Abstract
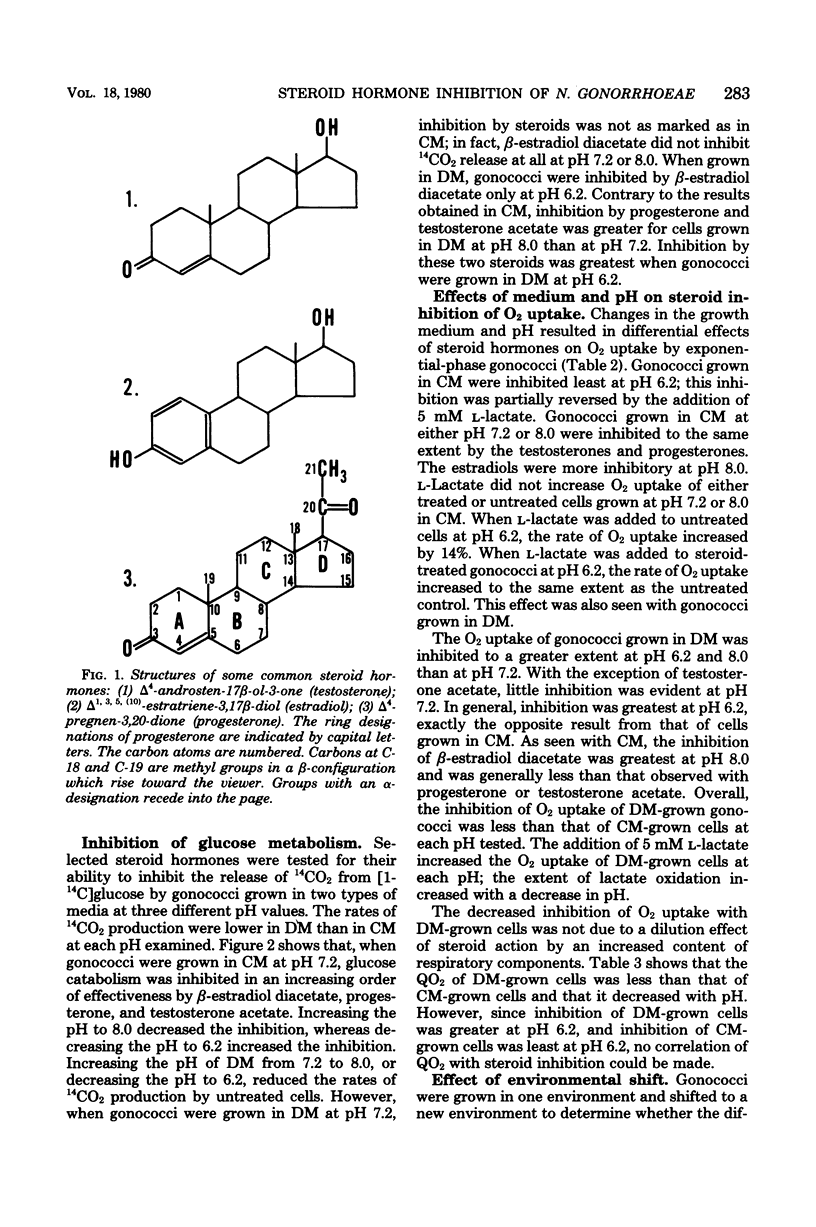
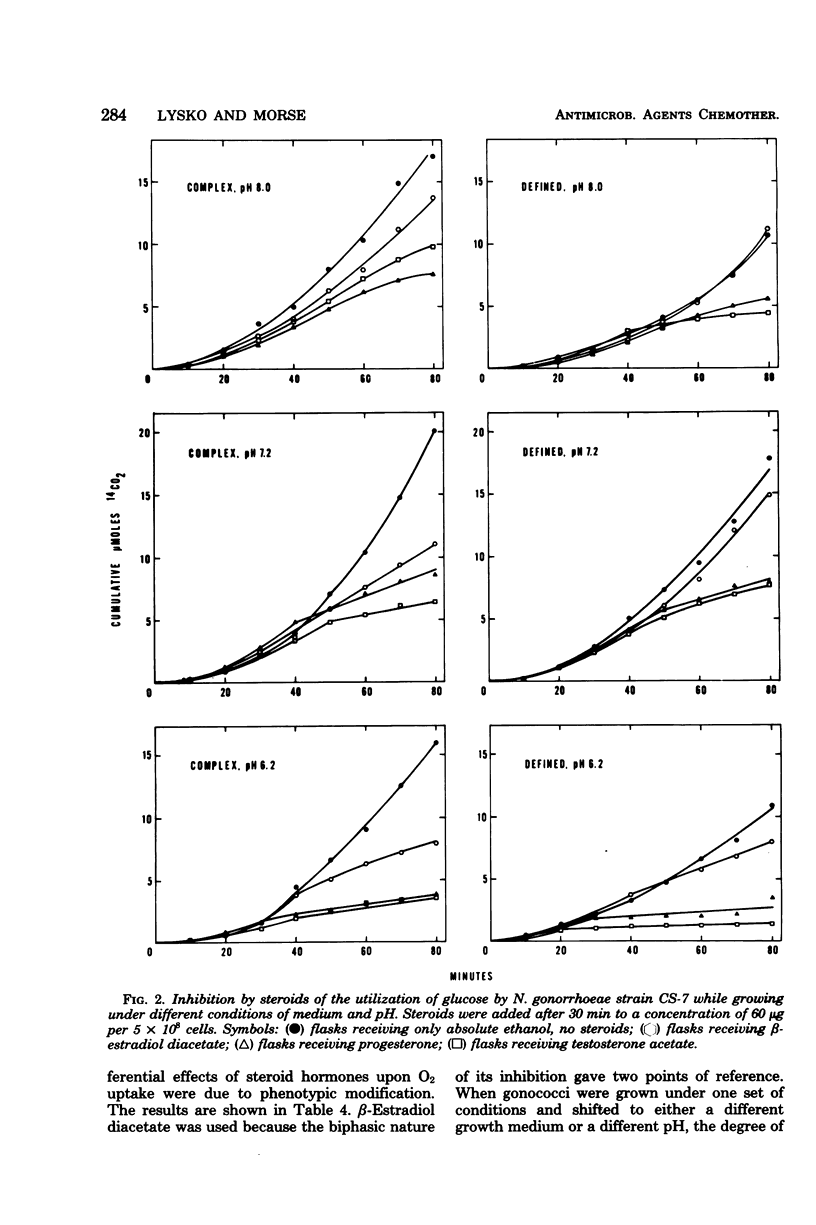
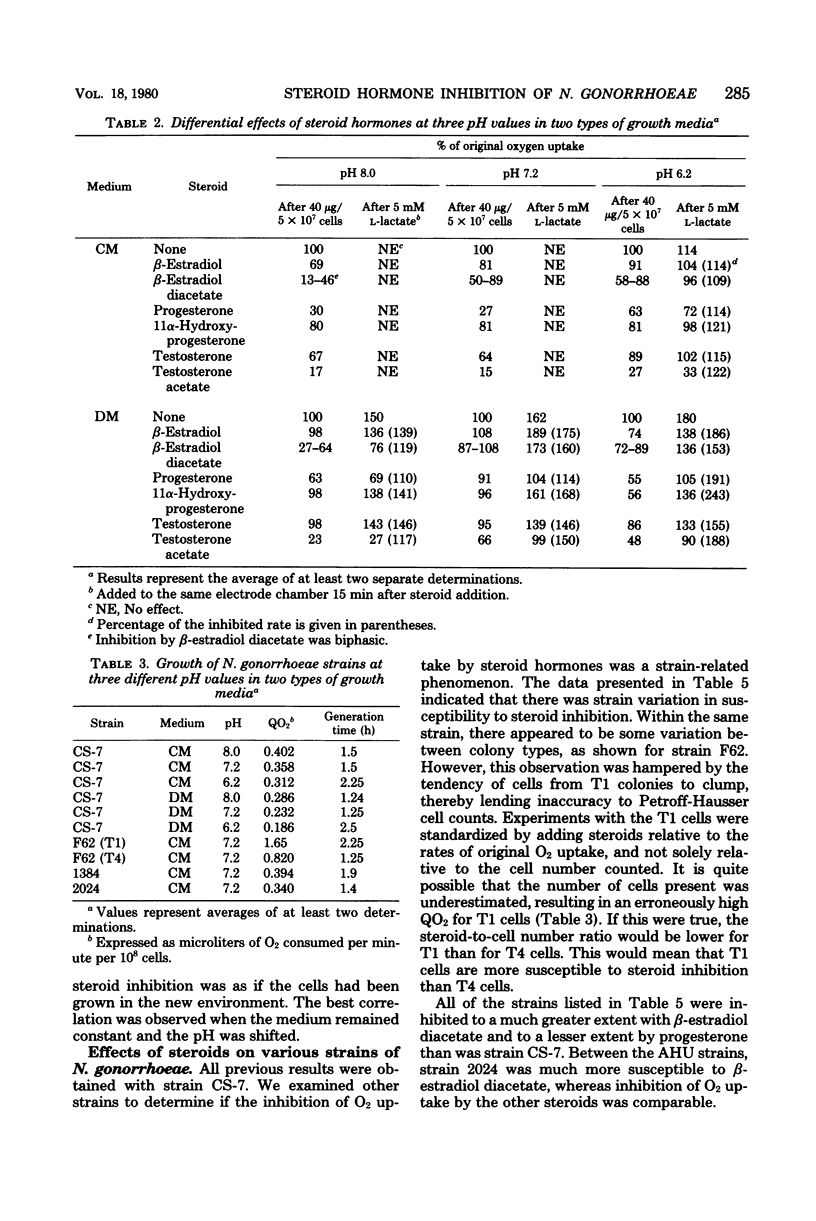
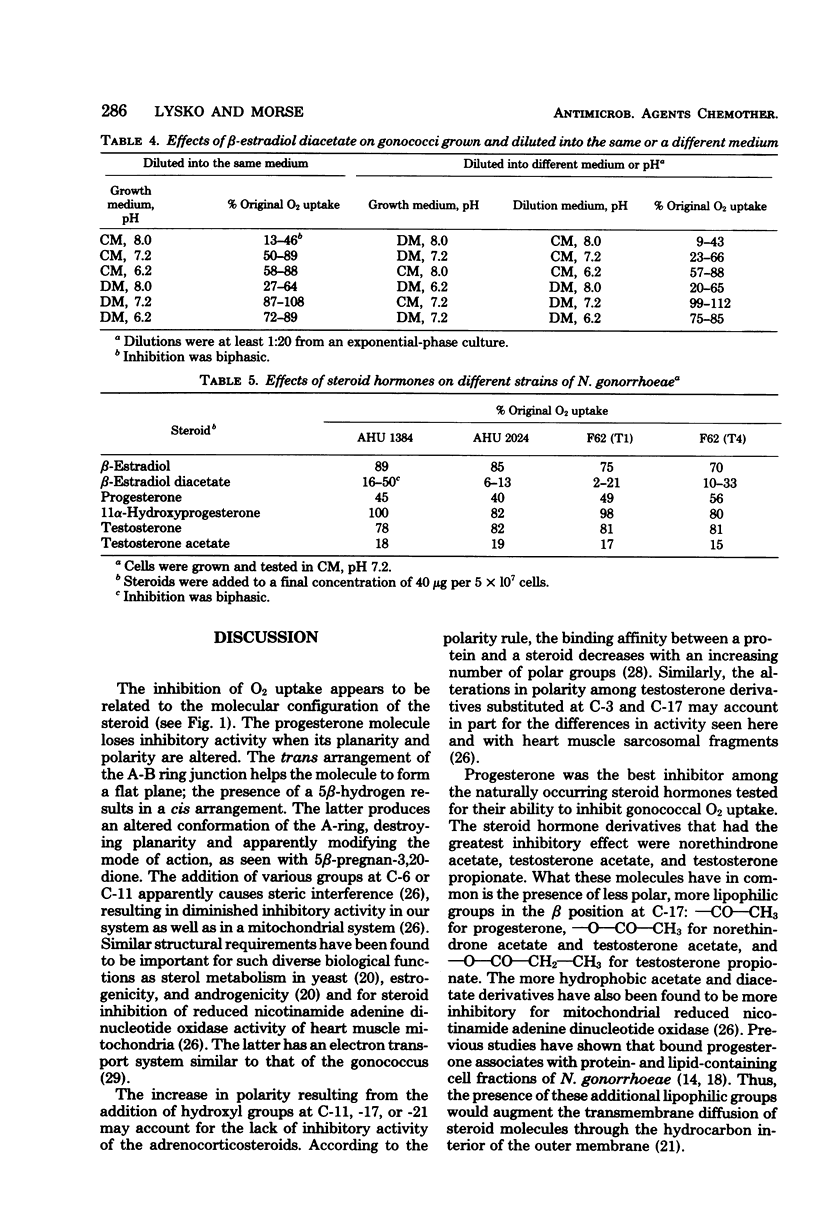
Various steroids were tested for their effects upon gonococcal O2 consumption and glucose catabolism. The ability to inhibit gonococcal O2 uptake appeared to be related to the molecular configuration of the steroid. The presence of lipophilic groups enhanced inhibition, whereas the addition of hydrophilic groups markedly diminished inhibition. Steroid inhibition decreased with an increasing number of polar groups. Glucose catabolism was inhibited by steroid hormones, and the degree of inhibition was influenced by pH and medium composition. Changes in growth medium and pH also resulted in differential steroid inhibition of O2 uptake. Under certain conditions, lactate partially relieved this inhibition. Gonococci that were grown in one environment and shifted to a new environment were inhibited by steroids to the same extent as if they had been originally grown in the new environment. The differential effects of medium and pH upon steroid inhibition may be due to structural rearrangements involving membrane phase transitions or to altered receptor affinity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandts J. F., Taverna R. D., Sadasivan E., Lysko K. A. Calorimetric studies of the structural transitions of the human erythrocyte membrane. Studies of the B and C transitions. Biochim Biophys Acta. 1978 Oct 4;512(3):566–578. doi: 10.1016/0005-2736(78)90166-9. [DOI] [PubMed] [Google Scholar]
- Fitzgerald T. J., Morse S. A. Alteration of growth, infectivity, and viability of Neisseria gonorrhoeae by gonadal steroids. Can J Microbiol. 1976 Feb;22(2):286–294. doi: 10.1139/m76-039. [DOI] [PubMed] [Google Scholar]
- Ganguly M., Carnighan R. H., Westphal U. Steroid-protein interactions. XIV. Interaction between human alpha 1-acid glycoprotein and progesterone. Biochemistry. 1967 Sep;6(9):2803–2814. doi: 10.1021/bi00861a022. [DOI] [PubMed] [Google Scholar]
- Huang L., Haug A. Regulation of membrane lipid fluidity in Acholeplasma laidlawii: effect of carotenoid pigment content. Biochim Biophys Acta. 1974 Jun 29;352(3):361–370. doi: 10.1016/0005-2736(74)90228-4. [DOI] [PubMed] [Google Scholar]
- Huang L., Lorch S. K., Smith G. G., Haug A. Control of membrane lipid fluidity in Acholeplasma laidlawii. FEBS Lett. 1974 Jul 1;43(1):1–5. doi: 10.1016/0014-5793(74)81090-2. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Papahadjopoulos D. Phase transitions and phase separations in phospholipid membranes induced by changes in temperature, pH, and concentration of bivalent cations. Biochemistry. 1975 Jan 14;14(1):152–161. doi: 10.1021/bi00672a026. [DOI] [PubMed] [Google Scholar]
- James J. F., Swanson J. Studies on gonococcus infection. XIII. Occurrence of color/opacity colonial variants in clinical cultures. Infect Immun. 1978 Jan;19(1):332–340. doi: 10.1128/iai.19.1.332-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson E. D. Progesterone levels in peripheral plasma during the luteal phase of the normal human menstrual cycle measured by a rapid competitive protein binding technique. Acta Endocrinol (Copenh) 1969 Aug;61(4):592–606. doi: 10.1530/acta.0.0610592. [DOI] [PubMed] [Google Scholar]
- Johnson D. W., Holmes K. K., Kvale P. A., Halverson C. W., Hirsch W. P. An evaluation of gonorrhea case findings in the chronically infected female. Am J Epidemiol. 1969 Nov;90(5):438–448. doi: 10.1093/oxfordjournals.aje.a121090. [DOI] [PubMed] [Google Scholar]
- Lowe T. L., Kraus S. J. Quantitation of Neisseria gonorrhoeae from women with gonorrhea. J Infect Dis. 1976 Jun;133(6):621–626. doi: 10.1093/infdis/133.6.621. [DOI] [PubMed] [Google Scholar]
- Miller R. D., Brown K. E., Morse S. A. Inhibitory action of fatty acids on the growth of Neisseria gonorrhoeae. Infect Immun. 1977 Aug;17(2):303–312. doi: 10.1128/iai.17.2.303-312.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. D., Morse S. A. Binding of progesterone to Neisseria gonorrhoeae and other gram-negative bacteria. Infect Immun. 1977 Apr;16(1):115–123. doi: 10.1128/iai.16.1.115-123.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. D., Warren W. J., Sizemore R. C., Morse S. A. Binding of cholesterol by Neisseria gonorrhoeae. Infect Immun. 1978 Dec;22(3):698–708. doi: 10.1128/iai.22.3.698-708.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Bartenstein L. Factors affecting autolysis of Neisseria gonorrhoeae. Proc Soc Exp Biol Med. 1974 Apr;145(4):1418–1421. doi: 10.3181/00379727-145-38025. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Bartenstein L. Purine metabolism in Neisseria gonorrhoeae: the requirement for hypoxanthine. Can J Microbiol. 1980 Jan;26(1):13–20. doi: 10.1139/m80-003. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Fitzgerald T. J. Effect of progesterone on Neisseria gonorrhoeae. Infect Immun. 1974 Dec;10(6):1370–1377. doi: 10.1128/iai.10.6.1370-1377.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Stein S., Hines J. Glucose metabolism in Neisseria gonorrhoeae. J Bacteriol. 1974 Nov;120(2):702–714. doi: 10.1128/jb.120.2.702-714.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Preti G., Huggins G. R., Silverberg G. D. Alterations in the organic compounds of vaginal secretions caused by sexual arousal. Fertil Steril. 1979 Jul;32(1):47–54. [PubMed] [Google Scholar]
- Robinson J., Cooper J. M. Method of determining oxygen concentrations in biological media, suitable for calibration of the oxygen electrode. Anal Biochem. 1970 Feb;33(2):390–399. doi: 10.1016/0003-2697(70)90310-6. [DOI] [PubMed] [Google Scholar]
- Ross G. T., Cargille C. M., Lipsett M. B., Rayford P. L., Marshall J. R., Strott C. A., Rodbard D. Pituitary and gonadal hormones in women during spontaneous and induced ovulatory cycles. Recent Prog Horm Res. 1970;26:1–62. doi: 10.1016/b978-0-12-571126-5.50005-4. [DOI] [PubMed] [Google Scholar]
- Saxena B. N., Dusitsin N., Poshyachinda V., Smith I. Luteinizing hormone, oestradiol, and progesterone levels in the serum of menstruating Thai women. J Obstet Gynaecol Br Commonw. 1974 Feb;81(2):113–119. doi: 10.1111/j.1471-0528.1974.tb00428.x. [DOI] [PubMed] [Google Scholar]
- Stoppani A. O., De Brignone C. M., Brignone J. A. Structural requirements for the action of steroids as inhibitors of electron transfer. Arch Biochem Biophys. 1968 Sep 20;127(1):463–475. doi: 10.1016/0003-9861(68)90251-8. [DOI] [PubMed] [Google Scholar]
- Träuble H., Eibl H. Electrostatic effects on lipid phase transitions: membrane structure and ionic environment. Proc Natl Acad Sci U S A. 1974 Jan;71(1):214–219. doi: 10.1073/pnas.71.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D. B., Morse S. A. Physiology and metabolism of pathogenic Neisseria: partial characterization of the respiratory chain of Neisseria gonorrhoeae. J Bacteriol. 1975 Aug;123(2):631–636. doi: 10.1128/jb.123.2.631-636.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kruyff B., Demel R. A., van Deenen L. L. The effect of cholesterol and epicholesterol incorporation on the permeability and on the phase transition of intact Acholeplasma laidlawii cell membranes and derived liposomes. Biochim Biophys Acta. 1972 Jan 17;255(1):331–347. doi: 10.1016/0005-2736(72)90032-6. [DOI] [PubMed] [Google Scholar]


