Abstract
The mitogen-activated protein kinase (MAPK) cascade is a paradigmatic signaling cascade, which plays a crucial role in many aspects of cellular events. The main initiator of the cascade in Xenopus oocytes is the oncoprotein Mos. After activation of the cascade, Mos activity is stabilized by MAPK via a feedback loop. Mos concentration levels are, however, not controlled by MAPK alone. In this paper we show, by imposing either a sustained or a peaked activity of M-phase promoting factor (MPF) (Cdc2-cyclin B), how the latter regulates the dynamics of Mos. Our experiments are supported by a detailed kinetic model for the Mos-MPF-MAPK network, which takes into account the three different phosphorylation states of Mos and, as a consequence, allows us to determine the time evolution of Mos under control of MPF. Our work opens a path toward a more complete and biologically realistic quantitative understanding of the dynamic interdependence of Mos and MPF in Xenopus oocytes.
The mitogen-activated protein kinase (MAPK) cascade is a paradigmatic signaling cascade, which plays a crucial role in many aspects of cellular events. Relaying extracellular stimuli at the plasma membrane to targets in the cytoplasm and the nucleus, the MAPK cascade consists of several levels in which the activated kinase at each level phosphorylates the kinase at the downstream level in the cascade. The MAPKK kinase—the activator at cascade entry—is either the protein Raf in somatic cells or the oncoprotein Mos in female gametes such as those of vertebrates like Xenopus, which underlies our present work.
In frog female gametes, the protein-protein interaction network organization drives an ultrasensitive and all-or-none response of MAPK. The functional organization of the MAPK signal transduction pathway greatly enhances the sensitivity of cellular targets to external stimuli such as hormones and leads to ultrasensitivity of MAPK to the input signal. The basis of the topological representation of the pathway was laid in (Huang and Ferrell, 1996). All MAPKs are recruited upon progesterone stimulation in Xenopus oocytes (Ferrell and Machleder, 1998) and this pathway has been demonstrated to be involved in at least three aspects of meiosis progression: (1) meiotic spindle formation (Bodart et al., 2002, 2005; Horne and Guadagno, 2003); (2) DNA replication inhibition (Gross et al., 2001;Dupré et al., 2002); and (3) establishment of the cytostatic activity in metaphase II-arrested oocytes (Dupré et al., 2002; Inoue et al., 2007).
Above a threshold value progesterone stimulates oocytes to resume meiosis in an irreversible manner (Xiong and Ferrell, 2003), dependent upon a burst of protein-kinase activities involving Cdk and MAPK. The key protein in the initiation module of the MAPK cascade is the oncoprotein Mos, a MAPK kinase whose function appears to be conserved from echinoderm models to vertebrates (echinoderms: Amiel et al., 2009; mammals: Colledge et al., 1994, Hashimoto et al., 1994; goldfish: Kajiura-Kobayashi et al., 2000; amphibians: Sagata et al., 1988, 1989). In immature amphibian oocytes, Mos mRNA is stored but not translated. Upon hormonal stimulation, Mos mRNA is polyadenylated and translated. In response to the activation of the polyadenylation and protein synthesis machineries, Mos level dramatically increases, leading to MEK1 phoshorylation and MAPK activation.
Mos is not the only molecule implied in the activation of the MAPK cascade. Another important actor is the M-phase promoting factor (MPF), which is activated simultaneously to MAPK at meiotic resumption. In their pioneering experiments (Masui and Markert, 1971) demonstrated that cytoplasm from an egg or metaphase II arrested oocyte was capable of inducing meiosis or M-phase entry. The biochemical nature of the meiotic promoting activity detected in egg-cytoplasm was later determined to be a heterodimer complex of Cdc2 and cyclin B; for a review, see Masui (2001). The MPF was found to be the universal factor responsible for the G2∕M cell cycle transition, regardless of the species considered.
Both Mos and MPF activities are interrelated and MPF plays a non-negligible role in the activation of the MAPK module. In Xenopus oocytes, Mos had appeared as the best candidate for a MPF-regulated stimulator of the MAPK activation during meiosis, although Mos-independent mechanisms cannot be excluded. Indeed, if the first MPF activation burst at metaphase I is impaired by chemical inhibitors such as roscovitine and olomoucine (Flament et al., 2000) or through the use of Cdc2 negative mutants or Cdc2 inactivating antibodies (Nebreda et al., 1995), no MAPK activation is observed in Xenopus oocytes. Oligonucleotide strategies targeting cyclin B have also been employed to address the role of MPF during meiosis, but failed to prevent the activation peak at meiosis I because the latter relies on cyclins associated to Cdc2 under an inactive stored stock of MPF (called pre-MPF) and is not dependent upon cyclin B synthesis. Such studies stressed the role of MPF reactivation and cyclin B synthesis in division spindle morphogenesis but also underlined the role of MPF activity in Mos maintenance (Hochegger et al., 2001, Haccard and Jessus, 2006). Consistent with this view, injection of nondegradable cyclin B in these conditions prevented the loss of Mos (Hochegger et al., 2001). The MPF activity inhibition can also be achieved at metaphase II arrest, a stage at which most vertebrates stop in anticipation for fertilization. When the metaphase II-block release occurs upon calcium release at fertilization, the physiological drop in MPF activity is followed by Mos degradation within 30 min (Watanabe et al., 1989; Bodart et al., 1999). Similarly, when chemical kinase inhibitors such as six dimethyl amino purine impair MPF activity, Mos disappears 30 min after inactivation of MPF (Bodart et al., 1999), suggesting that Mos maintenance is under the control of MPF. Metaphase II arrest has further been proposed to be set by the equilibrium between Mos and MPF activities at metaphase II: Mos prevents MPF inactivation through the inhibition of cyclin B proteolysis by modulating the activity of the anaphase promoting complex (Wu and Kornbluth, 2008) while MPF promotes Mos stability through its phosphorylation (Castro et al., 2001; Sheng et al., 2002). Taken together, these observations lead to the conclusion that MPF is a major component of both Mos activation and maintenance.
In this work, we address the roles of both Mos and MPF in the initiation steps of the MAPK cascade. We make explicit, by both experiment and a modeling approach, how Mos dynamics is driven by MPF. In order to achieve this we develop a detailed model for MAPK cascade activation in the presence of MPF. The key feature of the model is, aside from the inclusion of MPF, the distinction between the different states of stability and activity of the oncoprotein Mos, which, to our knowledge, is developed here for the first time into a mathematical model for the kinetics of the MAPK cascade. The model is confronted with experiments in which the MPF activity is turned on by hormonal stimulation or through the activation of the MPF auto-amplification loop either in presence or absence of the MAPK feedback loop.
The paper is organized as follows. After the introduction, a detailed description is given of the three phosphorylation states of Mos and how they can be built into a kinetic model represented by ordinary differential equations (ODEs) for the concentrations of the molecules. Subsequently, the model is parameterized and its equilibrium states are obtained from a bifurcation analysis. Finally, we describe our experiments on the time evolution of Mos under a number of different conditions and their comparison with the results obtained from numerical simulations from our model. We conclude and provide an outlook on future work based on our results.
RESULTS
A model for Mos-MPF-driven activation of the MAPK cascade
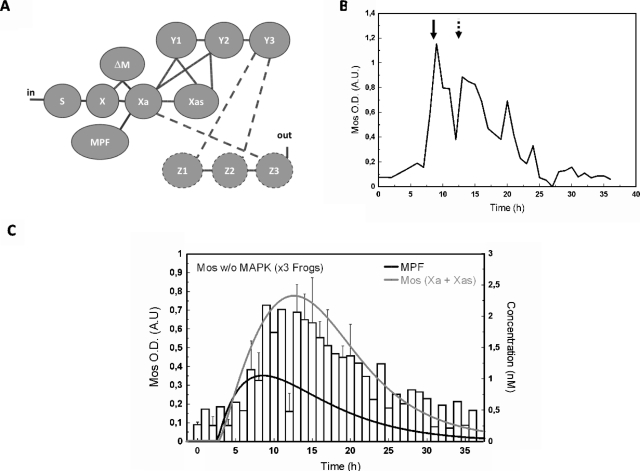
The essential feature of our model for the initiation steps of the MAPK cascade network is that it takes into account three different forms of Mos, which differ among each other in their phosphorylation state, see Fig. 1A.
Figure 1. (A) Mos phosphorylations states.
Folded Mos (X) is at first inhibited by phosphorylation of residue Ser-105, located in a α-helix domain and promoting its association with CK2β. Phosphorylated on Ser-3, X, as well as Xa, are targeted for proteasomal degradation, presumably through their association with the anaphase promoting complex. Beside its role in protein stabilization, Ser-3 phosphorylation enhances catalytic activity of Xas toward Y/MEK. (See text for further discussion.) (B) Network structure of the MAPK cascade activation in Xenopus oocytes as studied in the present work. Molecules are indicated by their abbreviating symbols in the kinetic model, whereby X,Y,Z with their indices standing for the three corresponding phosphorylation states of Mos, MEK, and MAPK, respectively.
First, a distinction between active versus nonactive Mos is made. Mos activation can be conceived as a double-hit process in which dephosphorylation of Ser-105 and folding proteins such as chaperones free Mos from an inactive status while phosphorylation of Ser-3 enables Mos to exert its full catalytic activity on MEK1. Indeed, when not phosphorylated on the Ser-3 residue, Mos appears to exhibit little or no catalytic activity. In accord with (Chen and Cooper, 1995) who proposed that the phosphorylation of Ser-3 promotes the interaction of Mos with MEK1 and promotes the activation of MEK1 by Mos, it was observed that Ser-3A mutation decreased Mos activity and ability to promote meiosis resumption (Yue and Ferrell, 2006). Other studies are consistent with this view (Matten et al., 1996), although Ser-3 mutations were initially not reported to impair Mos activity because Mos phosphorylation could be observed even in absence of catalytic activity of the protein (Freeman et al., 1992; Nishizawa et al., 1992). In contrast to active Mos, inactive Mos is characterized by phosphorylation of Ser-105 in the kinase domain of Mos and an unfolded structure (Yue and Ferrell, 2006): (i) dephosphorylation of Ser-105 has been proposed to drive the structural rearrangement of helix alpha-C and (ii) inhibition of chaperone Hsp90 by geldanamycine leads to accumulation of inactive Mos (Fisher et al., 1999). Active Mos appears as a folded protein dephosphorylated on Ser-105 but phosphorylated on Ser-3. The specificity constant for dephosphorylated Ser-105∕folded Mos toward MEK is expected to be generally inferior to the specificity constant of dephosphorylated Ser-105∕folded∕phosphorylated Ser-3.
Second, a distinction between stable versus nonstable Mos is made. The main residue involved in Mos stability appears to be Ser-3, although Ser-16 phosphorylation prevents Mos degradation in Cos-cells (Pham et al., 1999). A second-codon rule-based ubiquitin pathway was first hypothesized, where Ser-3 phosphorylation prevented the recognition of Mos by the ubiquitin-ligase driving its degradation (Freeman et al., 1992; Nishizawa et al., 1993, 1992). Replacement of the Ser-3 residue by Asp3 impairs Mos degradation by the proteasome (Ishida et al., 1993). More recently, unphosphorylated X-Ser-Mos was shown to be short-lived in immature stage VI oocytes, irrespective of the nature of the N-terminal residue, whereas phosphorylated X-Ser-Mos is metabolically stable (Sheng et al., 2002).
Figure 1B shows how this “Mos-module” integrates into the MAPK cascade. We denote by S the source for Mos, which stands for the stock of mRNA whose translation is regulated by polyadenylation mechanisms, details of which are neglected in our model. The latter step is a multistep-process including polyadenylation machinery from S to X; several actors have been identified, among them are the CPEB, its regulators, PAP machinery (Charlesworth et al., 2002; De Moor and Richter, 1997; Gebauer and Richter, 1997; Mendez et al., 2000; Sheets et al., 1995). This is also to note that the potential roles of feedback loops at this level are not considered here.
X stands for the unfolded, Ser-105 phosphorylated form of Mos. Because this form is not phosphorylated on Ser-3, it may be targeted by the degradation machinery and is assumed to have a short lifetime. This form is not expected to have any catalytic activity toward MEK (Y1,Y2).
Xa stands for a folded, Ser-3 and Ser-105 dephosphorylated form of Mos. This short-lived protein exhibits a low level of catalytic activity on MEK (Y1,Y2). Both X and Xa half-lives have been estimated to be about 30 min (Sagata et al., 1989; Sheng et al., 2002; Watanabe et al., 1989).
Xas stands for a folded, Ser-3 phosphorylated form of Mos. Shielded against degradation, this protein exerts its kinase activity in a fully efficient way. Among the kinases that prevent such degradation mechanism and stabilize Mos into a long-lived protein is the MPF [from MPF to Xa (Castro et al., 2001; Liu et al., 1990; Nebreda et al., 1995)]. Then, Mos phosphorylates MEK from Xa∕Xas to (Y1,Y2), which in turn activates MAPK from Y3 to Z1,Z2 by dual phosphorylation of a TEY motif (Ferrell and Bhatt, 1997; Nebreda and Hunt, 1993; Posada et al., 1993; Sagata et al., 1988). MAPK∕Erk are the main identified targets of MEK (Cowley et al., 1994; Mansour et al., 1994). Finally, this interaction network is embedded in a feedback loop, which enables MAPK to enhance Mos stability∕accumulation [from Z3 to Xa, (Matten et al., 1996)].
The kinetic model of the Mos-MPF-MAPK cascade
The initiation of the MAPK cascade by Mos has been modeled mathematically in several publications, most notably by the Ferrell group (Huang and Ferrell, 1996; Ferrell and Bhatt, 1997; Ferrell and Machleder, 1998; Xiong and Ferrell, 2003; Brandman et al., 2005; Angeli et al., 2004; Yue and Ferrell, 2006; Justman et al., 2009). Models for the MAPK cascade triggered by Mos generally do not distinguish as explicitly between the different phosphorylation states of Mos as we do here. As indicated in Figs. 1A, 1B, in our model each molecule of the cascade Mos-MEK-MAPK is considered in each of its three phosphorylation states. For Mos, we call these states X, Xa, and Xas, respectively. In our model, the upstream kinases Xa and Xas are effective activators of the downstream kinase Y1,2 by phosphorylation. Then, the active form Y3 phosphorylates Z1,2 and therefore produces Z3. Note that even in the presence of basal concentrations of active X (Xa, Xas) and Z3 is present at low concentration when the cascade is in its low concentration state. The symbols Y1,2,3 and Z1,2,3 account for the three phosphorylation states of the kinases MEK and MAPK. The subscript numbers represent in increasing order the nonphosphorylated form of the kinase, the singly phosphorylated form of the kinase and the doubly phosphorylated form of the kinase, the latter of which is the only active one. The symbols Px, Py, and Pz represent, respectively, the phosphatases of the Mos protein and the proteins Y2,3 and Z2,3.
The mathematical model representing the Mos-MPF-MAPK cascade kinetics is given by the following system of ODEs:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
| (9) |
For this set of equations two relations of mass conservation exist [see Eqs. 4 and 7, 8, 9]:
| (10) |
| (11) |
In our discussion the concentration of phosphatases are constants.
Tables 1, 2 contain the parameter values of the ODE model. Table 1 lists the paramerization of the cascade itself for which previous data exist in the literature. The papers by (Huang and Ferrell, 1996) and (Angeli et al., 2004) provided estimates of the concentrations of the studied proteins. The total concentration of [YT] and [ZT] is taken from Angeli’s study. The concentrations of phosphatases Px,y,z are identical to those used by Huang and Ferrell for their numerical simulation. Moreover, Angeli provides in the supplementary material the values of the catalytic constants of MEK phosphorylation by Mos (kcat=0.064 s−1) and MAPK phosphorylation by MEK (kcat=0.06 s−1), and the Michaelis–Menten constants associated with the affinity of the complex Mos∕MEK (KM=1,200 nM) and the complex MEK∕MAPK (KM=300 nM). We note that these KM values are high and equal to the total concentration of substrate, so the linear approximation of the dynamics is possible because by definition KM corresponds to the value of concentration of the substrate at which the reaction speed is at its midlevel. We further obtain from the Supplement of Angeli’s paper the experimental values related to the speed of dephosphorylation of MEK (5 nM s−1). For the dephosphorylation of MAPK, we used the reference proposed by Angeli in the Supplement to his paper. Within this reference, we find an experimental measurement of the dephosphorylation rate of about 0.1 phosphate groups removed per minute per molecule of MAPK.
Table 1.
Parameters related to the Y1,2,3∕Z1,2,3 cascade.
| Name | Value | Unit | Reference |
|---|---|---|---|
| k8, k9 | 0.0000533 | nM−1 s−1 | Angeli et al. (2004) |
| k10, k11 | 0.0139 | nM−1 s−1 | Angeli et al. (2004) |
| k12, k13 | 0.0002 | nM−1 s−1 | Angeli et al. (2004) |
| k14, k15 | 0.0000139 | nM−1 s−1 | Angeli et al. (2004) |
| [YT] | 1200 | nM | Huang and Ferrell (1996);Angeli et al. (2004) |
| [ZT] | 300 | nM | Angeli et al. (2004) |
| [Px] | 0.3 | nM | Huang and Ferrell (1996) |
| [Py] | 0.3 | nM | Huang and Ferrell (1996) |
| [Pz] | 120 | nM | Huang and Ferrell (1996) |
Table 2.
Parameters related to the X, Xa, and Xas regulation.
| Name | Value | Unit |
|---|---|---|
| k1 | 0.00025 | nM−1 s−1 |
| k2 | 1000 | s−1 |
| k3 | 0.062 | nM−1 s−1 |
| k4 | 0.001 | nM−1 s−1 |
| k5 | 0.001 | nM−1 s−1 |
| k6, k7 | 0.02 | s−1 |
With the experimental values taken from Angeli’s paper it is possible to infer the value of the k8,…,15 constants. We remark that k8=k9, k10=k11, k12=k13, and k14=k15 in his simulation and thus also in our numerical solutions. To determine the phosphorylation specificity constants (k8,k9,k12,k13), we use their definition: kcat∕KM with the values given above for MEK and MAPK, e.g., for k8, we obtain a value of 0.0000533 nM−1 s−1. To determine the dephosphorylation specificity constants (k10,k11) we identify the relation (e.g., k10[Y3][Py], which has the dimension of a reaction speed) with the experimentally measured reaction speed (here equal to 5 nM s−1). We fixed [Py]=0.3 nM and [Y3] has a maximum value of 1200 nM, therefore we extract a value of k10 as k10=0.0139 nM−1 s−1. A different procedure is used for the other specificity constants of dephosphorylation (k14,k15). With the estimated number of phosphate groups removed cited above, we convert its unit to obtain a dimension of per second per molecule of MAPK. We find the value of 0.00167 phosphate groups removed per seconds per molecule of MAPK. Then, since we fixed [Pz]=120 nM, we can extract the value of k14=0.00167∕120=0.0000139 nM−1 s−1.
While we determined the values related to the cascade Y1,2,3 and Z1,2,3 from experimental estimates, for Mos the parameters are not known experimentally. The value of the parameter k1 on the behavior of the system is chosen in order to obtain a bistable response of the Xas. The catalytic constant k2 related to the folding of the protein X is not known experimentally, but since this protein has a standard size, we can use a value of folding time of 1 ms, which is a common mean time (Zeeb and Balbach, 2005). For the parameters k3, k4, and k5, so far no study gives values for these specificity constants. We therefore chose values consistent with the experimentally observed bistable behavior of the cascade. The k6 and k7 parameters have an identical (arbitrary) value because we supposed that the proteasome machinery processes and degrades the two proteins in an identical manner. The value chosen corresponds to a degradation time such that the concentration of Xas is at 3 nM when the cascade is off. This concentration value had been used as total concentration of Mos by (Huang and Ferrell, 1996) in their simulation.
Bifurcation analysis of the ODE model
In a first step we have studied the behavior of the model under the assumption of a fixed (time constant) value of the concentration of MPF. The results of our calculations for constant MPF are summarized in Figs. 2A, 2B, 2C.
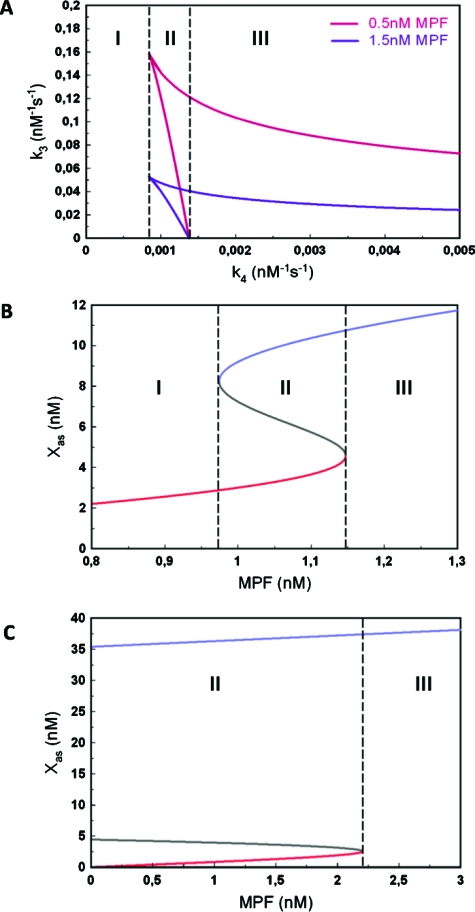
Figure 2. Bifurcation analysis of the Mos-MPF-MAPK ODE model.
(A) State diagram in the space of the kinetic parameters k3, (Mos-MPF) and k4 (Mos-MAPK). (B) State diagram in the space of variables [Xas; (MPF)] for the case of a bistable transition [region II in (B)]. (C) As (B) but for the irreversible case [region III in (B)].
Figure 2A shows the state diagram in the kinetic parameters k3 and k4, which correspond to the interactions between active Mos (Xa) and MPF and Xa and MAPK (Z3), respectively. Two curves are shown for two different fixed values of MPF-concentration. The area enclosed by the red and blue curves corresponds to the bistability regime. Three regions can be identified depending on the value k4. In region I, the system is always monostable. There is a minimal value at which bistability arises and region II begins. It is noteworthy that this value does not depend on MPF-concentration. Region II is limited by the value , which also does not depend on MPF-concentration. Therefore, region II in which the system can undergo a reversible, hysteretic transition, can only occur in a fixed interval of values . Beyond region II, in region III, the system is also bistable but it displays an irreversible transition of type I according to the nomenclature of (Giudi and Goldbeter, 1997).
The behaviors in regions II and III are further illustrated in Figs. 2B, 2C. Figures 2B, 2C show the state diagram in the space of concentrations of Mos in its active and stable form, Xas and MPF with all other parameters kept fixed. The diagram indeed has the classic shape of a reversible, bistable diagram. Figure 2C, by contrast, displays the form of the irreversible transition of type I. In this part of the analysis, we have considered MPF as a constant parameter and obtained a state diagram whose shape is a function of MPF-concentration. Since MPF in general varies in time, this diagram itself will be varying. However, irrespective of the MPF value, we can conclude from this analysis that (i) there is always a monostable region if either the MAPK feedback loop is suppressed or below threshold; (ii) depending in the strength of the feedback loop above threshold, there is reversible or irreversible bistability. In the following section, we pass on to study the time evolution of Mos by both experiment and numerical simulation.
The time evolution of Mos: experiment and modeling
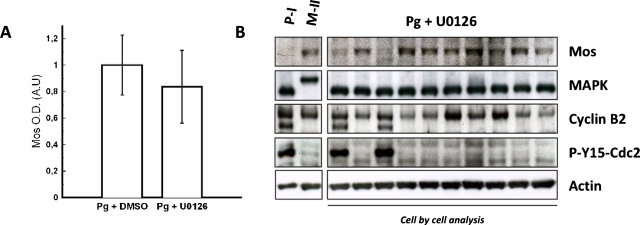
Mos concentration in oocytes was assessed by western blot analysis when the MAPK feedback loop driven by MAPK is active or not (shown in Fig. 3). In our experiments, Mos accumulation is observed in the absence of MAPK activity in accord with our assumption that, while contributing to Mos accumulation through a feedback loop, MAPK is not the main primer of the cascade. Mos accumulation can be determined by optical density measurement. Progesterone-stimulated oocyte data were used as a control for semiquantification and normalization and no significant differences were observed in Mos levels between U0126-treated oocytes and control oocytes treated with DMSO vehicle [Fig. 3A, n=6 females]. This analysis, performed on population, was also done cell-by-cell, which revealed that the oocytes exhibited a clear response, i.e., Mos production is “on” in each cell, in accord with data obtained on population shown in Fig. 3B. Nevertheless, the cells exhibit individual concentration variation (as we will also see below). These data are consistent with previous works reporting Mos detection in MAPK inhibiting conditions such as HSP-90-inhibitor geldanamycin incubation (Fisher et al., 2000) or U0126 treatment of oocytes stimulated by insulin through a Ras-dependent pathway (Baert et al., 2003). Considering that no significant differences in Mos levels appear in presence or in absence of MAPK activity, MAPK obviously does not contribute as a major component in the initiation of the cascade.
Figure 3. Mos accumulates in the absence of MAPK following progesterone addition.
(A) Histogram depicts normalized values of Mos concentration at GVBD time in control oocytes treated with DMSO vehicle (0%,1%) and in U0126-treated oocytes (50 μM). Mos levels are, respectively, 1±0.22 and 0.83±0.27. (B) Cell by cell analysis by western blot for contents in Mos, MAPK, cyclin B2, phospho-tyrosine 15 Cdc2, and actin. Oocytes were taken at GVBD time for biochemical analysis.
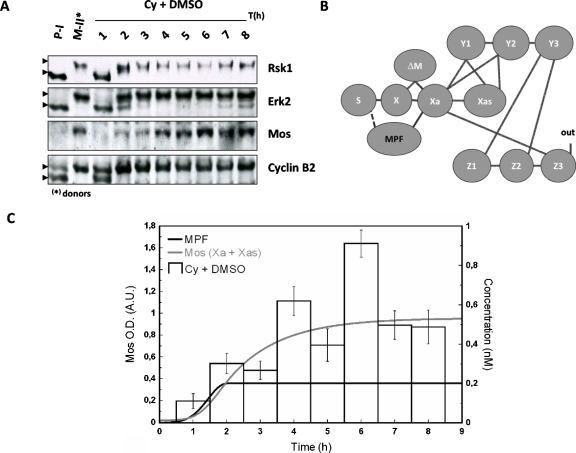
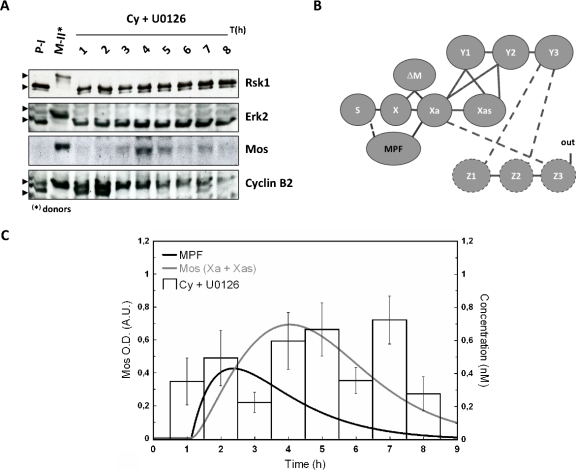
Another source than MAPK has to be considered to build up a biologically realistic model, in contrast to initial and previous works, which proposed MAPK itself as the trigger of the cascade. As argued earlier, MPF appears as the best candidate. Thus, the physiological mechanism of cytoplasm injection was used to turn on MPF activity in absence of hormonal stimulation [“in” in Fig. 1B] and the dynamic of Mos accumulation was monitored [Figs. 4A, 5A]. The cytoplasm injection was performed in oocytes treated with or without U0126. In our hands, GVBD50 [the time at which 50% of treated oocytes exhibited germinal vesicle breakdown (GVBD)] was about 4 h postinjection in control oocytes whereas it was approximately doubled in U0126-treated oocytes. Activation of MPF, as reported by cyclin B2 shift was, respectively, observed 2 h and 3 h postcytoplasm-injection in control and U0126-treated oocytes. Activity of MPF was sustained in control oocyte whereas it is observed to faint 7–8 h postinjection as attested by both rephosphorylation of cyclin B2 and decrease in cyclin B2 levels [Fig. 5A]. Such observation is in accord with loss of MPF activity in U0126-treated oocytes stimulated by progesterone (Fig. 6) and is explained by MAPK involvement in cyclin B synthesis and MPF upregulation (Palmer et al., 1998; Abrieu et al., 2001).
Figure 4. Sustained accumulation of Mos follows egg-cytoplasm injection.
Donors and recipient were subjected to western blot analysis (A). (B) Network structure of the MAPK cascade activation in Xenopus oocytes: MPF is proposed to interact with S (dashed line); (C) normalized values for Mos from western blots, theoretical time evolution of the concentration of MPF (bold) and total active form of Mos (Xa+Xas). The concentration of MPF has been chosen in order to reach a constant maximal value of Mos as in the experiment. For further discussion see text.
Figure 5. Mos accumulation dynamics in the absence of the MAPK-driven feedback loop.
In the absence of MAPK activity, Mos transiently accumulates following metaphase II egg (donor) cytoplasm injection. Meiotic resumption was stimulated in presence of U0126 by 50 nl injection of metaphase arrested oocytes, rinsed of progesterone. Donors and recipient oocytes were subjected to western blot analysis (A) for their contents in Mos, Erk2, Rsk1, and cyclin B2. Neither Erk nor Rsk exhibits active profiles; PI, prophase I arrested oocytes; MII, Metaphase II arrested oocytes; Pg, progesterone; Cy, MPF-containing cytoplasm from MII oocytes. (B) Network structure of the MAPK cascade activation in Xenopus oocytes: the interaction of Z3 with Xa is broken by U0126, a chemical inhibitor of MEK/Y (dashed lines). MPF activation is achieved in the absence of input as described in (A) and MPF is proposed to interact with S (dashed line). (C) Normalized values for Mos level are depicted for control and U0126-treated oocytes injected with MPF-containing cytoplasm. Numerically calculated time evolution of the concentration of MPF (bold), and total active form of Mos (Xa+Xas). The concentration of MPF follows an assumed χ2-shape leading to a profile of Mos qualitatively consistent with experiment. For further discussion see text.
Figure 6. Mos accumulation pattern following progesterone stimulation in presence of MEK-inhibitor U0216.
(A) Network structure of the MAPK cascade activation in Xenopus oocytes: Z3-interaction with Xa is broken by U0126, chemical inhibitor of MEK∕Y (dashed lines). MPF activation and in are brought by hormonal stimulation. (B) Representative experiment (n=3 females). Dashed arrow: GVBD50, arrow: MPK activity peak. (C) Normalized values for Mos and numerically calculated time evolution of the concentration of MPF (bold) and total active form of Mos (Xa+Xas).
Absence of MAPK activity in U0126-treated oocytes was confirmed by the absence of phosphorylation of its downstream effector, Rsk1. Noticeable levels of Mos were detected in absence of MAPK activity. Nontreated cytoplasm injected oocytes exhibited a level of Mos slightly inferior at 4 h and slightly superior at 6 h to the level of progesterone-treated oocytes at GVBD (respectively, 0.80±0.12 and 1.17±0.11). Average values for U0126-treated MII cytoplasm injected oocytes were lower to those of progesterone-treated oocytes at GVBD and peaked at 0.69±0.13 at 7 h. Thus, MPF appears as a major trigger for Mos accumulation and maintenance, independently of MAPK.
Parallel to our experiments we have calculated the time variation for Mos from our model. Panels B in Figs. 45 represent the network structure of the MAPK cascade activation in Xenopus oocytes. The line between S and X represents the translation of Mos from a pool of maternal mRNA stock. According to our model hypothesis the production velocity of Mos is identical for progesterone-stimulated oocytes and MPF-injected oocytes. In absence of progesterone stimulation, the dashed line corresponds to the influence of injected MPF to the protein synthesis, which has to be introduced in order to have a biologically realistic model. Indeed, injection of MPF causes activation of Aurora-A (Ma et al., 2003); the latter phosphorylates CPEB, causing Mos polyadenylation and its subsequent translation (Mendez et al., 2000).
Panels C in Figs. 45 illustrate the time evolution of the total active form of Mos (Xa+Xas) calculated with two time-dependent variations in MPF-concentration. In our ODE system, MPF is a variable parameter (no differential equation has been defined to estimate the dynamics of MPF). Therefore, according to the experimentally observed qualitative state of MPF (active vs nonactive), a corresponding concentration function was defined and applied to solve the ODE system with the tool SBML ODE solver implemented in CELLDESIGNER (see Materials and Methods). From Fig. 4A, we note that 2 h after injection, MPF-concentration reaches a plateau at its maximal value. As a consequence, a sigmoidal curve has been designed to correspond to the behavior of the observed values of MPF-concentration (gray curve). The same procedure has been applied to design a χ2-type function used to evaluate the numerical calculation of Mos concentration shown in Fig. 5C. In this set of experiments the variability of the experimental signal over a time scale of few hours, obtained over a population of ten oocytes per female (three in total), cannot be properly captured by a simple curve since no clear average signal emerges. Our numerical result in this case should be taken as indicative only; see the discussion in the conclusions.
Taken together, in control oocytes [Cy+DMSO, Fig. 4C], we found a good correlation between the quantified Mos amount and the expected one: both exhibit accumulation of the oncoprotein Mos. In the presence of the feedback loop, Mos appears thus to reach a stable concentration level. By contrast, in absence of the feedback loop no clear sustained accumulation of Mos is observed.
The Mos accumulation pattern following progesterone stimulation in the presence of MEK-inhibitor U0126 (50 μM) is depicted in Figs. 6A, 6B, 6C. This experimental condition allows turning MPF activation into a single peak: MPF activity had been reported to peak around GVBD time and drop 1 to 2 h after GVBD (Gross et al., 2001). In our hands, when oocytes were stimulated by progesterone in presence of U0126, fully phosphorylated cyclin B2 was observed at 0.718 GVBD50±0.046, attesting MPF activation. A decrease in the amount of cyclin B2 detected by western blot, due to its degradation, was observed at about 1.14 GVBD50±0.04. Mos accumulation was assessed as previously described and progesterone-stimulated oocytes at GVBD were used as a control for semiquantification and normalization. Mos semiquantification data exhibit a single-peak curve with asymmetric flanks. The latter agrees with computed Mos (Xa+Xas) from a simulation assuming MPF time evolution to be given by a single peak [Fig. 6C]. We note that on the extended time-scale of this experiment (ranging over 35 h rather than 9 h) we find a very satisfactory agreement between experiment and the numerical result for Mos concentration, starting from a χ2-type input signal of MPF.
The excellent quantitative agreement between experimental data and the numerical result in Fig. 6C is a consequence of the better statistics obtained over the time course, since the size of the oocyte population in Figs. 4C, 5C, 6C is identical. However, as shown by way of example in Fig. 6B, data on individual animals still show a marked variation in which typically two peaks were observedfor Mos accumulation: the first one occurs at 0.728 GVBD50±0.09, almost simultaneously to detection of MPF under its active state, while the second peak is observed at 1.039 GVBD50±0.085, 4.5 h later. Initial Mos mRNA translation is induced in a MAPK-independent manner (Gross et al., 2001; Bodart et al., 2005), downstream of progesterone-mediated inhibition of PKA. If the polyadenylation machinery activity may be tuned by MPF, this machinery is initially activated through MPF-independent mechanisms, e.g., upon activity of PRE binding protein like Musashi (Charlesworth et al., 2006). Then, the second peak of Mos may arise from MPF activity peak whereas the first may arise from the early initiation events driven by progesterone, independently of MPF.
CONCLUSIONS
In this paper we have studied the role of MPF and Mos in the initiation of the MAPK cascade in a combined experimental and modeling approach. In our experiments we have looked at the dynamics of Mos by provoking a sustained activity of MPF, or by stimulating a single peak of MPF activity. Our experiments show that the dynamics is clearly different in the presence or absence of the MAPK feedback loop. Mos does not depend on MAPK for its initiation and accumulation as long as MPF is active. The peak of Mos activity follows the peak of MPF activity in time. Our rate equation model takes into account the coupling of MPF to Mos, by explicitly describing the three different phosphorylation states of Mos, to our knowledge, for the first time. From the comparisons of experiments and numerical solutions of our model, we can conclude that the model is capable to not only qualitatively but also quantitatively capture well the average features observed over a population of oocytes.
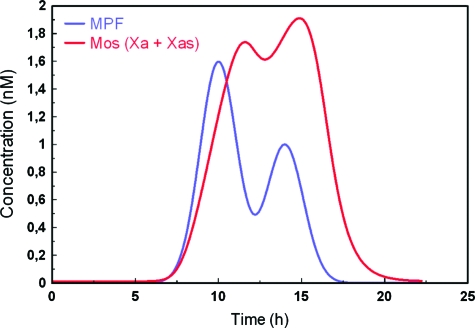
We have not tried to advance further on the individual features of the Mos concentration signals, but a simple test of our model nevertheless reveals a behavior potentially important for the explanation of such features. Going back to the case of hormonal stimulation shown in Fig. 6, while we observe an excellent agreement of the overall behavior of Mos over an extended time scale shown in Fig. 6C, still a significant variability on the level of individual animals persists, as exemplified in Fig. 6B. Figure 7 illustrates a possible reason contributing to this variability suggested from our model. If MPF-concentration is assumed to change strongly over time, as shown by the blue curve in Fig. 7, the resulting time course of Mos concentration shown in red appears to be a strongly modulated function of its input signal. The model, and we believe therefore the real network, appears to be highly sensitive to MPF variation.
Figure 7. Supposed bimodal time evolution of the concentration of MPF and resulting total active form of Mos.
(Xa+Xas). The resulting curve for Mos clearly shows a complex nonlinear variation with the input signal.
In sum, we have presented a study in which we have elucidated the interplay between MAPK and MPF at the entry of the MAPK cascade by combining experiment, literature-guided model building and numerical simulations. We believe that our model lays the basis for more detailed investigations of the initiation of the MAPK cascade in Xenopus oocytes, both at the entry level and for the intertwined feedback loops that it is subjected to. In future work we will aim at elucidating further the interplay of Mos and MPF. So far, the influence of MAPK on MPF dynamics has not been considered explicitly in our model since we impose only a hypothesized time evolution of MPF. The next step therefore is to couple our model to an explicit model for the kinetics of MPF (see, e.g., Tyson, 1991). It remains to be seen how far a detailed kinetics of Mos will allow to capture more of the details of the experimental signals. In parallel, we aim at measuring MPF activity at the single-cell level.
Materials and methods
Animals, chemicals, and bioreagents: Adult Xenopus females were purchased from the University of Rennes I (France). Tricaine methane sulfonate was purchased from Sandoz (Levallois–Perret, France). Collagenase A and protease inhibitors were purchased from Roche Applied Science (Meylan, France). Mouse monoclonal anti-Erk2 (D-2) and rabbit polyclonal anti-Mos(xe) (C237), anti-Rsk1 (C-21), and anti-actin (I-19) antibodies were obtained from Santa Cruz Biotechnologies (Santa Cruz, CA). Rabbit polyclonal antiphospho-Tyr15-Cdc2 antibody was purchased from Cell Signaling (Cell Signaling TechnologyTM, USA) and rabbit polyclonal anticyclin B2 antibody JG103 is a gift of Dr. J. Gannon (ICRF, South Mimms, United Kingdom). MEK inhibitor U0126 ethanolate was obtained from sigma.
Handling of oocytes: After anesthetizing Xenopus females by immersion in 1 gal of 1 MS222 solution (tricaine methane sulfonate), ovarian lobes were surgically removed and placed in ND96 medium (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM HEPES-NaOH, pH 7.5). Fully grown stage VI oocytes according to Dumont’s classification, were isolated and follicle cells were partially removed by a collagenase treatment for 30 min (1 mg ml of 1 collagenase A) followed by a manual microdissection with forceps. Oocytes were stored at 14°C in ND96 medium until experiments.
Ovariectomies were performed in accordance with guidelines and regulations regarding living animals. Protocols have accepted by Veterinary Departments and J.F. Bodart is authorized for husbandry and amphibian experimentation procedures (authorization number 59-009117; Arrêté préfectoral 05∕04∕2006).
Xenopus oocytes treatment, stimulation, and analysis of G2∕M transition (meiotic resumption): Meiotic resumption (M-phase entry) was induced by incubating G2-arrested oocytes in ND96 medium containing 10 μM of progesterone. Alternatively to hormonal stimulation, meiotic resumption was stimulated by crude cytoplasm injection from mature oocyte (50 nl per cell). Cytoplasm from mature metaphase II-stage oocytes present MPF activated complex able to activate pool of pre-MPF inactive complex of immature oocyte (Masui, 2001). U0126 was prepared in DMSO and used at a final concentration of 50 μM. Treatments began overnight before progesterone addition or cytoplasm injection. Control oocytes were treated with an identical amount of DMSO (1‰). Kinetic of GVBD achievement, witness of the M-phase entry, was scored by the appearance of a white spot (WS) at the animal pole of the oocyte. Every hour, the representative of the WS score, ten oocytes were frozen at −20°C for later biochemical analysis.
SDS-PAGE and western blotting: Proteins (the equivalent of a third of an oocyte was loaded per lane) were run on a 12,5% or a 15% modified SDS-PAGE (Baert et al., 2003, Chesnel et al., 1997) for Erk2, Rsk1, cyclin B2, and electroblotted onto nitrocellulose sheet. While the quantity of proteins remains rather constant in the Xenopus oocyte, equal loading and transfer efficiency were checked using the Ponceau red staining. Blots were saturated with 5% (w∕v) nonfat milk in tris-buffered saline (TBS)-tween (15 mM Tris, 140 mM NaCl, 0.05% (v∕v) Tween) for 45 min. Rabbit polyclonal anti-Mos(xe) (1∕500), antiphospho-Tyr15-Cdc2 (1∕5000) (not shown), antitubulin (1∕5000) primary antibodies were incubated overnight at 4°C. Mouse monoclonal anti-Erk2 (1∕500), Rabbit polyclonal anti-Rsk1 (1∕500) and anticyclin B2 (1∕1250) antibodies were incubated 2 h at room temperature. Membranes were washed three times for 10 min in TBS-Tween and incubated 1 h with either an antimouse (IgG or IgM) horseradish peroxidase-labeled secondary antibody or an antirabbit IgG at a dilution of 1:2500. Detection was carried out with enhanced chemiluminescence on Amersham hyperfilms.
Western blotting analysis and data normalization: Two types of analysis were performed to distinguish the states of Mos synthesis: (i) Western blotting of a population of ten oocytes representative of the WS score and (ii) cell-by-cell western blotting analysis. Films were scanned and Mos protein semi-quantification was realized by optical density (OD) measurement using QUANTITY ONE V4.21 one—dimensional gel analysis software from BioRad. To quantify Mos, similar rectangular areas were first made to surround each Mos band of the gel. Then volume intensity is quantified in each plot. It corresponds to the intensity data (Mos OD) inside a defined boundary drawn on our image. Also, the QUANTITY ONE V4.21 software is able to determine the local background. Local background is defined as the intensity of the nondata background pixels included in the volume quantification after area drawing. The data treatment is multistep: (i) we subtracted local background from volume intensity for each plot; (ii) we subtracted global gel background from every volume intensity; and (iii) we finally normalized Mos OD=1 relative to a standardized positive control sample (i.e., progesterone-stimulated control oocytes). We chose OD for untreated oocytes as a value for global gel background. In these oocytes, Mos protein is not detectable. We investigated total MAPK∕Erk immunoblot intensity through the same process. Since degradation of MAPK does not occur duringXenopus oocyte maturation, we considered this protein as a representative loading control to adjust Mos semiquantification data.
The ODE model and its analysis: The ODE model described in the paper was developed in CELLDESIGNER, a process diagram editor, with the graphical notation proposed by Kitano (Kitano, 2003, Funahashi et al., 2003). This software is SBML compliant. Therefore our model has been done in level 2 version 1 SBML format and can be used by other software to able to import the SBML file. Simulations were done with SBML ODE Solver implemented within CELLDESIGNER.
The bifurcation analysis of the model was done withXPPAUT (Ermentrout, 2007). This is a software used to simulate and analyze dynamical systems. There are three main steps in the analysis. The first step consists in writing down the ODE-equations and in determining the variables and the parameters with their initial values. Since we know that MPF has a time evolution of its concentration value, it is defined as a variable parameter with initial value of 1 nM. This latter value is also the value of cyclin B concentration in fully grown stage VI Xenopus oocytes (Kanatsu-Shinohara et al., 2000). Therefore, when the cascade is at its highest stable steady-state, we obtain a value of Xa+Xas of about 9 nM similar to the estimate concentration of active Mos in mature oocytes (Yew et al., 1992). During the second step, the software solves the ODE-equations system to reach a steady-state for all the variables. Then, the third step begins with a perturbation of one parameter chosen by the user. It is also possible to perform a bifurcation analysis with two parameters. As a function of the perturbed parameter value, the software supplies the steady-state values for the variables. We chose to study the variable Xas as its concentration values are dependent of MPF and Z3.
The numerical solution of system of ODE equations has been performed with the parameters values described in the supplementary material. We chose as initial conditions: [Y1]=1200 nM, [Y2]=[Y3]=0 nM, [Z1]=300 nM, [Z2]=[Z3]=0 nM, and [X]=[Xa]=[Xas]=0 nM. This choice corresponds to an initiation of the cascade in absence of Mos protein and without phosphorylated forms of MEK and MAPK.
ACKNOWLEDGMENTS
The work by C. Russo was supported by a doctoral grant from the French National Cancer Institute (INCa). R. Beaujois is a recipient of a fellowship from the “Ministère de la Recherche et de l’Enseignement.” We thank Arlette Rousseau-Lescuyer for technical assistance. This work was supported by a CNRS grant from the PEPS program 2008. C. Russo and R. Beaujois contributed equally to the work.
DISCLAIMER
The authors declare that they have no conflict of interest.
REFERENCES
- Abrieu, A, Doree, M, and Fisher, D (2001). “The interplay between cyclin-B-Cdc2 kinase (MPF) and MAP kinase during maturation of oocytes.” J. Cell Sci. 114, 257–267. [DOI] [PubMed] [Google Scholar]
- Amiel, A, Leclere, L, Robert, L, Chevalier, S, and Houliston, E (2009). “Conserved functions for Mos in eumetazoan oocyte maturation revealed by studies in a cnidarian.” Curr. Biol. 19, 305–311. 10.1016/j.cub.2008.12.054 [DOI] [PubMed] [Google Scholar]
- Angeli, D, Ferrell, J E, Jr., and Sontag, E D (2004). “Detection of multistability, bifurcations, and hysteresis in a large class of biological positive-feedback systems.” Proc. Natl. Acad. Sci. U.S.A. 101, 1822–1827. 10.1073/pnas.0308265100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baert, F Y, Bodart, J F, Bocquet-Muchembled, B, Lescuyer-Rousseau, A, and Vilain, J P (2003). “Xp42 (Mpk1) activation is not required for germinal vesicle breakdown but for Raf complete phosphorylation in insulin-stimulated Xenopus oocytes.” J. Biol. Chem. 278, 49714–49720. 10.1074/jbc.M308067200 [DOI] [PubMed] [Google Scholar]
- Bodart, J F, Baert, F Y, Sellier, C, Duesbery, N S, Flament, S, and Vilain, J P (2005). “Differential roles of p39Mos-Xp42Mpk1 cascade proteins on Raf1 phosphorylation and spindle morphogenesis in Xenopus oocytes.” Dev. Biol. 283, 373–383. 10.1016/j.ydbio.2005.04.031 [DOI] [PubMed] [Google Scholar]
- Bodart, J F, Bechard, D, Bertout, M, Gannon, J, Rousseau, A, Vilain, J P, and Flament, S (1999). “Activation of Xenopus eggs by the kinase inhibitor 6-DMAP suggests a differential regulation of cyclin B and p39(Mos) proteolysis.” Exp. Cell Res. 253, 413–421. 10.1006/excr.1999.4662 [DOI] [PubMed] [Google Scholar]
- Bodart, J F, Flament, S, and Vilain, J P (2002). “Metaphase arrest in amphibian oocytes: interaction between CSF and MPF sets the equilibrium.” Mol. Reprod. Dev. 61, 570–574. 10.1002/mrd.10112 [DOI] [PubMed] [Google Scholar]
- Brandman, O, Ferrell, J E, Jr., Li, R, and Meyer, T (2005). “Interlinked fast and slow positive feedback loops drive reliable cell decisions.” Science 310, 496–498. 10.1126/science.1113834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro, A, Peter, M, Lorca, T, and Mandart, E (2001). “C-Mos and cyclin B/cdc2 connections during Xenopus oocyte maturation.” Biol. Cell 93, 15–25. 10.1016/S0248-4900(01)01128-5 [DOI] [PubMed] [Google Scholar]
- Charlesworth, A, Ridge, J A, King, L A, MacNicol, M C, and MacNicol, A M (2002). “A novel regulatory element determines the timing of Mos mRNA translation during Xenopus oocyte maturation.” EMBO J. 21, 2798–2806. 10.1093/emboj/21.11.2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth, A, Wilczynska, A, Thampi, P, Cox, L L, and MacNicol, A M (2006). “Musashi regulates the temporal order of mRNA translation during Xenopus oocyte maturation.” EMBO J. 25, 2792–801. 10.1038/sj.emboj.7601159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, M, and Cooper, J A (1995). “Ser-3 is important for regulating Mos interaction with and stimulation of mitogen-activated protein kinase kinase.” Mol. Cell. Biol. 15, 4727–4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnel, F, Bonnec, G, Tardivel, A, and Boujard, D (1997). “Comparative effects of insulin on the activation of the Raf/Mos-dependent MAP kinase cascade in vitellogenic versus postvitellogenic Xenopus oocytes.” Dev. Biol. 188, 122–133. 10.1006/dbio.1997.8631 [DOI] [PubMed] [Google Scholar]
- Colledge, W H, Carlton, M B, Udy, G B, and Evans, M J (1994). “Disruption of c-Mos causes parthenogenetic development of unfertilized mouse eggs.” Nature (London) 370, 65–68. 10.1038/370065a0 [DOI] [PubMed] [Google Scholar]
- Cowley, S, Paterson, H, Kemp, P, and Marshall, C J (1994). “Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells.” Cell 77, 841–852. 10.1016/0092-8674(94)90133-3 [DOI] [PubMed] [Google Scholar]
- De Moor, C H, and Richter, J D (1997). “The Mos pathway regulates cytoplasmic polyadenylation in Xenopus oocytes.” Mol. Cell. Biol. 17, 6419–6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupré, A, Jessus, C, Ozon, R, and Haccard, O (2002). “Mos is not required for the initiation of meiotic maturation in Xenopus oocytes.” EMBO J. 21, 4026–4036. 10.1093/emboj/cdf400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermentrout, B (2007). “XPPAUT.” Scholarpedia J. 2, 1399. [Google Scholar]
- Ferrell, J E, Jr., and Bhatt, R R (1997). “Mechanistic studies of the dual phosphorylation of mitogen-activated protein kinase.” J. Biol. Chem. 272, 19008–19016. 10.1074/jbc.272.30.19008 [DOI] [PubMed] [Google Scholar]
- Ferrell, J E, Jr., and Machleder, E M (1998). “The biochemical basis of an all-or-none cell fate switch in Xenopus oocytes.” Science 280, 895–898. 10.1126/science.280.5365.895 [DOI] [PubMed] [Google Scholar]
- Fisher, D L, Brassac, T, Galas, S, and Doree, M (1999). “Dissociation of MAP kinase activation and MPF activation in hormone-stimulated maturation of Xenopus oocytes.” Development 126, 4537–4546. [DOI] [PubMed] [Google Scholar]
- Fisher, D L, Mandart, E, and Doree, M (2000). “Hsp90 is required for c-Mos activation and biphasic MAP kinase activation in Xenopus oocytes.” EMBO J. 19, 1516–1524. 10.1093/emboj/19.7.1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flament, S, Bodart, J F, Bertout, M, Browaeys, E, Rousseau, A, and Vilain, J P (2000). “Differential effects of 6-DMAP, olomoucine and roscovitine on Xenopus oocytes and eggs.” Zygote 8, 3–14. 10.1017/S0967199400000770 [DOI] [PubMed] [Google Scholar]
- Freeman, R S, Meyer, A N, Li, J, and Donoghue, D J (1992). “Phosphorylation of conserved serine residues does not regulate the ability of mosxe protein kinase to induce oocyte maturation or function as cytostatic factor.” J. Cell Biol. 116, 725–735. 10.1083/jcb.116.3.725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi, A, Tanimura, N, Morohashi, M, and Kitano, H (2003). “CellDesigner: a process diagram editor for gene-regulatory and biochemical networks.” BIOSILICO 1, 159–162. 10.1016/S1478-5382(03)02370-9 [DOI] [Google Scholar]
- Gebauer, F, and Richter, J D (1997). “Synthesis and function of Mos: the control switch of vertebrate oocyte meiosis.” BioEssays 19, 23–28. 10.1002/bies.950190106 [DOI] [PubMed] [Google Scholar]
- Giudi, G M, and Goldbeter, A (1997). “Bistability without hysteresis in chemical reaction systems: a theoretical analysis of irreversible transitions between multiple steady states.” J. Phys. Chem. 101, 9367–9376. [Google Scholar]
- Gross, S D, Lewellyn, A L, and Maller, J L (2001). “A constitutively active form of the protein kinase p90Rsk1 is sufficient to trigger the G2/M transition in Xenopus oocytes.” J. Biol. Chem. 276, 46099–46103. 10.1074/jbc.C100496200 [DOI] [PubMed] [Google Scholar]
- Haccard, O, and Jessus, C (2006). “Redundant pathways for Cdc2 activation in Xenopus oocyte: either cyclin B or Mos synthesis.” EMBO Rep. 7, 321–325. 10.1038/sj.embor.7400611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto N, Watanabe N, Furuta Y, Tamemoto H, Sagata N, Yokoyama M, Okazaki K, Nagayoshi M, Takeda N, Ikawa, Y, and Aizawai, S (1994). “Parthenogenetic activation of oocytes in c-Mos-deficient mice.” Nature (London) 370, 68–71. 10.1038/370068a0 [DOI] [PubMed] [Google Scholar]
- Hochegger, H, Klotzbucher, A, Kirk, J, Howell, M, le Guellec, K, Fletcher, K, Duncan, T, Sohail, M, and Hunt, T (2001). “New B-type cyclin synthesis is required between meiosis I and II during Xenopus oocyte maturation.” Development 128, 3795–3807. [DOI] [PubMed] [Google Scholar]
- Horne, M M, and Guadagno, T M (2003). “A requirement for MAP kinase in the assembly and maintenance of the mitotic spindle.” J. Cell Biol. 161, 1021–1028. 10.1083/jcb.200304144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C Y, and Ferrell, J E, Jr. (1996). “Ultrasensitivity in the mitogen-activated protein kinase cascade.” Proc. Natl. Acad. Sci. U.S.A. 93, 10078–10083. 10.1073/pnas.93.19.10078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue, D, Ohe, M, Kanemori, Y, Nobui, T, and Sagata, N (2007). “A direct link of the Mos-MAPK pathway to Erp1/Emi2 in meiotic arrest of Xenopus laevis eggs.” Nature (London) 446, 1100–1104. 10.1038/nature05688 [DOI] [PubMed] [Google Scholar]
- Ishida, N, Tanaka, K, Tamura, T, Nishizawa, M, Okazaki, K, Sagata, N, and Ichihara, A (1993). “Mos is degraded by the 26S proteasome in a ubiquitin-dependent fashion.” FEBS Lett. 324, 345–348. 10.1016/0014-5793(93)80148-N [DOI] [PubMed] [Google Scholar]
- Justman, Q A, Serber, Z, Ferrell, J E, Jr., El-Samad, H, and Shokat, K M (2009). “Tuning the activation threshold of a kinase network by nested feedback loops.” Science 324, 509–512. 10.1126/science.1169498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiura-Kobayashi, H, Yoshida, N, Sagata, N, Yamashita, M, and Nagahama, Y (2000). “The Mos/MAPK pathway is involved in metaphase II arrest as a cytostatic factor but is neither necessary nor sufficient for initiating oocyte maturation in goldfish.” Dev. Genes Evol. 210, 416–425. 10.1007/s004270000083 [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara, M, Schultz, R M, and Kopf, G S (2000). “Acquisition of meiotic competence in mouse oocytes: absolute amounts of p34(cdc2), cyclin B1, cdc25C, and wee1 in meiotically incompetent and competent oocytes.” Biol. Reprod. 63, 1610–1616. 10.1095/biolreprod63.6.1610 [DOI] [PubMed] [Google Scholar]
- Kitano, H (2003). “A graphical notation for biochemical networks.” BIOSILICO 1, 169–176. 10.1016/S1478-5382(03)02380-1 [DOI] [Google Scholar]
- Liu, J X, Singh, B, Wlodek, D, and Arlinghaus, R B (1990). “Cell cycle-mediated structural and functional alteration of P85gag-Mos protein kinase activity.” Oncogene 5, 171–178. [PubMed] [Google Scholar]
- Ma, C, Cummings, C, and Liu, X J (2003). “Biphasic activation of Aurora-A kinase during the meiosis I—meiosis II transition in Xenopus oocytes.” Mol. Cell. Biol. 23, 1703–1716. 10.1128/MCB.23.5.1703-1716.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour, S J, Resing, K A, Candi, J M, Hermann, A S, Gloor, J W, Herskind, K R, Wartmann, M, Davis, R J, and Ahn, N G (1994). “Mitogen-activated protein (MAP) kinase phosphorylation of MAP kinase kinase: determination of phosphorylation sites by mass spectrometry and site-directed mutagenesis.” J. Biochem. (Tokyo) 116, 304–314. [DOI] [PubMed] [Google Scholar]
- Masui, Y (2001). “From oocyte maturation to the in vitro cell cycle: the history of discoveries of maturation-promoting factor (MPF) and cytostatic factor (CSF).” Differentiation 69, 1–17. 10.1046/j.1432-0436.2001.690101.x [DOI] [PubMed] [Google Scholar]
- Masui, Y, and Markert, C L (1971). “Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes.” J. Exp. Zool. 177, 129–145. 10.1002/jez.1401770202 [DOI] [PubMed] [Google Scholar]
- Matten, W T, Copeland, T D, Ahn, N G, and Vande Woude, G F (1996). “Positive feedback between MAP kinase and Mos during Xenopus oocyte maturation.” Dev Biol. 179, 485–492. 10.1006/dbio.1996.0277 [DOI] [PubMed] [Google Scholar]
- Mendez, R, Hake, L E, Andresson, T, Littlepage, L E, Ruderman, J V, and Richter, J D (2000). “Phosphorylation of CPE binding factor by Eg2 regulates translation of c-Mos mRNA.” Nature (London) 404, 302–307. 10.1038/35005126 [DOI] [PubMed] [Google Scholar]
- Nebreda, A R, Gannon, J V, and Hunt, T (1995). “Newly synthesized protein(s) must associate with p34cdc2 to activate MAP kinase and MPF during progesterone-induced maturation of Xenopus oocytes.” EMBO J. 14, 5597–5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebreda, A R, and Hunt, T (1993). “The c-Mos proto-oncogene protein kinase turns on and maintains the activity of MAP kinase, but not MPF, in cell-free extracts of Xenopus oocytes and eggs.” EMBO J. 12, 1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa, M, Furuno, N, Okazaki, K, Tanaka, H, Ogawa, Y, and Sagata, N (1993). “Degradation of Mos by the N-terminal proline (Pro2)-dependent ubiquitin pathway on fertilization of Xenopus eggs: possible significance of natural selection for Pro2 in Mos.” EMBO J. 12, 4021–4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa, M, Okazaki, K, Furuno, N, Watanabe, N, and Sagata, N (1992). “The ‘second-codon rule’ and autophosphorylation govern the stability and activity of Mos during the meiotic cell cycle in Xenopus oocytes.” EMBO J. 11, 2433–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, A, Gavin, A C, and Nebreda, A R (1998). “A link between MAP kinase and p34(cdc2)/cyclin B during oocyte maturation: p90(rsk) phosphorylates and inactivates the p34(cdc2) inhibitory kinase Myt1.” EMBO J. 17, 5037–5047. 10.1093/emboj/17.17.5037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham, C D, Vuyyuru, V B, Yang, Y, Bai, W, and Singh, B (1999). “Evidence for an important role of serine 16 and its phosphorylation in the stabilization of c-Mos.” Oncogene 18, 4287–4294. 10.1038/sj.onc.1202804 [DOI] [PubMed] [Google Scholar]
- Posada, J, Yew, N, Ahn, N G, Vande Woude, G F, and Cooper, J A (1993). “Mos stimulates MAP kinase in Xenopus oocytes and activates a MAP kinase kinase in vitro.” Mol. Cell. Biol. 13, 2546–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagata, N, Oskarsson, M, Copeland, T, Brumbaugh, J, and Vande Woude, G F (1988). “Function of c-Mos proto-oncogene product in meiotic maturation in Xenopus oocytes.” Nature (London) 335, 519–525. 10.1038/335519a0 [DOI] [PubMed] [Google Scholar]
- Sagata, N, Watanabe, N, Vande Woude, G F, and Ikawa, Y (1989). “The c-Mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs.” Nature (London) 342, 512–518. 10.1038/342512a0 [DOI] [PubMed] [Google Scholar]
- Sheets, M D, Wu, M, and Wickens, M (1995). “Polyadenylation of c-Mos mRNA as a control point in Xenopus meiotic maturation.” Nature (London) 374, 511–516. 10.1038/374511a0 [DOI] [PubMed] [Google Scholar]
- Sheng, J, Kumagai, A, Dunphy, W G, and Varshavsky, A (2002). “Dissection of c-Mos degron.” EMBO J. 21, 6061–6071. 10.1093/emboj/cdf626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson, J J (1991). “Modeling the cell division cycle: cdc2 and cyclin interactions.” Proc. Natl. Acad. Sci. U.S.A. 88, 7328–7332. 10.1073/pnas.88.16.7328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, N, Vande Woude, G F, Ikawa, Y, and Sagata, N (1989). “Specific proteolysis of the c-Mos proto-oncogene product by calpain on fertilization of Xenopus eggs.” Nature (London) 342, 505–511. 10.1038/342505a0 [DOI] [PubMed] [Google Scholar]
- Wu, J Q, and Kornbluth, S (2008). “Across the meiotic divide—CSF activity in the post-Emi2/XErp1 era.” J. Cell Sci. 121, 3509–3514. 10.1242/jcs.036855 [DOI] [PubMed] [Google Scholar]
- Xiong, W, and Ferrell, J E, Jr. (2003). “A positive-feedback-based bistable ‘memory module’ that governs a cell-fate decision.” Nature (London) 426, 460–465. 10.1038/nature02089 [DOI] [PubMed] [Google Scholar]
- Yew N, Mellini M L, and Vande Woude G F (1992). “Meiotic initiation by the Mos protein in Xenopus.” Nature 355, 649–652. 10.1038/355649a0 [DOI] [PubMed] [Google Scholar]
- Yue, J, and Ferrell, J E, Jr. (2006). “Mechanistic studies of the mitotic activation of Mos.” Mol. Cell. Biol. 26, 5300–5309. 10.1128/MCB.00273-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb, M, and Balbach, J (2005). “NMR spectroscopic characterization of millisecond protein folding by transverse relaxation dispersion measurements.” J. Am. Chem. Soc. 127, 13207–13212. 10.1021/ja051141+ [DOI] [PubMed] [Google Scholar]