Abstract
Trichomoniasis was diagnosed in multiple incidents of mortality in wild purple finch (Carpodacus purpureus) and American goldfinch (Carduelis tristis) in the Canadian Maritimes. Birds exhibited regurgitation, emaciation, and hyperplastic oropharyngitis, ingluvitis, and esophagitis. Trichomonas gallinae was identified by histopathology and polymerase chain reaction (PCR). Trichomoniasis (trichomonosis) is an emerging disease in wild finches of eastern Canada.
Résumé
Trichomoniase chez les roselins des provinces maritimes du Canada — Une maladie émergente. La trichomoniase a été diagnostiquée lors d’incidents multiples de mortalité chez les roselins pourprés (Carpodacus purpureus) et les chardonnerets jaunes (Carduelis tristis) dans les provinces maritimes du Canada. Les oiseaux manifestaient de la régurgitation, de l’émaciation ainsi que de l’oropharyngite hyperplasique, de l’ingluvite et de l’œsophagite. Trichomonas gallinae a été identifié par histopathologie et amplification en chaîne par la polymérase (PCR). La trichomoniase (trichomonose) est une maladie émergente chez les roselins sauvages de l’Est du Canada.
(Traduit par Isabelle Vallières)
Infection with Trichomonas gallinae has recently been recognized as an important cause of mortality in finches in the United Kingdom (1–3). Here we describe similar mortalities in finches of the Maritime provinces of Canada (New Brunswick, Nova Scotia, and Prince Edward Island) beginning in 2007. The disease caused by T. gallinae has long been known as trichomoniasis in the literature and will be referred to as such in this manuscript, although the World Association for the Advancement of Veterinary Parasitology (WAAVP) has recently recommended use of the term trichomonosis (4).
Case description
Two separate incidents of purple finch (Carpodacus purpureus) mortality were reported by the bird-feeding public of Nova Scotia in the fall of 2007. The mortalities were subsequently diagnosed as due to trichomoniasis by pathologists of the Canadian Cooperative Wildlife Health Centre (CCWHC), at the Atlantic Veterinary College, University of Prince Edward Island. Subsequently, a call for submissions of dead finches was made by CCWHC to the bird-watching and bird-feeding public of the Maritimes in early 2008 (5).
From the incidents in Nova Scotia in 2007, 3 purple finches, 1 (male) from the first incident and 2 (1 male, 1 female) from the second incident, were submitted and examined at CCWHC. Throughout the summer and fall of 2008, 14 purple finches (4 males, 10 females) and 4 American goldfinches (Carduelis tristis) (2 males, 1 female, 1 undetermined sex) from all 3 Maritime provinces were submitted for examination (Table 1). In most cases, the birds submitted were only a subsample of a larger group of dead birds, up to 8 individuals, found at the same location. In 2007, mortalities began during September–October, while in 2008 reports of sick and dying purple finches began circulating amongst bird watchers and avid bird feeders of the Maritime provinces in mid-June.
Table 1.
Mortalities of purple finch, Carpodacus purpureus, and American goldfinch, Carduelis tristis, in the Canadian Maritime provinces during 2007–2008
| Species | Sex/Age | Date of death (Y/M/D) | Body condition | Primary diagnosisa | Secondary diagnosisa | Feederb |
|---|---|---|---|---|---|---|
| Purple finch | M/A | 2007–09–11 | emaciated | Trichomoniasis-crop | — | |
| Purple finch | F/I | 2007–10–01 | emaciated | Trichomoniasis-crop | — | |
| Purple finch | F/I | 2008–07–03 | emaciated | Trichomoniasis-crop | — | |
| Purple finch | F/A | 2008–07–17 | moderate | Trichomoniasis-crop | Y | |
| Purple finch | F/A | 2008–07–17 | emaciated | Trichomoniasis-crop | Y | |
| Purple finch | M/A | 2008–07–29 | emaciated | Trichomoniasis-crop | Gastric megabacteriosis | Y |
| Purple finch | F/A | 2008–08–07 | emaciated | Trichomoniasis-crop | Osteomyelitis skull | — |
| A. goldfinch | F/I | 2008–09–10 | emaciated | Trichomoniasis-liver & intestine | Y | |
| A. goldfinch | M/A | 2008–09–22 | emaciated | Trichomoniasis-crop | Osteomyelitis skull | Y |
| Purple finchc | F/A | 2007–09–26 | good | Trauma | Y | |
| A. goldfinchc | M/A | 2007–10–01 | good | Trauma | N | |
| Purple finchc | M/A | 2008–08–31 | good | Trauma | N | |
| A. goldfinchc | M/A | 2008–09–25 | good | Trauma | Y | |
| Purple finchc | M/I | 2007–10–01 | emaciated | Starvation | Microfillariasis | — |
| Purple finchc | M/I | 2008–06–21 | emaciated | Starvation | — | |
| Purple finchc | F/I | 2008–08–28 | emaciated | Starvation | — | |
| A. goldfinchc | — | 2008–09–22 | emaciated | Starvation | Y | |
| Purple finch | F/I | 2008–09–10 | good | Drowning | Trichomoniasis-liver | N |
| Purple finchc | F/I | 2008–09–10 | good | Drowning | N | |
| Purple finchc | F/I | 2008–09–10 | good | Drowning | N | |
| Purple finch | F/I | 2008–09–10 | moderate | Cat attack | Cloacal bursitis, bacteria | N |
| Purple finch | F/A | 2008–06–13 | emaciated | Mycoplasmosis | — | |
| Purple finch | F/I | 2007–09–28 | moderate | No diagnosis | — | |
| Purple finch | M/A | 2008–09–10 | good | No diagnosis | Y |
F (female), M (male), I (immature), A (adult), — (not recorded).
Primary diagnosis is the cause of death, secondary diagnosis includes other pathologic conditions that may have had ill effects on the bird.
Feeder indicates whether the birds were found dead under or around a bird feeder. Y (yes), N (no), — (not recorded).
Birds without an underlying infectious disease that were used as representative of the normal finch population when evaluating effect of trichomoniasis on body condition.
Birds that were subsequently diagnosed with trichomoniasis were described by the submitters as lethargic, with ruffled feathers, labored breathing, and poor flight ability. The birds would sit on a bird feeder for several minutes at a time with eyes closed before resuming feeding. Pictures submitted by the public revealed birds with debris attached by saliva to the feathers of their head and neck and small amounts of what was interpreted as regurgitated material at the commissures of their beaks (Figure 1).
Figure 1.
Immature purple finch, Carpodacus purpureus, that later died of trichomoniasis. Note the regurgitated material at the beak commissure and vegetable material adhering to the saliva-coated plumage. Photo courtesy of Sheryl and Doug Wilson.
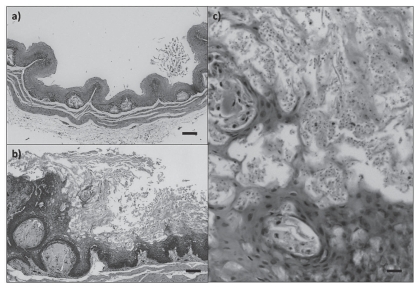
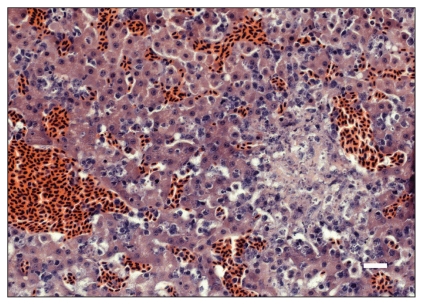
At necropsy, birds with trichomoniasis were emaciated, and some of them had a slightly to markedly thickened crop; rarely, there were white to yellow masses in the lumen of the oropharynx (cankers). Microscopically, the mucosa of the oropharynx, ingluvies (crop) and/or esophagus was severely thickened due to epithelial hyperplasia and a necrotic pseudomembrane. This hyperplastic epithelium had multifocal areas of erosive to ulcerative necrosis and variable amounts of inflammation composed of heterophils, macrophages, and lymphocytes. Opportunistic coccoid and cocco-bacillary bacteria proliferated on the surface of the exudate. Amongst the keratinized epithelial cells were numerous ovoid to leaf-shaped protozoan parasites that stained pale pink with a Periodic Acid Schiff’s stain, measured 5 to 8 mm in length, had a pale eosinophilic cytoplasm and a round basophilic nucleus, morphology consistent with Trichomonas sp. (Figure 2). Necrotizing and proliferative oropharyngitis, ingluvitis, and/or esophagitis due to trichomoniasis was diagnosed in 7 purple finches and 1 A. goldfinch. Additionally, an A. goldfinch, was diagnosed with hepatic trichomoniasis. The A. goldfinch that died of hepatic trichomoniasis was emaciated but had no other gross lesions. Microscopically, the liver had randomly scattered foci of necrosis with rare macrophages, plasma cells and lymphocytes (Figure 3). In the necrotic foci and free in the sinusoids were many protozoans identified morphologically as Trichomonas sp.
Figure 2.
Esophagi/crops of purple finches, Carpodacus purpureus. a) Normal tissue. Bar = 100 μm. b) Severe erosive and proliferative esophagitis/ingluvitis due to trichomoniasis. Note the intense staining of the basal layers that correspond to active replication (hyperplasia). Bar = 100 μm. c) Higher magnification of the lesion described in b, showing numerous protozoans (Trichomonas gallinae) among the keratinized epithelial layers. Bar = 20 μm.
Figure 3.
Focus of necrosis in the liver of an American goldfinch, Carduelis tristis, with hepatic trichomoniasis. Protozoans identified morphologically as Trichomonas sp. are present in the necrotic area and adjacent sinusoids. Bar = 20 μm.
The parasite was confirmed to be Trichomonas gallinae through polymerase chain reaction (PCR) (HealthGene, Toronto, Ontario). The primers Trich-1F (5′-ATGAGTCAACACACGCC ATCAG-3′) and Trich-1R (5′-CACCTGGACGTCTGTGA CCTTC-3′) were used to amplify a 290-bp fragment of the T. gallinae DNA. All PCR amplifications were performed in a solution containing 20 mM Tris-HCl (pH 8.3), 50 mM KCl, 2.5 mM MgCl2, 200 μM (each) dATP, dCTP, dGTP, and dTTP, 0.4 μM each primer, 0.5 U of Taq DNA polymerase (Qiagen, Mississauga, Ontario) and 2.5 μL of extracted DNA. Amplifications were performed in an automated thermal cycler (GeneAmp PCR System 2700; Applied Biosystems, Foster City, California) with initial denaturation (95°C, 2 min), followed by 35 cycles of denaturation (94°C, 1 min), annealing (50°C, 30 s), and extension (72°C, 30 s), with a single final extension at 72°C for 5 min. After the reaction, 20 μL of the PCR product was separated on a 2% agarose gel, stained with ethidium bromide, visualized on a UV transilluminator, and photographed with Polaroid 667 film. The DNA molecular weight marker was ΦX-174-RF DNA HincII (Invitrogen, Burlington, Ontario) (6).
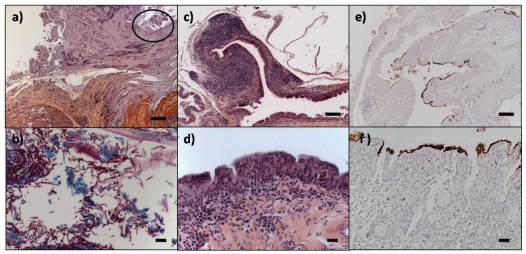
In total, trichomoniasis was the cause of death of 9/24 (37.5%) finches, 7 purple finches, and 2 American goldfinches, submitted in 2007–2008. In addition to ingluvial trichomoniasis in these birds, bacterial osteomyelitis of the skull was present in 1 purple finch and 1 American goldfinch, and infection of the proventriculus and gizzard with the fungus Macrorhabdus ornithogaster (previously known as megabacterium), identified by its characteristic morphology and location, was present in 1 purple finch (Figure 4, A–B).
Figure 4.
Miscellaneous lesions in purple finches, Carpodacus purpureus. a) Proventricular-ventricular junction with abundant fungal hyphae identified morphologically as Macrorhabdus ornithogaster (circle). Hematoxylin & eosin stain. b) Hyphae of M. ornithogaster. Periodic acid Schiff stain. c and d) Lymphoplasmacytic conjunctivitis due to Mycoplasma gallisepticum. Hematoxylin & eosin stain. e and f) Immunohistochemistry for M. gallisepticum. Bars a, c, e = 100 μm; Bars b, d, f = 20 μm.
Causes of death of the remaining 15 finches examined in 2007–2008 were: trauma (3 purple finches and 1 American goldfinch), primary starvation (3 purple finches and 1 American goldfinch), drowning in a water trough (3 purple finches), predation (1 purple finch), mycoplasmal conjunctivitis (1 purple finch), and unknown (2 purple finches). Mycoplasma gallisepticum was confirmed as the cause of the conjunctivitis through immunohistochemistry (California Animal Health and Food Safety Laboratory System, San Bernardino, California, USA) (Figure 4, C–F). Interestingly, 1 of the purple finches that died from drowning also had hepatic trichomoniasis; the infection was mild, perhaps in the early stages, and not the ultimate cause of death.
In total during 2007–2008, 24 finches were examined by CCWHC, 19 purple finches and 5 American goldfinches (Table 1). Fisher’s exact test and a significance level of 0.05 were used for statistical analyses. There were no statistically significant differences (P > 0.05) in the cause of death between male and female finches. However, there was a trend in birds that died of emaciation with no underlying disease (thus due to primary starvation from a failure to forage efficiently) to be immature individuals (P = 0.093). There was no significant difference in the age (adult versus immature) of the birds that died of trichomoniasis (P = 0.68). Most of the finches that were found dead near feeders died of trichomoniasis (5/9 = 55.6%) while none of the birds found away from feeders (0/6 = 0%) died of trichomoniasis (P = 0.044).
All but one of the finches that died of trichomoniasis in 2007–2008 (8/9 = 88.9%) were emaciated. In contrast, birds representative of a normal finch population (those that died from trauma, predation, drowning or primary starvation), with no evidence of an underlying disease and submitted during the same period of time (9 birds), were in good, moderate, or emaciated body condition (P = 0.049).
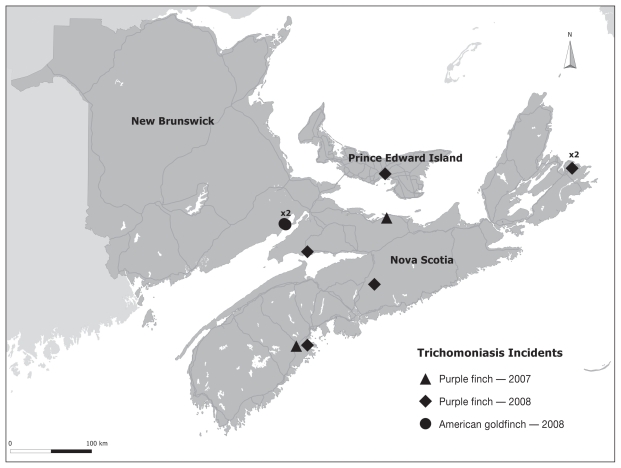
The incident locations of trichomoniasis were scattered throughout the Maritime provinces, indicating a widespread problem in the purple and American goldfinch populations (Figure 5).
Figure 5.
Locations of trichomoniasis incidents in purple goldfinch, Carpodacus purpureus, and American goldfinch, Carduelis tristis, in the Canadian Maritime provinces in 2007–2008. Note: the purple finch in Prince Edward Island died from drowning, hepatic trichomoniasis was the additional diagnosis.
Discussion
Trichomoniasis is caused by the flagellated protozoan parasite Trichomonas gallinae (Rivolta, 1878). It is a well-documented illness affecting many bird species, primarily diagnosed in columbiforms (pigeons and doves) (commonly known as canker), but also found in raptors (commonly known as frounce) and budgerigars (7). The parasite inhabits the upper digestive tract, mainly the crop and esophagus, but may also infect the liver, bones, and sinuses of the skull, lungs, air sacs, peritoneum and pancreas (8,9). Natural transmission of trichomoniasis amongst columbiforms occurs when adults feed nestlings with regurgitated food during the breeding season (9,10). Raptors acquire the disease when consuming infected prey birds (9). Food or water contaminated with recently regurgitated saliva or droppings from an infected individual are potential sources of infection (7,9), although the parasite is only viable in the environment for short periods of time (9,11). Trichomoniasis has also been reported in various species of passerine birds, particularly American robins (Turdus migratorius) (10), has emerged as a new cause of significant mortality in populations of greenfinch (Carduelis chloris), and chaffinch (Fringella coelebs) frequenting bird feeders in the UK (1–3), and is reported as the cause of high levels of mortality in the endangered pink pigeon (Columba mayeri) of Mauritius (12).
Trichomoniasis causes severe tissue damage in the crop and esophagus of birds and, at least in budgerigars, it is reported to cause weight loss and death due to constant vomiting or regurgitation and anorexia (7). Findings suggestive of regurgitation were present in finches dead of trichomoniasis in the Maritimes and those in the UK (13), and there was a significant association between trichomoniasis and emaciation in the Maritime finches (P = 0.049). Trichomoniasis may also cause lesions large enough to occlude the esophagus and result in death from starvation (9). Lesions of sufficient size to preclude ingestion of food were identified in some Maritime finches. Secondary bacterial infection can occur (14), and may perhaps exacerbate the clinical signs and severity of the lesions and thus contribute to the emaciation. Typically, gross lesions include dry exudate in the oropharyngeal cavity, thickening of the crop and/or esophagus and emaciation. Proliferative and necrotizing stomatitis, ingluvitis and esophagitis are the most common microscopic findings. Speciation of trichomonads can be achieved through PCR testing (6,15,16).
Trichomoniasis in finches has been reported in North America but only in a captive collection (14) and in experimentally immunosuppressed birds (17). To our knowledge, trichomoniasis in wild finches has not been previously reported in North America. The CCWHC has been conducting wildlife health surveillance in the Canadian Atlantic provinces for the past 17 y, and the cases in 2007 were the first instances of trichomoniasis diagnosed in finches. Similar to the recently described situation in the UK, this suggests that finch trichomoniasis is an emerging disease in eastern Canada.
Since it was first reported in 2005, the incidence of trichomoniasis in finches in the UK has increased (18,19). The reason for the emergence in finches of what is usually a columbiform disease is uncertain.
Infection with T. gallinae in birds in general can be asymptomatic or cause severe disease suggesting, amongst other things, the existence of different strains with variable pathogenicity (15). A study on the T. gallinae organisms present in the close population of pink pigeons of the island of Mauritius found no variation between isolates at the ITS1/5.8/ITS2 region of rDNA, but significant genotypic variation between isolates through the use of Random Amplified Polymorphic DNA (RAPD) analysis (15). The variability seemed correlated to geographical distribution of the birds, but the authors indicate that the small sample examined precludes statistical analysis to confirm a geographical association. There is no indication as to whether the difference in the isolates was associated with their pathogenicity. The Mauritian population of pink pigeons has an average prevalence of infection with T. gallinae of 50.3% (19.6% to 82.4%), with prevalence increasing with the age of the host (20). Although observed pathogenicity (active clinical signs found at sampling) of T. gallinae was low, birds that persistently tested positive for the parasite were less likely to survive in a 2-year interval (20). Recently, a study in the United States that examined crop isolates of trichomonads from various bird species, mainly columbiforms and hawks, found through sequence analysis of 5.8 rRNA and flanking loci (ITS1 and ITS2) differences that suggest at least 2 species may exist within the T. gallinae morphologic complex (16). Whether the increased incidence of trichomoniasis in the UK and its emergence in the Canadian Maritimes is associated with a highly pathogenic isolate, or indeed a different and more virulent species, remains to be determined.
In the UK, finch mortalities begin in July but are more common in late summer and early fall. Climatic factors, particularly high precipitation, were initially thought to have played a role in the emergence of the disease in the UK (18), but this hypothesis was all but abandoned due to the inconsistency between weather events and trichomoniasis outbreaks. More recently, dry weather and low rainfall have been suggested as factors involved with the emergence of the disease in the UK (19). The prevalence of T. gallinae infection in doves in Mauritius is higher at sites and times of warmer temperatures and lower rainfall (21), supporting this hypothesis. Similar to the UK, when Maritime weather data for the last 2 summers was reviewed, consistent patterns of precipitation and/or temperature were not identified in relation to specific locations of finch mortality (22). Further research and continued surveillance for the disease will be necessary to determine if weather patterns are involved in the emergence and spread of the disease.
Another factor that is shared between the cases in the UK and the Maritimes and which may prove to be important in the epidemiology of the disease, is that most of the mortality from trichomoniasis was identified around feeding stations. Granted that finches around feeders may have been submitted more assiduously since the call to submit dead finches had reached mostly bird-feeding enthusiasts, the finding is still significant and noteworthy as there was a definite association between bird feeders and finches with trichomoniasis (P = 0.044) in the Maritimes. Of all finches found dead around feeders in the Maritimes, 55.6% died of trichomoniasis, while none of the finches found away from feeders died of the disease. These findings suggest that this is a real association and not merely a reflection of the ease in observing mortalities at such locations, usually by avid bird watchers. Historically, bird feeding was a winter activity in the Maritimes, but in recent years it has become a year-round activity. Perhaps the disease is being transmitted to finches, birds not usually affected, simply by the concentration of such birds at specific locations also frequented by carriers of trichomoniasis (pigeons and doves). Transmission may be more likely during the summer months as trichomonads have better survival in warmer temperatures.
Bird baths and drinking water sources provided by the general public may also play a role in transmission. Because T. gallinae is highly susceptible to desiccation, water can serve to promote survival of the organism. High densities of birds aggregating at limited natural water sources may increase transmission of the infection (21). During dry summer periods, bird baths may become the only source of water in a given area, attracting many species of birds in large numbers and increasing the possibility for transmission of trichomoniasis. This remains speculative as water samples collected during outbreaks of trichomoniasis in doves in Arizona were routinely negative for the parasite (23), in spite of using a method considered as ideal for detection of T. gallinae (24).
Factors involved in finch trichomoniasis in the UK and the Canadian Maritimes require further investigation before the epidemiology of the disease is understood and prevention methods can be suggested. The CCWHC is currently encouraging reports and submission of any finch mortalities from all Canadian Atlantic provinces (including Newfoundland and Labrador). Surveillance and proactive investigation of the source of contagion will help determine guidelines for the bird-feeding public that may help reduce the impact of trichomoniasis in the finch population.
Acknowledgments
The authors thank Dr. Alfonso López for his invaluable help with the microscopic photography, Dr. López and Dr. Pierre-Yves Daoust for their thorough critical review of the manuscript, and Fiep de Bie for her assistance with the composition of digital images. The provinces of New Brunswick, Nova Scotia, and Prince Edward Island, and the United States Geological Survey are thanked for providing online mapping data for the region.
Footnotes
Reprints will not be available from the authors.
The diagnostic work was done as part of the wildlife health surveillance program of the Canadian Cooperative Wildlife Health Centre which operates with support from several Canadian federal, provincial, and territorial government departments, and nongovernmental organizations.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office ( hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Pennycott T, Lawson B, Cunningham A, Simpson V, Chantrey J. Necrotic ingluvitis in wild finches. Vet Rec. 2005;157:360. doi: 10.1136/vr.157.12.360. [DOI] [PubMed] [Google Scholar]
- 2.Lawson B, Cunningham A, Chantrey J, et al. Epidemic finch mortality. Vet Rec. 2006;159:367. doi: 10.1136/vr.159.11.367-a. [DOI] [PubMed] [Google Scholar]
- 3.Duff JP, Pennycott TW, Willmington JA, Roberston SL. Emergence of garden bird trichomonosis. Vet Rec. 2007;161:828. [PubMed] [Google Scholar]
- 4.Kassai T, Cordero del Campillo M, Euzeby J, Gaafar S, Hiepe Th, Himonas CA. Standardized Nomenclature of Animal Parasitic Diseases (SNOAPAD) Vet Parasitol. 1988;29:299–326. doi: 10.1016/0304-4017(88)90148-3. [DOI] [PubMed] [Google Scholar]
- 5.McBurney S, Forzán MJ. Trichomoniasis in purple finches — An emerging disease? Wildlife Health Centre Newsletter, Canadian Cooperative Wildlife Health Centre. 2008;13:6. [Google Scholar]
- 6.Padilla LR, Santiago-Alarcon D, Merkel J, Miller RE, Parker PG. Survey for Haemoproteus spp., Trichomonas gallinae, Chlamydophila psittaci, and Salmonella spp. in Galapagos islands columbiformes. J Zoo Wildl Med. 2004;35:60–64. doi: 10.1638/03-029. [DOI] [PubMed] [Google Scholar]
- 7.Baker JR. Trichomoniasis, a major cause of vomiting in budgerigars. Vet Rec. 1986;118:447–449. doi: 10.1136/vr.118.16.447. [DOI] [PubMed] [Google Scholar]
- 8.Narcisi EM, Sevoian M, Honigberg BM. Pathologic changes in pigeons infected with a virulent Trichomonas gallinae strain (Eiberg) Avian Dis. 1991;35:55–61. [PubMed] [Google Scholar]
- 9.Kocan RM, Herman CM. Trichomoniasis. In: Davis JW, Anderson RC, Karstad L, Trainer DO, editors. Infectious and parasitic diseases of wild birds. Ames, Iowa: Iowa State Univ Pr; 1971. pp. 282–290. [Google Scholar]
- 10.Burton DL, Doblar KA. Morbidity and mortality of urban wildlife in the Midwestern United States. Proc 4th International Urban Wildlife Symposium. 2004:171–181. [Google Scholar]
- 11.Greiner EC, Ritchie BW. Parasites. In: Ritchie BW, Harrison GJ, Harrison LR, editors. Avian Medicine: Principles and Application. Florida, USA: Wingers Publishing Inc; 1994. pp. 1005–1029. [Google Scholar]
- 12.Bunbury N, Stidworthy MF, Greenwood A, et al. Causes of mortality in free-living Mauritian pink pigeons Columba mayeri, 2002–2006. Endang Species Res. 2008 doi: 10.3354/esr00088. [DOI] [Google Scholar]
- 13.Garden Bird Initiative [monograph on the Internet] [Last accessed February 17, 2010]. Available from http://www.ufaw.org.uk/documents/GBHI_Trichomonas_sheet_180707.pdf.
- 14.Chalmers GA. Trichomoniasis in finches. Can Vet J. 1992;33:616–617. [PMC free article] [PubMed] [Google Scholar]
- 15.da Silva DG, Barton E, Bungury N, Lunness P, Bell DJ, Tyler KM. Molecular identity and heterogeneity of trichomonad parasites in a closed avian population. Infect Genet Evol. 2007;7:433–440. doi: 10.1016/j.meegid.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Gerhold RW, Yabsley MJ, Smith AJ, et al. Molecular characterization of the Trichomonas gallinae morphologic complex in the United States. J Parasitol. 2008;94:1335–1341. doi: 10.1645/GE-1585.1. [DOI] [PubMed] [Google Scholar]
- 17.Reisen WK, Chiles RE, Green EN, et al. Effects of immunosuppression on encephalitis virus infection in the house finch, Carpodacus mexicanus. J Med Entomol. 2003;40:206–214. doi: 10.1603/0022-2585-40.2.206. [DOI] [PubMed] [Google Scholar]
- 18.Anonymous Veterinary Laboratory Surveillance Report for August. Vet Rec. 2006;159:507–510. [Google Scholar]
- 19.Simpson V, Molenaar F. Increase in trichomonosis in finches. Vet Rec. 2006;159:606. doi: 10.1136/vr.159.18.606-a. [DOI] [PubMed] [Google Scholar]
- 20.Bunbury N, Jones CG, Greenwood A, Bell DJ. Epidemiology and conservation implications of Trichomonas gallinae infection in the endangered Mauritian pink pigeon. Biol Conserv. 2008;141:153–161. [Google Scholar]
- 21.Bunbury N, Jones CG, Greenwood AG, Bell DJ. Trichomonas gallinae in Mauritian columbids: Implications for an endangered endemic. J Widl Dis. 2007;43:399–407. doi: 10.7589/0090-3558-43.3.399. [DOI] [PubMed] [Google Scholar]
- 22.Environment Canada’s National Climate Data and Information Archive [database on the Internet] [Last accessed February 17, 2010]. Available from http://www.climate.weatheroffice.ec.gc.ca/Welcome_e.html.
- 23.Rosentock SS, Rabe MJ, O’Brien CS, Waddell RB. Studies of wildlife water developments in southwestern Arizona: Wildlife use, water quality, wildlife diseases, wildlife mortalities and influences on native pollina-tors. Arizona Game and Fish Department, Technical Guidance Bulletin No. 8; Phoenix, USA: 2004. pp. 10–11. [Google Scholar]
- 24.Bunbury N, Bell D, Jones C, Greenwood A, Hunter P. Comparison of InPouch TF culture system and wet-mount microscopy for diagnosis of Trichomonas gallinae in pink pigeon Columba mayeri. J Clin Microbiol. 2005;43:1005–1006. doi: 10.1128/JCM.43.2.1005-1006.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]