Abstract
Long-term caloric restriction (CR) has been repeatedly shown to increase life span and delay the onset of age-associated pathologies in laboratory mice and rats. The purpose of the current study was to determine whether the CR-associated increase in life span occurs in all strains of mice or only in some genotypes and whether the effects of CR and ad libitum (AL) feeding on mortality accrue gradually or are rapidly inducible and reversible. In one experiment, groups of male C57BL/6, DBA/2, and B6D2F1 mice were fed AL or CR (60% of AL) diets beginning at 4 months of age until death. In the companion study, separate groups of mice were maintained chronically on AL or CR regimens until 7, 17, or 22–24 months of age, after which, half of each AL and CR group was switched to the opposite regimen for 11 wk. This procedure yielded four experimental groups for each genotype, namely AL→AL, AL→CR, CR→CR, and CR→AL, designated according to long-term and short-term caloric regimen, respectively. Long-term CR resulted in increased median and maximum life span in C57BL/6 and B6D2F1 mice but failed to affect either parameter in the DBA/2 mice. The shift from AL→CR increased mortality in 17- and 24-month-old mice, whereas the shift from CR→AL did not significantly affect mortality of any age group. Such increased risk of mortality following implementation of CR at older ages was evident in all three strains but was most dramatic in DBA/2 mice. Results of this study indicate that CR does not have beneficial effects in all strains of mice, and it increases rather than decreases mortality if initiated in advanced age.
Keywords: caloric restriction, aging, C57BL/6, DBA/2, B6D2F1
Caloric restriction (CR), compared with ad libitum (AL) feeding, has been shown to increase the life span of laboratory mice and rats (e.g., 1, 2–4). For instance, a 40% decrease in total caloric intake, implemented after 3 months of age, has been found to increase the life span of C57BL/6 mice by more than 35% (5), and comparable findings have been made in Fischer 344 rats (4). Because CR produces such robust extensions in both the average and maximum life span, this regimen is widely assumed to provide a model for understanding the fundamental causes of the aging process. Notwithstanding, understanding of the mechanism by which CR retards the progression of age-related alterations has remained elusive even though several hypotheses have been proffered. A fundamental, but as yet, unresolved issue pertaining to the elucidation of the putative mechanism is whether the effects of CR accrue gradually or are they rapidly inducible and reversible upon shifting from AL feeding to the CR regimen and vice versa.
Another issue critical to understanding the mechanistic basis of CR effects on aging is whether CR causes life span extension in virtually all animal species or only in some genotypes. The majority of previous studies on the effects of CR have been conducted on relatively few strains of rats and mice in which CR has been well established to result in robust extension of life span. Whether or not this assumption is valid needs to be critically examined, because the outcome will determine whether the CR effects on life span are dependant on the genetic peculiarities of a particular organism or whether CR invariably prolongs life span and can thus be reliably assumed to alter the rate of aging.
In context of this reasoning, the present study was conducted to determine whether the effects of long-term CR or AL feeding regimens on mortality rates of different strains of mice are affected by short-term transfers to the opposite regimens and whether the long-term imposition of CR extends the life span of all the various genetic strains of mice. The results suggest that CR does not extend the life span of all mouse strains and may indeed be deleterious if initiated late in life. Furthermore, life span extension by CR is neither quickly reversible nor inducible but instead requires a long-term reduction in calorie intake.
MATERIALS AND METHODS
Animals
We used a total of 988 male mice of three different strains, 331 DBA/2Nnia, 338 C57BL/6Nnia, and 319 B6D2F1Nnia, that were obtained from the National Institute on Aging (NIA) CR colonies. Following their arrival, the mice were housed individually in 29.5 × 19.2 × 12.8 cm polycarbonate cages with wire tops, modified into two mouse units by insertion of a stainless steel divider. This caging system was identical to that used at the NIA facilities. Within the University of North Texas Health Science Center (UNTHSC) Vivarium, the mice were maintained on a 12-h light/dark cycle, with the light portion beginning at 0600 h. These conditions were similar to those in the NIA colonies, with the exception that CR mice in our colonies were placed on a night feeding regimen in which they were fed at 2200 h. This time was ~2 h before the acrophase of the normal circadian feeding cycle of AL mice. The purpose of the night feeding procedure was to provide parallel phasing of circadian cycles in the AL and CR groups. The mouse cages were maintained in positive laminar flow units, and standard serological testing performed on sentinels verified specific pathogen-free conditions for the time period of these studies.
CR
One half of the mice of each genotype had AL access to the diet (NIH-31, Purina Feeds) beginning at weaning, whereas the other half, beginning at 4 months of age, was maintained on a CR regimen permitting daily access to only 60% of the intake of the AL mice. The restricted mice were fed a NIH-31 formulation providing a correction for intake of essential nutrients. The CR mice surviving to advanced age were maintained on a fixed amount of food based on the average intake of AL mice at earlier ages as described previously (6). This procedure began at 26, 30, or 32 months for DBA/2, C57BL/6, and B6D2F1 mice, respectively. All mice had access to tap water ad libitum. Groups of 20–25 mice of each genotype, obtained from NIA when 7 months of age, were maintained at UNTHSC under the AL or CR conditions until dead or moribund. The CR regimen has been described in detail previously (6).
In a separate study, additional groups of AL and CR mice, obtained from NIA at ~7, 17, or 22–25 months of age, were divided into four subgroups in order to discern short- and long-term consequences of the different levels of caloric intake. One half of the original AL mice of each genotype and age were switched and maintained under the CR regimen (AL→CR); conversely, one half of the original restricted mice were switched and maintained with AL access (CR→AL) to the diet. These diet groups were maintained for at least 11 wk along with control groups that were maintained under their original long-term diet condition (AL→AL and CR→CR groups). Beginning 4 wk following implementation of the new feeding regimens, all mice received a 5-wk series of behavioral tests as described and discussed previously (7, 8). The cages were checked daily, and body weights were recorded each week throughout the experiment.
Statistical analysis
For the life span study, age at death was considered in a two-way ANOVA, with caloric intake and genotype as the factors. Body weights of mice in both experiments were also considered in analyses of variance. For analysis of the effects of shifts in caloric intake, Kaplan-Meier survival distributions were calculated and log-rank X2 statistics (Tarone-Ware) were used to compare mortality rates within each genotype (AL→AL vs. AL→CR and CR→CR vs. CR→AL).
RESULTS
Effects of long-term CR and genotype on life span
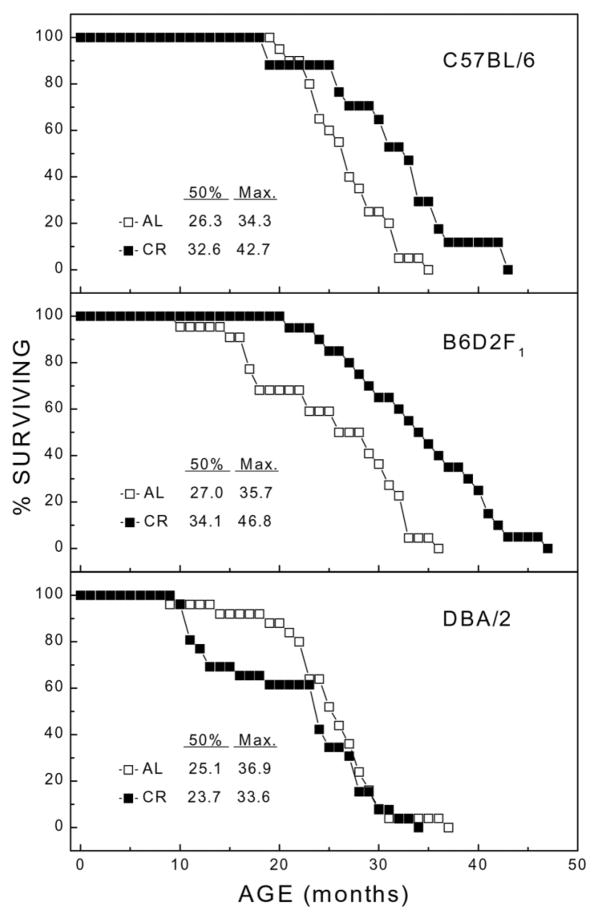
The survival characteristics for groups of C57BL/6, B6D2F1, and DBA/2 mice, maintained on the AL or CR regimen, are shown in Figure 1. Under AL conditions, the genotypes differed only slightly in their median life span, with B6D2F1 and C57BL/6 mice living 1–2 months longer than DBA/2. However, under conditions of reduced caloric intake, the C57BL/6 and B6D2F1 mice differed markedly from DBA/2. The former showed increases of 6–7 months in median and 8–11 months in maximum (oldest death) life span under the CR regimen, whereas the CR regimen resulted in a slight overall decrease in the median and maximum life span in the DBA/2 mice. The small overall decrease in longevity reflected an increase in mortality rate of DBA/2 mice on CR between 10 and 20 months. However, the mortality rate for AL and CR groups did not differ after 20 months of age. A two-way ANOVA of the life span indicated a significant interaction of genotype with caloric intake [F(2,124)=10.5; P<0.001]. Thus, when caloric intake was reduced throughout adult life, a life-prolonging effect was evident in two of the mouse genotypes, but absent in the DBA/2 strain.
Figure 1. Survivorship of C57BL/6, B6D2F1, and DBA/2 mice as a function of age.
Separate groups of each genotype were maintained from 4 months of age under ad libitum (AL) or 40% calorie-restricted (CR) feeding regimens as described in Materials and Methods.
Body weight
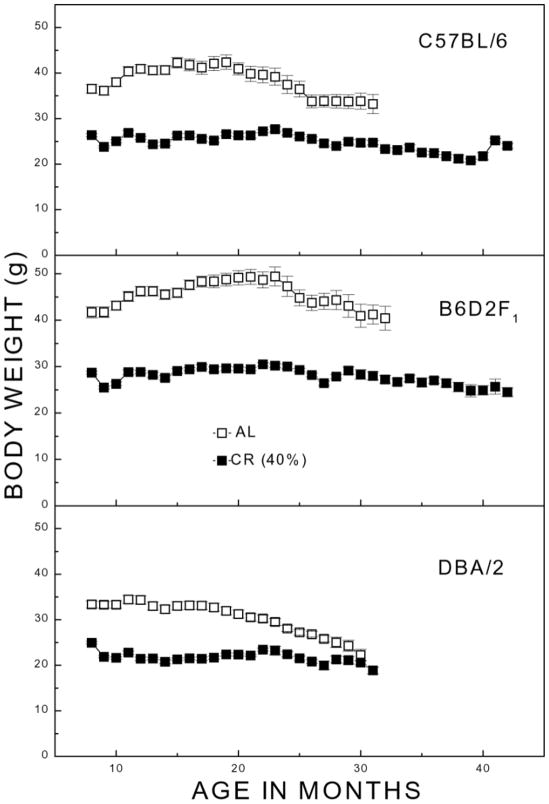
Average body weight of the AL groups differed at all ages among the genotypes, with B6D2F1 > C57BL/6 > DBA/2. The weight of the AL groups peaked from 10 to 20 months of age; in contrast, the CR mice showed stable, relatively lower weights throughout life. From 8 to 18 months, the absolute difference in weight between the AL and CR groups was larger for C57BL/6 (−14 g) and B6D2F1 (−17 g) when compared with DBA/2 (−11 g). However, relative to their average AL weights over this period, the weight reduction attributable to CR was nearly equivalent in all three genotypes (34–37% reduction). After 18 months of age, the weights of the AL groups tended to decline in all genotypes, approaching those of the CR mice with increasing age. ANOVA on body weights of mice surviving through 22 months indicated significant main effects of genotype and caloric intake [F>42.6; P<0.001] but failed to indicate significant two- or three-way interactions involving genotype, caloric intake, or age [F<2.6; P>0.077]. In short, the analysis of body weight indicated that whereas the body weights of AL mice of the different genotypes varied at different ages, the restriction of caloric intake resulted in a relative decrease in weight that was comparable among the different genotypes.
Effects of shifts in caloric intake on mortality at different ages
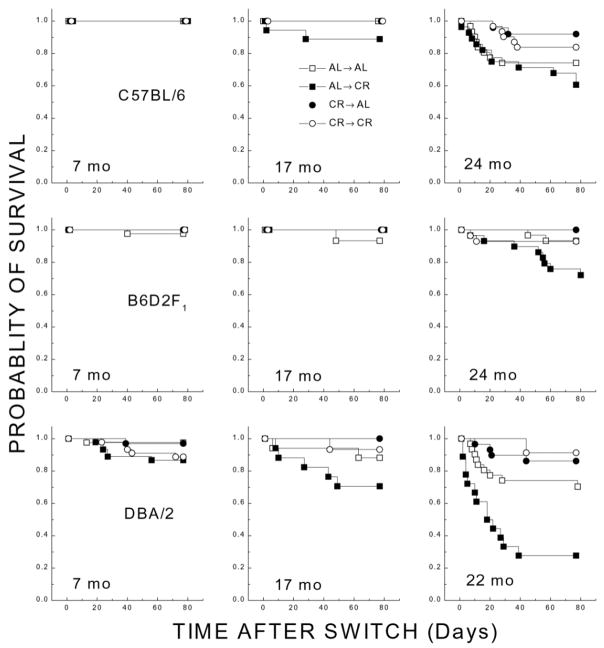
The characteristics of survival of mice kept on different caloric regimens during 11 wk following the shifts in caloric intake from AL to CR, and vice versa, are plotted as a function of age in Figure 3. Overall, the probability of survival declined with age for the AL→AL groups, with the greatest effects occurring in DBA/2 and C57BL/6. Cumulative survival during the 11 wk was greater in the CR→CR than in the AL→AL group for the DBA/2 and C57BL/6 genotypes at the oldest ages. Switching the old AL mice to the CR regimen (AL→CR) tended to decrease their probability of survival relative to the AL→AL groups. This effect was pronounced for the DBA/2 mice, but the same trend was evident in the C57BL/6 and B6D2F1 mice. The decrease in probability of survival was evident by 17 months in the DBA/2 and C57BL/6 mice but not until 24 months in B6D2F1. Transfer of CR mice to the AL regimen (CR→AL) had little or no effect on survival (relative to CR→CR groups) in the three genotypes.
Figure 3. Probability of survival over a period of 11 wk following shifts in caloric intake for separate age groups (left to right panels) of C57BL/6, B6D2F1, and DBA/2 mice.
Separate groups of each genotype were maintained chronically under AL or CR before the shifts.
A log-rank test considering all genotypes and ages indicated an overall difference in survival distributions of AL→AL and AL→CR groups (X2=12.1; P=0.001) but did not suggest a survival difference between CR→CR and CR→AL (P=0.153). Separate log-rank analyses for AL→AL vs. AL→CR groups at the oldest age of each genotype indicated significant effects for DBA/2 (P=0.002) and B6D2F1 (P=0.033). Thus, the results of this experiment indicated that CR was without benefit and tended to increase mortality when implemented at advanced age in all genotypes and that the beneficial effects of long-term CR in C57BL/6 and B6D2F1 mice were not rapidly reversible.
Effects of short-term shifts in caloric intake on body weight at different ages
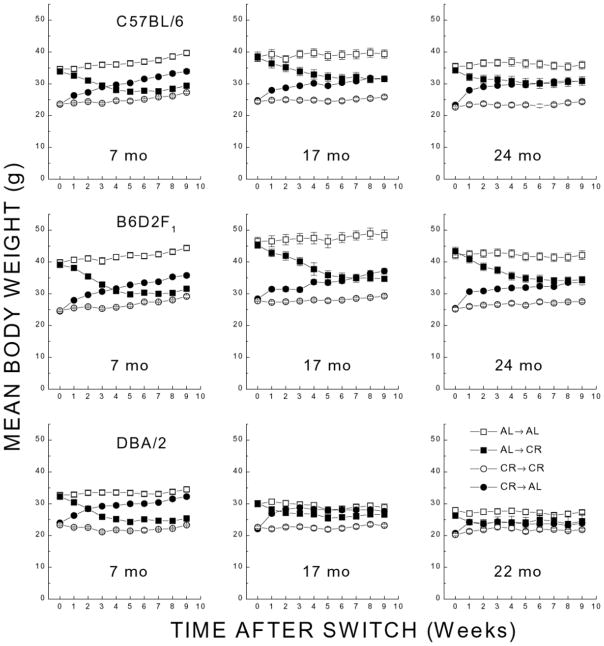
Figure 4 shows mean body weight for all ages and genotypes following shifts in caloric intake. The weights of nonswitched mice (AL→AL and CR→CR) in these studies were similar to those observed in the longevity experiment (Fig. 2). The 7-month-old AL→AL and CR→CR groups showed an overall weight gain during the period following the switch, whereas the weight of the older mice in these groups remained stable within the same period. Body weights of AL→CR and CR→AL groups of all ages showed weight loss or gain that reached the maximum within 5 wk following switching of the diets. With the exception of the 7- and 17-month-old DBA/2 mice, the weights of the CR→AL groups tended to stabilize at levels that were substantially lower than those of AL→AL groups. Whereas the stable weights of the 7-month-old AL→CR groups matched that of the appropriate CR→CR groups, the stable weights of older AL→CR groups were higher than the matching CR→CR mice. The DBA/2 mice were lighter than the other strains (also evident in Fig. 2), with the weight of the AL→AL groups of DBA/2 showing a decline from 7 to 22 months. As a result, DBA/2 mice exhibited smaller changes in weight following AL→CR and CR→AL, particularly in the 17- and 22-month-old groups. A five-way ANOVA on body weights (with time as a within-groups factor) resulted in main effects of time, genotype, age, long-term caloric intake, and short-term caloric intake [F>19.5; P<0.001]. Whereas the genotypes showed the same basic pattern of weight change following shifts in caloric intake, the age- and genotype-dependent differences in magnitude of the weight changes resulted in significant two-, three-, and four-way interactions involving these factors [F>1.9; P<0.001]. In summary, these results suggested that although the genotypes differed in their AL weights, there was no qualitative difference in the effect of shifts in caloric intake on their body weights.
Figure 4. Mean body weight (grams; ±SE) following two-way shifts in caloric intake for different age groups (left to right panels) of C57BL/6, B6D2F1, and DBA/2 mice.
Separate groups of each genotype were maintained chronically under AL or CR before the shifts.
Figure 2. Mean body weight (grams; ±SE) of surviving C57BL/6, B6D2F1, and DBA/2 mice as a function of age.
Separate groups of each genotype were maintained from 4 months of age under ad libitum (AL) or 40% calorie-restricted (CR) feeding regimens as described in Materials and Methods. Data not shown for cells with less than four mice surviving.
DISCUSSION
Results of this study indicate that both the age and the genotype of the mouse are independent determinants of the effects of CR on life span. The role of age is indicated by the finding that, if initiated at an advanced age, CR tends to increase rather than decrease the mortality rate. The role of the genotype is demonstrated by the finding that long-term reduction of caloric intake, that is, throughout the adult life, prolongs life span in C57BL/6 and B6D2F1 strains but not in the DBA/2 strain. This particular strain of mice not only exhibited an absence of an effect of long-term CR on mortality, but also showed relatively more enhanced mortality compared with the other genotypes, if CR was initiated at relatively old ages (17 and 22 months).
Although genotype-related variance in the effectiveness of CR to influence the progression of age-related alteration has been recognized previously (3, 9, 10), few attempts have been made to systematically identify the strains or the causes of this variation. Understandably, most of the studies have focused on those genotypes that exhibit a strong positive effect of CR on life span, because the apparent purpose of such studies was to understand biological consequences of CR. Thus, although the effectiveness of CR has been amply demonstrated in C57BL/6 mice, a popular strain in aging research, the DBA/2 strain, has been virtually ignored. The few findings for this strain that exist are mostly in agreement with the results of the current study. For instance, Fernandez et al. (11) reported that male DBA/2 mice showed a slight decrease in mean and maximum life span following CR, which is in agreement with the current investigation. In a large study sponsored by the NIA (from which the current animals were obtained), mortality rate of male DBA/2 mice was unaffected by CR up to 700 days of age, albeit, a relatively small decrease in mortality rate was evident thereafter (6). The fact that a slight increase in life span was observed in the NIA study, but not in the current study, may be due to the difference in maximum life span of AL mice, which was 4–5 months longer in the present study as compared with the NIA study. Regardless of the apparent small discrepancy between these studies, the main finding that DBA/2 mice are relatively insensitive to the life-prolonging effects of CR, was evident in both the current and the NIA study. For example, in the NIA study, the percent of increase in life span under CR for male C57BL/6 and B6D2F1 mice was three- to fivefold greater compared with DBA/2 mice. Thus, cumulatively, the existing data support the conclusion that DBA/2 mice are CR-insensitive, at least when fed on diets that contain 40% fewer calories than the AL group.
Whereas the life span of DBA/2 mice was not affected by CR, age-related pathological lesions were indeed decreased to the same extent as observed in C57BL/6 and B6D2F1 mice (12). Thus, it is unlikely that the lack of effect of CR on life span of DBA/2 mice is due to a relatively higher incidence of any specific age-related pathology.
One relevant question concerning the strain differences in response to CR may be that CR by 40% is too severe for certain strains that have comparatively lower body mass, such as the DBA/2. Indeed, it has been pointed out by Ingram and Reynolds (13) that CR might be less effective or even deleterious in relatively leaner mouse strains. However, results of other studies have failed to find a relationship between body weight and CR-induced effects on life extension (10, 13). This inference is strengthened by a finding in the NIA study, which reported that female DBA/2 mice weighed up to 10 g less than males, yet showed extension of life span under 40% CR (6). Thus, it seems unlikely that the lack of effect of CR on life span in male DBA/2 mice was due to their relatively low weight.
Another notable finding of this study was that the transfer of mice, kept continuously on CR, to the AL regimen, either had no effect (at 17 months of age) or lowered the rate of mortality if the transfers were made at a relatively old age (25 months of age). This is in contrast to the transfers in reverse (i.e., AL→CR), which decreased life span in all strains of mice, albeit to varying degrees. Thus, the beneficial effects of CR on mortality seem to accrue gradually over a long period and are neither relatively quickly reversible nor inducible.
In conclusion, the results of this investigation support three main points: Life-long restriction of calories does not increase the longevity of all of the mouse strains; CR, instituted at a relatively advanced age, can significantly increase mortality; and the effects of CR on mortality are neither rapidly inducible nor reversible. These findings do not support the popular conception that CR might be a practical tool for the retardation of the aging process in all of the various animal species, including humans. The nature of the mechanisms by which CR affects life span of rodents, or why different strains of laboratory mice and rats respond differently to CR, is presently unknown. It has been variously speculated that attenuation of oxidative stress, modulation of glucose and insulin levels, modulation of energy metabolism responses, effects on body composition, or hormesis is responsible for the anti-aging action of CR (e.g., 14, 15). The variation among genotypes in their sensitivity to CR reported herein may serve as an important tool in establishing the specificity of the relationship between these CR effects and the prolongation of life span. Furthermore, the CR-resistant/intolerant strains of mice may be useful as negative controls for the elucidation of mechanisms by which CR affects the aging process.
Acknowledgments
This research was supported by grants R01 AG 7695 and R01 13563 from the National Institute on Aging, National Institutes of Health.
References
- 1.Fishbein L. Biological effects of dietary restriction. Springer-Verlag; New York: 1991. [Google Scholar]
- 2.McCarter RJM. Role of caloric restriction in the prolongation of life. Clin Geriatr Med. 1995;11:553–565. [PubMed] [Google Scholar]
- 3.Weindruch R, Walford RL. The retardation of aging and disease by dietary restriction. Charles C. Thomas; Springfield, IL: 1988. [Google Scholar]
- 4.Weindruch R. The retardation of aging by caloric restriction: Studies in rodents and primates. Toxicol Pathol. 1996;24:742–745. doi: 10.1177/019262339602400618. [DOI] [PubMed] [Google Scholar]
- 5.Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev. 1994;74:121–133. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 6.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the biomarkers of aging program. J Gerontol Biol Sci. 1999;54A:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 7.Forster MJ, Lal H. Neurobehavioral biomarkers of aging: Influence of genotype and dietary restriction. Biomed Environ Sci. 1991;4:144–165. [PubMed] [Google Scholar]
- 8.Forster MJ, Lal H. Estimating age-related changes in psychomotor function: Influence of practice and level of caloric intake in different genotypes. Neurobiol Aging. 1999;20:167–176. doi: 10.1016/s0197-4580(99)00041-x. [DOI] [PubMed] [Google Scholar]
- 9.Harrison DE, Archer JR. Genetic effects on responses to food restriction in aging mice. J Nutr. 1987;117:376–382. doi: 10.1093/jn/117.2.376. [DOI] [PubMed] [Google Scholar]
- 10.Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider N. Effects of intermittent feeding upon body weight and lifespan in inbred mice: Interaction of genotype and age. Mech Ageing Dev. 1990;55:69–87. doi: 10.1016/0047-6374(90)90107-q. [DOI] [PubMed] [Google Scholar]
- 11.Fernandes G, Yunis EJ, Good RA. Influence of diet on survival of mice. Proc Natl Acad Sci USA. 1976;73:1279–1283. doi: 10.1073/pnas.73.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bronson R, Lipman R. Reduction in rate of occurrence of age related lesions in dietary restricted laboratory mice. GrowthDev Aging. 1991;55:169–184. [PubMed] [Google Scholar]
- 13.Ingram DK, Reynolds MA. The relationship of body weight to longevity within laboratory rodent species. In: Woodhead AD, Thompson KH, editors. Evolution of longevity in animals: A comparative approach. Plenum; New York: 1987. pp. 247–282. [DOI] [PubMed] [Google Scholar]
- 14.Masoro EJ. Caloric restriction and aging: an update. Exp Gerontol. 2000;35:299–305. doi: 10.1016/s0531-5565(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 15.Masoro EJ. Hormesis and the antiaging action of dietary restriction. Exp Gerontol. 1998;33:61–66. doi: 10.1016/s0531-5565(97)00071-5. [DOI] [PubMed] [Google Scholar]