Summary
Phytochelatins (PCs) are glutathione-derived peptides that function in heavy metal detoxification in plants and certain fungi. Recent research in Arabidopsis has shown that PCs undergo long-distance transport between roots and shoots. However, it remains unknown which tissues or vascular systems, xylem or phloem, mediate PC translocation and whether PC transport contributes to physiologically relevant long-distance transport of cadmium (Cd) between shoots and roots. To address these questions, xylem and phloem sap were obtained from Brassica napus to quantitatively analyze which thiol species are present in response to Cd exposure. High levels of PCs were identified in the phloem sap within 24 h of Cd exposure using combined mass spectrometry and fluorescence HPLC analyses. Unexpectedly, the concentration of Cd was more than four-fold higher in phloem sap compared to xylem sap. Cadmium exposure dramatically decreased iron levels in xylem and phloem sap whereas other essential heavy metals such as zinc and manganese remained unchanged. Data suggest that Cd inhibits vascular loading of iron but not nicotianamine. The high ratios [PCs]/[Cd] and [glutathione]/[Cd] in the phloem sap suggest that PCs and glutathione (GSH) can function as long-distance carriers of Cd. In contrast, only traces of PCs were detected in xylem sap. Our results suggest that, in addition to directional xylem Cd transport, the phloem is a major vascular system for long-distance source to sink transport of Cd as PC–Cd and glutathione–Cd complexes.
Keywords: cadmium translocation, iron content in xylem, long-distance transport, heavy metals, thiol-peptides, mass spectrometry
Introduction
Heavy metal pollution has become a serious environmental and health problem and bioremediation has been proposed as a sustainable technology for the clean-up of sites contaminated with heavy metals and other toxic compounds (Lasat, 2002; Omichinski, 2007). An efficient phytoextraction strategy will require significant amounts of heavy metals to be translocated to the aerial, harvestable, tissues of plants. Genetic manipulation of plants to achieve this goal requires a mechanistic understanding of how heavy metals are mobilized within the plant. The chemical nature of cadmium (Cd) complexes during long-distance transport between shoots and roots remains incompletely understood. It is also unknown which vascular system, xylem or phloem, is more active in transporting this heavy metal. In xylem sap, oxygen- and nitrogen-containing compounds have been identified as ligands able to bind Cd (Salt et al., 1995) but neither the full identity of these compounds nor the relative contributions of the xylem and phloem to long-distance transport of Cd have been quantified.
Cadmium is an extremely reactive heavy metal with affinity towards the functional groups of biomolecules (i.e. phosphate, carboxy, amino and thiol groups; Sillén and Martell, 1964). Among these molecules, thiols have the highest affinity towards Cd, and this reactivity has led to the hypothesis that, within the cell environment, Cd is more likely to form complexes with biomolecules than to be present as a free ion (Mendoza-Cózatl et al., 2005; Vatamaniuk et al., 2000).
Phytochelatin-mediated heavy metal detoxification is a major mechanism for Cd resistance in plants, some yeast and photosynthetic protists (Grill et al., 1985; Hayashi et al., 1991; for reviews see Clemens et al., 2002; Mendoza-Cózatl et al., 2005). Phytochelatins (PCs) are glutathione (GSH)-derived peptides synthesized by the transpeptidase phytochelatin synthase (Clemens et al., 1999; Ha et al., 1999; Vatamaniuk et al., 1999). Originally, PCs were considered solely as an intracellular mechanism for Cd detoxification by shuttling PC–Cd complexes into plant cell vacuoles. However, recent studies have shown that in transgenic Arabidopsis thaliana and in grafted Arabidopsis plants, PCs also have the ability to undergo long-distance transport in the root-to-shoot and shoot-to-root directions (Chen et al., 2006; Gong et al., 2003). As PC synthesis is strictly dependent on the presence of heavy metals, it is conceivable that PCs may be involved in the long-distance transport of Cd, and possibly other heavy metals. However, the physiological relevance and relative contribution of PCs and other thiols to long-distance transport of Cd has not yet been directly examined.
Thiol transport from shoots to roots seems to be a very efficient process. Shoot-specific expression of the key enzyme of GSH biosynthesis, γ-glutamylcysteine synthetase (γ-EC synthetase), in an Arabidopsis GSH-deficient mutant restored the levels of all thiols [γ-glutamylcysteine (γ-EC), GSH and PCs] in roots (Li et al., 2006). However, whether γ-EC, GSH, PCs or any other thiol-peptide can be transported through the phloem of plants has not yet been directly quantified.
In this study, experiments were pursued to determine whether xylem and/or phloem mediates PC, γ-EC and GSH transport and to analyze the role of these thiols in the long-distance transport of Cd. We apply a well-developed technique in Brassica napus, a close relative of Arabidopsis, to extract large amounts of pure phloem and xylem sap and determine the concentrations of different thiol species and Cd. The results show that PCs are present in the phloem sap in large quantities that are sufficient to form stable complexes with Cd. The [thiol]/[Cd] stoichiometries also reveal that GSH may contribute to the long-distance transport of Cd. The Cd concentration and abundance of thiols suggest that the phloem plays a major role in the long-distance source-to-sink transport of Cd as thiol–Cd complexes.
Results
Analysis of purity of phloem sap
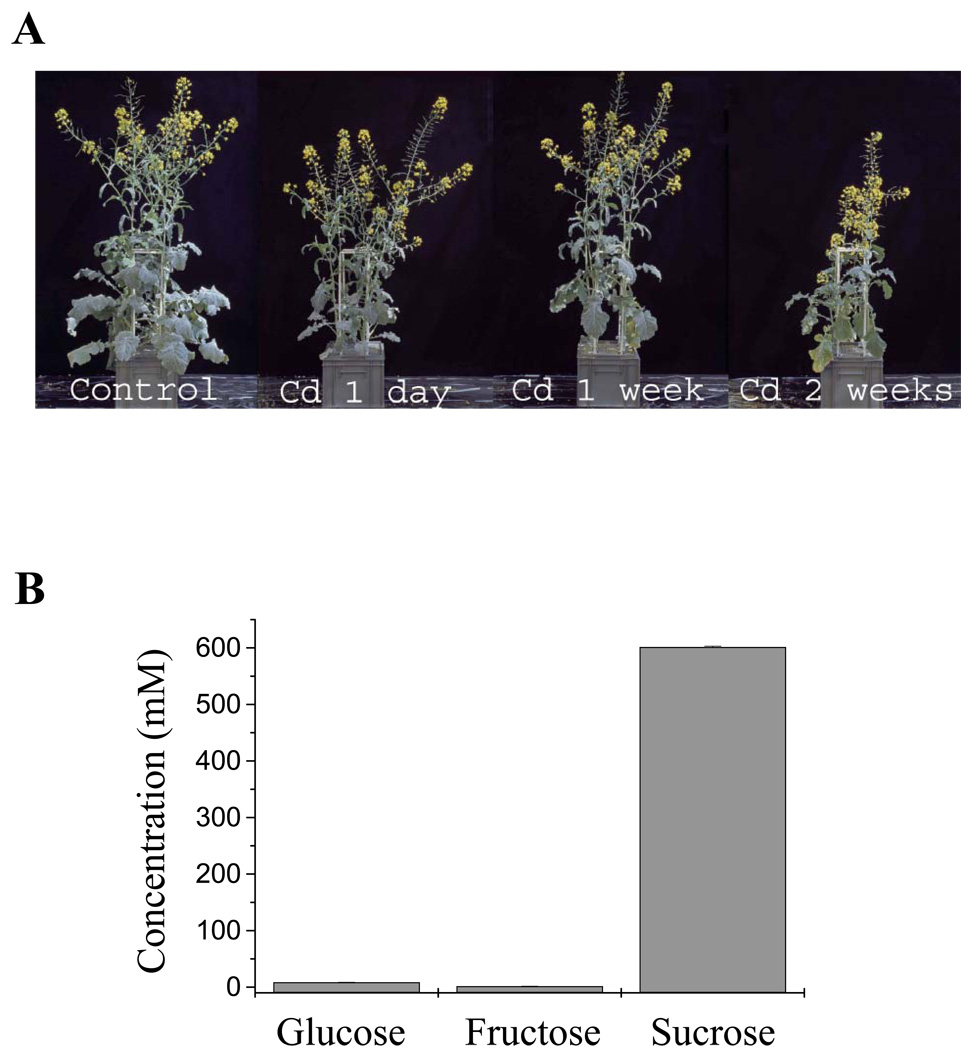
Brassica napus was chosen as a model, because the method for obtaining large quantities of highly pure phloem is well established (Giavalisco et al., 2006). The Cd concentration used in the present study (75 µm), induced strong phytochelatin synthesis but allowed the plants to flower for phloem sampling (Figure 1a). As a first step, the purity of phloem sap used for thiol and Cd measurements was evaluated. This was determined by measuring the ratio of reducing sugars (glucose, fructose) to total sugars present in the sap. The levels of glucose, fructose and sucrose in phloem sap samples are shown in Figure 1(b). From these values, a reducing sugars/total sugars ratio of 1.8% was calculated. This low ratio was indicative of a highly pure phloem sap, as the same ratio in the surrounding stem tissues can be up to 72% (Geigenberger et al., 1993; Giavalisco et al., 2006).
Figure 1.
Phloem and xylem sap samples were obtained from Brassica napus grown in hydroponic cultures.
(a) Plants were grown under greenhouse conditions. After 9 weeks plants were exposed to 75 µm CdSO4 in the hydroponic solution for 1 day, 1 week or 2 weeks before phloem and xylem sap sampling.
(b) Sugar content in phloem sap samples was measured enzymatically to assess the purity of the sap. The ratio of [reducing sugars]/[total sugars] obtained from these concentrations was 1.8%, showing highly pure phloem sap. Values shown are means ± SE (n = 5).
Identification of thiol in the phloem sap
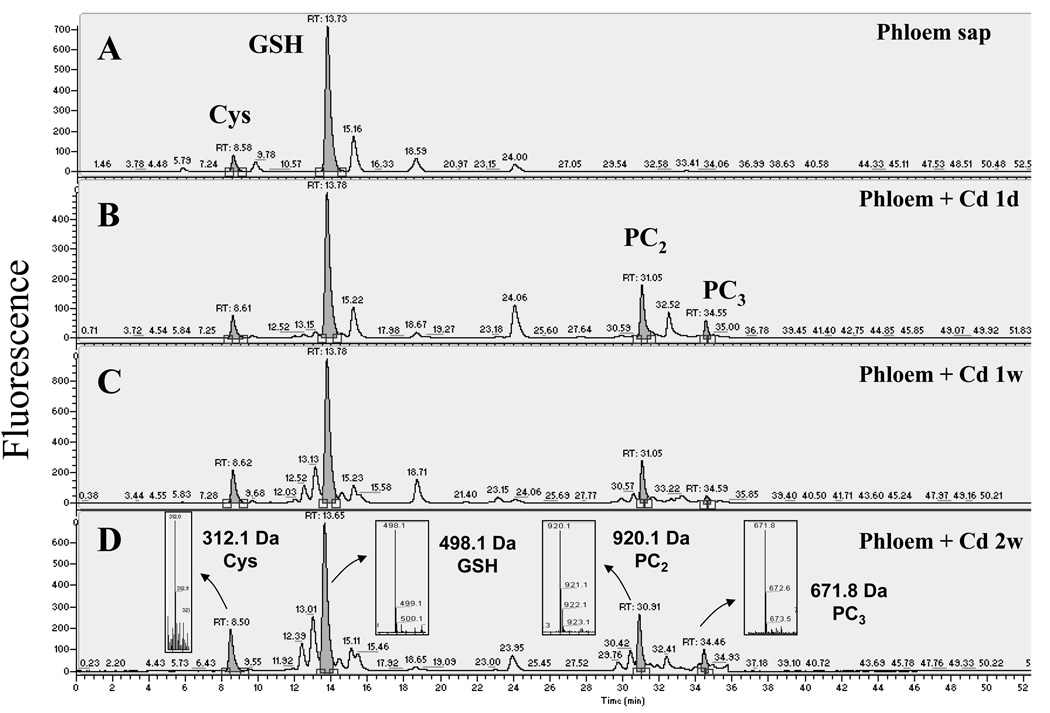
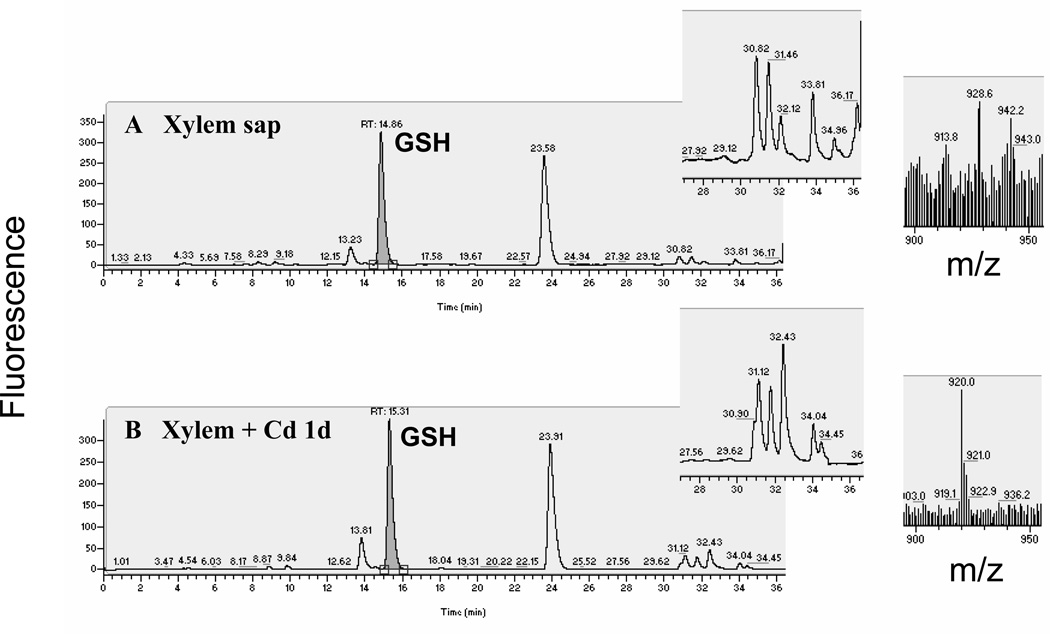
Thiols in the phloem sap were separated and quantified by fluorescence HPLC (Figure 2). In phloem sap obtained from control plants (non-Cd treated), GSH was the most abundant thiol (Figure 2a, n = 4 experiments; two to three samples measured per experiment). Cysteine (Cys) was also present, although at a much lower level (≈10% relative to GSH; Figure 2a). No PCs were detected in phloem sap obtained from control plants (Figure 2a). Interestingly, PCs were clearly detected in the phloem sap 1 day after the onset of Cd exposure and after 1 and 2 weeks of Cd treatment. (Figure 2b–d, n = 3–4 experiments; two or three samples measured per experiment). The identity of the PCs was directly determined by parallel mass spectrometry analyses of the thiol-labeled samples (Figure 2d, insets). The bimane label increases the mass of the thiol compounds by 190 Da per thiol labeled. Therefore, PC2, which has a molecular mass of 539.1 Da and contains two thiols per molecule, was detected as a singly charged ion at m/z 920.1. Phytochelatin PC3 was detected as a doubly charged ion at m/z 671.8 (Figure 2d). Phytochelatin PC4 would be observed as a doubly charged ion at m/z 882.9, as determined by the use of a PC4 standard, but no PC4 was detected in any of the phloem sap samples analyzed (n = 3 experiments for phloem sap from 1 day and 1 week of Cd exposure and n = 4 experiments for plants exposed to Cd for 2 weeks). Glutathione was the most abundant thiol at all time points measured, even during Cd exposure (Figure 2a–d, n = 3–4).
Figure 2.
Cadmium exposure results in the appearance of phytochelatins (PCs) in the phloem sap of Brassica napus. Thiols from (a) control and plants exposed to 75 µm CdSO4 for (b) 1 day, (c) 1 week and (d) 2 weeks were labeled with monobromobimane, separated by reverse phase HPLC and detected by fluorescence. Thiols were identified with a mass spectrometer coupled to the HPLC. Phytochelatins appeared after 1 day of cadmium exposure and their concentrations increased with the time of exposure. Note the difference in the Y-axis scales in panels (c) and (d). The insets in (d) show the mass spectrum of the corresponding monobromobimane-labeled thiols. Bimane label accounts for the addition of 190 Da per thiol to the mass of the unlabeled compounds.
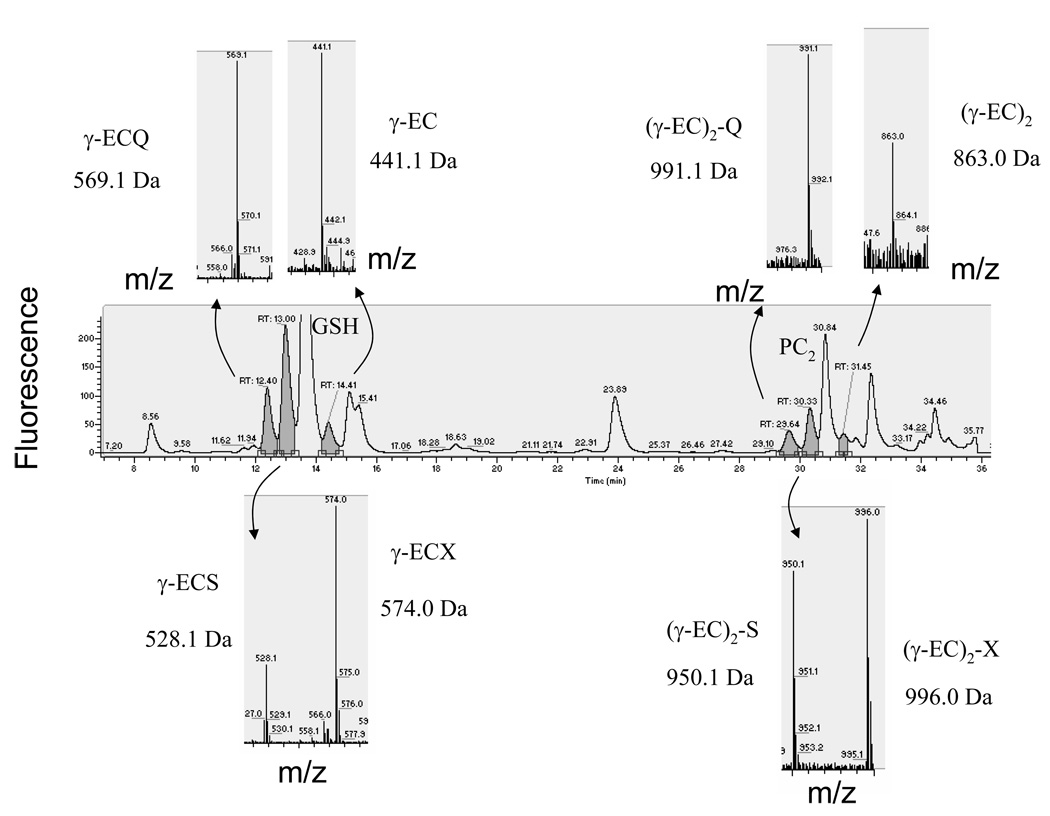
After 1 week of Cd exposure, additional thiols with retention times close to GSH and PC2 were also detected (Figure 3). The peak eluting after GSH was identified as γ-EC (m/z 441.1, singly charged ion; Figure 3). The peaks eluting before GSH were identified as GSH-related peptides. The thiol compound at m/z 569.1 corresponded to γ-glutamyl-cysteinyl-glutamine (γ-ECQ), often called homoglutathione, where glutamine substitutes for the glycine at the C-terminus (Kubota et al., 2000). The glutamine substitution at the C-terminus was confirmed using tandem mass spectrometry (Figure S1). The thiol compound at m/z 528.1 showed the expected mass for hydroxymethyl-glutathione (γ-ECS), where serine substitutes for the glycine in the GSH molecule. The same amino acid modifications were found for the compounds near PC2 (Figure 3). The m/z 863.0 ion was identified as desGly-PC2 [(γ-EC)2], the m/z 991.1 ion corresponded to glutamine-PC2 [(γ-EC)2-Q] and the m/z 950.1 ion was assigned as hydroxymethyl-PC2 [(γ-EC)2-S]. Interestingly, both serine-containing thiol-peptides (hydroxymethyl-GSH and hydroxymethyl-PC2) were accompanied by an ion that was 46 Da heavier (m/z 574 and m/z 996; Figure 3). The presence of the b2 ion (corresponding to γ-EC) in the tandem MS of them/z 574 ion confirmed that this compound is a γ-EC-related peptide with a modified amino acid at the C-terminus that differed from a serine, glutamic acid, glutamine or β-alanine (Figure S2). The precise chemical structure of this modified amino acid was not determined in this study (see Discussion). These analyses directly demonstrated the presence of PCs in the phloem sap during Cd exposure and led us to further quantify the different thiol compounds and the Cd levels in the phloem sap during Cd exposure.
Figure 3.
Glutathione (GSH) and phytochelatin (PC) related peptides were induced after 1 week of Cd exposure.
Thiol-containing peptides with retention times close to GSH and PC2 were detected after Cd treatment. In the upper part of the chromatogram from left to right the compounds were identified as: γ-glutamyl-cysteinyl-glutamine (γ-ECQ; 569.1 Da), γ-glutamylcysteine (γ-EC; 441 Da), glutamine-PC2 [(γ-EC)2-Q; 991.1 Da], desGly-PC2 [(γ-EC)2; 863.0 Da]. The mass spectra corresponding to hydroxymethyl-GSH (γ-ECS; 528 Da) and hydroxymethyl-PC2 [(γ-EC)2-S; 950.1 Da] are shown below the main chromatogram. These ions were accompanied by an ion that is 46 Da heavier. In the case of the ion with a mass of 574.0 Da, tandem mass spectrometry confirmed that this compound is a γ-EC peptide with a modified amino acid residue (133 Da) at the C-terminus.
Thiols and heavy metal content in the phloem sap after Cd exposure
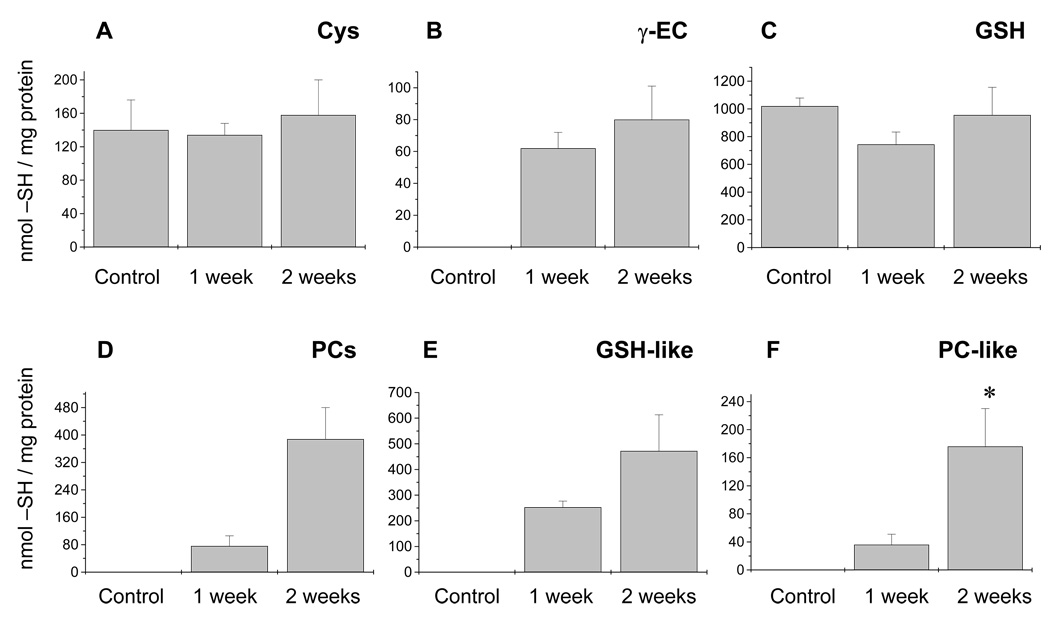
The quantification of acid-soluble thiols from the phloem sap is shown in Figure 4. Levels of Cys and GSH remained constant over the duration of Cd exposure (Figure 4a,c; n = 3–4). The γ-EC and PCs were undetectable under control conditions and increased dramatically in response to Cd exposure (Figure 4b,d). Interestingly, the glutathione-and PC-related peptides (see above, Figure 3) were induced only upon Cd exposure and their levels increased with the time of Cd exposure (Figure 4e,f; n = 3–4). However, canonical GSH and PCs were more abundant at all times than their corresponding modified peptides (Figure 4c–f).
Figure 4.
Thiol content in the phloem sap of Brassica napus. The contents of (a) cysteine (Cys), (b) γ-glutamylcysteine (γ-EC), (c) glutathione (GSH), (d) phytochelatins (PCs), (e) GSH-like peptides, and (f) PC-like peptides were measured in the phloem sap of non-treated and Cd exposed plants (see Results).
(a, c) Cysteine and GSH levels remained constant independently of the length of Cd treatment.
(b) The level of γ-EC in phloem sap samples increased dramatically upon Cd exposure. The GSH-like (sum of γ-glutamyl-cysteinyl-serine and γ-glutamyl-cysteinyl-glutamine, γ-ECQ) and PC-like (sum of hydroxymethyl-PC2 [(γ-EC)2-S] and glutamine-PC2 [(γ-EC)2-Q]) peptides were also induced only after Cd exposure (d–f). Levels of PCs and their homologs are expressed in thiols mg−1 of protein. The phloem sap contained on average 0.174 ± 0.09 mg protein ml−1 (mean ± SE, n = 4); this value was used to express GSH in concentration (196 µm after 2 weeks of Cd exposure), allowing direct comparison with the xylem sap measurements.
The asterisk in the content of PC-like peptides after 2 weeks of Cd exposure denotes a significant difference compared with the levels of PC-like peptides after 1 week of treatment. Values shown are n = 3–4 experiments; two or three samples were measured per experiment.
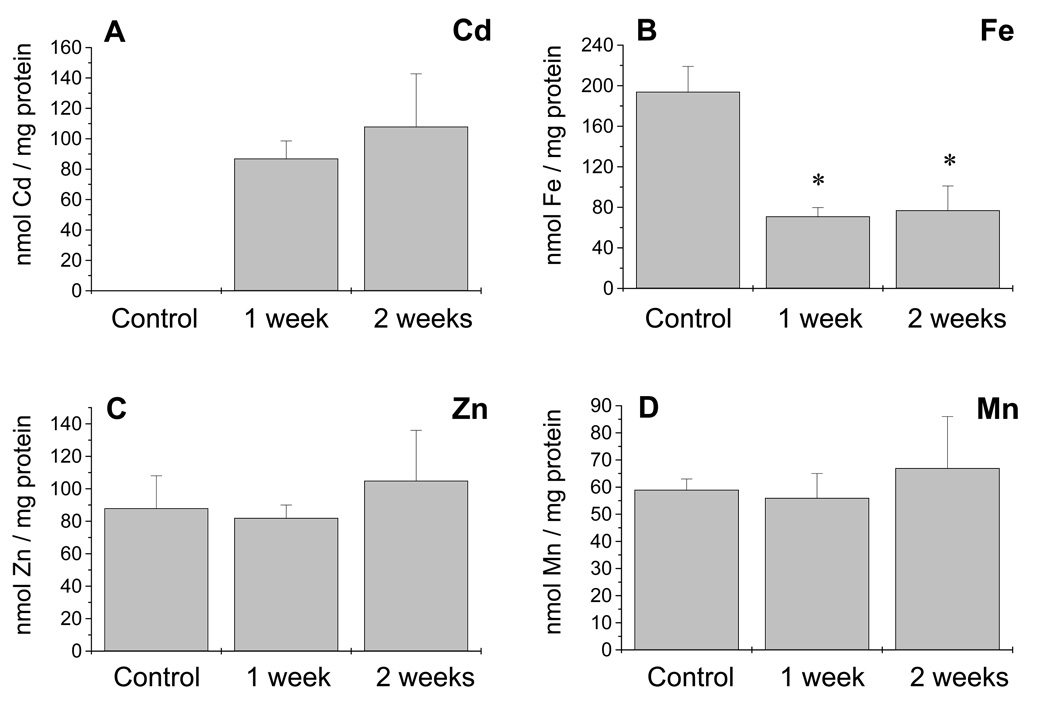
The levels of cadmium, iron, zinc and manganese in the phloem sap were determined by inductively coupled plasma optical emission spectroscopy (ICP-OES) and are shown in Figure 5. The Cd content of the phloem sap was similar after 1 and 2 weeks of continuous Cd exposure (Figure 5a, n = 3–4), reaching a concentration of 20 µm after 2 weeks of treatment. Whereas zinc and manganese remained constant both before and during Cd exposure (Figure 5c,d), iron showed a dramatic decrease after 1 and 2 weeks of Cd exposure to 36% of the iron content in the phloem sap under control conditions (Figure 5b, n = 3–4). This decrease in iron was found to be independent of the level of the phytosiderophore nicotianamine (NA), as determined by NA measurements performed by fluorescence HPLC. In fact, Cd induced a significant increase (P < 0.01) in NA levels in phloem sap obtained from plants exposed to Cd for 2 weeks (132.65 ± 25.6 nmol NA mg−1 protein; mean ± SD, n = 3) compared with control plants (54.2 ± 10.2 nmol NA mg−1 protein; mean ± SD, n = 3). Therefore, NA is not responsible for the decreased levels of iron found in the phloem sap during Cd exposure.
Figure 5.
Heavy metal content in the phloem sap of Brassica napus. The levels of (a) cadmium, (b) iron, (c) zinc and (d) manganese were measured in the phloem sap from control and Cd exposed plants.
(a) Using the relationship 0.174 ± 0.09 mg protein ml−1, a Cd concentration of 15.5 µm for 1 week of Cd exposure and 20 µm for 2 weeks of exposure was calculated. Cadmium content in the phloem sap after 1 day if Cd exposure was 35 nmol Cd mg−1 protein (n = 2).
(b) Iron concentration dramatically decreased during Cd exposure (*, P < 0.01) with respect to plants not exposed to Cd.
(c, d) No significant differences were found in the zinc and manganese content. Values shown are n = 3–4 experiments; two or three samples were measured per experiment.
Iron content in leaves and roots during cadmium exposure
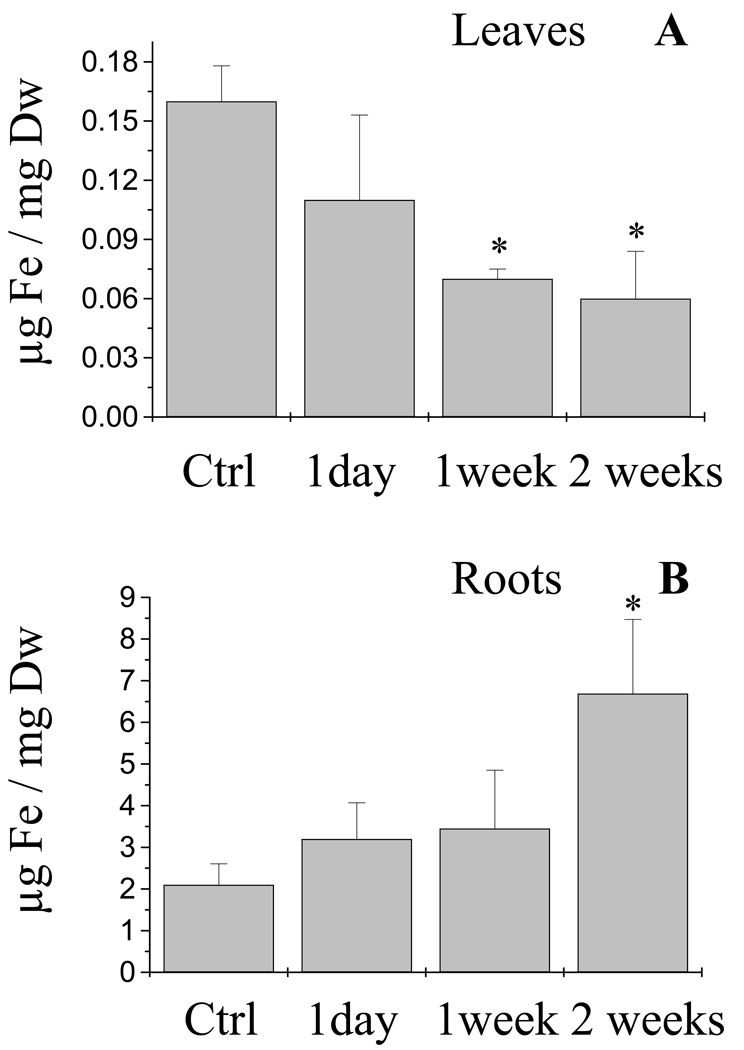
To further establish whether the decrease in iron content in the phloem sap was due to a decreased uptake of iron into roots or an impaired translocation from roots to shoots, the iron content in roots and leaves from B. napus was determined (Figure 6, n = 3–4). The iron content in leaves from plants exposed to Cd decreased dramatically after 1 and 2 weeks of Cd treatment to 40% of the iron content from leaves grown without Cd (Figure 6a). Interestingly, iron content in roots increased with the time of Cd exposure (Figure 6b). After 2 weeks of Cd treatment iron concentrations showed a three-fold increase in roots relative to plants grown without Cd. These results suggest that the decrease in iron in the phloem sap (Figure 5b) is more likely to be related to an impaired vascular loading and root to shoot translocation of iron, rather than due to a Cd-induced blocking of iron uptake into roots.
Figure 6.
Iron levels in leaves and roots of Brassica napus were affected by Cd exposure. Iron levels in (a) leaves and (b) roots from non-exposed and Cd-treated plants were measured by inductively coupled plasma optical emission spectroscopy. Iron content in leaves was dramatically reduced after 1 and 2 weeks of Cd exposure (P < 0.05; a), whereas Fe showed significant overaccumulation in roots after 2 weeks of Cd exposure (P < 0.05; b). Asterisks denote statistically significant differences compared with non-treated plants. Values shown are n = 3–4 experiments; two or three samples were measured per experiment.
Thiols are more abundant than cadmium in the phloem sap
With the Cd content and thiol measurements shown in Figure 4 and Figure 5 it was possible to establish the [GSH]/[PCs] and [PCs]/[Cd] stoichiometries present in the phloem sap. These ratios are important for understanding which stable complexes can be formed in the phloem sap and to determine whether thiols can function in the chelation and long-distance transport of Cd. Phloem sap obtained from Cd-exposed plants contained a higher concentration of thiols than Cd ([thiols]/[Cd] ratio > 10; Figure 4 and Figure 5). Of the different thiol species, GSH and PCs were the most abundant (Figure 4). The ratio of [GSH]/[PCs] after 1 week of Cd exposure was 8:1, whereas the [GSH]/[PCs] ratio after 2 weeks of treatment was 2:1. Furthermore, the ratio of [PCs]/[Cd] was 1.5:1 after 1 week of Cd treatment and 5:1 after 2 weeks of Cd exposure.
To determine the contribution of thiols from proteins to the total thiol content in the phloem sap, the thiols derived from proteins were also measured in the phloem sap. In control plants, thiols from proteins account for 18% (271 nmol −SH mg−1 protein; n = 2) of the total thiols present in the phloem, whereas in the phloem sap from plants exposed to Cd for 2 weeks this percentage increases to 28% (917 nmol −SH mg−1 protein). This increase in protein-thiols appears to indicate a toxic interaction between Cd and protein thiol moieties, leading to an increased demand and consequently an increased loading of these proteins into the phloem sap. It is worth noting that in both samples, non-protein soluble thiols (Cys, GSH and PCs) accounted for more than 70% of the total thiols present in the phloem sap.
Thiol and heavy metal content in the xylem sap
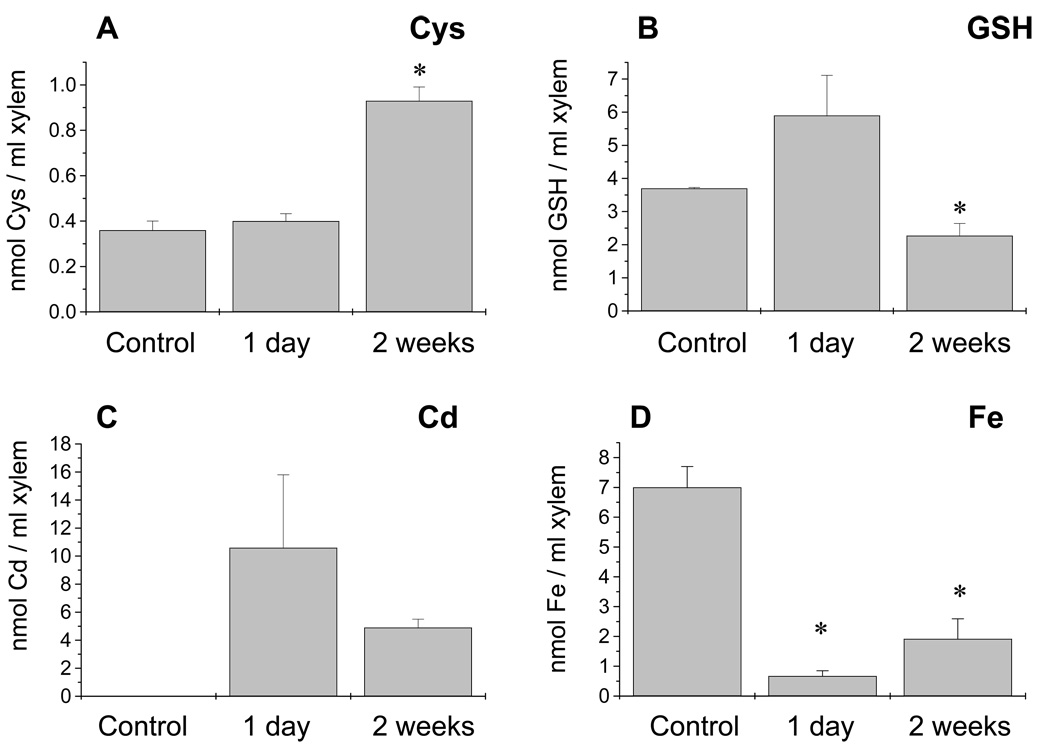
The concentration of thiol compounds in the xylem sap was also determined (Figure 7; n = 3–4 experiments, two or three measurements per experiment). Glutathione was the most abundant thiol found in the xylem sap (Figure 7 and Figure 8). Fluorescence HPLC was unable to detect measurable amounts of PC2 in the xylem sap after 1 day, 1 week and 2 weeks of Cd exposure (Figure 7b and data not shown; n = 3–4). However, mass spectrometry showed the characteristic ion corresponding to PC2 at the retention time expected in four of the seven xylem sap samples measured (Figure 7b inset; m/z 920 at 31 ± 1 min). This peak accounted for <1% of the thiols compared with the GSH peak (Figure 7). The Cys concentration was significantly increased in the xylem sap after 2 weeks of Cd exposure, while the GSH content showed a moderate but statistically significant decrease (P < 0.05) after 2 weeks of treatment (Figure 8a,b; n = 3–4). Cadmium was found in the xylem sap after the first day of Cd exposure and the concentration was significantly lower after 2 weeks of Cd treatment, decreasing to a concentration of 4 µm (Figure 8c). Of the different essential heavy metals measured in the xylem sap (iron, zinc, manganese) only the iron content was significantly affected (Figure 8d). A similar decrease in the iron content during Cd exposure has also been found in maize and has been linked to an inhibition by Cd of the phytosiderophoreiron complex loading of the xylem, disrupting iron homeostasis in the whole plant (Meda et al., 2007). Interestingly, the ratio of [thiols]/[Cd] in the xylem sap was <1 after 1 day (0.63:1) and 2 weeks (0.9:1) of Cd exposure. These ratios suggest that thiols in the xylem sap are not present in sufficient quantities to bind most of the Cd (see Discussion) and that other molecules, such as histidine, may be involved in the root-to-shoot transport of Cd through the xylem sap (Kerkeb and Kramer, 2003).
Figure 7.
Glutathione (GSH) was the most abundant thiol in the xylem sap of Brassica napus. Non-protein thiols in the xylem sap from (a) control and (b) Cd-exposed plants were measured by fluorescence HPLC. No obvious phytochelatin (PC2) peak was detected, but mass spectrometry revealed a compound with the retention time and mass expected for PC2 (m/z 920). This peak was found in four out of seven xylem sap samples measured (three independent samples for 1 week of treatment and four independent samples for 2 weeks of Cd exposure). The PC2 peak accounts for <1% of the thiols present from GSH.
Figure 8.
Thiol and heavy metal content in the xylem sap of Brassica napus.
(a) The cysteine content in xylem sap increased after 2 weeks of Cd exposure but
(b) glutathione (GSH) was the most abundant thiol at all times measured.
(c) Cadmium was present in the xylem in significant amounts after 1 day of Cd treatment, decreasing to a concentration of 4 µm after 2 weeks of Cd exposure.
(d) Iron content in the xylem sap significantly decreased after 1 day of cadmium exposure. Asterisks denote statistically significant differences from untreated plants. Values shown are n = 3–4 experiments; two to three samples were measured per experiment.
It has been reported for other plant species that the pH of the xylem sap is more acidic than that of the phloem sap (Shelp, 1987). As pH is an important factor for metal–ligand complex formation we determined the pH values in our sap samples. Phloem sap had a basic pH (pH 7.7, n = 2) compared with the acidic xylem sap (pH 5.8, n = 2), suggesting that the environment within the xylem sap is not as favorable as in the phloem sap for stable thiol–Cd complex formation.
Discussion
Phytochelatin accumulation in the phloem sap in response to cadmium
Long-distance transport of PCs among roots and shoots in transgenic Arabidopsis is a phenomenon that was discovered only recently (Chen et al., 2006; Gong et al., 2003). The present study shows that PCs occur naturally and at high levels in the phloem sap of B. napus upon Cd exposure. The fact that wild-type plants, rather than transgenic or manipulated plants, were used in the present study strengthens the notion that long-distance transport of PCs is a physiological process and that PCs can function in source-to-sink transport of metals. Analyses of phloem and xylem thiols and Cd concentrations suggest distinct mechanisms of Cd transport in both vascular pathways.
Glutathione and PCs as molecules involved in long-distance transport of cadmium
Long-distance transport of organic thiols through the phloem has previously been reported (Lappartient and Touraine, 1996; Li et al., 2006; Rennenberg et al.,1979). However, it remained unknown whether phloem sap thiols are present in sufficient quantities during Cd exposure to account for physiologically relevant Cd chelation and long-distance source-to-sink transport of Cd through the phloem.
Cadmium is a highly reactive heavy metal with high affinity towards the major functional groups of biomolecules (i.e. amino, carboxy, phosphate and thiol groups). These interactions are the biochemical basis of the Cd toxicity. Within biological environments, Cd is expected to be found as complexes rather than existing as a free ion (Mendoza-Cózatl et al., 2005; Sillén and Martell, 1964; Vatamaniuk et al., 2000). It can then be predicted that if Cd is being transported over a long distance within the plant, such transport may take place in the form of complexes. Among the different biochemical moieties, thiols have the highest affinity towards Cd (Sillén and Martell, 1964). Furthermore, among the thiols, PCs are the strongest Cd chelators. The dissociation constant (Kd) for a stable PC–Cd complex is 7.9 × 10−17 m (Dorcák and Krezel, 2003), lower than the dissociation constants for the Cd-chelating proteins metallothioneins (3.44 × 10−11 m) or monothiol molecules such as Cys (1.28 × 10−10 m), GSH (3.16 × 10−11 m) and from other physiological Cd-chelating compounds such as histidine (2.23 × 10−6 m), glutamate (1.25 × 10−4 m) and phosphate-containing compounds such as ATP or pyrophosphate (2.51 × 10−6 m; Erk and Raspor, 1998; Sillén and Martell, 1964). Due to the thiol–Cd affinity constants and because the ratio of ([GSH] + [PCs]) to [Cd] in the phloem sap was >10 at all measured times (Figure 4 and Figure 5), it is likely that GSH and PCs form physiologically relevant levels of stable complexes with Cd in the phloem sap. Other heavy metals such as iron, zinc, or manganese may also form complexes with thiols, but Cd has higher affinity for the thiol moiety (Sillén and Martell, 1964). For example, the Kd of Cys–metal complexes are: cadmium (1.28 × 10−10 m), zinc (1.38 × 10−10 m), iron (6.3 × 10−7 m) and manganese (7.9 × 10−5 m; Sillén and Martell, 1964). The Kd of Cys–copper complexes is lower (6.3 × 10−20 m); however, under our conditions copper was below the detection limit (157 nm) in the phloem sap. In phloem sap samples obtained from plants exposed to Cd for 2 weeks, the ratio of [thiols from PCs]/[Cd] was 5:1 (Figure 4 and Figure 5). This ratio satisfies the 4:1 stoichiometry needed for stable PC–Cd complex formation (Dorcák and Krezel, 2003; Pickering et al., 1999; Salt et al., 1995). On the other hand, the ratio of [PCs]/[Cd] after 1 week of Cd exposure was significantly lower (1.5:1), suggesting that other molecules, most likely GSH, may participate in Cd chelation and transport through the phloem sap.
Glutathione and PC derivatives were detected in the phloem sap after 1 week of Cd exposure (Figure 3). Synthesis of glutathione and PC derivatives seems to be dependent on substrate availability rather than on the expression of specific enzymes (Iturbe-Ormaetxe et al., 2002; Oven et al., 2002). For example, both Arabidopsis phytochelatin synthase and Glycine max homo-phytochelatin synthase are able to synthesize PC derivatives, with a C-terminal amino acid other than glycine, with the only apparent requirement that GSH be the donor of the γ-EC moiety and the modified GSH the acceptor (Oven et al., 2002). On the other hand, homo-glutathione synthetase cloned from pea nodules has a higher catalytic efficiency with β-alanine as a substrate than glycine, but the same enzyme is able to synthesize both GSH and γ-glutamyl-cysteinyl-β-alanine (γ-ECA; Iturbe-Ormaetxe et al., 2002).
It has also been documented that Cd exposure induces protein degradation, leading to an increase in the pool of free amino acids (Wu et al., 2004). It is plausible that after several days of Cd exposure the substrates for the synthesis of GSH derivatives are more available, leading to synthesis of the corresponding modified peptides. Interestingly, mass spectrometry also showed a serine-like GSH and PC homolog, 46 Da heavier than the corresponding hydroxymethyl–GSH and hydroxymethyl–PC2 (Figure 3). These peptides were confirmed to be γ-EC derivatives by tandem mass spectrometry (Figure S2). The tandem mass spectrometry of the m/z 574 peptide showed a neutral loss of 64 units, consistent with a methane sulfenic acid loss (CH3SOH). The only 46 Da modification reported to date corresponds to the addition of methylsulfide (SCH3) to the β-carbon of an aspartic acid (Kowalak and Walsh, 1996). Further investigation is needed to confirm a similar modification in a serine, which in turn would result in β-methylthioserine.
Cadmium impairs iron homeostasis in xylem and phloem sap
Of the heavy metals measured in the xylem and phloem sap, only iron showed a significant decrease in both the phloem and the xylem in response to Cd exposure (Figure 5b and Figure 8d). This decrease was independent of the NA content in the phloem sap. In Arabidopsis, Cd can enter the root through the iron transporter IRT1 (Rogers et al., 2000; Vert et al., 2002), but iron accumulation in B. napus roots was not reduced by Cd (Figure 6). The significant decrease in the iron content of phloem, leaves and xylem (Figure 5b, Figure 6 and Figure 8d) suggests that Cd impairs vascular loading and/or root-to-shoot iron translocation. It is possible that iron loading into the xylem is inhibited by Cd, thus reducing iron accumulation in leaves and iron loading into the phloem. A similar observation was made in maize (Zea mays), where it has been suggested that Cd inhibits the phytosiderophore–Fe root-to-shoot translocation system. It should be noted that Cd inhibited the transport system itself and was not attributed to Cd–phytosiderophore complex formation (Meda et al., 2007). No value has been reported for the dissociation constant of the phytosiderophore–Cd complex. However, 2′-deoxymugineic acid is able to form a 1:1 complex with Cd. This complex is weak, and Cd can be easily replaced by adding equimolar concentrations of iron or zinc (Meda et al., 2007). Thus, phytosiderophores appear not to be good candidates for long-distance transport of Cd in plants.
Distinct functions and mechanisms of phloem and xylem Cd transport
Three findings in this study suggest that phloem is an important route for long-distance source-to-sink transport of Cd in B. napus: (i) Cd was more concentrated in phloem sap (20 µm) than in xylem sap (4 µm; Figure 5a and Figure 8c). (ii) Thiols were more than 50-fold more abundant in phloem sap than in xylem sap samples (Figure 3 and Figure 7a,b) and PCs were almost exclusively located in the phloem (Figure 2 and Figure 7). (iii) The slightly basic pH of phloem sap would allow for greater stability of metal–ligand complexes compared with the xylem, which exhibited an acidic pH. More stable Cd–ligand complexes would reduce the interaction of Cd with other molecules (i.e. proteins, RNA), preventing further toxicity induced by Cd. The role of the phloem in long-distance source-to-sink mobilization of Cd is also supported by a recent study in Arabidopsis, where significant amounts of Cd where found in the cytoplasm of the phloem and companion cells (Van Belleghem et al., 2007). Several studies have also found that Cd can be redistributed from leaves to other parts of the plant including roots, implicating a role of the phloem in Cd transport (Cakmak et al., 2000; Reid et al., 2003). In contrast, only traces of PCs were found in the xylem, which is in agreement with previous reports showing that Cd in the xylem sap is mainly present in unknown complexes that include oxygen- and nitrogen containing compounds (Salt et al., 1995). Moreover, the fact that the ratio of [thiols]/[Cd] in the xylem sap was below 1 (Figure 8) suggests that molecules other than thiols are responsible for the root to shoot translocation of Cd through the xylem sap.
It should be noted that the relative contribution of xylem sap and phloem sap in the Cd partitioning through the plant will be determined not only by the concentration of metabolites (thiols and Cd) but also by the flux through each vascular system. Several studies have pointed out the role of xylem in the root-to-shoot translocation of heavy metals (Kerkeb and Kramer, 2003; Salt et al., 1995). Further, our data correlate with this model, as we found 4 µm Cd in xylem sap samples and the flux through the xylem can be usually expected to be higher than in the phloem. Further, our findings show that transport of Cd through the xylem is not likely to be in the form of thiol–Cd complexes. Once Cd has been transported in the xylem, the phloem functions in the redistribution of Cd to roots and younger leaves (sink-to-source transport) and this transport is highly likely to be as thiol–Cd complexes.
Characterization of the precise structures that PCs, GSH and Cd form in the phloem sap, as well as the identification of transporters capable of loading and unloading thiols, Cd or thiol–Cd complexes into and from the phloem, represent important challenges in the study of the long-distance transport of Cd. Several transporters of different families have been identified as Cd2+ transporters in plants, including CAX, Nramp, P1B-ATPases and ZIP transporters (Mäser et al., 2001; Williams and Mills, 2005), as well as the membrane protein low-affinity cation transporter 1 (LCT1) from wheat (Triticum aestivum) and rice (Oryza sativa; Clemens et al., 1998; rice LCT1 homolog Os06g38120). However, no PC or PC–Cd transporter genes have been identified in plants to date. A member of the Arabidopsis oligopeptide transporter family (AtOPT6) was shown to have the capacity to transport glutathione–Cd complexes in yeast (Saccharomyces cerevisiae; Cagnac et al., 2004). However, this result could not be reproduced using a different yeast expression system or the two-electrode voltage-clamp technique in oocytes (Osawa et al., 2006). Therefore, future work is needed to fully understand the mechanisms by which thiols, cadmium and/or thiol-cadmium complexes are loaded and unloaded from the phloem.
Experimental procedures
Plant growth and sap sampling
Brassica napus (cv. Drakkar, Serasem GIE, la Chapelle d’Armentiers, France) seeds were germinated with tap water and grown in hydroponic culture under controlled conditions, 16-h light/8-h dark, 25.7°C day, 20.7°C night and 55% relative air humidity. The hydroponic solution contained (final concentrations) 0.6 mm NH4NO3, 1 mm Ca(NO3)2, 40 µm Fe-EDTA, 0.5 mm K2HPO4, 0.5 mm K2SO4, 0.4 mm Mg(NO3)2, 0.8 µm ZnSO4, 9 µm MnCl2, 0.1 µm Na2MoO4, 23 µm H3BO3, 0.3 µm CuSO4 and pH was adjusted to 4.7 with H2SO4. After the fifth week an air-pump was used for aeration of the hydroponic solution and the hydroponic solution was changed every week. Plants were grown for 9 weeks before being exposed to 75 µm of CdSO4 for the indicated lengths of time. This Cd concentration was chosen since it is high enough to induce strong PC synthesis but still allows the plants to flower for phloem sampling. Phloem sap was obtained as described previously (Giavalisco et al., 2006). Unless otherwise stated, collected phloem was diluted 1:1 with a solution of 1 mm DTT to avoid thiol oxidation. Xylem sap was obtained by decapitation of the stems (Horie et al., 2006) and the sample was also mixed with a 1 mm DTT solution to allow the thiols to remain in a reduced state. Phloem and xylem samples were collected at the Max Plank Institute, Potsdam, Germany and shipped frozen (−78.5°C) to the University of California, San Diego, USA, for fluorescence HPLC, mass spectrometry and ICP-OES analyses. Concentrations of glucose, fructose and sucrose in phloem sap were determined enzymatically as described by Galtier et al. (1993).
The HPLC analysis and mass spectrometry
Thiols were derivatized using monobromobimane (mBBr) as described previously (Chen et al., 2006) with slight modifications. Ten to 50 µg of protein (phloem sap) or 50–100 µl (xylem sap) were labeled with 3.5 mm of mBBr (final concentration) for 30 min and the reaction was stopped with the addition of perchloric acid 3% final concentration. Protein was precipitated by centrifugation, 5 min at 20 000 g, in a bench microfuge and the supernatant was filtered through 0.45 µm membranes (Millex, Millipore, http:// www.millipore.com/) before HPLC separation. Protein was determined by the Bradford assay (Sigma, http://www.sigmaaldrich.com/) using bovine serum albumin as a standard. Fifty microliters of mBBr-labeled samples were injected into a 4.6 × 250 mm SunFire C18 column, 5 µm particle size (Waters, http://www.waters.com/) and separated with a gradient from 20% to 95% methanol in 0.05% trifluoroacetic acid (TFA). Excitation and emission wavelengths for the mBBr derivatives were 380 and 470 nm, respectively. After column separation 95% of the flow was analyzed with a fluorometer and the remaining 5% was electrosprayed for mass spectrometry analysis in the positive mode using a ThermoFinnigan LCQ Advantage system (Chen et al., 2006). The bimane label accounts for 190 Da on each peptide per thiol labeled. For tandem mass spectrometry, thiol-peptides were separated by HPLC as described above and each thiol was collected independently. After evaporation of the mobile phase, peptides were resuspended in 50% methanol, 1% acetic acid and analyzed on a QSTAR XL ESI Mass Spectrometer (Applied Biosystems, http://www.appliedbiosystems. com/) in the positive mode. All mass spectrometry and fluorescence HPLC experiments were conducted using the UCSD Superfund Mass Spectrometry Facility. Phytochelatin standards PC2, PC3 and PC4 were purchased from Anaspec (http://www.anaspec.com/).
For quantification of thiols derived from proteins in the phloem sap, 5–10 µg of protein were acidified with an equal volume of TFA 0.2% (v/v, pH < 2) containing 10 mm diethylenetriaminepentaacetic acid (DTPA) and mixed vigorously. The protein sample was divided into two and one tube was centrifuged at 20 000 g for 10 min in a bench top centrifuge to precipitate proteins. The resulting supernatant was transferred to a new tube. The pH of the non-centrifuged sample and the supernatant of the centrifuged sample were adjusted to pH 8 by adding 950 µl of 2-amino-2-(hydroxymethyl)-1,3-propanediol (TRIS) 100 mm, pH 8. Total thiols were titrated with 5,5′-dithio-bis-(2-nitrobenzoic acid; DTNB), 1 mm final concentration and the thiols were quantified by measuring the absorbance at 412 nm and using the ∊TNB 412 nm = 13 600 m −1 cm−1 (Ellman, 1959). The difference of thiols between the non-centrifuged sample and the centrifuged sample was considered as the thiol content from proteins in the phloem sap.
Nicotianamine measurements
For NA determination, 10–50 µg of protein (phloem sap) was heated at 80°C for 20 min and then centrifuged at 20 000 g in a bench top microfuge for 10 min and filtered through 0.45 µm membranes (Millex, Millipore) before HPLC separation. One to five microliters of sample was diluted 1:1 with an equal volume of a o-phthaldialdehyde (OPA, Pierce, http://www.piercenet.com/) incubated in the dark for 1 min and the reaction was terminated by the addition of 1 µl of a sulfosalicylic acid 50% (w/v) before injection. The HPLC separation was carried out on the same column described for thiol separation with the mobile phase conditions described by Le Jean et al. (2005). The NA standard was a kind gift from Dr Erin L. Connolly (University of South Carolina, USA).
Heavy metal measurements
For elemental analysis, 10–50 µg of protein (phloem sap) or 50–100 µl (xylem sap) was digested overnight in 70% HNO3, metal trace grade, and then diluted in Milli-Q water (Millipore) to reach 3.5% HNO3 final concentration. Leaves and roots tissue from B. napus were dried in an oven overnight (60°C) and 10–25 mg of dry weight was used for the acid digestion as described above. Roots were harvested directly from the hydroponic culture, therefore iron levels in roots represent the sum of apoplastic and symplastic iron content. Cadmium, iron, zinc and manganese were determined by ICP-OES at the UCSD/Scripps Institution of Oceanography analytical facility.
Supplementary Material
Acknowledgements
We thank the Superfund Mass Spectrometry Core at UCSD for allowing the use of mass spectrometers and fluorescence HPLC instrumentation. We also thank Ina Talke, Max Planck Institute of Molecular Plant Physiology, Potsdam, for help with setting up B. napus growth. This research was supported by NIEHS Superfund grant 1 P42 ESI0337 to JIS and EAK and in part by Department of Energy grant DOE-DE-FG02-03ER15449 and NSF grant IBN-0419695 to JIS. DGMC is recipient of a PEW Latin American fellowship 2006.
Footnotes
Supplementary Material
The following supplementary material is available for this article online:
Figure S1. Tandem mass spectrometry of the GSH-like peptide γ-ECQ.
Figure S2. Tandem mass spectrometry of the ion that is 46 Da heavier than hydroxymethyl glutathione (γ-ECS).
This material is available as part of the online article from http://www.blackwell-synergy.com
Please note: Blackwell publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Cagnac O, Bourbouloux A, Chakrabarty D, Zhang MY, Delrot S. AtOPT6 transports glutathione derivatives and is induced by primisulfuron. Plant Physiol. 2004;135:1378–1387. doi: 10.1104/pp.104.039859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakmak I, Welch RM, Hart J, Norvell WA, Ozturk L, Kochian LV. Uptake and re translocation of leaf-applied cadmium (109Cd) in diploid, tetraploid and hexaploid wheats. J Exp. Bot. 2000;51:221–226. doi: 10.1093/jexbot/51.343.221. [DOI] [PubMed] [Google Scholar]
- Chen A, Komives EA, Schroeder JI. An improved grafting technique for mature Arabidopsis plants demonstrates long-distance shoot-to-root transport of phytochelatins in Arabidopsis. Plant Physiol. 2006;141:108–120. doi: 10.1104/pp.105.072637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Antosiewicz DM, Ward JM, Schachtman DP, Schroeder JI. The plant cDNA LCT1 mediates the uptake of calcium and cadmium in yeast. Proc. Natl Acad. Sci. USA. 1998;95:12043–12048. doi: 10.1073/pnas.95.20.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Kim EJ, Neumann D, Schroeder JI. Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. EMBO J. 1999;18:3325–3333. doi: 10.1093/emboj/18.12.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Thomine S, Schroeder JI. Molecular mechanisms that control plant tolerances to heavy metals and possible roles in manipulating metal accumulation. In: Oksman-Caldentey KM, Barz MH, editors. Plant Biotechnology and Transgenic Plants. New York: Marcel Kekker, Inc.; 2002. pp. 665–691. [Google Scholar]
- Dorcák V, Krezel A. Correlation of acid-base chemistry of phytochelatin PC2 with its ccordination properties towards the toxic metal ion Cd(II) Dalton Trans. 2003;11:2253–2259. [Google Scholar]
- Ellman GL. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Erk M, Raspor B. Evaluation of cadmium-metallothionein stability constants based on voltammetric measurements. Anal. Chim. Acta. 1998;360:189–1943. [Google Scholar]
- Galtier N, Foyer CH, Huber J, Voelker TA. Effects of elevated sucrose-phosphate synthase activity on photosynthesis, assimilate partitioning and growth in tomato (Lycopersicon esculentum var UC82B) Plant Physiol. 1993;101:535–543. doi: 10.1104/pp.101.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P, Langenberger S, Wilke I, Heineke D. Sucrose is metabolised by sucrose synthase and glycolysis within the phloem complex of Ricinus communis L. seedlings. Planta. 1993;190:446–453. [Google Scholar]
- Giavalisco P, Kapitza K, Kolasa A, Buhtz A, Kehr J. Towards the proteome of Brassica napus phloem sap. Proteomics. 2006;6:896–909. doi: 10.1002/pmic.200500155. [DOI] [PubMed] [Google Scholar]
- Gong JM, Lee DA, Schroeder JI. Long-distance root-to-shoot transport of phytochelatins and cadmium in Arabidopsis. Proc. Natl Acad. Sci. USA. 2003;100:10118–10123. doi: 10.1073/pnas.1734072100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill E, Winnacker EL, Zenk MH. Phytochelatins: the principal heavy-metal complexing peptides of higher plants. Science. 1985;230:674–676. doi: 10.1126/science.230.4726.674. [DOI] [PubMed] [Google Scholar]
- Ha SB, Smith AP, Howden R, Dietrich WM, Bugg S, O’Connell MJ, Goldsbrough PB, Cobbett CS. Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell. 1999;11:1153–1164. doi: 10.1105/tpc.11.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Isobe M, Mutoh N, Nakagawa CW, Kawabata M. Cadystins: small metal-binding peptides. Methods Enzymol. 1991;205:348–358. doi: 10.1016/0076-6879(91)05117-e. [DOI] [PubMed] [Google Scholar]
- Horie T, Horie R, Chan WY, Leung HY, Schroeder JI. Calcium regulation of sodium hypersensitivities of sos3 and athkt1 mutants. Plant Cell Physiol. 2006;47:622–633. doi: 10.1093/pcp/pcj029. [DOI] [PubMed] [Google Scholar]
- Iturbe-Ormaetxe I, Heras B, Matamoros MA, Ramos J, Moran JF, Becana M. Cloning and functional characterization of a homoglutathione synthetase from pea nodules. Physiol Plant. 2002;115:69–73. doi: 10.1034/j.1399-3054.2002.1150107.x. [DOI] [PubMed] [Google Scholar]
- Kerkeb L, Kramer U. The role of free histidine in xylem loading of nickel in Alyssum lesbiacum and Brassica juncea. Plant Physiol. 2003;131:16–24. doi: 10.1104/pp102.010686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalak JA, Walsh KA. β-Methylthio-aspartic acid: Identification of a novel posttranslational modification in ribosomal protein S12 from Escherichia coli. Protein Sci. 1996;5:1625–1632. doi: 10.1002/pro.5560050816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H, Sato K, Yamada T, Maitani T. Phytochelatin homologs induced in hairy roots of horseradish. Phytochemistry. 2000;53:239–245. doi: 10.1016/s0031-9422(99)00493-8. [DOI] [PubMed] [Google Scholar]
- Lappartient AG, Touraine B. Demand-driven control of root ATP sulfurylase activity and SO42− uptake in intact canola (The role of phloem-translocated glutathione) Plant Physiol. 1996;111:147–157. doi: 10.1104/pp.111.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasat MM. Phytoextraction of toxic metals: a review of biological mechanisms. J. Environ. Qual. 2002;31:109–120. [PubMed] [Google Scholar]
- Le Jean M, Schikora A, Mari S, Briat JF, Curie C. A loss-of-function mutation in AtYSL1 reveals its role in iron and nicotianamine seed loading. Plant J. 2005;44:769–782. doi: 10.1111/j.1365-313X.2005.02569.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Dankher OP, Carreira L, Smith AP, Meagher RB. The shoot-specific expression of gamma-glutamylcysteine synthetase directs the long-distance transport of thiol-peptides to roots conferring tolerance to mercury and arsenic. Plant Physiol. 2006;141:288–298. doi: 10.1104/pp.105.074815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäser P, Thomine S, Schroeder JI, et al. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 2001;126:1646–1667. doi: 10.1104/pp.126.4.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda AR, Scheuermann EB, Prechsl UE, Erenoglu B, Schaaf G, Hayen H, Weber G, von Wiren N. Iron acquisition by phytosiderophores contributes to cadmium tolerance. Plant Physiol. 2007;143:1761–1773. doi: 10.1104/pp.106.094474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Cózatl D, Loza-Tavera H, Hernandez-Navarro A, Moreno-Sanchez R. Sulfur assimilation and glutathione metabolism under cadmium stress in yeast, protists and plants. FEMS Microbiol. Rev. 2005;29:653–671. doi: 10.1016/j.femsre.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Omichinski JG. Toward methylmercury bioremediation. Science. 2007;317:205–206. doi: 10.1126/science.1145810. [DOI] [PubMed] [Google Scholar]
- Osawa H, Stacey G, Gassmann W. ScOPT1 and AtOPT4 function as proton-coupled oligopeptide transporters with broad but distinct substrate specificities. Biochem. J. 2006;393:267–275. doi: 10.1042/BJ20050920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oven M, Page JE, Zenk MH, Kutchan TM. Molecular characterization of the homo-phytochelatin synthase of soybean Glycine max: relation to phytochelatin synthase. J. Biol. Chem. 2002;277:4747–4754. doi: 10.1074/jbc.M108254200. [DOI] [PubMed] [Google Scholar]
- Pickering IJ, Prince RC, George GN, Rauser WE, Wickramasinghe WA, Watson AA, Dameron CT, Dance IG, Fairlie DP, Salt DE. X-ray absorption spectroscopy of cadmium phytochelatin and model systems. Biochim. Biophys. Acta. 1999;1429:351–364. doi: 10.1016/s0167-4838(98)00242-8. [DOI] [PubMed] [Google Scholar]
- Reid RJ, Dunbar KR, McLaughlin MJ. Cadmium loading into potato tubers: the roles of the periderm, xylem and phloem. Plant Cell Environ. 2003;26:201–206. [Google Scholar]
- Rennenberg H, Schmitz K, Bergmann L. Long-distance transport of sulfur in Nicotiana tabacum. Planta. 1979;147:57–62. doi: 10.1007/BF00384591. [DOI] [PubMed] [Google Scholar]
- Rogers EE, Eide DJ, Guerinot ML. Altered selectivity in an Arabidopsis metal transporter. Proc. Natl Acad. Sci. USA. 2000;97:12356–12360. doi: 10.1073/pnas.210214197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt DE, Prince RC, Pickering IJ, Raskin I. Mechanisms of cadmium mobility and accumulation in indian mustard. Plant Physiol. 1995;109:1427–1433. doi: 10.1104/pp.109.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelp BJ. The composition of phloem exudates and xylem sap from broccoli (Brassica oleracea var. italica) supplied with NH4+, NO−3 or NH4NO3. J. Exp. Bot. 1987;38:1619–1636. [Google Scholar]
- Sillén LG, Martell AE. London: The Chemical Society; Stability Constants of Metal-ion Complexes. 1964:754. Special publication no. 17.
- Van Belleghem F, Cuypers A, Semane B, Smeets K, Vangronsveld J, d’Haen J, Valcke R. Subcellular localization of cadmium in roots and leaves of Arabidopsis thaliana. New Phytol. 2007;173:495–508. doi: 10.1111/j.1469-8137.2006.01940.x. [DOI] [PubMed] [Google Scholar]
- Vatamaniuk OK, Mari S, Lu YP, Rea PA. AtPCS1, a phytochelatin synthase from Arabidopsis: isolation and in vitro reconstitution. Proc. Natl Acad. Sci. USA. 1999;96:7110–7115. doi: 10.1073/pnas.96.12.7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatamaniuk OK, Mari S, Lu YP, Rea PA. Mechanism of heavy metal ion activation of phytochelatin (PC) synthase: blocked thiols are sufficient for PC synthase-catalyzed transpeptidation of glutathione and related thiol peptides. J. Biol. Chem. 2000;275:31451–31459. doi: 10.1074/jbc.M002997200. [DOI] [PubMed] [Google Scholar]
- Vert G, Grotz N, Dedaldechamp F, Gaymard F, Guerinot ML, Briat JF, Curie C. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell. 2002;14:1223–1233. doi: 10.1105/tpc.001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LE, Mills RF. P1B-ATPases – an ancient family of transition metal pumps with diverse functions in plants. Trends Plant Sci. 2005;10:491–502. doi: 10.1016/j.tplants.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Wu FB, Chen F, Wie K, Zhang GP. Effect of cadmium on free amino acid, glutathione and ascorbic acid concentrations in two barley genotypes (Hordeum vulgare L.) differing in cadmium tolerance. Chemosphere. 2004;57:447–454. doi: 10.1016/j.chemosphere.2004.06.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.