QIP IRC, Royal Society, and the Wolfson Foundation, National Research Foundation (Singapore) and the Ministry of Education (Singapore) for financial support.
REFERENCES
- Abbot, D, Davies, P C, and Pati, A K (2008). Quantum Aspects of Life. Imperial College Press. [Google Scholar]
- Amico, L, Fazio, R, Osterloh, A, and Vedral, V (2008). “Entanglement in many-body systems.” Rev. Mod. Phys. 80, 517–576. 10.1103/RevModPhys.80.517 [DOI] [Google Scholar]
- Anderson, P (1958). “Absence of diffusion in certain random lattices.” Phys. Rev. 109, 1492–1505. 10.1103/PhysRev.109.1492 [DOI] [Google Scholar]
- Arndt, M, Nairz, O, Voss-Andreae, J, Keller, C, der Zouw, G V, and Zeilinger, A (1999). “Wave-particle duality of C60 molecules.” Nature (London) 401, 680–682. 10.1038/44348 [DOI] [PubMed] [Google Scholar]
- Balabin, I, and Onuchic, J (2000). “Dynamically controlled protein tunneling paths in photosynthetic reaction centers.” Science 290, 114–117. 10.1126/science.290.5489.114 [DOI] [PubMed] [Google Scholar]
- Balasubramanian, G, et al. (2008). “Nanoscale imaging magnetometry with diamond spins under ambient conditions.” Nature (London) 455, 648–651. 10.1038/nature07278 [DOI] [PubMed] [Google Scholar]
- Blankenship, R E (1989). “Special issue—tunneling processes in photosynthesis.” Photosynth. Res. 22, 1. 10.1007/BF00114760 [DOI] [Google Scholar]
- Blankenship, R E (2002). Molecular Mechanisms of Photosynthesis, Blackwell, Oxford, U.K. [Google Scholar]
- Bouwmeester, D, Ekert, A, and Zeilinger, A (eds) (2000). The physics of quantum information, Springer, Berlin. [Google Scholar]
- Brixner, T, Stenger, J, Vaswani, H, Cho, M, Blankenship, R E, and Fleming, G (2005). “Two-dimensional spectroscopy of electronic couplings in photosynthesis.” Nature (London) 434, 625–628. 10.1038/nature03429 [DOI] [PubMed] [Google Scholar]
- Brookes, J C, Hartoutsiou, F, Horsfield, A P, and Stoneham, A M (2007). “Could humans recognize odor by phonon assisted tunneling?.” Phys. Rev. Lett. 98, 038101. 10.1103/PhysRevLett.98.038101 [DOI] [PubMed] [Google Scholar]
- Brougham, D F, Caciuffo, R, and Horsewill, A J (1999). “Coordinated proton tunnelling in a cyclic network of four hydrogen bonds in the solid state.” Nature (London) 397, 241–243. 10.1038/16672 [DOI] [Google Scholar]
- Brukner, C, Vedral, V, and Zeilinger, A (2006). “Crucial role of quantum entanglement in bulk properties of solids.” Phys. Rev. A 73, 012110. 10.1103/PhysRevA.73.012110 [DOI] [Google Scholar]
- Buck, L, and Axel, R (1991). “A novel multigene family may encode odorant receptors—a molecular basis for odor recognition.” Cell 65, 175–187. 10.1016/0092-8674(91)90418-X [DOI] [PubMed] [Google Scholar]
- Cai, J, Guerreschi, G G, and Briegel, H J (2009). “Quantum control and entanglement in a chemical compass.” arXiv:0906.2383. [DOI] [PubMed]
- Cai, J, Popescu, S, and Briegel, H J (2008). “Dynamic entanglement in oscillating molecules.” arXiv:0809.4906. [DOI] [PubMed]
- Caruso, F, Chin, A, Datta, A, Huelga, S, and Plenio, M (2009). “Fundamental mechanisms of noise supported energy transfer in biological systems.” arXiv:0901.4454.
- Cha, Y, Murray, C, and Klinman, J (1989). “Hydrogen tunneling in enzyme reactions.” Science 243, 1325–1330. 10.1126/science.2646716 [DOI] [PubMed] [Google Scholar]
- Cheng, Y C, and Fleming, G R (2009). “Dynamics of light harvesting in photosynthesis.” Annu. Rev. Phys. Chem. 60, 241–262. 10.1146/annurev.physchem.040808.090259 [DOI] [PubMed] [Google Scholar]
- Clauser, J (1997). “De Broglie-wave interference of small rocks and live viruses.” In Experimental Metaphysics, Cohen, R, Horne, M, and Stachel, J (eds), pp 1–11, Kluwer Academic, Dordrecht, Germany. [Google Scholar]
- Closs, G L (1969). “Mechanism explaining nuclear spin polarizations in radical combination reactions.” J. Am. Chem. Soc. 91, 4552–4554. 10.1021/ja01044a043 [DOI] [Google Scholar]
- Collini, E, and Scholes, G D (2009). “Coherent intrachain energy migration in a conjugated polymer at room temperature.” Science 323, 369–373. 10.1126/science.1164016 [DOI] [PubMed] [Google Scholar]
- Cory, D G, Price, M D, Maas, W, Knill, E, Laflamme, R, Zurek, W H, Havel, T F, and Somaroo, S S (1998). “Experimental quantum error correction.” Phys. Rev. Lett. 81, 2152–2155. 10.1103/PhysRevLett.81.2152 [DOI] [Google Scholar]
- Cronin, A D, Schmiedmayer, J, and Pritchard, D E (2009). “Optics and interferometry with atoms and molecules.” Rev. Mod. Phys. 81, 1051–1129. 10.1103/RevModPhys.81.1051 [DOI] [Google Scholar]
- Dahan, M, Levi, S, Luccardini, C, Rostaing, P, Riveau, B, and Triller, A (2003). “Diffusion dynamics of glycine receptors revealed by single-quantum dot tracking.” Science 302, 442–445. 10.1126/science.1088525 [DOI] [PubMed] [Google Scholar]
- Dahlbom, M, Pullerits, T, Mukamel, S, and Sundstrom, V (2001). “Exciton delocalization in the B850 light-harvesting complex: comparison of different measures.” J. Phys. Chem. B 105, 5515–5524. 10.1021/jp004496i [DOI] [Google Scholar]
- De Vault, D, and Chance, B (1966). “Studies of photosynthesis using a pulsed laser. I: Temperature dependence of cytochrome oxidation rate in chromatium. evidence for tunneling.” Biophys. J. 6, 825–847. 10.1016/S0006-3495(66)86698-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diosi, L (1989). “Models for universal reduction of macroscopic quantum fluctuations.” Phys. Rev. A 40, 1165–1174. 10.1103/PhysRevA.40.1165 [DOI] [PubMed] [Google Scholar]
- Dyson, G M (1938). “The scientific basis of odour.” Chem. Ind. 57, 647–651. 10.1002/jctb.5000572802 [DOI] [Google Scholar]
- Eisert, J, Wilkens, M, and Lewenstein, M (1999). “Quantum games and quantum strategies.” Phys. Rev. Lett. 83, 3077–3080. 10.1103/PhysRevLett.83.3077 [DOI] [Google Scholar]
- Eisert, J, and Wiseman, H, (2007). “Quantum aspects of life,” In Nontrivial Quantum Effects in Biology: A Skeptical Physicists’ View, World Scientific, Singapore. [Google Scholar]
- Engel, G S, Calhoun, T R, Read, E L, Ahn, T Y, Mančal, T, Cheng, Y C, Blankenship, R E, and Fleming, G R (2007). “Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems.” Nature (London) 446, 782–786. 10.1038/nature05678 [DOI] [PubMed] [Google Scholar]
- Felker, P M, Lambert, W R, and Zewail, A H (1982). “Picosecond excitation of jet-cooled pyrazine: magnetic field effects on the fluorescence decay and quantum beats.” Chem. Phys. Lett. 89, 309–314. 10.1016/0009-2614(82)83505-7 [DOI] [Google Scholar]
- Förster, T (1948). “Zwischenmolekulare energiewanderung und fluoreszenz.” Ann. Phys. 2, 55–75. 10.1002/andp.19484370105 [DOI] [Google Scholar]
- Gai, F, Hasson, K C, McDonald, J C, and Anfinrud, P A (1998). “Chemical dynamics in proteins: the photoisomerization of retinal in bacteriorhodopsin.” Science 279, 1886–1891. 10.1126/science.279.5358.1886 [DOI] [PubMed] [Google Scholar]
- Ghirardi, G C, Rimini, A, and Weber, T (1986). “Unified dynamics for microscopic and macroscopic systems.” Phys. Rev. D 34, 470–491. 10.1103/PhysRevD.34.470 [DOI] [PubMed] [Google Scholar]
- Glauber, R J (2006). “Nobel lecture: one hundred years of light quanta.” Rev. Mod. Phys. 78, 1267–1278. 10.1103/RevModPhys.78.1267 [DOI] [PubMed] [Google Scholar]
- Glickman, M, Wiseman, J, and Klinman, J (1994). “Extremely large isotope effects in the soybean lipoxygenase-linoleic acid reaction.” J. Am. Chem. Soc. 116, 793–794. 10.1021/ja00081a060 [DOI] [Google Scholar]
- Goldanskii, V (1979). “Facts and hypotheses of molecular chemical tunnelling.” Nature (London) 279, 109–115. 10.1038/279109a0 [DOI] [Google Scholar]
- Gray, H, and Winkler, J (2003). “Electron tunneling through proteins.” Q. Rev. Biophys. 36, 341–372. 10.1017/S0033583503003913 [DOI] [PubMed] [Google Scholar]
- Hackermüller, L, Hornberger, K, Brezger, B, Zeilinger, A, and Arndt, M (2004). “Decoherence of matter waves by thermal emission of radiation.” Nature (London) 427, 711–714. 10.1038/nature02276 [DOI] [PubMed] [Google Scholar]
- Hackermüller, L, Uttenthaler, S, Hornberger, K, Reiger, E, Brezger, B, Zeilinger, A, and Arndt, M (2003). “Wave nature of biomolecules and fluorofullerenes.” Phys. Rev. Lett. 91, 090408. 10.1103/PhysRevLett.91.090408 [DOI] [PubMed] [Google Scholar]
- Hameroff, S, and Penrose, R (1996). “Orchestrated reduction of quantum coherence in brain microtubules: a model for consciousness.” Math. Comput. Simul. 40, 453–480. 10.1016/0378-4754(96)80476-9 [DOI] [Google Scholar]
- Harrow, A W, Hassidim, A, and Lloyd, S (2009). “Quantum algorithm for solving linear systems of equations.” arXiv:0811.3171. [DOI] [PubMed]
- Hecht, S, Shlaer, S, and Pirenne, M (1942). “Energy, quanta and vision.” J. Gen. Physiol. 25, 819–840. 10.1085/jgp.25.6.819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger, K, Uttenthaler, S, Brezger, B, Hackermüller, L, Arndt, M, and Zeilinger, A (2003). “Collisional decoherence observed in matter wave Interferometry.” Phys. Rev. Lett. 90, 160401. 10.1103/PhysRevLett.90.160401 [DOI] [PubMed] [Google Scholar]
- Johnsen, S, and Lohmann, K J (2008). “Magnetoreception in animals.” Phys. Today 61, 29–35. 10.1063/1.2897947 [DOI] [Google Scholar]
- Jones, J, and Mosca, M (1999). “Approximate quantum counting on an NMR ensemble quantum computer.” Phys. Rev. Lett. 83, 1050–1053. 10.1103/PhysRevLett.83.1050 [DOI] [Google Scholar]
- Jones, J A, Vedral, V, Ekert, A, and Castagnoli, G (2000). “Geometric quantum computation using nuclear magnetic resonance.” Nature (London) 403, 869–871. 10.1038/35002528 [DOI] [PubMed] [Google Scholar]
- Joos, E, and Zeh, H D (1985). “The emergence of classical properties through interaction with the environment.” Z. Phys. B 59, 223–243. 10.1007/BF01725541 [DOI] [Google Scholar]
- Joos, E, Zeh, H D, Kiefer, C, Giulini, D, Kupsch, J, and Stamatescu, I O (2003). Decoherence and the Appearance of a Classical World in Quantum Theory, 2nd ed., Springer, Berlin. [Google Scholar]
- Kaptein, R, and Oosterhoff, J L (1969). “Chemically induced dynamic nuclear polarization II: (relation with anomalous ESR spectra).” Chem. Phys. Lett. 4, 195–197. 10.1016/0009-2614(69)80098-9 [DOI] [Google Scholar]
- Kasprzak, J, et al. (2006). “Bose-Einstein condensation of exciton polaritons.” Nature (London) 443(7110), 409–414. 10.1038/nature05131 [DOI] [PubMed] [Google Scholar]
- Keller, A, and Vosshall, L (2004). “A psychophysical test of the vibration theory of olfaction.” Nat. Neurosci. 7, 337–338. 10.1038/nn1215 [DOI] [PubMed] [Google Scholar]
- Kwiat, P, Mattle, K, Weinfurter, H, Zeilinger, A, Sergienko, A, and Shih, Y (1995). “New high-intensity source of polarization-entangled photon pairs.” Phys. Rev. Lett. 75(24), 4337–4341. 10.1103/PhysRevLett.75.4337 [DOI] [PubMed] [Google Scholar]
- Lauterbur, P (1973). “Image formation by induced local interactions: examples employing nuclear magnetic resonance.” Nature (London) 242, 190–191. 10.1038/242190a0 [DOI] [PubMed] [Google Scholar]
- Lidar, D, Chuang, I, and Whaley, K (1998). “Decoherence free subspaces for quantum computation.” arXiv:quant-ph/9807004.
- Longuet-Higgins, H C (1962). “Quantum mechanics and biology.” Biophys. J. 2, 207–215. 10.1016/S0006-3495(62)86957-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounis, B, and Orrit, M (2005). “Single-photon sources.” Rep. Prog. Phys. 68, 1129–1179. 10.1088/0034-4885/68/5/R04 [DOI] [Google Scholar]
- Löwdin, P O (1963). “Proton tunneling in DNA and its biological implocations.” Rev. Mod. Phys. 35, 724–732. 10.1103/RevModPhys.35.724 [DOI] [Google Scholar]
- Maeda, K, Henbest, K B, Cintolesi, F, Kuprov, I, Rodgers, C T, Liddell, P A, Gust, D, Timmel, C R, and Hore, P J (2008). “Chemical compass model of avian magnetoreception.” Nature (London) 453, 387–390. 10.1038/nature06834 [DOI] [PubMed] [Google Scholar]
- Marcus, R A (1956). “Theory of oxidation-reduction reactions involving electron transfer.” J. Chem. Phys. 24(5), 966–978. 10.1063/1.1742723 [DOI] [Google Scholar]
- Markham, D, Anders, J, Vedral, V, Murao, M, and Miyake, A (2008). “Survival of entanglement in thermal states.” Eur. Phys. Lett. 81, 40006. 10.1209/0295-5075/81/40006 [DOI] [Google Scholar]
- Masgrau, L, Roujeinikova, A, Johannissen, L, Hothi, P, Basran, J, Ranaghan, K, Mulholland, A, Sutcliffe, M, Scrutton, N, and Leys, D (2006). “Atomic description of an enzyme reaction dominated by proton tunneling.” Science 312, 237–241. 10.1126/science.1126002 [DOI] [PubMed] [Google Scholar]
- Matsuno, K (1999). “Cell motility as an entangled quantum coherence.” BioSystems 51, 15–19. 10.1016/S0303-2647(99)00009-X [DOI] [PubMed] [Google Scholar]
- McFadden, J (2000). Quantum Evolution: How Physics’ Weirdest Theory Explains Life’s Biggest Mystery, Harper Collins, New York. [Google Scholar]
- Mohseni, M, Rebentrost, P, Lloyd, S, and Aspuru-Guzik, A (2008). “Environment-assisted quantum walks in energy transfer of photosynthetic complexes.” J. Chem. Phys. 129, 174106. 10.1063/1.3002335 [DOI] [PubMed] [Google Scholar]
- Nielsen, M A, and Chuang, I L (2000). Quantum Computation and Quantum Information, Cambridge University Press, Cambridge, U.K. [Google Scholar]
- Olaya-Castro, A, Lee, C F, Olsen, F F, and Johnson, N F (2008). “Efficiency of energy transfer in a light-harvesting system under quantum coherence.” Phys. Rev. B 78, 085115. 10.1103/PhysRevB.78.085115 [DOI] [Google Scholar]
- Penrose, R (1989). The Emperor’s New Mind: Concerning Computers, Minds and the Laws of Physics, Oxford University Press, New York. [Google Scholar]
- Penrose, R (1996). “On gravity’s role in quantum state reduction.” Gen. Relativ. Gravit. 28, 581–600. 10.1007/BF02105068 [DOI] [Google Scholar]
- Polson, R C, and Vardeny, Z V (2004). “Random lasing in human tissues.” Appl. Phys. Lett. 85, 1289–1291. 10.1063/1.1782259 [DOI] [Google Scholar]
- Prokhorenko, V, Nagy, A, Waschuk, S, Brown, L, Birge, R, and Miller, R (2006). “Coherent control of retinal isomerization in bacteriorhodopsin.” Science 313, 1257–1261. 10.1126/science.1130747 [DOI] [PubMed] [Google Scholar]
- Rebentrost, P, Mohseni, M, Kassal, I, Lloyd, S, and Aspuru-Guzik, A (2009). “Environment-assisted quantum transport.” New J. Phys. 11, 033003. 10.1088/1367-2630/11/3/033003 [DOI] [Google Scholar]
- Reiger, E, Hackermüller, L, Berninger, M, and Arndt, M (2006). “Exploration of gold nanoparticle beams for matter wave interferometry.” Opt. Commun. 264, 326–332. 10.1016/j.optcom.2006.02.060 [DOI] [Google Scholar]
- Rieke, F, and Baylor, D (1998). “Single-photon detection by rod cells of the retina.” Rev. Mod. Phys. 70, 1027–1036. 10.1103/RevModPhys.70.1027 [DOI] [Google Scholar]
- Rieper, E, Gauger, E, Morton, J JL, Benjamin, S C, and Vedral, V (2009). “Quantum coherence and entanglement in the avian compass.” arXiv:0906.3725. [DOI] [PubMed]
- Ritz, T, Adem, S, and Schulten, K (2000). “A model for photoreceptor-based magnetoreception in birds.” Biophys. J. 78, 707–718. 10.1016/S0006-3495(00)76629-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Isart, O, Juan, M L, Quidant, R, and Cirac, J I (2009). “Towards quantum superposition of living organisms.” arXiv:0909.1469.
- Sanchez-Mosteiro, G, Koopman, M, van Dijk, EMHP, Hernando, J, van Hulst, N F, and García-Parajó, M F (2004). “Photon antibunching proves emission from a single subunit in the autofluorescent protein DsRed.” ChemPhysChem 5, 1782–1785. 10.1002/cphc.200400216 [DOI] [PubMed] [Google Scholar]
- Schrödinger, E (1944). What Is Life? The Physical Aspect of the Living Cell, Cambridge University Press, Cambridge, U.K. [Google Scholar]
- Schulten, K, Swenberg, C, and Weller, A (1978). “Biomagnetic sensory mechanism based on magnetic-field modulated coherent electron-spin motion.” Z. Phys. Chem. 111(1), 1–5. [Google Scholar]
- Sherson, J, Krauter, H, Olsson, R, Julsgaard, B, Hammerer, K, Cirac, I, and Polzik, E (2006). “Quantum teleportation between light and matter.” Nature (London) 443, 557–560. 10.1038/nature05136 [DOI] [PubMed] [Google Scholar]
- Shor, P (1995). “Scheme for reducing decoherence in quantum computer memory.” Phys. Rev. A 52, 2493–2496. 10.1103/PhysRevA.52.R2493 [DOI] [PubMed] [Google Scholar]
- Sivozhelezov, V, and Nicolini, C (2007). “Prospects for octopus rhodopsin utilization in optical and quantum computation.” Phys. Part. Nucl. Lett. 4, 189–196. 10.1134/S1547477107020227 [DOI] [Google Scholar]
- Solov’yov, I A, and Schulten, K (2009). “Magnetoreception through cryptochrome may involve superoxide.” Biophys. J. 96, 4804–4813. 10.1016/j.bpj.2009.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegmark, M (2000). “The importance of quantum decoherence in brain processes.” Phys. Rev. E 61, 4194–4206. 10.1103/PhysRevE.61.4194 [DOI] [PubMed] [Google Scholar]
- Tronrud, D, Wen, J, Gay, L, Fenna, R, and Blankenship, RE (2009). “Unique bidentate ligation of a bacteriochlorophyll found in an FMO antenna protein.” to be published.
- Trost, J, and Hornberger, K (2009). “Hund’s paradox and the collisional stabilization of chiral molecules.” Phys. Rev. Lett. 103, 023202. 10.1103/PhysRevLett.103.023202 [DOI] [PubMed] [Google Scholar]
- Turin, L (1996). “A spectroscopic mechanism for primary olfactory reception.” Chem. Senses 21(6), 773–791. 10.1093/chemse/21.6.773 [DOI] [PubMed] [Google Scholar]
- Turin, L (2002). “A method for the calculation of odor character from molecular structure.” J. Theor. Biol. 216(3), 367–385. 10.1006/jtbi.2001.2504 [DOI] [PubMed] [Google Scholar]
- van Grondelle, R, and Novoderezhkin, V (2006). “Energy transfer in photosynthesis: experimental insights and quantitative models.” Phys. Chem. Chem. Phys. 8(7), 793–807. 10.1039/b514032c [DOI] [PubMed] [Google Scholar]
- Vedral, V (2003). “Entanglement hits the big time.” Nature (London) 425, 28–29. 10.1038/425028a [DOI] [PubMed] [Google Scholar]
- Vedral, V, and Plenio, M (1998). “Entanglement measures and purification procedures.” Phys. Rev. A 57(3), 1619–1633. 10.1103/PhysRevA.57.1619 [DOI] [Google Scholar]
- Watson, J DH, and Crick, F HC (1953). “Molecular structure of nucleic acids: a structure for deoxyribose nucleic acid.” Nature (London) 171, 737–738. 10.1038/171737a0 [DOI] [PubMed] [Google Scholar]
- Weaver, J, Vaughan, T, and Astumian, R (2000). “Biological sensing of small field differences by magnetically sensitive chemical reactions.” Nature (London) 405, 707–709. 10.1038/35015128 [DOI] [PubMed] [Google Scholar]
- Wieśniak, M, Vedral, V, and Brukner, Č (2008). “Heat capacity as an indicator of entanglement.” Phys. Rev. B 78, 064108. 10.1103/PhysRevB.78.064108 [DOI] [Google Scholar]
- Wiltschko, R, and Wiltschko, W (1995). Magnetic Orientation in Animals Springer, Berlin. [Google Scholar]
- Wiltschko, R, and Wiltschko, W (2006). “Magnetoreception.” BioEssays 28, 157–168. 10.1002/bies.20363 [DOI] [PubMed] [Google Scholar]
- Winkler, J, Gray, H, Prytkova, T, Kurnikov, I, and Beratan, D (2005). “Electron transfer through proteins.” Bioelectronics, pp 15–33, Wiley-VCH, Weinheim, Germany. [Google Scholar]
- Zarzo, M (2007). “The sense of smell: molecular basis of odorant recognition.” Biol. Rev. Cambridge Philos. Soc. 82, 455–479. 10.1111/j.1469-185X.2007.00019.x [DOI] [PubMed] [Google Scholar]
- Zurek, W H (1991). “Decoherence and the transition from quantum to classical.” Phys. Today 44, 36–44. 10.1063/1.881293 [DOI] [Google Scholar]