Abstract
Homeostatic synaptic plasticity (HSP) is important for maintaining neurons' excitability within the dynamic range and for protecting neurons from unconstrained LTP that can cause breakdown of synapse specificity (Turrigiano, 2008). Knowledge of the molecular mechanism underlying this phenomenon remains incomplete, especially for the rapid form of HSP. In order to test whether HSP in adulthood depends on an F-actin binding protein – drebrin A, mice deleted of the adult isoform of drebrin (DAKO) but retaining the embryonic isoform (drebrin E) were generated. HSP was assayed by determining whether the NR2A subunit of NMDA receptors (NMDARs) can rise rapidly within spines following the application of an NMDAR antagonist, D-APV, onto the cortical surface. Electron microscopic immunocytochemistry revealed that, as expected, the D-APV treatment of WT mouse cortex increased the proportion of NR2A-immunolabeled spines within 30 min, relative to basal levels in hemispheres treated with an inactive enantiomer, L-APV. This difference was significant at the postsynaptic membrane and postsynaptic density (i.e., synaptic junction) as well as at non-synaptic sites within spines and was not accompanied by spine size changes. In contrast, the D-APV treatment of DAKO brains did not augment NR2A labeling within the spine cytoplasm or at the synaptic junction, even though basal levels of NR2A were not significantly different from those of WT cortices. These findings indicate that drebrin A is required for the rapid (<30 min) form of HSP at excitatory synapses of adult cortices while drebrin E is sufficient for maintaining basal NR2A levels within spines.
INTRODUCTION
Neurons throughout the CNS are endowed with mechanisms that integrate activity over time and convert these into signals that regulate the maintenance and up/down changes in the expression of genes encoding receptors and channels. Some of the mechanisms underlying this self-regulation are achieved locally and rapidly at synapses (Malenka and Bear, 2004; Perez-Otano and Ehlers, 2005). Without these checks-and-balances, steady maintenance of synaptic strength (homeostatic synaptic plasticity) is lost, and this could lead to unconstrained LTP, excessive excitation of neurons, and degradation of synapse specificity (Turrigiano, 2008).
In cortex and hippocampus, excitatory synapses form almost exclusively at spines, a specialized structure, typically less than 1 μm in diameter, where glutamate receptors, their scaffolding proteins and signaling molecules, such as αCaMKII, are organized (Kennedy and Ehlers, 2006). Through quantitative electron microscopic-immunocytochemistry (EM-ICC), we have demonstrated that spines of adult rat cortex can respond rapidly (<30 min) to blockade of NMDA receptors (NMDAR) by increasing the levels of the NMDAR subunit, NR2A, precisely at axo-spinous synaptic junctions and within the spine cytoplasm (Aoki et al., 2003). Such a response would be useful for returning excitability of NMDAR-antagonized synapses towards original set-point. This form of homeostatic synaptic plasticity was first observed for cultured hippocampal neurons (Rao and Craig, 1997), although the response observed there may have been more sluggish, since NMDAR's NR1 puncta were reported to increase only after exposing neurons to D-APV for a minimum of 7 days. For any of these examples of activity-dependent plasticity, rapid or slower, our understanding of the molecular mechanisms underlying NMDAR insertion at synapses is incomplete. However, converging evidence indicates that receptor turnover at synapses involves the interaction of plasmalemmal mechanisms to capture receptors at synapses and the cytoplasmic organelles that deliver receptor cargos into and out of spines and to the postsynaptic membrane (Groc and Choquet, 2006; Kennedy and Ehlers, 2006; Perez-Otano and Ehlers, 2005). Those studies exploring the molecular mechanisms underlying plasticity of excitatory synapses indicate that F-actin plays a central role, in that both the synaptic capturing and translocation of receptor cargos to synapses involve F-actin (Allison et al., 2000; Allison et al., 1998; Halpain, 2006; Halpain et al., 1998; Kennedy and Ehlers, 2006; Krupp et al., 1999; Star et al., 2002; Wyszynski et al., 1997). These observations suggest that candidate molecules linking synaptic activity to receptor localization are likely to be enriched at the postsynaptic side of excitatory synapses and exhibit F-actin-binding characteristics.
More recently, we showed that the increase of NR2A in dendritic spines is accompanied by increases of F-actin and an F-actin binding protein, drebrin A (Fujisawa et al., 2006). Drebrin A is the only neuron-specific, F-actin binding protein that is found exclusively on the postsynaptic side of excitatory synapses (Aoki et al., 2005). In that study, we were prompted to examine whether synaptic activity regulates the localization of drebrin A within spines, because a number of studies (Shirao and Sekino, 2001) had indicated that drebrin (the embryonic/E- or adult/A-isoforms) has properties suitable for modulating the trafficking of proteins into and out of spines, as well as to modify the shape and even the stability of spines. One of drebrin's interesting properties is to reduce the sliding velocity of actin filaments on immobilized myosin and inhibit the actin-activated ATPase activity of myosin (Hayashi et al., 1996). Such a property could underlie drebrin's ability to regulate the accumulation of synaptic molecules within spines. Because drebrin can bind to F-actin, it can displace α-actinin's binding to F-actin and in this way, also liberate the link between NMDARs and F-actin (Shirao and Sekino, 2001). If drebrin resides precisely at the synapse, this property could help unload receptors at the postsynaptic membrane and also liberate NMDARs that are tethered to the subsynaptic F-actin lattice. By displacing α-actinin from F-actin, drebrin can also make F-actin accessible to gelsolin. Gelsolin, in turn, severs F-actin into shorter fragments in a calcium-dependent manner (Kinosian et al., 1998) and this action may alter the fluidity of the cytoplasm, thereby facilitating the diffusion of cytoplasmic organelles and proteins into, out of and within the spine cytoplasm. In accordance with both of these properties that are linked to the displacement of α-actinin, drebrin A knock-down of cultured hippocampal neurons by antisense treatment leads to impairment in the NMDAR-blockade-evoked up-regulation of the NR1 subunits within spines (Takahashi et al., 2006). The action of gelsolin upon F-actin, which is promoted by the presence of drebrin, can also increase spine shape flexibility. Indeed, knock-down of drebrin within cultured hippocampal neurons by RNAi promotes stabilization of dendritic protrusions, leading to an increased population of globular, mature, stable spines in an F-actin/Ras dependent manner (Biou et al., 2008).
While these studies indicate that drebrin is an excellent candidate for linking NMDAR activity to NMDAR localization at synapses, all of these experiments were performed using acellular systems or hippocampal neurons grown in vitro. Thus, whether drebrin A exerts activity-dependent regulation upon the redistributions of receptors within intact tissue and, especially, in fully mature brains has yet to be tested. Nevertheless, this question is important, because drebrin is one protein that declines in brains of patients diagnosed with Alzhemier's disease or mild cognitive impairment (Counts et al., 2006; Harigaya et al., 1996; Hatanpaa et al., 1999; Shim and Lubec, 2002) and in animal models of Alzhemier's disease (Aoki et al., 2007; Calon et al., 2004) in the early stages (Aoki et al., 2007; Calon et al., 2004).
The in vitro pharmacological manipulations described above rendered the spine shapes less stable, thereby precluding analysis of receptor redistribution separately from spine shape stability. Moreover, without the use of EM, the distinction of receptor immunoreactivity precisely at the surface versus non-surface portions of the spine is difficult, particularly for neurons within intact tissue. Therefore, in the current study, we examined the impact of the knockout of the adult isoform of drebrin (drebrin A) upon the responsiveness of spines to NMDAR blockade. Drebrin A knockout (DAKO) mice were generated by targeted knockout of exon 11A, encoding the drebrin-A specific insertion. These animals continue to express the embryonic isoform, drebrin E, instead of switching to drebrin A in adulthood. We demonstrate that DAKO eliminates the rapid NMDAR blockade-evoked rise of NR2A levels within spines and at the PSD, even though resting levels of NR2A at the postsynaptic membrane is comparable to the levels observed within control hemispheres of age-matched WT animals. These observations indicate that drebrin A is required for homeostatic synaptic plasticity, whereas drebrin E is sufficient for maintaining adult levels of NR2A at postsynaptic membranes. Moreover, the rapidity with which spines respond, in the presence of drebrin A, indicates that spines of intact neurons possess the mechanism to regulate receptor levels at synapses without evoking transcriptional mechanisms.
METHODS
Generation of drebrin A knockout (DAKO) mice
All DAKO and WT littermates were bred at the animal facility of Gunma University Graduate School of Medicine, Gunma, Japan in accordance with the guidelines published in the NIH Guide for the Care and Use of Laboratory Animals and Gunma University's Animal Care and Experimentation Committee. All ante mortem experiments were carried out according to the Animal Care and Experimentation Committee of Gunma University, Showa Campus and of NYU's University Animal Welfare Committee.
All mice were F4 generation adult male. DAKO mice were generated by targeted knockout of exon 11A, encoding the drebrin-A specific insertion, while control animals were 11A homozygous wild type (+/+). An outline of the procedure for generating DAKO mice is presented here, while a more complete description appears elsewhere (Kojima et al., manuscript in preparaption). Briefly, a rat drebrin A cDNA (clone Drh102, (Shirao et al., 1992) was used as the probe for screening the C57BL/6J mouse genomic DNA BAC library (BACPAC Resources Center, Oakland, CA) for the drebrin gene. From one of these BAC clones, DNA fragments covering the genomic locus containing exon 11A were subcloned and characterized. For construction of the targeting vector, exon 11A, encoding the drebrin A-specific insertion (ins2 (Jin et al., 2002)), was flanked by loxP sites (Sternberg and Hamilton, 1981). For the positive and negative selections, the PGK-neo cassette (Wu et al., 1994) flanked by frt sites (Broach et al., 1982) and the pMC1DT-A were inserted into the 3' direction of exon 11A and the 5'-end of the targeting vector, respectively. This construct was linearized and electroporated into C57BL/6 mouse embryonic stem (ES) cells (line MS12 (Kawase et al., 1994)) as described previously (Homanics et al., 1997). The recombinant ES clones were screened by Southern blot analysis. ES cell clones that harbored the targeted locus were injected into C57BL/6J blastocysts to produce chimeric mice. Chimeric animals were mated with C57BL/6J females to establish germ line transmission. Heterozygous (F/+) male mice of the F1 generation were subsequently mated with EIIa-Cre transgenic mice (Lakso et al., 1996) to delete the drebrin exon 11A flanked by loxP sites. Mice carrying the deletion of exon 11A were selected by PCR of the tail DNA. Mice heterozygous for the 11A null allele were backcrossed to C57BL/6J females for obtaining the breeder, and intercrossed for obtaining mice homozygous for the 11A null allele (−/−). The KO homozygous mice continue to express the embryonic isoform, drebrin E, instead of switching to drebrin A in adulthood (Fig. 1).
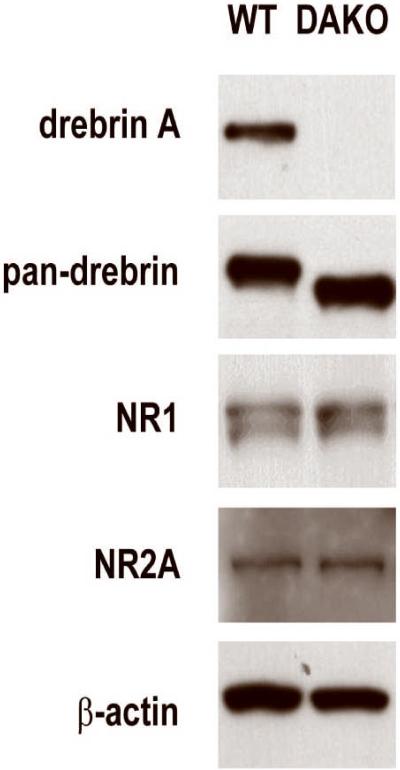
Fig. 1. Persistence of drebrin E and absence of drebrin A within adult DAKO brain homogenates.
Whole homogenates of two adult WT and two adult DAKO cerebral cortices were analyzed for the presence of drebrin and NMDAR subunits by Western blotting. When probed using the drebrin A-specific antibody (DAS2), brain homogenates prepared from WT mice exhibited a single band corresponding to drebrin A. In contrast, the homogenate from DAKO mice exhibited no immunoreactive band. When probed using the monoclonal antibody that recognizes both embryonic and adult isoforms of drebrin (M2F6), the brain homogenate prepared from WT mice exhibited a band corresponding to drebrin A concomitantly with a faint band corresponding to drebrin E. In contrast, brain homogenate prepared from age-matched DAKO mice exhibited only a single band with apparent molecular weight corresponding to that of drebrin E. Western blots probed with anti-NR1 and anti-NR2A subunits of NMDARs and with anti-β-actin yielded identical bands in lanes loaded with WT and DAKO homogenates.
DAKO and WT male mice were marked by ear punching at the Gunma University facility, so as to be able to identify each animal according to its ID number and genotype. At the age of three months, those animals to be used for light and electron microscopy and homeostatic synaptic plasticity assay were transported from Japan to US, using World Courier. The genotype of each animal was confirmed in three ways: by genotyping the DNA prior to shipping, genotyping the DNA again, after shipping and by the EM-ICC detection of drebrin A within spines (Fig. 2). NYU investigators were kept blind regarding the genotype of the animals until all parts of the experimental procedure (surgery, immunocytochemistry and EM quantification) were completed, so as to minimize any potential for the NYU team to be biased in their treatment of the animals or in the tissue sampling or the quantification procedure. Throughout the experimental procedures at NYU, the animals were referred to by new ID numbers - #5, #6, #7, #8, #9, #10 and #11, given in the US, so as to conceal the genotype to all participating investigators at NYU except for one.
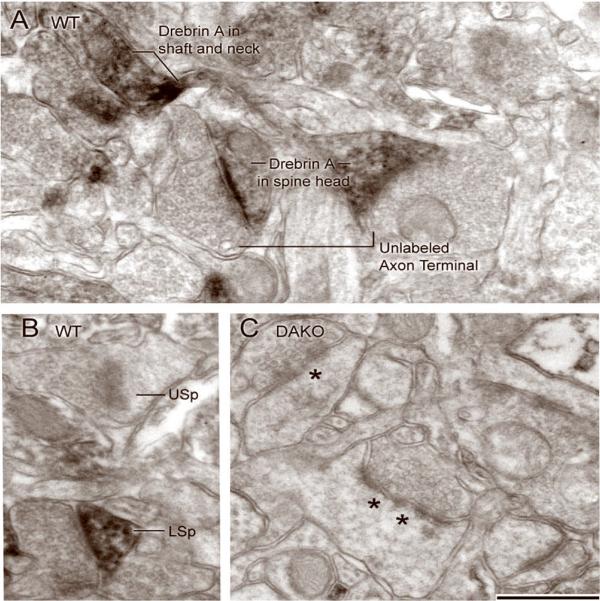
Fig. 2. Drebrin A immunolabeling within WT and DAKO cortices.
EM-ICC was performed using the drebrin A-specific antibody, DAS2. All panels show electron micrographs taken from layer 1 of the cerebral cortex. In the WT tissue, HRP-DAB immunolabeling is evident within the spine head, dendritic shaft and portions of the spine neck (Panel A). Within spine heads, the immunoreactivity can be diffuse (spine to the right in Panel A), occur concentrated at the postsynaptic density (PSD) (spine to the left in Panel A) or be distributed throughout the spine cytoplasm and at PSDs (LSp in Panel B). Panel B also shows an example on an unlabeled spine head (USp). The DAKO tissue shown in panel C was processed in parallel to those shown in Panels A and B. Immunoreactivity is not detectable within spines. Asterisks point to examples of interruptions in the PSDs, indicating that the PSDs are perforated. These samples were obtained by an investigator kept blind to the genotype of the animal. The collection of such micrographs was used to determine the frequency of drebrin A labeling within spines for the purpose of confirming the genotype of each animal. Calibration bar = 500 nm and applies to all panels.
Antibody Information and chemicals
Information about the primary antibodies used in this study appears in Table 1. The anti-drebrin A antibody, DAS2 (IBL, Takasaki, Japan), was generated and characterized previously for its specificity (Aoki et al., 2005), based on elimination of immunoreactivity within whole-brain homogenates obtained from DAKO mice, as assessed by Western blotting, and recognition of a single band in homogenates from WT mouse brains at the expected molecular weight of 116 kD (Fig. 1). DAS2 is generated using the immunogen, Phe-Ile-Lys-Ala Ser-Asp-Ser-Gly-Pro-Ser-Ser-Ser, which corresponds to a sequence of residues 325–336 of drebrin that is unique to the adult form, drebrin A (Shirao et al., 1992). DAS2 was produced in rabbits, and purified by epitope selection, using the above polypeptide. This antibody is able to discriminate drebrin A from its isomer, drebrin E, as is shown in Fig. 1. The pan-drebrin antibody was a mouse monoclonal antibody M2F6, produced using the protein, drebrin, that was purified electrophoretically from the soluble fraction of 11day-old chick embryo brains (Shirao and Obata, 1986) (Fig. 1), as the immunogen. By Western blotting, the pan-drebrin antibody recognizes two bands within whole homogenates of WT mouse brains, corresponding to drebrin A and drebrin E (Fig. 1).
Table 1.
Primary Antibodies Used
| Antigen | Immunogen | Manufacturer, Species antibody was raised in, mono- vs polyclonal, catalog or lot number | Dilution used |
|---|---|---|---|
| NR2A subunit of NMDA receptors | amino acids 1265–1464 of the C-terminus of mouse NR2A subunit | Upstate Biotechnology, (now Millipore, Billerica, Massachusetts) Catalog number 07-632, polyclonal antibody raised in rabbit | 1 μg/ml for HRP-DAB; 10 μg/ml for post-embed gold immunolabeling; 1:1000 for Western blot |
| NR1 subunit of NMDA receptors | Amino acids 660–811 | BD Biosciences Japan (originally Pharmingen) monoclonal antibody 54.1 | 1:2000 |
| β-actin | slightly modified β-cytoplasmic actin N-terminal peptide, Ac-Asp-Asp-Asp-Ile-Ala-Ala-Leu-Val-Ile-Asp-Asn-Gly-Ser-Gly-Lys, conjugated to KLH | Sigma-Aldrich Japan, monoclonal antibody AC-15 | 1:10000 |
| Drebrin A | Amino acid residues 325–336, which is unique to drebrin A and absent in drebrin E | DAS2, generated and provided by the Shirao laboratory, polyclonal antibody raised in rabbit | 1:1000 for EM-ICC; 1:500 for Western blot. |
| Drebrin E | Whole drebrin protein purified from E11 chick embryos (Shirao and Obata, 1986) | M2F6, MBL International, monoclonal antibody | Undiluted supernatant |
The anti-NR2A subunit antibody was purchased from Upstate Biotechnology (Lake Placid, NY, now part of Millipore, Catalog No. 07-632). The antibody was produced using a peptide corresponding to amino acids 1265–1464 of the C-terminus as the immunogen and was generated by immunizing rabbits. Specificity of this antibody has been demonstrated previously by Western blot, which shows that this antibody recognizes a single band corresponding to the molecular weight, ~170 kD of the NR2A subunit and neither the NR2B nor NR1 subunits of NMDAR (Rinaldi et al., 2007). Previously and currently conducted electron microscopic immunocytochemistry (EM-ICC) study from this laboratory determined that this antibody recognizes asymmetric synapses but not the symmetric synapses (Kobayashi et al., 2007), thereby indicating synapse- and pathway-specificity in staining pattern generated by this antibody. The NR1 antibody was produced using an immunogen that was a fusion protein encoding the glutathione S-transferase frame with the amino acid sequence corresponding to residues 660 to 811 of the intracellular loop between putative transmembrane regions III and IV of the NR1 subunit of NMDARs. This is a mouse monoclonal antibody purchased from BD Biosciences (clone 54.1, catalog # 56308, originally distributed by Pharmingen). The NR1 antibody has been shown to be specific, based on reactivity to a single band of MW 120 kDa by Western blot and the absence of reactivity to the NR2A subunit expressed in 293 cells (BD Sciences and Pharmingen's data sheets and previously described (Brose et al., 1994). Our previous EM study also showed that this antibody recognizes asymmetric (presumably excitatory) synapses, but not the symmetric (presumably inhibitory) synapses (Farb et al., 1995).
The β-actin antibody was a mouse monoclonal antibody purchased from Sigma (Clone AC-15). This antibody has been shown to recognize a single band at the expected molecular weight of 42kDa (manufacturer's data sheet). The immunogen was a slightly modified β-cytoplasmic actin N-terminal peptide, Ac-Asp-Asp-Asp-Ile-Ala-Ala-Leu-Val-Ile-Asp-Asn-Gly-Ser-Gly-Lys, conjugated to KLH (keyhole limpet hemocyanin).
Secondary antibodies were biotinylated goat anti-rabbit IgG (Vector's Elite kit catalog no. #PK-6200) or gold-conjugated goat anti-rabbit IgG, where the colloidal golds were either 0.8 nm or 10 nm in diameter (Aurion, EMSciences Catalog Nos. #800.011 and #810.011, respectively). D- and L-APV and bovine serum albumin (BSA) were purchased from Sigma Chemicals (OR). Glutaraldehyde, osmium tetroxide, paraformaldehyde and EMBED-812 were purchased from EMSciences. The Silver IntesEM kit was purchased from Amersham (now part of GE, Catalog No. RPN 491).
NMDAR blockade
Homeostatic plasticity was tested in three DAKO and four WT mice. All were adult males, ranging in age from 105 to 111 days postnatal. The animals received the NMDAR blocker, D-APV, unilaterally over the cerebral cortex, using the procedure detailed previously (Aoki et al., 2003; Fujisawa and Aoki, 2003; Fujisawa et al., 2006) but with minor changes described here. All procedures were performed using aseptic techniques, while maintaining the animal anesthetized with isoflurane (2–3%) and normothermic with a water-circulating heating pad. NMDAR blocker, D-aminophosphovaleric acid (D-APV) (5 mM), was delivered to the cortical surface of anesthetized mice for 30 min, immediately prior to euthanasia. The active enantiomer, D-AP5, was delivered to the cortical surface of one hemisphere, only, by first thinning a 1mm × 3mm skull region overlying the dorsal neocortex with dental drill, then using forceps to lift the thinned disk of bone (ca 100 μm thick). The underlying dura was lifted, then nicked using a bent, sterile 28-guage hypodermic needle tip. Immediately thereafter, a piece of sterile gelfoam, small enough to fit inside the drilled hole, and soaking in sterile artificial cerebral spinal fluid (115 mM NaCl, 3.3 mM KCl, 1 mM MgSO4, 2 mM CaCl2, 25.5 mM NaHCO3, 1.2 mM NaH2PO4, 5 mM lactic acid, and 25 mM glucose) containing 5 mM of D-APV, was placed gently over the cortical surface. In order to deliver the control solution to the contralateral cortex, the skull overlying the contralateral cortical surface was removed similarly. Then, a piece of gelfoam soaked with 5 mM of L-APV, an inactive enantiomer, instead of D-APV, was placed over the contralateral cortical surface. Based on results from previous studies, we have verified that the contralateral cortex treated with L-APV serves as the intra-animal control brain region, equivalent in NR2A levels to portions of the cortex receiving no drug treatment (Aoki et al., 2003; Fujisawa and Aoki, 2003; Fujisawa et al., 2006). The drug concentrations used - 5 mM - is 1/10th of the concentrations used most commonly for iontophoresis and long-term delivery of the drug using an osmotic minipump (Aguilar et al., 2003; Bear et al., 1990; Cline and Constantine-Paton, 1990; Malmierca and Nunez, 2004; Miller et al., 1989; Rabacchi et al., 1992).
Preparation of brain tissue for light and electron microscopy
At the end of the 30-min D/L-APV delivery, mice received a supplement of anesthetic, consisting of 50 mg/kg Nembutal, i.p. Two to three minutes following i.p. delivery of supplement, the depth of anesthesia was ascertained by testing for reflex to increasing pinching pressure to the paw and light touch of the cornea. Transcardial perfusion was achieved by using a peristaltic pump to control the flow-rate of the perfusates. The perfusates were the following: (1) 10 – 50 ml of saline containing heparin (1000 U/ml), delivered for 1 min at a flow-rate of 50 ml/min; (2) 200 ml of 0.10 % glutaraldehyde, mixed with 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4), delivered at a flow-rate of 50 ml/min during the initial 1 min, then slowed down to a rate of 15 ml/min during the subsequent 10 min. During the subsequent hours, the brain was cut into 40 μm-thick sections using a vibratome. On the 5th hour following the perfusion, the sections were immersed in PBS containing 1% sodium borohydride, so as to terminate residual cross-linking activities of glutaraldehyde with precise timing. Subsequently, the sections were stored for up to 1 month, free-floating at 4°C in PBS containing 0.05% sodium azide.
Two DAKO and one WT animal that did not receive NMDAR blockade were also perfused transcardially with aldehydes for morphological analyses, only. These sections did not undergo immunocytochemical procedures and were post-fixed with 1% osmium tetroxide.
NR2A-immunocytochemistry and the rationale for the choice of the three immunolabels
Immunocytochemistry was performed to immunolabel the NR2A subunits of NMDAR, using three immunolabeling procedures for EM. The three immunolabels we employed were: horseradish peroxidase/3,3'-diaminobenzidine (HRP-DAB), silver intensified immunogold (SIG) and post-embed immunogold (PEG). HRP-DAB was employed, because the enzymatic amplification step allows for the lowest threshold for detection. However, the enzymatic amplification also generates a diffusible label, and this causes degradation of the information regarding the precise localization of the antigen within spines. SIG complements HRP-DAB by providing better localization, but at the expense of high threshold for detection. With both HRPDAB and SIG, antigens residing directly over the PSDs can be difficult to detect, due to the heavy matrix of proteins surrounding the synaptic cleft that interferes with antibody access. The PEG approach complemented the HRP-DAB and SIG approaches for localizing antigens over the PSD, although some of the antigens may have been lost during EM-tissue processing. Because of these known advantages and short-comings of each EM-immunolabel, we opted to test the NR2A levels using all three procedures. Details of the immunocytochemical procedures were as described previously (Aoki et al., 2000) with slight modifications, as described recently (Sarro et al., 2008).
Two of the immunolabels were applied upon free-floating sections prior to embedding in the resin, EM812. One was horse-radish peroxidase, using 3,3'-diaminobenzidine and H2O2 as substrates (HRP-DAB), so as to optimize detection of immunolabels within spines. The second was silver-intensified 0.8 nm colloidal gold (SIG), conjugated to secondary antibodies (1:100). Although the SIG procedure is less sensitive than the enzymatically amplified HRP-DAB, the SIG procedure was employed, so as to identify the location of antigens at synaptic versus non-synaptic sites within spines. These pre-emedding procedures employed the NR2A antibody at a concentration of 1 μg/ml, incubated overnight at room temperature under constant agitation. The HRP-DAB labeled sections were post-fixed with 1% osmium tetroxide. The SIG-labeled sections were post-fixed using Phend's procedure, which excludes fixation with osmium tetroxide (Phend et al., 1995), so as to protect the SIG particles from oxidation. The third immunolabel was post-embed immunogold (PEG). Although antigenicity is frequently lost during the EM procedures required for plastic embedding, the ultrathin sectioning step can also reveal antigens that are embedded within large proteinaceous matrix such as the PSD. The PEG method was used to quantify the level of NR2A-immunolabels positioned directly over the PSD. The concentration of the NR2A antibody used for the PEG procedure was 10μg/ml, incubated overnight at room temperature. The PEG procedure employed anti-rabbit IgG antibodies (1:100), conjugated to 10 nm colloidal gold. For the PEG procedure, ultrathin sections were collected on formvar-coated, 200-mesh EM grids, and followed Phend's general procedure for PEG labeling, using vibratome sections that were fixed in the absence of osmium tetroxide (Phend et al., 1995) with modifications described specifically for PEG-labeling of NR2A subunits (Erisir and Harris, 2003).
Drebrin A-immunocytochemistry was achieved using the drebrin A antibody, DAS2, at a concentration of 1:1000, incubated overnight at room temperature, and HRP-DAB as the immunolabel. Tissue was post-fixed with osmium tetroxide and processed for EM as described previously (Aoki et al., 2005).
Immunocytochemical controls
Besides the Western blotting controls described under `Antibody information and chemicals,' each of the three EM-ICC procedures were accompanied by immunocytochemical controls. These consisted of verifications that omission of the primary antibody or use of secondary antibodies that were mismatched for species yielded no immunolabeling that could be detected by light or electron microscopy. In addition, EM examination revealed that neither the NR2A- nor the drebrin A-immunolabeling occurred postsynaptic to symmetric (presumably inhibitory) synapses, thereby indicating specificity of labeling at the subsynaptic level and in a pathway-specific manner, as were described previously (Aoki et al., 2005; Erisir and Harris, 2003).
Image capturing and areas sampled for electron microscopy
The person capturing the images was kept blind to the genotype and pharmacological treatment that the animal received, so as to eliminate bias in the sampling of neuropil. Images used for quantitative analyses were captured digitally using a Hamamatsu CCD camera attached to a JEOL 1200XL EM and a software developed by AMT, Inc. (Boston, MA). Adjustments to the images, including size, brightness, and contrast, were carried out in Adobe Photoshop 7.0 (San Jose, CA) to optimize discrimination of immunolabel from postsynaptic densities.
The images were taken at a magnification of 40,000×, with each digitized frame spanning 29 μm2 of area. Systematic sweeps were made across the tissue, so as to avoid capturing the same field more than once. Micrographs used for quantitative analyses were taken from regions of the neuropil that fulfilled the following criteria. (1) The neuropil resided in layer 1 of the cortex, where penetration of the drug, D/L-APV, that was applied from the cortical surface into the underlying neuropil would be maximal (Aoki et al., 2003). (2) For the HRP-DAB and SIG labeled tissue, the neuropil resided within portions of vibratome sections forming the razor-blade-cut surface, where penetration of immunoreagents into the vibratome sections would be maximal. (3) The synaptic neuropil was free of ultrastructural features reflective of trauma associated with surgery, such as swollen mitochondria, swollen neurites and/or electron-dense cytoplasms. The fields shown in the Figures 2, 3, 4, and 6 are examples of ultrastructure that were deemed satisfactory for use in quantitative analysis, as they fulfilled all three of the criteria.
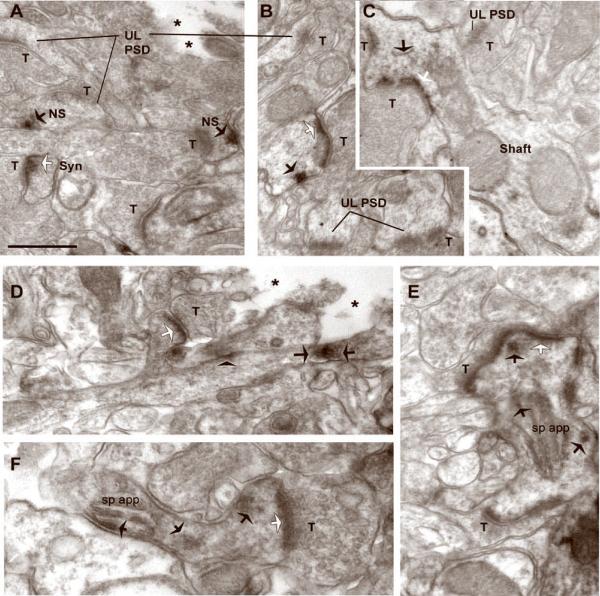
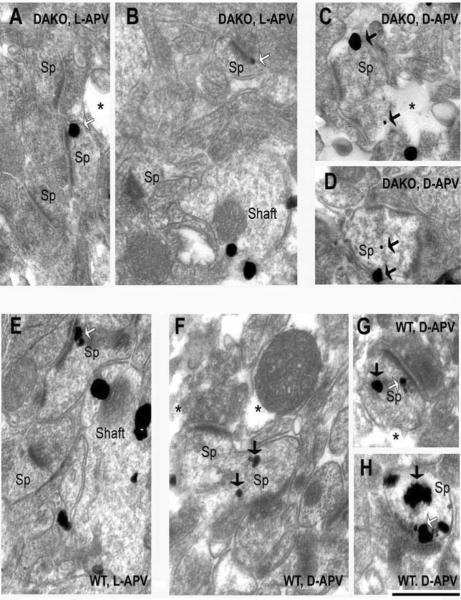
Fig. 3. DAB-immunolabeling reveals synaptic and nonsynaptic NR2A within cortical spines of WT mice.
Synapses in layer 1 were identified as excitatory, based on the presence of thick postsynaptic densities (PSDs) that align the intracellular surfaces of plasma membranes abutting vesicle-filled axon terminals (T). Asterisks point to portions of the ultrathin sections that are devoid of tissue, indicating the proximity of the sampled region relative to the vibratome surface. Panel A was taken from a control hemisphere (L-APV-treated) of WT animal #8, while the rest of the panels were taken from D-APV-treated cortices of two WT animals (Animals #6 and #8). In the experimental as well as control hemispheres, NR2A immunolabeling occurs synaptically (white arrows). Immunolabeling is also found at nonsynaptic sites (NS, black arrows) within spines, adjacent to unlabeled PSDs (UL PSD, panel A), labeled PSDs (panels B, C and F), and spine apparatus (sp app, in panels E and F). Yet other HRP-DAB clusters occur removed from spines but are along the plasma membrane of dendritic shafts (black arrowhead in panel D) or reside at sites removed from the plasma membrane in dendritic shafts (between two arrows in panel D). Calibration bar = 500 nm in panels A–E and 688 nm for panel F.
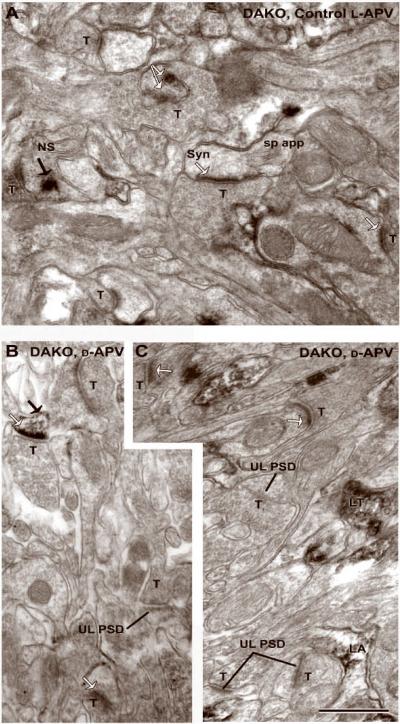
Fig. 4. DAB-labeling of DAKO cortices reveals NR2A labeling at synaptic and nonsynaptic sites within spines.
All panels were taken from layer 1 of DAKO Animal #9. Synaptic (Syn, white arrows) and nonsynaptic (NS, black arrows) labeling as well as unlabeled PSDs (UL PSD) persist following the application of the NMDAR antagonist, D-APV. The ultrastructural features of cortical spines and NR2A immunolabeling pattern within dendritic spines are indistinguishable from those of the age-matched WT animals. LT=labeled terminal; T=unlabeled terminal; LA=labeled astrocyte. The calibration bar = 500 nm and applies to all panels.
Fig. 6. SIG-labeling confirms similarity in the localization of NR2A.
The SIG procedure was employed, so as to be able to distinguish spines with labeling in the vicinity of the postsynaptic membrane versus those removed from the postsynaptic membrane. DAKO tissue shown here were obtained from Animal #10, while the WT tissue shown here are from Animal #11. SIG occurs at the PSD (white arrows) and removed from the PSD but still within the dendritic spine (Sp, black arrows). Yet others are found in the dendritic shaft, removed from the plasma membrane (Shaft, panels B and E). Asterisks point to portions of ultrathin sections that are devoid of tissue, confirming that the sampling is at the surface-most portions of the vibratome section. Calibration bar = 500 nm for all panels.
Quantitative electron microscopic analyses
For all parts of the quantitative analysis, the person performing the tallying was kept blind to the genotype and pharmacological treatment of the animal. Within 2-dimensional, digitized image frames, synapses were identified as excitatory, based on the presence of thick PSD that aligned the intracellular surfaces of plasma membranes abutting vesicle-filled axon terminals. Excitatory synapses were numbered in the order encountered. Each excitatory synapse was subsequently categorized as being associated with or without NR2A or drebrin A immuno-label. A minimum of 100 synapses and in most cases, more than 200 synapses were sampled from each hemisphere of each animal. For every group of 10 synapses that were encountered, the number of synapses associated with immunolabel was tallied. This tallying was repeated 10 to 20 times for each hemisphere of each cortical hemisphere, and the mean and SEM of the percent of synapses associated with labeled was calculated for each hemisphere.
For HRP-DAB and especially for the SIG- and PEG-labeled tissue, the immunolabeling was sufficiently discrete to allow discrimination of the immunoreactive sites as contiguous or not contiguous with the postsynaptic specialization. For quantification of SIG- labeled tissue, the position of the immunolabel was categorized further as being `at or near the postsynaptic membrane,' if the center of any of the SIG cluster was found directly over the PSD or within a distance equal to the thickness of the PSD in its plane of section. For PEG-labeled tissue, the position of immunolabel was categorized as being at or near the PSD, if the immunolabeling was directly over the PSD (`at PSD'), within a distance equal to the thickness of the PSD in its plane of section, or within the synaptic cleft. Labeling that occurred within the spine head but removed from the PSD by a distance greater than the thickness of the PSD was categorized as occurring `within the spine but not at or near the synapse' (i.e., `non-synaptic'). For all three immunolabels (HRP-DAB, SIG or PEG), labeling on the presynaptic side was also categorized as being `at the presynaptic membrane' or `near the presynaptic membrane' or `within the axon terminal but removed from the presynaptic membrane.
The images used to determine NR2A-immunoreactivity were re-analyzed to determine the density of synapses in layer 1 of cortex. Frequency of encounter with synaptic profile was determined by calculating the mean of the number of synaptic profile encountered in each electron micrograph spanning a synaptic neuropil area equal to 29 μm2 and free of blood vessels, somata and large dendritic shafts (Table 2). For estimating the density of synapses per unit volume, the Abercrombie correction was applied, using the following formula:
in which Nv = corrected estimate of object number per unit of volume (numerical density) NA = uncorrected profile estimate per unit of area T equals the mean tissue thickness of the section and d equals the mean diameter of the object (Guillery, 2002). In order to make this estimation, the mean thickness, T, of ultrathin sections were measured, using multiple 29 μm2 framed images that were acquired using the same digitizing camera, software and magnification (40,000×) and in which folded portions of the ultrathin sections were included. Image J was used to measure the thickness of folds, from which thicknesses of single layers of ultrathin sections could be derived.
Table 2.
Morphology of Synapses in Layer 1 of WT and DAKO Cortices
| WT | DAKO | p-value | |
|---|---|---|---|
| Encounter with synapse profile (per micrograph of area 29 μm2, n is number of micrographs) | 5.34 ± 0.18 (n=142) | 5.13 ± 0.18 (n=143) | 0.398 |
|
| |||
| Synapse density, per 10 μm3 estimated using Abercrombie's correction | 5.58 ± 0.19 | 5.35 ± 0.19 | |
|
| |||
| Freq. of Perforated Synapses (of 2770 synapses, encountered In 5 WT and 5 DAKO cortices) | 7.1 ± 0.8% | 10.8 ± 0.8% | 0.002 |
|
| |||
| Non-perforated synapses | |||
| Spine width | 451 ± 18 nm (n=174) | 443 ± 13 nm (n=320) | 0.982 |
| PSD width | 254 ± 8 nm (n=214) | 249 ± 6 nm (n=408) | 0.975 |
|
| |||
| Perforated synapses | |||
| Spine width | 705 ± 49 nm (n=24) | 682 ± 26 nm (n=70) | 0.978 |
| PSD width | 523 ± 22 nm (n=31) | 482 ± 13 nm (n=88) | 0.376 |
The images used to determine NR2A-immunoreactivity were also analyzed to determine whether the synapses appeared perforated or non-perforated. The frequency of perforated synapses, i.e., excitatory synapses with interruptions in the PSDs, was determined for every group of 10 excitatory synapses encountered. An additional two DAKO and one WT tissue that did not undergo the homeostatic plasticity assay were also used to determine the frequency of perforated synapses. The widths of spine and PSD profiles captured in 2-D digitized images from osmium tetroxide-post-fixed tissue were used to assess the relative sizes of spines and synapses in DAKO and WT cortices. Tissue immunolabeled for the NR2A subunit and post-fixed using osmium tetroxide were also used to compare the widths of PSDs of NR2A-immunolabeled and unlabeled synapses. The software, Image J (US NIH, Bethesda, MD, http://rsb.info.nih.gov/ij), was used to measure these lengths, as described previously (Fujisawa et al., 2006).
Statistical analyses
All statistical analyses were performed using the software, Statistica (Statsoft). When comparing data across the two hemispheres of one animal, Student's t-test was used. When comparing data across more than two groups, ANOVA and Tukey's HSD test was used. Significance was accepted for p-values less than 0.05.
Western blotting
Neocortical homogenates of two 11-week old DAKO and two WT littermates were prepared using a Teflon-glass homogenizer and 10× weight-to-volume ratio of SDS sample buffer, consisting of 2% SDS, 5% 2-mercaptoethanol, 10% glycerol, 1 mM EDTA, 40 mM Tris, and 240 mM glycine at pH 8.5 (Laemmli, 1970). The homogenate was then boiled for 5 min. Aliquots of whole homogenates were equalized to 100 μg wet weight of cortical tissue/lane), then subjected to SDS-PAGE, using 8%-polyacrylamide gels. Proteins were transferred to PVDF-membranes (Immobilon transfer membrane, Millipore, Bedford, MA), then probed either with the anti-pan-drebrin monoclonal antibody (clone M2F6 hybridoma supernatant, undiluted) (Shirao and Obata, 1986), the anti-drebrin A-specific polyclonal antibody (DAS2, diluted to a concentration of 1:500) (Aoki et al., 2005; Song et al., 2008), the NR2A antibody (1:1000 dilution), the anti-NR1 antibody (1:2000), or the β-actin antibody (1:10,000). Following incubation with the HRP-DAB-conjugated second antibody, immunoreactive signals were visualized on X-ray films (Hyperfilm-ECL; GE Healthcare) using ECL detection reagents (GE Healthcare, Piscataway, NJ).
RESULTS
Ultrastructure of the drebrin A knockout (DAKO) cortex
EM-ICC using horseradish peroxidase-diaminobenzidine (HRP-DAB) as the label revealed that 71 ± 10% of the spines in the WT cortex exhibit immunoreactivity for drebrin A. Immunoreactivity was also present within dendritic shafts and spine necks (Fig. 2A & B). This was as observed earlier for rat cortices (Aoki et al., 2005; Fujisawa et al., 2006). In contrast, immunolabeling for drebrin A was very slight within individual spines of DAKO cortex, with none exhibiting immunolabeling within spines that were comparable in intensity to those seen in WT cortex. Only 18 ± 5% of the spines exhibited any kind of HRP-DAB labeling and these were of very low levels and diffuse (Fig. 2C). These are likely to reflect weak reactivity of this antibody with proteins other than drebrin A.
EM analysis indicated drebrin A KO does not cause any overt ultrastructural changes. No statistically significant difference was detected in the density of axospinous synapses per unit area (p=0.4), the diameter of spine profiles (p =0.98) or the diameter of PSD profiles (p=0.98) (Table2). Both the DAKO and WT cortices exhibited perforated synapses that were approximately twice the diameter of non-perforated synapses (Table 2). The widths of the PSDs and spines exhibiting perforated synapses also were not different across genotypes (p=0.38 and 0.98, respectively). However, one notable difference was in the frequency of perforated synapses. Whereas approximately 7% of the excitatory synapses of WT cortices appeared perforated within 2-D digitally captured images, frequency of perforated synapses within the micrographs collected from DAKO cortices (e.g., Fig. 2C) was increased significantly (p<0.005) to 10% (Table 2). The frequency of encounter with particular profiles is influenced by their size and shape (Mouton, 2002). However, since the mean diameter of perforated synapse profiles was not different across the genotypes (Table 2), the increased encounter with perforated synapse profiles within DAKO tissue is likely to reflect an increase in the number of perforated synapses per unit volume. Together, these observation indicates that the genetic deletion of drebrin A may impact upon the cycling of synapses between larger and smaller ones (Nieto-Sampedro et al., 1982), without interfering with spinogenesis, synapse growth or synapse stabilization.
Comparisons of spine density and perforated synapse density across the D-versus L-APV treated hemispheres of WT cortices revealed no change (p=0.311, ANOVA with post hoc Tukey HSD test). Similarly, spine density across the two hemispheres of DAKO cortices revealed no change (p=0.976). These findings indicate that 30-min of drug application did not induce any net change in the overt aspects of synapse stability.
NR2A-immunoreactivity of spines in the cortex of DAKO and WT mice, as revealed by pre-embed immunolabels
In both the WT and DAKO cortices, excitatory synapses were identifiable by the presence of thick postsynaptic densities (PSD) on one side (postsynaptic), clustering of small clear vesicles within the profile opposing it (presynaptic), and parallel alignment of the two plasma membranes facing the synaptic cleft. Within spines of WT cortex, HRP-DAB labeling reflecting NR2A-immunoreactivity occurred along the postsynaptic plasma membrane. However, the majority of postsynaptic membranes were devoid of HRP-DAB labeling (e.g., “UL PSD” in Fig 3A–C). Some of those spines lacking NR2A immunoreactivity at the PSD exhibited HRP-DAB at non-synaptic portions of the spine. The non-synaptic portions consisted of plasma membranes removed from the synaptic cleft (“NS” in Fig. 3A), intracellular membranes of dendritic shafts (between two arrows in Fig. 3D), and surfaces of the spine apparatus facing the cytoplasm (“sp app” in Fig. 3E and F). These subcellular distribution patterns are in accord with previous reports of NR2A labeling in the cortex of ferrets (Erisir and Harris, 2003), mice (Kobayashi et al., 2007) and rats (Aoki et al., 2003). NR2A immunoreactivity within spines of DAKO cortices were indistinguishable from the pattern described for the WT brains, in that the labeling was both synaptic (white arrows in Fig 4) and non-synaptic (black arrows in Fig. 4).
Although the subcellular distribution of NR2A within spines of DAKO cortices appeared remarkably similar to that of WT cortex, quantitative analysis revealed that the responsiveness of spines to D-APV was strikingly different across genotypes (p<0.05, t = 2.68, Student's t-test). Following the 30 min treatment with D-APV, spines in the cortex of WT animals were much more immunoreactive to the NR2A antibody, relative to the synapses in the contralateral hemisphere that received L-APV. Collectively, the inter-hemispheric comparisons of NR2A-immunoreactivity revealed a statistically significant difference for all four WT cortices tested (Fig. 5), with the mean of the four values of synaptic NR2A-enhancement being 94% ± 28% (mean ± SEM). In contrast, the same analysis of the three DAKO brains revealed no intra-animal, inter-hemispheric difference (Fig. 5). The degree of enhancement in NR2A-immunoreactivity for the DAKO brains was 0% ± 13% (mean ±SEM of the three DAKO brains). There was a trend for the basal levels of synaptic NR2A to be slightly higher within DAKO brains than of WT brains, but this difference across the genotypes did not reach statistical significance. The D-APV-evoked responsiveness that was clearly different across the genotypes indicates that drebrin A is a protein required for the rapid, NMDAR-dependent influx of NR2A-containing NMDARs into spines.
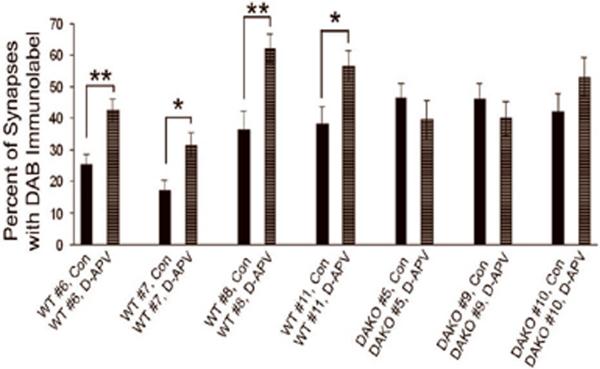
Fig. 5. Quantification of NR2A immunolabeling by HRP-DAB in layer 1 of WT and DAKO brains following 30 min exposure to D- and L-APV.
Vibratome sections containing the neocortex of DAKO and WT mice were immunolabeled for the NR2A subunit of NMDARs by the HRP-DAB procedure. For every 10 synapses encountered within layer 1, the proportion with DAB-immunulabeling was assessed. This counting procedure was repeated at least 10 times for each hemisphere treated with D-APV and at least another 10 times for the hemisphere that received L-APV. The graph shows the mean value ±SEM of the proportion of spines with immunolabeling. For each of the four WT brains, the proportion immunolabeled for NR2A in the D-APV treated hemisphere was significantly different from the proportion observed in the L-APV-treated control (“Con”) hemisphere (* indicates p<0.05; ** indicates p<0.005, comparing across the two hemispheres within animal). None of the brains from the three DAKO animals exhibited differences across the two hemispheres.
The results obtained from HRP-DAB immunolabeling suggested the D-APV-induced enhancement within spines of WT cortex reflects changes at both the synaptic and non-synaptic portions of spines. However, since HRP-DAB labeling is diffusible, precision of antigen localization within spines is limited. So as to improve the localization of NR2A within spines, we repeated the NR2A-immunolabeling using an alternative label - silver-intensified gold (SIG). SIG-immunolabeling confirmed that, within spines of WT cortex, NR2A immunolabeling is detectable at synaptic (white arrows in Fig. 6E, G and H) and non-synaptic sites (black arrows, Fig. 6F, G and H). The cortex of DAKO animals also exhibited labeling at synapses (white arrows in Fig. 6A, B) and at non-synaptic portions within spine heads (black arrows in Fig. 6C and D). Quantitative analysis of three WT brains revealed consistent augmentation of spinous NR2A following the treatment with D-APV, relative to the L-APV-treated cortices (68%, 83% and 177%). These increments were at synaptic and non-synaptic sites within spines. In contrast, analysis of two DAKO animals revealed inconsistent small changes (−4% and 21%, relative to control hemispheres).
D-APV effect upon spine sizes
Within brains of WT mice, the encounter with NR2A-immunoreactive synapses was more frequent within the D-APV treated hemispheres than in the contralateral control hemisphere. Although the simplest interpretation of this result is that the proportion of synapses expressing NR2A had increased, another possibility was that the synapses containing NR2A had increased in size following the D-APV treatment. In general, within single, 2-D EM images, processes that are large are encountered more frequently than are the smaller processes, even if they occur in equal number (Guillery, 2002; Mouton, 2002). Moreover, although our past experiments had shown that 30-min exposures of the cortex to D-APV does not alter the sizes of asymmetric synapses (Fujisawa et al., 2006), a possibility remained that D-APV enlarges the NR2A-containing synapses specifically. In order to test for these possibilities, we quantified the relative sizes of asymmetric synapses by measuring PSD widths. This analysis was performed using sections that had undergone NR2A-immunolabeling by the HRP-DAB procedure and post-fixation using osmium tetroxide that protected tissue from shrinkage during tissue processing for EM. We first compared PSD widths of labeled versus unlabeled synapses. Results of this analysis indicated that NR2A-containing synapses are larger than the unlabeled synapses. Although the synapse width differences were small (49 nm), amounting to a 19% enlargement, relative to the PSD length of unlabeled synapses, these differences were highly significant (p<0.000001; t-test, t-value -8.41640) and consistent across every one of the WT and DAKO brains (not shown).
The synapses encountered from the quantification of NR2A labeling were then separated into eight groups (WT or DAKO; NR2A-labeled or unlabeled; D-APV-treated or control) to obtain the mean values for assessing genotype and drug-treatment effects. The mean values were compared by ANOVA and post hoc Tukey's HSD test (df=2351). Comparisons of PSD widths belonging to labeled versus unlabeled spines of 4 WT brains revealed significant differences for both the D-APV-treated hemispheres (p=0.00007) and for the control hemispheres (p=0.013). Similarly, comparisons of PSD widths of labeled versus unlabeled spines of DAKO brains revealed significant differences for both the D-APV-treated hemispheres (p=0.0007) and for the control hemispheres (p=0.004). In sharp contrast to these differences across labeled versus unlabeled synapses, comparisons of the PSD widths of NR2A-labeled synapses across genotype and drug-treatment revealed no difference (Table 3). Specifically, the mean value of PSD widths belonging to NR2A-labeled synapses in the D-APV-treated hemispheres was not significantly different from the mean value of PSD widths belonging to labeled synapses from the control hemispheres, whether compared within WT brains (p=0.9999) or in DAKO brains (p=0.947). These measurements indicated that the apparent increase in encounter with NR2A-labeled synapses in D-APV treated hemispheres of WT brains is not likely to have resulted from increases in synapse sizes there. Rather, the NR2A levels at synapses and within spines are increased by the D-APV exposure in WT brains, without alterations in the size of synapses. Similarly, the apparent lack of increase of NR2A-immunolabeling within the D-APV-treated hemispheres of DAKO brains is not likely to be a result of an actual increase of NR2A immunoreactivity that is counterbalanced by shrinkage of those synapses.
Table 3.
PSD Width Comparisons Mean ± SEM
| Labeled 307±5 |
Unlabeled 259±4 |
p-value <0.00001 |
|---|---|---|
| Genotype effects: | ||
|
| ||
| APV-treated Labeled WT 297±7 |
APV-treated Labeled DAKO 315±10 |
p-value 0.826 |
|
| ||
| Control Labeled WT 294±11 |
Control Labeled DAKO 331±510 |
p-value 0.146 |
|
| ||
| APV-treated Unlabeled WT 249±7 |
APV-treated Unlabeled DAKO 263±7 |
p-value 0.830 |
|
| ||
| Control Unlabeled WT 250±7 |
Control Unlabeled DAKO 282±9 |
p-value 0.084 |
|
| ||
| Pharmacological treatment effects: | ||
|
| ||
| APV-treated Labeled WT 297±7 |
Control Labeled WT 294±11 |
p-value 0.999 |
|
| ||
| APV-treated Labeled DAKO 315±10 |
Control Labeled DAKO 331±510 |
p-value 0.947 |
|
| ||
| APV-treated Unlabeled WT 249±7 |
Control Unlabeled WT 250±7 |
p-value 1.000 |
|
| ||
| APV-treated Unlabeled DAKO 263±7 |
Control Unlabeled DAKO 282±9 |
p-value 0.683 |
Post-embed immunogold (PEG) immunolabeling, to verify the localization of NR2A subunits at PSDs
The PSD is comprised of a high density of proteins (Carlin et al., 1980; Kennedy and Ehlers, 2006). Therefore, the apparent absence of responsiveness observed for the DAKO brains may have been due to the inaccessibility of the antibodies to the NR2A subunits that are embedded most deeply in the subsynaptic protein lattice (Bresler et al., 2004). In order to test for this possibility, we re-examined the synaptic and non-synaptic population of NR2A using a third EM-ICC procedure, namely the post-embed immunogold (PEG) method, which is a procedure that allows access of antibodies through the deeply embedded proteinaceous matrix. This is achieved by ultrathin-sectioning through the PSD. Indeed, the PEG procedure allowed greatest level of detection of NR2A over the PSD (Fig. 7, graph). However, even by this procedure, DAKO brains (Animal #5, #9 and #10, Fig.7) revealed no D-APV-evoked increase of NR2A immunolabeling, while the WT brains responded with robust increases, both at the synapse (Animal #6) and at non-synaptic sites within spines (Animal #7). These results confirm that the lack of D-APV-evoked increase of NR2A immunoreactivity within spines of DAKO cortices is not due to failures in the EM-ICC detection of the synaptic population of NR2A subunits. The PEG immunolabeling data confirms that spines of DAKO brains are not responsive to the D-APV treatment. Together, the EM-ICC data demonstrate that DAKO brains are impaired in the rapid form of homeostatic synaptic plasticity.
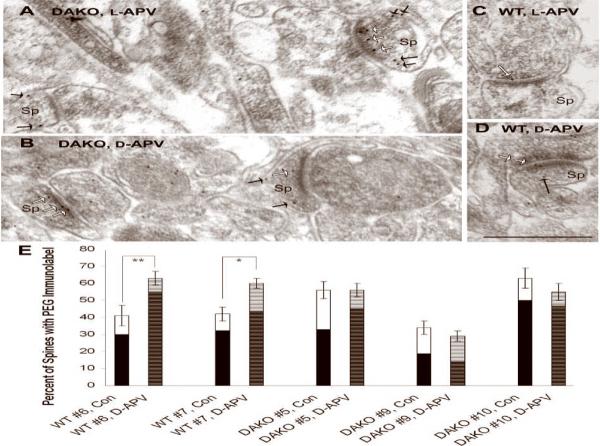
Fig. 7. PEG-labeling reveals the localization of NR2A at and near thick PSDs of both genotypes but responsiveness to NMDAR blockade only within the WT cortex.
The post-embed immunogold (PEG) procedure was employed, so as to identify the portion of receptor subunits residing precisely over the PSD. Images from DAKO mouse brain were taken from Animal #5, while the images from WT mouse brain were collected from Animal #6. PEG particles are found residing at or near the synapse (white arrow) and also at sites removed from the PSD but still in within dendritic spine (Sp, black arrows). Calibration bar = 500 nm for all panels.
The bar graph in panel E shows the percent of spines immunolabeled for the NR2A subunit by the PEG procedure. Comparison of this value between the L-APV (control) versus D-APV-treated hemispheres of WT brains (Animal #6 and 7) reveal an inter-hemispheric difference in the proportion of spines exhibiting NR2A-immunoreactivity over the entire cytoplasm of spines (represented by the total height of bars) and in the immediate vicinity of PSDs (darkened portion of the bars). In contrast, spines of DAKO brains (Animal #5, #9 and #10) exhibited no significant difference in the distribution of the NR2A subunits at regions surrounding or removed from PSDs.
* indicates p<0.05; ** indicates p<0.005.
DISCUSSION
The application of D-APV upon cortical surfaces of adult WT mice evoked a rapid increase of NR2A immunoreactivity in the spine cytoplasm and at the synaptic junction. This was a response we observed previously for the adult rat cortex. In contrast, neither the synaptic nor the non-synaptic portions of DAKO spines increased NR2A-immunoreactivity following the D-APV blockade. This difference between the genotypes indicates that drebrin A is required for the NMDAR activity-dependent up-regulation of the NR2A-subunits within spines and at synapses. Since NR2A subunits form functional heteromers with NR1 subunits before exiting the endoplasmic reticulum (Groc and Choquet, 2006), the elevation of NR2A subunits at synapses is likely to reflect the elevation of NR2A-containing NMDARs at synapses. It has been reported that APV-blockade of organotypic hippocampal slices also causes increased influx of NR2A subunits into spines, but importantly, that the insertion of NR2A-subunit containing receptors into the synaptic membrane requires receptor activation (Barria and Malinow, 2002). Our findings are compatible with this interpretation and indicate that trafficking of NR2A-containing NMDARs to within 10 nm of the synaptic membrane can be accomplished without activation of the NMDARs.
The functions of drebrin A versus drebrin E
It was a surprise to us that knockout of drebrin A does not affect basal levels of NR2A or the density of spinous profiles. This observation indicates that drebrin A is not required for spinogenesis or synaptogenesis. It is possible that the embryonic isoform of drebrin - drebrin E, which continues to be expressed by DAKO brains, is able to fulfill some of the cellular functions pertaining to the stable structure of spines and synapses. Apparently, the function reserved specifically for drebrin A is to enable the activity-dependent, rapid and local regulation of glutamate receptor distributions within spines via a mechanism involving exocytosis and endocytosis of vesicles containing NMDARs. The lateral diffusion of NMDARs from extrajunctional plasmalemmal sites to the synaptic junction may also be facilitated by drebrin A, based on our PEG-immunolabeling data that revealed an increase of NR2A specifically at PSDs.
We cannot rule out the possibility that the unresponsiveness of DAKO synapses to D-APV is due to the persistence of drebrin E, rather than the absence of drebrin A, and that drebrin E that interferes with APV-induced increases in NR2A expression levels at synapses. However, we favor the former interpretation. Cultured hippocampal neurons that are grown in vitro for 21 days exhibit the APV-evoked up-regulation of NR1 subunits into spines, even though approximately half of the total drebrin expressed by these neurons are of the drebrin E-isoform (Takahashi et al., 2006). This indicates that drebrin E does not interfere with the cellular mechanisms that lead to the up-regulation of NMDARs into spines. At the very least, if drebrin E does interfere with homeostatic synaptic plasticity, this inhibitory effect is not sufficiently strong to over-ride the facilitatory role played by drebrin A. Western blot analysese indicate that, at ages younger than P21, intact cortex also exhibits a mixture of drebrin E and drebrin A (Aoki et al., 2005). Assuming that the Western blot result reflects individual neurons expressing a mixture of drebrin E and drebrin A, these neurons are also likely to be able to exhibit the drebrin A-dependent rapid homeostatic synaptic plasticity.
Methodological considerations
We observed some inter-animal differences in basal levels of NR2A within spines. Regardless of these differences, we were able to detect differences in the D-APV responses across genotypes by making homeostatic synaptic plasticity measurements that relied on intra-animal, inter-hemispheric measurements for comparing single brain's reactivity to the D- versus L-APV.
We cannot rule out the possibility that the differences in baseline levels arose post mortem, due to differences in the treatment of brain sections. However, we think the post mortem introduction of artifacts was minimized to the limit of the methodology, since considerable effort was put into treating the brains uniformly. For example, transcardial perfusion of animals used the same peristaltic pump, set at a single flow rate and perfusate volume. The post-perfusion fixation period was set to be 5 hours, by using sodium borohydride to control the termination time point of tissue fixation. Finally, all tissue underwent the same steps of EM-ICC, in parallel.
On the other hand, what we observed may actually reflect inter-animal variability in behavior and synaptic structure, even those these were derived from a single strain and genotype. It has been shown that rats from a single strain and breeding colony exhibit a wide range of fear reactivity, as is measured by the number of seconds that the animals freeze to a conditioned tone following fear conditioning (Bush et al., 2007). Whether such behavioral differences reflect quantitative differences in receptor density at synapses within discrete regions of the brain remain to be explored. Also, these animals underwent a somewhat unusual rearing environment, consisting of the vivarium in Japan and the US, as well as an inter-continental shipment experience and group housing. The experiences that these mice received during the 3 postnatal months may have contributed towards inter-animal variability.
Mechanism underlying the rapid rise of NR2A in spines and at synaptic junctions
Another in vivo assay revealed that the NR2B-to-NR2A switching of NMDAR subunits can occur rapidly - within 1 hour, but no less than 30 min – in the visual cortex, following the initial exposure of developing rodents to visual stimulus (Quinlan et al., 1999). Such a delay may be due to the need for the sequential events involving transcription, translation and finally trafficking of the newly synthesized proteins to critical sites to enable trafficking of NR2A subunits. Although 1 hour is a delay sufficiently long to evoke transcriptional mechanisms, whether or not transcriptional mechanisms can account for the augmentation of the NR2A-containing NMDARs that we observed within the first half hour remains to be tested. Even Arc, one of the immediate early genes that can be induced by neural activity and is thought to play a role in receptor trafficking, exhibits a delay of about 2 hours before the proteins rise to levels detectable within stimulated regions of the brain (Ploski et al., 2008). On the other hand, since Arc mRNA can reside near spine necks (Bramham 2008), it is possible that the newly synthesized Arc protein facilitates the trafficking of NMDARs to the synaptic junction within 30 min of receptor blockade. Since we have no reason to suspect that Arc-mRNA levels are affected by DAKO, the loss of the rapid response observed within DAKO cortices indicates that drebrin A is at least as important as Arc in the activity-dependent redistribution of NMDARs to synapses. If NMDAR subunit-mRNAs already reside in the vicinity of spine heads, such as the parent dendritic shaft (Martin and Zukin, 2006), then it is possible that NR2A-mRNA trafficking towards spine heads and a highly localized de novo synthesis of NR2A-subunits also contribute to the rise of NR2A-immunoreactivity within spine heads and at the synaptic junction. A decrease in NR2A proteolysis may also have contributed to the rise in immunoreactivity at synapses. In summary, the rapidity with which the excitatory synapses responded in the current study suggests that the rise of synaptic NR2A-immunoreactivity may have occurred without the participation of transcriptional mechanisms, while de novo synthesis of proteins and decreased proteolysis, in addition to the trafficking of already existing receptor proteins to synaptic sites may all have contributed to the observed rise.
In healthy brains, long-term sensory deprivation or TTX-blockade of action potentials can lead to compensatory increases in cortical excitability through shifts in the balance of excitatory-to-inhibitory synapses or of the intrinsic excitability of neurons (Kotak et al., 2005; Maffei and Turrigiano, 2008; Sarro et al., 2008). On the other hand, aberrant set points of homeostasis can cause chronic hyper-excitability that lead to illnesses, such as the central pain syndrome, where non-painful tactile stimuli are perceived as excruciating pain (Wang and Thompson, 2008). Experiments are underway to determine whether DAKO animals exhibit impairment in homeostatic plasticity over longer time scale and in learning paradigms.
Putative role of F-actin in drebrin A's action
We observed previously that NMDAR blockade leads to an increase of F-actin and drebrin A in spines, together with NR2A, within the first half hour and without evoking overt changes in spine size or density (Aoki et al., 2003; Fujisawa et al., 2006). Similarly, mouse cortex also responds to NMDAR-blockade with the rise of NR2A that is unaccompanied by spine size changes. Conversely, activation of glutamate receptors can depolymerize F-actin (Star et al., 2002) and if intense enough, lead to the collapse of spines (Halpain et al., 1998). Even without activation of glutamate receptors, perturbation of F-actin can lead to the collapse of spines and the loss of synaptic proteins, including a subpopulation of NMDARs (Allison et al., 2000; Allison et al., 1998). Since NMDARs can be associated with F-actin via α-actinin (Krupp et al., 1999; Wyszynski et al., 1997), NMDAR and other proteins may be anchored to subsynaptic surface or be liberated from synapses through F-actin dynamics. The F-actin depolymerization experiments also indicate that the F-actin lattice within spines could be serving dual roles – one, of anchoring receptors and scaffolding proteins along the postsynaptic membrane facing the synaptic cleft, and another for maintaining the overall shape of spines. If so, or perhaps particularly because of the dual roles ascribed to F-actin, it remains unclear whether the loss of glutamate receptors at the synapses following receptor stimulation occurs as a consequence of spine collapse or due to depolymerization of F-actin that is restricted to the subsynaptic membrane surface.
Results from the current study indicate that NR2A levels change independently of spine size changes. Therefore, it is possible that the drebrin A-mediated rise of NR2A immunoreactivity involves liberation of NR2A-containing NMDARs that are tethered in the vicinity of spines, such as the neck and dendritic shaft, via the F-actin lattice there. In support of this idea, we (Fig. 3D) and others (Allison et al., 1998) have observed NMDAR-subunit immunoreactivity within dendritic shafts near spine heads, and these have been shown to be tethered to the plasma membrane via F-actin lattices and/or α-actinin. It is also possible that the influx of receptor-containing vesicles into the spine head is facilitated by the influx of drebrin into the spine head that leads to the relaxation of F-actin cross-links formed by α-actinin. Conversely, in the absence of drebrin A, such as in DAKO brains, the entry of receptor-containing vesicles into the spine head and the liberation of NMDARs tethered to F-actin for the subsequent mobilization may be impaired, due to the relative rigidity of spine heads and necks.
An increased proportion of perforated synapse – enhanced connectivity but at the expense of plasticity?
One notable structural difference in DAKO cortices was the prevalence of perforated axo-spinous synapses. Correlative ultrastructural-immunocytochemical studies indicate that perforated synapses are particularly efficacious, due to the enrichment of AMPA receptors and large spine head size, but without concomitant increases in the number of NMDARs (Kharazia et al., 1996; Nicholson and Geinisman, 2009). Apparently, synapse enlargement does not require the concomitant influx of NMDARs. The prevalence of perforated synapses, together with the similar basal levels of NMDARs found within DAKO spines, indicate that DAKO cortex retains functional synaptic pathways. Whether or not the perforated synapses of DAKO cortex also exhibit enrichment of AMPARs remains to be tested but if they are, the prevalence of perforated synapses within DAKO cortex would suggest that synaptic pathways are strong and stable. On the other hand, the literature on perforated synapses also indicate that these synapses occur at certain immature stages that precede robust synaptogenesis (Nieto-Sampedro et al., 1982) and following episodes of robust synaptic activity, such as epileptic seizure (Henry et al., 2008), cortical stimulation (Adkins et al., 2008), passive avoidance test (Platano et al., 2008) and water-maze training (Hongpaisan and Alkon, 2007). These are examples of experiences that are likely to evoke synaptogenesis and synapse pruning. The prevalence of perforated synapses within DAKO cortices may reflect the `freezing' of synapse cycling at a stage preceding synapse division (Carlin and Siekevitz, 1983) or pruning, and with it, the loss of mechanisms that ensure synaptic strengths to be self-regulated in ways that mirror sensory experience and synaptic activity.
Is impairment in the activity-dependent NMDAR recruitment causal to the increased incidence of perforated synapses? We do not know. However, one possible scenario is that, in spines of WT cortex, the activity-dependent recruitment of NMDARs can augment the activity-dependent influx of calcium, which in turn promotes severing of F-actin into shorter fragments by the protein, gelsolin. These may be the steps enabling spines of WT cortex to cycle between large and small spines and with varying concentrations of AMPARs and NMDARs. Live, in vivo imaging of adult cortices has shown that 5 to 8% (Zuo et al., 2005) or up to 30% (Holtmaat et al., 2005) of the dendritic spines turn over within 1 month, leaving most of the excitatory synapses stable (Pan and Gan, 2008). A prediction consistent with this idea is that the turnover rate of spines in DAKO cortex will be less than that observed for spines in WT cortex.
Mice with conditional ablation of the β-catenin gene also exhibit elevated frequency of perforated synapses (Bamji et al., 2003), while triple knock-in of genes linked to Alzheimer's disease (Bertoni-Freddari et al., 2008) and knock-out of the GluR2 subunit of AMPA receptors (Medvedev et al., 2008) exhibit decreased levels of perforated synapses. Both the reduction and elevation of perforated synapses may reflect impairments in the activity-dependent synapse cycling mechanism. Whether or not the increased frequency of perforated synapses is associated with the phenotypes of reduced synaptic plasticity, reduced mental capacity and neurodegeneration are topics that can be addressed experimentally in future studies that examine the behavior and brain histology of DAKO animals. In support of this idea, we noted that hippocampal spines of an Alzheimer's disease mouse model exhibit reduction of drebrin A and an increase in the mean area of spine head profiles within the hippocampus (Aoki et al., 2007). Brains of patients diagnosed either with AD or mild cognitive impairment also show reduction of drebrin (Counts et al., 2006; Harigaya et al., 1996; Hatanpaa et al., 1999; Shim and Lubec, 2002). While results of the current study indicated that NMDAR blockade leads to the up-regulation of NMDARs, together with drebrin itself (Fujisawa et al., 2006), previous studies have indicated an opposite effect following NMDAR activation: efflux of drebrin A from spines (Sekino et al., 2006) and PSD-95-regulated endocytosis of NR2A-containing NMDARs from synapses (Sornarajah et al., 2008). Altogether, these findings support the idea that impairment in synapse turnover and synaptic plasticity associated with Alzheimer's disease may be caused, at least in part, by the reduction of drebrin which leads directly to the loss of bi-directional homeostatic synaptic plasticity – i.e., activity-dependent recruitment of NMDARs into spines or removal of NMDARs out of spines. Loss of this drebrin A-dependent, bi-directional homeostatic synaptic plasticity may ultimately lead to cognitive impairments and excitotoxicity. If so, then manipulations that boost drebrin A levels may be beneficial for enhancing cognitive abilities of patients afflicted by Alzheimer's disease. Although highly speculative, this is a working model that can be tested by examining the consequence of drebrin A over-expression within mouse models of Alzheimer's disease.
ACKNOWLEDGEMENTS
We thank Dr. Sho Fujisawa, Dr. Robert Levy and Veera Mahadomrongkul for their assistance with the pilot studies. We thank Dr Robert Levy, Gerardo Moreno and Hilda M Fernandez for their technical assistance and Hermina Nedelescu for reading the manuscript. This research was supported, in part, by NIH grants 1 P30 EY13079, NIH 5 R01 DA009618-09, R01 NS41091, R01 13145, NYU's Research Challenge Fund, and the NSF-REU Site grant to C. Aoki and by the Grants-in-Aid for Scientific Research (19200029) and on Priority Areas-Elucidation of neural network function in the brain-from the Ministry of Education, Culture, Sports, Science and Technology of Japan (20021002).
LITERATURE CITED
- Adkins DL, Hsu JE, Jones TA. Motor cortical stimulation promotes synaptic plasticity and behavioral improvements following sensorimotor cortex lesions. Exp Neurol. 2008;212(1):14–28. doi: 10.1016/j.expneurol.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar J, Rivadulla C, Soto C, Canedo A. New corticocuneate cellular mechanisms underlying the modulation of cutaneous ascending transmission in anesthetized cats. J Neurophysiol. 2003;89(6):3328–3339. doi: 10.1152/jn.01085.2002. [DOI] [PubMed] [Google Scholar]
- Allison DW, Chervin AS, Gelfand VI, Craig AM. Postsynaptic scaffolds of excitatory and inhibitory synapses in hippocampal neurons: maintenance of core components independent of actin filaments and microtubules. J Neurosci. 2000;20(12):4545–4554. doi: 10.1523/JNEUROSCI.20-12-04545.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison DW, Gelfand VI, Spector I, Craig AM. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. J Neurosci. 1998;18(7):2423–2436. doi: 10.1523/JNEUROSCI.18-07-02423.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki C, Fujisawa S, Mahadomrongkul V, Shah PJ, Nader K, Erisir A. NMDA receptor blockade in intact adult cortex increases trafficking of NR2A subunits into spines, postsynaptic densities, and axon terminals. Brain Res. 2003;963(1–2):139–149. doi: 10.1016/s0006-8993(02)03962-8. [DOI] [PubMed] [Google Scholar]
- Aoki C, Mahadomrongkul V, Fujisawa S, Habersat R, Shirao T. Chemical and morphological alterations of spines within the hippocampus and entorhinal cortex precede the onset of Alzheimer's disease pathology in double knock-in mice. J Comp Neurol. 2007;505(4):352–362. doi: 10.1002/cne.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki C, Rodrigues S, Kurose H. Use of electron microscopy in the detection of adrenergic receptors. Methods Mol Biol. 2000;126:535–563. doi: 10.1385/1-59259-684-3:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki C, Sekino Y, Hanamura K, Fujisawa S, Mahadomrongkul V, Ren Y, Shirao T. Drebrin A is a postsynaptic protein that localizes in vivo to the submembranous surface of dendritic sites forming excitatory synapses. J Comp Neurol. 2005;483(4):383–402. doi: 10.1002/cne.20449. [DOI] [PubMed] [Google Scholar]
- Bamji SX, Shimazu K, Kimes N, Huelsken J, Birchmeier W, Lu B, Reichardt LF. Role of beta-catenin in synaptic vesicle localization and presynaptic assembly. Neuron. 2003;40(4):719–731. doi: 10.1016/s0896-6273(03)00718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35(2):345–353. doi: 10.1016/s0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- Bear MF, Kleinschmidt A, Gu QA, Singer W. Disruption of experience-dependent synaptic modifications in striate cortex by infusion of an NMDA receptor antagonist. J Neurosci. 1990;10(3):909–925. doi: 10.1523/JNEUROSCI.10-03-00909.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoni-Freddari C, Sensi SL, Giorgetti B, Balietti M, Di Stefano G, Canzoniero LM, Casoli T, Fattoretti P. Decreased presence of perforated synapses in a triple-transgenic mouse model of Alzheimer's disease. Rejuvenation Res. 2008;11(2):309–313. doi: 10.1089/rej.2008.0660. [DOI] [PubMed] [Google Scholar]
- Biou V, Brinkhaus H, Malenka RC, Matus A. Interactions between drebrin and Ras regulate dendritic spine plasticity. Eur J Neurosci. 2008;27(11):2847–2859. doi: 10.1111/j.1460-9568.2008.06269.x. [DOI] [PubMed] [Google Scholar]
- Bramham CR. Local protein synthesis, actin dynamics, and LTP consolidation. Current Opinion in Neurobiology. 2008;18:524–531. doi: 10.1016/j.conb.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Bresler T, Shapira M, Boeckers T, Dresbach T, Futter M, Garner CC, Rosenblum K, Gundelfinger ED, Ziv NE. Postsynaptic density assembly is fundamentally different from presynaptic active zone assembly. J Neurosci. 2004;24(6):1507–1520. doi: 10.1523/JNEUROSCI.3819-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broach JR, Guarascio VR, Jayaram M. Recombination within the yeast plasmid 2mu circle is site-specific. Cell. 1982;29(1):227–234. doi: 10.1016/0092-8674(82)90107-6. [DOI] [PubMed] [Google Scholar]
- Brose N, Huntley GW, Stern-Bach Y, Sharma G, Morrison JH, Heinemann SF. Differential assembly of coexpressed glutamate receptor subunits in neurons of rat cerebral cortex. J Biol Chem. 1994;269(24):16780–16784. [PubMed] [Google Scholar]
- Bush DE, Sotres-Bayon F, LeDoux JE. Individual differences in fear: isolating fear reactivity and fear recovery phenotypes. J Trauma Stress. 2007;20(4):413–422. doi: 10.1002/jts.20261. [DOI] [PubMed] [Google Scholar]
- Calon F, Lim GP, Yang F, Morihara T, Teter B, Ubeda O, Rostaing P, Triller A, Salem N, Jr., Ashe KH, Frautschy SA, Cole GM. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer's disease mouse model. Neuron. 2004;43(5):633–645. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin RK, Grab DJ, Cohen RS, Siekevitz P. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J Cell Biol. 1980;86(3):831–845. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin RK, Siekevitz P. Plasticity in the central nervous system: do synapses divide? Proc Natl Acad Sci U S A. 1983;80(11):3517–3521. doi: 10.1073/pnas.80.11.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline HT, Constantine-Paton M. NMDA receptor agonist and antagonists alter retinal ganglion cell arbor structure in the developing frog retinotectal projection. J Neurosci. 1990;10(4):1197–1216. doi: 10.1523/JNEUROSCI.10-04-01197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts SE, Nadeem M, Lad SP, Wuu J, Mufson EJ. Differential expression of synaptic proteins in the frontal and temporal cortex of elderly subjects with mild cognitive impairment. J Neuropathol Exp Neurol. 2006;65(6):592–601. doi: 10.1097/00005072-200606000-00007. [DOI] [PubMed] [Google Scholar]
- Erisir A, Harris JL. Decline of the critical period of visual plasticity is concurrent with the reduction of NR2B subunit of the synaptic NMDA receptor in layer 4. J Neurosci. 2003;23(12):5208–5218. doi: 10.1523/JNEUROSCI.23-12-05208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb CR, Aoki C, Ledoux JE. Differential localization of NMDA and AMPA receptor subunits in the lateral and basal nuclei of the amygdala: a light and electron microscopic study. J Comp Neurol. 1995;362(1):86–108. doi: 10.1002/cne.903620106. [DOI] [PubMed] [Google Scholar]
- Fujisawa S, Aoki C. In vivo blockade of N-methyl-D-aspartate receptors induces rapid trafficking of NR2B subunits away from synapses and out of spines and terminals in adult cortex. Neuroscience. 2003;121(1):51–63. doi: 10.1016/s0306-4522(03)00341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa S, Shirao T, Aoki C. In vivo, competitive blockade of N-methyl-D-aspartate receptors induces rapid changes in filamentous actin and drebrin A distributions within dendritic spines of adult rat cortex. Neuroscience. 2006;140(4):1177–1187. doi: 10.1016/j.neuroscience.2006.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Choquet D. AMPA and NMDA glutamate receptor trafficking: multiple roads for reaching and leaving the synapse. Cell Tissue Res. 2006;326(2):423–438. doi: 10.1007/s00441-006-0254-9. [DOI] [PubMed] [Google Scholar]
- Guillery RW. On counting and counting errors. J Comp Neurol. 2002;447(1):1–7. doi: 10.1002/cne.10221. [DOI] [PubMed] [Google Scholar]
- Halpain S. They're plastic, but they recycle. Neuron. 2006;52(5):746–748. doi: 10.1016/j.neuron.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Halpain S, Hipolito A, Saffer L. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J Neurosci. 1998;18(23):9835–9844. doi: 10.1523/JNEUROSCI.18-23-09835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harigaya Y, Shoji M, Shirao T, Hirai S. Disappearance of actin-binding protein, drebrin, from hippocampal synapses in Alzheimer's disease. J Neurosci Res. 1996;43(1):87–92. doi: 10.1002/jnr.490430111. [DOI] [PubMed] [Google Scholar]
- Hatanpaa K, Isaacs KR, Shirao T, Brady DR, Rapoport SI. Loss of proteins regulating synaptic plasticity in normal aging of the human brain and in Alzheimer disease. J Neuropathol Exp Neurol. 1999;58(6):637–643. doi: 10.1097/00005072-199906000-00008. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Ishikawa R, Ye LH, He XL, Takata K, Kohama K, Shirao T. Modulatory role of drebrin on the cytoskeleton within dendritic spines in the rat cerebral cortex. J Neurosci. 1996;16(22):7161–7170. doi: 10.1523/JNEUROSCI.16-22-07161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry LC, Goertzen CD, Lee A, Teskey GC. Repeated seizures lead to altered skilled behaviour and are associated with more highly efficacious excitatory synapses. Eur J Neurosci. 2008;27(8):2165–2176. doi: 10.1111/j.1460-9568.2008.06153.x. [DOI] [PubMed] [Google Scholar]
- Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45(2):279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Homanics GE, Ferguson C, Quinlan JJ, Daggett J, Snyder K, Lagenaur C, Mi ZP, Wang XH, Grayson DR, Firestone LL. Gene knockout of the alpha6 subunit of the gamma-aminobutyric acid type A receptor: lack of effect on responses to ethanol, pentobarbital, and general anesthetics. Mol Pharmacol. 1997;51(4):588–596. doi: 10.1124/mol.51.4.588. [DOI] [PubMed] [Google Scholar]
- Hongpaisan J, Alkon DL. A structural basis for enhancement of long-term associative memory in single dendritic spines regulated by PKC. Proc Natl Acad Sci U S A. 2007;104(49):19571–19576. doi: 10.1073/pnas.0709311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Tanaka S, Sekino Y, Ren Y, Yamazaki H, Kawai-Hirai R, Kojima N, Shirao T. A novel, brain-specific mouse drebrin: cDNA cloning, chromosomal mapping, genomic structure, expression, and functional characterization. Genomics. 2002;79(5):686–692. doi: 10.1006/geno.2002.6764. [DOI] [PubMed] [Google Scholar]
- Kawase E, Suemori H, Takahashi N, Okazaki K, Hashimoto K, Nakatsuji N. Strain difference in establishment of mouse embryonic stem (ES) cell lines. Int J Dev Biol. 1994;38(2):385–390. [PubMed] [Google Scholar]
- Kennedy MJ, Ehlers MD. Organelles and Trafficking Machinery for Postsynaptic Plasticity. Annu Rev Neurosci. 2006 doi: 10.1146/annurev.neuro.29.051605.112808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharazia VN, Phend KD, Rustioni A, Weinberg RJ. EM colocalization of AMPA and NMDA receptor subunits at synapses in rat cerebral cortex. Neurosci Lett. 1996;210(1):37–40. doi: 10.1016/0304-3940(96)12658-6. [DOI] [PubMed] [Google Scholar]
- Kinosian HJ, Newman J, Lincoln B, Selden LA, Gershman LC, Estes JE. Ca2+ regulation of gelsolin activity: binding and severing of F-actin. Biophys J. 1998;75(6):3101–3109. doi: 10.1016/S0006-3495(98)77751-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi C, Aoki C, Kojima N, Yamazaki H, Shirao T. Drebrin a content correlates with spine head size in the adult mouse cerebral cortex. J Comp Neurol. 2007;503(5):618–626. doi: 10.1002/cne.21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Fujisawa S, Lee FA, Karthikeyan O, Aoki C, Sanes DH. Hearing loss raises excitability in the auditory cortex. J Neurosci. 2005;25(15):3908–3918. doi: 10.1523/JNEUROSCI.5169-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp JJ, Vissel B, Thomas CG, Heinemann SF, Westbrook GL. Interactions of calmodulin and alpha-actinin with the NR1 subunit modulate Ca2+-dependent inactivation of NMDA receptors. J Neurosci. 1999;19(4):1165–1178. doi: 10.1523/JNEUROSCI.19-04-01165.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci U S A. 1996;93(12):5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Turrigiano GG. Multiple modes of network homeostasis in visual cortical layer 2/3. J Neurosci. 2008;28(17):4377–4384. doi: 10.1523/JNEUROSCI.5298-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Malmierca E, Nunez A. Primary somatosensory cortex modulation of tactile responses in nucleus gracilis cells of rats. Eur J Neurosci. 2004;19(6):1572–1580. doi: 10.1111/j.1460-9568.2004.03256.x. [DOI] [PubMed] [Google Scholar]
- Martin KC, Zukin RS. RNA trafficking and local protein synthesis in dendrites: an overview. J Neurosci. 2006;26(27):7131–7134. doi: 10.1523/JNEUROSCI.1801-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev NI, Rodriguez-Arellano JJ, Popov VI, Davies HA, Tigaret CM, Schoepfer R, Stewart MG. The glutamate receptor 2 subunit controls post-synaptic density complexity and spine shape in the dentate gyrus. Eur J Neurosci. 2008;27(2):315–325. doi: 10.1111/j.1460-9568.2007.06005.x. [DOI] [PubMed] [Google Scholar]
- Miller KD, Chapman B, Stryker MP. Visual responses in adult cat visual cortex depend on N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A. 1989;86(13):5183–5187. doi: 10.1073/pnas.86.13.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton PR. Principles and Practices of Unbiased Stereology: An Introduction for BIoscientists. Johns Hopkins University; Baltimore: 2002. p. 214. [Google Scholar]
- Nicholson DA, Geinisman Y. Axospinous synaptic subtype-specific differences in structure, size, ionotropic receptor expression, and connectivity in apical dendritic regions of rat hippocampal CA1 pyramidal neurons. J Comp Neurol. 2009;512(3):399–418. doi: 10.1002/cne.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Sampedro M, Hoff SF, Cotman CW. Perforated postsynaptic densities: probable intermediates in synapse turnover. Proc Natl Acad Sci U S A. 1982;79(18):5718–5722. doi: 10.1073/pnas.79.18.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Gan WB. Two-photon imaging of dendritic spine development in the mouse cortex. Dev Neurobiol. 2008;68(6):771–778. doi: 10.1002/dneu.20630. [DOI] [PubMed] [Google Scholar]
- Perez-Otano I, Ehlers MD. Homeostatic plasticity and NMDA receptor trafficking. Trends Neurosci. 2005;28(5):229–238. doi: 10.1016/j.tins.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Phend KD, Rustioni A, Weinberg RJ. An osmium-free method of epon embedment that preserves both ultrastructure and antigenicity for post-embedding immunocytochemistry. J Histochem Cytochem. 1995;43(3):283–292. doi: 10.1177/43.3.7532656. [DOI] [PubMed] [Google Scholar]
- Platano D, Fattoretti P, Balietti M, Giorgetti B, Casoli T, Di Stefano G, Bertoni-Freddari C, Aicardi G. Synaptic remodeling in hippocampal CA1 region of aged rats correlates with better memory performance in passive avoidance test. Rejuvenation Res. 2008;11(2):341–348. doi: 10.1089/rej.2008.0725. [DOI] [PubMed] [Google Scholar]
- Ploski JE, Pierre VJ, Smucny J, Park K, Monsey MS, Overeem KA, Schafe GE. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of pavlovian fear conditioning in the lateral amygdala. J Neurosci. 2008;28(47):12383–12395. doi: 10.1523/JNEUROSCI.1662-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan EM, Olstein DH, Bear MF. Bidirectional, experience-dependent regulation of N-methyl-D-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc Natl Acad Sci U S A. 1999;96(22):12876–12880. doi: 10.1073/pnas.96.22.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabacchi S, Bailly Y, Delhaye-Bouchaud N, Mariani J. Involvement of the N-methyl D-aspartate (NMDA) receptor in synapse elimination during cerebellar development. Science. 1992;256(5065):1823–1825. doi: 10.1126/science.1352066. [DOI] [PubMed] [Google Scholar]
- Rao A, Craig AM. Activity regulates the synaptic localization of the NMDA receptor in hippocampal neurons. Neuron. 1997;19(4):801–812. doi: 10.1016/s0896-6273(00)80962-9. [DOI] [PubMed] [Google Scholar]
- Rinaldi T, Kulangara K, Antoniello K, Markram H. Elevated NMDA receptor levels and enhanced postsynaptic long-term potentiation induced by prenatal exposure to valproic acid. Proc Natl Acad Sci U S A. 2007;104(33):13501–13506. doi: 10.1073/pnas.0704391104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarro EC, Kotak VC, Sanes DH, Aoki C. Hearing loss alters the subcellular distribution of presynaptic GAD and postsynaptic GABAA receptors in the auditory cortex. Cereb Cortex. 2008;18(12):2855–2867. doi: 10.1093/cercor/bhn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekino Y, Tanaka S, Hanamura K, Yamazaki H, Sasagawa Y, Xue Y, Hayashi K, Shirao T. Activation of N-methyl-D-aspartate receptor induces a shift of drebrin distribution: disappearance from dendritic spines and appearance in dendritic shafts. Mol Cell Neurosci. 2006;31(3):493–504. doi: 10.1016/j.mcn.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Shim KS, Lubec G. Drebrin, a dendritic spine protein, is manifold decreased in brains of patients with Alzheimer's disease and Down syndrome. Neurosci Lett. 2002;324(3):209–212. doi: 10.1016/s0304-3940(02)00210-0. [DOI] [PubMed] [Google Scholar]
- Shirao T, Kojima N, Obata K. Cloning of drebrin A and induction of neurite-like processes in drebrin-transfected cells. Neuroreport. 1992;3(1):109–112. doi: 10.1097/00001756-199201000-00029. [DOI] [PubMed] [Google Scholar]
- Shirao T, Obata K. Immunochemical homology of 3 developmentally regulated brain proteins and their developmental change in neuronal distribution. Brain Res. 1986;394(2):233–244. doi: 10.1016/0165-3806(86)90099-4. [DOI] [PubMed] [Google Scholar]
- Shirao T, Sekino Y. Clustering and anchoring mechanisms of molecular constituents of postsynaptic scaffolds in dendritic spines. Neurosci Res. 2001;40(1):1–7. doi: 10.1016/s0168-0102(01)00209-7. [DOI] [PubMed] [Google Scholar]
- Song M, Kojima N, Hanamura K, Sekino Y, Inoue HK, Mikuni M, Shirao T. Expression of drebrin E in migrating neuroblasts in adult rat brain: coincidence between drebrin E disappearance from cell body and cessation of migration. Neuroscience. 2008;152(3):670–682. doi: 10.1016/j.neuroscience.2007.10.068. [DOI] [PubMed] [Google Scholar]
- Sornarajah L, Vasuta OC, Zhang L, Sutton C, Li B, El-Husseini A, Raymond LA. NMDA receptor desensitization regulated by direct binding to PDZ1-2 domains of PSD-95. J Neurophysiol. 2008;99(6):3052–3062. doi: 10.1152/jn.90301.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Star EN, Kwiatkowski DJ, Murthy VN. Rapid turnover of actin in dendritic spines and its regulation by activity. Nat Neurosci. 2002;5(3):239–246. doi: 10.1038/nn811. [DOI] [PubMed] [Google Scholar]
- Sternberg N, Hamilton D. Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. J Mol Biol. 1981;150(4):467–486. doi: 10.1016/0022-2836(81)90375-2. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Mizui T, Shirao T. Down-regulation of drebrin A expression suppresses synaptic targeting of NMDA receptors in developing hippocampal neurones. J Neurochem. 2006;97(Suppl 1):110–115. doi: 10.1111/j.1471-4159.2005.03536.x. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135(3):422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Thompson SM. Maladaptive homeostatic plasticity in a rodent model of central pain syndrome: thalamic hyperexcitability after spinothalamic tract lesions. J Neurosci. 2008;28(46):11959–11969. doi: 10.1523/JNEUROSCI.3296-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Liu X, Jaenisch R. Double replacement: strategy for efficient introduction of subtle mutations into the murine Col1a-1 gene by homologous recombination in embryonic stem cells. Proc Natl Acad Sci U S A. 1994;91(7):2819–2823. doi: 10.1073/pnas.91.7.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyszynski M, Lin J, Rao A, Nigh E, Beggs AH, Craig AM, Sheng M. Competitive binding of alpha-actinin and calmodulin to the NMDA receptor. Nature. 1997;385(6615):439–442. doi: 10.1038/385439a0. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Lin A, Chang P, Gan WB. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005;46(2):181–189. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]