Abstract
Objective
To assess the predictive value of baseline measures of impairment, disability, and quality of life for the timing of initiation of symptomatic treatment in early Parkinson disease (PD).
Design
Inception cohort analysis.
Setting
Ambulatory population from multiple sites in the United States and Canada.
Participants
Four hundred thirteen patients with early, untreated PD who participated in 2 double-blind trials that assessed the potential of experimental drugs to serve as disease-modifying agents in PD.
Intervention
Participants were randomized into treatment groups: creatine (n=67), minocycline (n=66), coenzyme Q10(n=71), GPI-1485(n=71), and placebo(n=138).
Main Outcome Measure
Time between baseline assessment and need for the initiation of symptomatic treatment for PD. The following baseline variables were assessed for their relation to the main outcome measure, while adjusting for possible treatment effect: sex; age; level of education; race/ethnicity; disease duration; occupational status; and Unified Parkinson Disease Rating Scale (UPDRS), Medical Outcomes Study Short Form Survey, Modified Rankin Scale, Schwab and England Activities of Daily Living Scale, Total Functional Capacity Scale, 39-item Parkinson Disease Questionnaire, and Geriatric Depression Scale scores. Variables reaching statistical threshold in univariate analyses (α=.15) were entered into a multivariable Cox proportional hazards regression model using time to symptomatic treatment as the dependent variable.
Results
Approximately half (48.5%) of the participants reached end point within 12 months. Higher baseline impairment and disability, as determined by UPDRS III (motor section), UPDRS II (activities of daily living section, participant rating), and Modified Rankin Scale scores and level of education were independently associated with an earlier need for symptomatic treatment.
Conclusions
In early PD, greater impairment and disability and higher level of education are independently associated with an earlier need for symptomatic treatment.
Parkinson disease (PD) IS A chronic progressive neurodegenerative disease that leads to significant morbidity, disability, and increased likelihood of institutionalization.1,2 In view of the potential for short-term and long-term drug complications, symptomatic treatment is customarily delayed in PD until the severity of motor symptoms results in functional impairment.3 Therefore, the initiation of symptomatic treatment is considered an early indicator of disease progression in PD and is used as an important benchmark in clinical trials.4-7 As part of the National Institutes of Health Exploratory Trials in Parkinson Disease (NET-PD), 2 futility drug studies8,9 explored the potential of 4 compounds to serve as disease-modifying agents in early, untreated PD. The primary outcome measure of these studies was change in the total Unified Parkinson Disease Rating Scale (UPDRS) score from baseline to the time at which there was sufficient disability to warrant symptomatic treatment. Participants in these 2 studies were assessed with a number of impairment, disability, and quality of life measures at baseline visit. The usefulness of such measures in the earliest stages of PD is not well understood, and their relationship to functional impairment is unknown.4 In the present exploratory analysis, we examined the relative value of these baseline measures as predictors of the time of symptomatic treatment initiation. We hypothesized that quality of life and emotional factors as well as factors measuring physical disability would be associated with time to initiation of symptomatic therapy.
 CME available online at www.jamaarchivescme.com and questions on page 1055
CME available online at www.jamaarchivescme.com and questions on page 1055
METHODS
PARTICIPANTS
Data from the 413 participants in the 2 NET-PD futility trials (Futility Study 1 [FS-1] and Futility Study 2 [FS-TOO])8,9 were used for this analysis. Both trials were multicenter, randomized, double-blind studies designed to assess the futility of candidate drugs as disease-modifying agents in outpatients with early, untreated PD. Participants were men and women aged 30 years and older with a diagnosis of PD for 5 years or less, who did not require any medications for the treatment of their PD symptoms at the time of study entry. Two of 3 cardinal manifestations of PD (tremor, rigidity, and bradykinesia) were required for the diagnosis; these signs had to be asymmetric. Other potential causes of parkinsonism or prior brain surgery for PD were exclusion criteria. Women of childbearing potential had a negative pregnancy test result at baseline and were required to use adequate birth control for the duration of the study. All participants gave written informed consent. The protocols and consents for the drug trials were approved by a National Institute of Neurological Disorders and Stroke oversight board, an independent data safety monitoring board, and the institutional review boards of the participating sites.
BASELINE ASSESSMENTS
At the baseline visit, demographic data were collected, including sex, age, level of education, race/ethnicity, disease duration (from diagnosis), and (for FS-TOO participants only) occupational status. The UPDRS III (motor section) was administered as a measure of impairment.10 The following scales were administered to measure disability: UPDRS II (activities of daily living [ADL] section; completed by both the investigator and the participant)10; Modified Rankin Scale11; Schwab and England ADL Scale (completed by both the investigator and the participant)12; Total Functional Capacity Scale13; and the ADL Subscale of the 39-item Parkinson Disease Questionnaire (PDQ-39).14 The PDQ-39 (all subscales and the summary index) and the Medical Outcomes Study Short Form Survey (SF-12)15 were administered as indicators of quality of life. The UPDRS I (mentation, behavior, and mood section), completed both by the investigator and participant, and the Geriatric Depression Scale16 were also administered to provide information on mental and cognitive functions at baseline. In calculating the total UPDRS score, only sections completed by the investigator were used.
TREATMENTS
In each of the futility studies, participants were randomized in a 1:1:1 ratio to receive 1 of the 2 active experimental drug treatment arms or placebo. Of the 200 FS-1 participants, 67 received creatine, 66 minocycline, and 67 placebo, while 71 of 213 FS-TOO participants received coenzyme Q10, 71 GPI-1485, and 71 placebo.
END POINT DETERMINATION
The primary end point for the analyses in this study was time to initiation of symptomatic treatment. Symptomatic treatments included levodopa, dopamine agonists, amantadine, anticholinergics, and selegiline. Cases were censored at 12 months of follow-up if they had not reached the end point by that time. Site investigators used their clinical judgment to determine when participants had reached a level of dysfunction sufficient to require symptomatic therapy in any 1 of 3 areas: ambulation, activities of daily living, or occupational status.5,8,9
STATISTICAL ANALYSIS
Baseline variables were screened to assess their relationship with time to the initiation of symptomatic therapy using Cox proportional hazard regression. Regression models adjusting for experimental drug group assignment were used to test the association of each independent variable separately with the primary end point according to the Hosmer and Lemeshow model of development strategy17: any baseline variable associated with the initiation of symptomatic therapy at an α level of .15 was selected for inclusion in multivariable model construction. We adjusted for experimental drug group assignment, because we hypothesized that the drugs studied could potentially impact time to symptomatic treatment. Spearman rank correlations among all pairwise combinations of selected variables were also calculated to assess potential colinearity prior to proceeding with the multivariable model development.
A multivariable Cox proportional hazard regression model with time to initiation of symptomatic treatment as the dependent variable was constructed using the selected variables and experimental drug group. Restricted backwards selection was used to remove baseline variables meeting the exclusion criteria of α greater than .05 while forcing the experimental drug group assignments to remain in the model, as we did not assume absence of an experimental drug effect. Proportional hazard assumptions were checked after each step and at the derivation of the final model. The Harrell concordance index18 was also estimated after the final model as a measure of agreement between the model’s prediction and the observed events: a concordance of 0.5 indicates no agreement between prediction and observation and 1.0 indicates perfect agreement. Kaplan-Meier curves were produced for each of the independent covariates remaining in the final model. The analysis was completed using SAS, version 9.2 (SAS Institute Inc, Cary, North Carolina), and Stata, version 9.2 (Stata Corp, College Station, Texas).
RESULTS
BASELINE DEMOGRAPHICS AND DISEASE CHARACTERISTICS
Table 1 summarizes the baseline demographics of the patient population along with baseline impairment, disability, and quality of life measures. Two hundred of a total sample of 413 participants (48.5%) started symptomatic treatment within 12 months of baseline.
Table 1.
Baseline Variables Classified by End Point
| Variable | Mean (SD) |
P Value | |
|---|---|---|---|
| No Symptomatic Therapya (n=213) |
Symptomatic Therapy Initiatedb (n=200) |
||
| Age, yc | 62.0 (10.7) | 61.3 (10.1) | .77 |
| Education, y | 14.8 (3.3) | 15.7 (3.0) | .01d |
| Time from PD diagnosis, y | 0.7 (0.9) | 0.6 (0.8) | .77 |
| Male sex, % | 60.1 | 68.5 | .12d |
| White race, % | 88.3 | 94.0 | .36 |
| Working full-time, %e | 42.6 | 43.4 | .52 |
| Scale score | |||
| UPDRS III | 14.3 (6.3) | 17.6 (6.8) | <.001d |
| UPDRS I | 1.0 (1.4) | 1.2 (1.3) | .09d |
| UPDRS II | 5.1 (2.9) | 6.9 (3.3) | <.001d |
| UPDRS, total | 20.4 (8.5) | 25.8 (9.1) | <.001d |
| UPDRS I, participant reported | 1.7 (1.7) | 1.6 (1.5) | .97 |
| UPDRS II, participant reported | 5.8 (3.4) | 7.5 (3.8) | <.001d |
| SF-12, Physical Summary | 50.7 (7.4) | 49.3 (7.8) | .01c |
| SF-12, Mental Summary | 51.6 (9.4) | 51.2 (8.7) | .66 |
| Participants with Modified Rankin Scale scores >1, % | 6.6 | 17.0 | <.001d |
| Schwab and England ADL Scale | 94.1 (5.3) | 91.9 (5.1) | <.001d |
| Schwab and England ADL Scale, participant reported | 94.1 (6.5) | 91.9 (6.4) | <.001d |
| TFC | 12.5 (0.9) | 12.2 (1.5) | .01d |
| PDQ-39, Mobility | 8.1 (12.8) | 12.2 (13.9) | <.001d |
| PDQ-39, ADL | 13.1 (14.0) | 19.0 (15.9) | <.001d |
| PDQ-39, Emotional | 19.1 (18.7) | 20.3 (17.5) | .21 |
| PDQ-39, Stigma | 14.1 (18.9) | 17.4 (18.3) | .01d |
| PDQ-39, Social | 8.3 (16.6) | 5.6 (11.8) | .35 |
| PDQ-39, Cognition | 17.0 (17.9) | 17.1 (16.5) | .93 |
| PDQ-39, Communication | 12.3 (18.5) | 14.9 (18.5) | .08d |
| PDQ-39, Discomfort | 26.0 (22.1) | 26.4 (20.5) | .65 |
| PDQ-39, summary index | 14.8 (12.2) | 16.8 (11.1) | .01d |
| GDS | 2.0 (2.4) | 2.3 (2.7) | .11d |
Abbreviations: ADL, activities of daily living; GDS, Geriatric Depression Scale; PD, Parkinson disease; PDQ-39, 39-item Parkinson Disease Questionnaire; SF-12, Medical Outcomes Study Short Form Survey; TFC, Total Functional Capacity Scale; UPDRS, Unified Parkinson Disease Rating Scale.
End point not reached.
End point reached.
Ages older than 89 years were collapsed to 90 years.
Variable reaching threshold for inclusion in the multivariable analyses.
Measured only in Futility Study 2 (N=200).
DEMOGRAPHICS, IMPAIRMENT, DISABILITY, AND TIME TO END POINT
In the exploratory screening phase of the analyses, greater baseline impairment and disability, lower quality of life, and higher education level were associated with earlier need for symptomatic treatment (Table 1). More specifically, the following variables met criteria and were selected for inclusion in the multivariable model: level of education; sex; and UPDRS I, UPDRS II, UPDRS II (participant report), UPDRS III, total UPDRS, SF-12 (Physical Summary Subscale), Modified Rankin Scale (stratified as ≤1 vs >1), Schwab and England ADL Scale (investigator and participant reports), Total Functional Capacity Scale, PDQ-39 (summary index and Mobility, ADL, Stigma, and Communication Subscales), and Geriatric Depression Scale scores. An association was not detected for age; disease duration (from diagnosis); race/ethnicity; UPDRS I (participant report); SF-12 (Mental Summary Subscale); the Emotional, Social, Cognitive, or Discomfort subscales of the PDQ-39; or occupational status; these latter variables were excluded from the multivariable analyses.
Strong correlations were identified between total UPDRS and UPDRS II and III scores, with Spearman correlation coefficients of 0.75 for section II and 0.92 for section III. The correlation coefficient between the PDQ-39 Mobility Subscale and the PDQ-39 summary index was 0.70. Pairwise correlations between the subscale scores were weak; therefore, the UPDRS section scores and PDQ-39 subscale scores were retained, while the total UPDRS score and PDQ-39 summary index were excluded from subsequent consideration in the multivariable model. Despite a strong correlation between the investigator- and participant-reported UPDRS II scores (correlation coefficient, 0.80), both variables were retained in the multivariable model, as the data were derived from different sources (investigator vs participant).
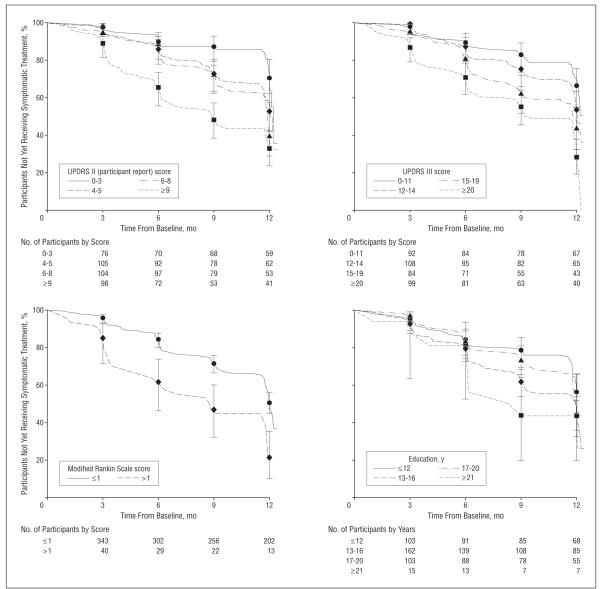
The final model, based on completion of the restricted backward selection of variables, adjusted for experimental drug assignment and included education and UPDRS III, UPDRS II (participant report), and Modified Rankin Scale scores (Table 2). Eight participants (1.9%) were excluded from this analysis owing to incomplete data. This model was confirmed to produce the largest overall Pearson χ2 statistic of other competing models. The Harrell concordance index was 0.665. Proportional hazard assumptions were satisfied at every backward step and the final model. Kaplan-Meier curves of the final 4 covariates are shown in the Figure.
Table 2.
Final Cox Regression Model (n=405)a
| Parameter | HR (95% CI) | P Value |
|---|---|---|
| Education, 4-year increment | 1.38 (1.16-1.65) | <.001 |
| UPDRS III, 1-point increment | 1.04 (1.01-1.06) | .001 |
| UPDRS II, participant reported, 1-point increment |
1.08 (1.04-1.12) | <.001 |
| Modified Rankin Scale, >1 vs ≤1 | 1.73 (1.15-2.61) | .009 |
Abbreviations: CI, confidence interval; HR, hazard ratio; UPDRS, Unified Parkinson Disease Rating Scale.
Adjusted for experimental drug assignment, P=.78.
Figure.
Kaplan-Meier curves of time to symptomatic therapy initiation by baseline variables in the final Cox model. Number of participants in each group remaining in the study are indicated beneath the graphs. Error bars represent 95% confidence intervals; UPDRS, Unified Parkinson Disease Rating Scale.
COMMENT
In the 2 NET-PD futility trials,8,9 a greater degree of impairment and disability at baseline was associated with an earlier need for symptomatic treatment. This finding was consistent across the 5 experimental drug assignment groups. Among the various measures of impairment and disability studied, scores on UPDRS III (motor), UPDRS II (ADL, completed by the participant), and the Modified Rankin Scale were independently linked to time to symptomatic treatment. None of the quality of life measures were retained in the final model. Among demographic characteristics, higher level of education was independently associated with earlier symptomatic treatment. The disease characteristics of PD among the NET-PD futility trial participants were similar to those of participants in previous studies of putative disease-modifying agents, and they were typical of early PD.5,6,19
Although only 3 of the impairment and disability measures used in the NET-PD futility trials showed strong, independent correlations with time to end point in a multivariable model, all such measures obtained at baseline as well as the quality of life measures used (ie, total UPDRS, Modified Rankin Scale, Schwab and England ADL Scale, Total Functional Capacity Scale, SF-12 Physical Summary Subscale, and PDQ-39 summary index) tracked well with time to end point in the exploratory univariate analyses. Scores on subscales related to motor functioning, such as UPDRS III, UPDRS II, UPDRS II (participant report), SF-12 Physical Summary Subscale, PDQ-39 Mobility Subscale, and PDQ-39 ADL Subscale, showed significant associations with time to end point. However, subscales associated with cognitive, psychiatric, and social functioning, including UPDRS I, UPDRS I (participant report), SF-12 Mental Summary Subscale, and the Emotional, Social, Cognitive, Communications, and Discomfort Subscales of the PDQ-39 were not associated with the end point, with the exception of the PDQ-39 Stigma Subscale. This dichotomy suggests that motor dysfunction is the most prominent baseline determinant of how soon the need for symptomatic treatment may arise.
Similar to the 2 NET-PD futility studies, data from the Deprenyl and Tocopherol Antioxidant Therapy of Parkinsonism (DATATOP) Study19 have been analyzed using time to symptomatic treatment as a primary end point.20 Analyses of baseline variables among participants in DATATOP have also identified greater disability (UPDRS II score, Schwab and England ADL Scale score, and self-reported difficulty with ADL and domestic capacity) and worse motor function (total, motor, and tremor, rigidity, postural stability, and bradykinesia scores on the UPDRS; perceived threat to gait and balance; Purdue Pegboard Test result; and Hoehn and Yahr stage) as significant predictors of earlier need for symptomatic treatment in early PD.20 Contrary to DATATOP, we found no association between participant-reported UPDRS I scores and only a statistically nonsignificant trend between higher investigator UPDRS I scores and shorter time to end point. This apparent discrepancy may be a consequence of the different statistical treatment of this variable (it was treated as dichotomous in the DATATOP analyses), or it might reflect the somewhat less advanced disease state among the NET-PD futility trial participants than in the DATATOP participants.8,9,19
While both impairment and disability assessments were predictive of the timing of symptomatic treatment, we found no significant correlations between UPDRS III (the impairment measure used in this analysis) and the disability measures. It is likely that this paradox arises from the relative insensitivity of disability measures in early PD. To illustrate the point, only 11% of our sample population had a Modified Rankin Scale score greater than 1, and the median Schwab and England ADL Scale score was 95%. This resulted in heavily skewed distributions of the disability ratings, which failed to attain robust correlations with the more evenly distributed impairment measure (UPDRS III).
The only PDQ-39 subscale that correlated highly with the PDQ-39 summary index was the Mobility Subscale, suggesting that mobility is the most important determinant of quality of life in patients with early PD as measured by this scale. The quality of life measures related to mobility, ADL, and stigma showed associations with time to end point, while the emotional, social, cognition, communications, and discomfort subscales did not. Stigma has been reported as an important determinant of quality of life, particularly among patients with younger-onset PD.21 In the multivariable model, motor impairment and disability were retained, while stigma and depression were not. This suggests that stigma is more likely related to motor impairment, rather than independent emotional or psychiatric factors.
The relatively lesser role of emotional factors at baseline in determining the need for symptomatic treatment is supported by the absence of a significant association between baseline Geriatric Depression Scale score and time to symptomatic treatment. However, a recent analysis of the same data set22 found that a higher degree of depression (when Geriatric Depression Scale score was treated as a time-dependent variable) was a significant predictor of earlier symptomatic treatment. The prior analysis assessed the impact of increasing depression, rather than the severity of depression at baseline, on the same outcome. Taken together, the results of these 2 analyses suggest that the severity of depression at baseline has no impact on the timing of the need for symptomatic therapy; however, a subsequent increase in depressive symptoms is associated with earlier need for symptomatic treatment. Given the lack of association between baseline Geriatric Depression Scale scores and time to symptomatic treatment in the present analysis and the strong association between Geriatric Depression Scale score and UPDRS II score progression found in the previous analysis,22 an alternative interpretation is that both the increasing severity of depression and an earlier decision to initiate symptomatic treatment are the parallel consequences of increasing disability, rather than increasing depression solely driving the decision to initiate symptomatic treatment. An interesting observation stemming from the comparison of the 2 analyses is that while some measures may be more informative at baseline, others may be more useful in monitoring disease activity during follow-up.
The level of education was the only demographic feature that was associated with time to initiation of symptomatic treatment, with higher education correlating with earlier symptomatic treatment. Such an association was not evident in the earlier analysis of the DATATOP data set.20 This discrepancy may be due to the variable being statistically treated as dichotomous in the earlier study. A possible explanation is that higher education may be associated with greater occupational demands and an increased need for symptomatic control. However, one might expect that occupations placing higher demands on physical abilities (usually associated with lower education levels) would be associated with a more pressing need for symptomatic control. An alternate possibility is that patients with higher education are likely to be better advocates for their health care needs and play a more active role in medical decision-making. The results of this study are consistent with the association between higher education and increased use of health care services previously found in PD2 and indicate that education level has a complex relationship with PD outcomes.
Although we found no association between disease duration at baseline and time to symptomatic treatment initiation, time from symptom onset longer than 2 years was reported as a significant predictor of earlier treatment in the DATATOP study.20 The variable used in our study is not directly comparable with that from the DATATOP study, as we analyzed duration from disease diagnosis. Sex and employment status were not statistically significant in either study.
In conclusion, in this early stage of the disease, baseline measures associated with earlier introduction of symptomatic impairment included UPDRS III (motor), UPDRS II (ADL, participant rating), and Modified Rankin Scale scores and education. Emotional, psychiatric, and quality of life factors at baseline played a smaller role in determining need for symptomatic treatment and did not enter the final model when baseline impairment, disability, and education were taken into account. The impact of the patient’s education level on clinical management is an unexpected finding and merits further investigation.
Acknowledgments
Funding/Support: This study was supported by the National Institute of Neurological Disorders and Stroke, National Institutes of Health.
Additional Contributions: The authors acknowledge the NET-PD investigators, NET-PD study sites, and NET-PD funding sources for providing the data that are analyzed in this study.
Footnotes
Financial Disclosure: None reported.
REFERENCES
- 1.Suchowersky O, Reich S, Perlmutter J, Zesiewicz T, Gronseth G, Weiner W. Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: diagnosis and prognosis of new onset Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66(7):968–975. doi: 10.1212/01.wnl.0000215437.80053.d0. [DOI] [PubMed] [Google Scholar]
- 2.Parashos SA, Maraganore DM, O’Brien PC, Rocca WA. Medical services utilization and prognosis in Parkinson disease: a population-based study. Mayo Clin Proc. 2002;77(9):918–925. doi: 10.4065/77.9.918. [DOI] [PubMed] [Google Scholar]
- 3.Miyasaki JM, Martin W, Suchowersky O, Weiner WJ, Lang AE. Practice parameter: initiation of treatment for Parkinson’s disease: an evidence-based review: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2002;58(1):11–17. doi: 10.1212/wnl.58.1.11. [DOI] [PubMed] [Google Scholar]
- 4.Elm JJ, Goetz CG, Ravina B, et al. NET-PD Investigators. A responsive outcome for Parkinson’s disease neuroprotection futility studies. Ann Neurol. 2005;57(2):197–203. doi: 10.1002/ana.20361. [DOI] [PubMed] [Google Scholar]
- 5.Parkinson Study Group DATATOP: a multicenter controlled clinical trial in early Parkinson’s disease. Arch Neurol. 1989;46(10):1052–1060. doi: 10.1001/archneur.1989.00520460028009. [DOI] [PubMed] [Google Scholar]
- 6.Shults CW, Oakes D, Kieburtz K, et al. Parkinson Study Group Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol. 2002;59(10):1541–1550. doi: 10.1001/archneur.59.10.1541. [DOI] [PubMed] [Google Scholar]
- 7.LeWitt P, Oakes D, Cui L, Parkinson Study Group The need for levodopa as an end point of Parkinson’s disease progression in a clinical trial of selegiline and a-tocopherol. Mov Disord. 1997;12(2):183–189. doi: 10.1002/mds.870120208. [DOI] [PubMed] [Google Scholar]
- 8.NINDS NET-PD Investigators A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology. 2006;66(5):664–671. doi: 10.1212/01.wnl.0000201252.57661.e1. [DOI] [PubMed] [Google Scholar]
- 9.NINDS NET-PD Investigators A randomized clinical trial of coenzyme Q10 and GPI-1485 in early Parkinson’s disease. Neurology. 2007;68(1):20–28. doi: 10.1212/01.wnl.0000250355.28474.8e. [DOI] [PubMed] [Google Scholar]
- 10.Fahn S, Elton RL, Members of the UPDRS Development Committee . Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CO, Calne DB, Goldstein M, editors. Recent Development in Parkinson’s Disease. Macmillan Health Care Information; Florham Park, NJ: 1987. pp. 153–164. [Google Scholar]
- 11.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interob-server agreement for the assessment of handicap stroke patients. Stroke. 1988;19(5):604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 12.Schwab RS, England ACJ. Projection technique for evaluating surgery in Parkinson’s disease. In: Gillingham FJ, Donaldson IML, editors. Third Symposium on Parkinson’s Disease. E & S Livingstone; Edinburgh, Scotland: 1969. pp. 152–157. [Google Scholar]
- 13.Shoulson I, Kurlan R, Rubin A, et al. Assessment of functional capacity in neuro-degenerative disorders: Huntington’s disease as a prototype. In: Munsat TL, editor. Quantification of Neurologic Deficit. Butterworth; Boston, MA: 1989. pp. 271–283. [Google Scholar]
- 14.Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson’s Disease Questionnaire (PDQ-39): development and validation of a Parkinson’s disease summary index score. Age Ageing. 1997;26(5):353–357. doi: 10.1093/ageing/26.5.353. [DOI] [PubMed] [Google Scholar]
- 15.Ware JE, Jr, Kosinski M, Turner-Bowker DM, Gandek B. How to Score Version 2 of the SF-12 Health Survey (With a Supplement Documenting Version 1) QualityMetric Inc; Lincoln, RI: 2002. [Google Scholar]
- 16.Meara J, Mitchelmore E, Hobson P. Use of the GDS-15 geriatric depression scale as a screening instrument for depressive symptomatology in patients with Parkinson’s disease and their careers in the community. Age Ageing. 1999;28(1):35–38. doi: 10.1093/ageing/28.1.35. [DOI] [PubMed] [Google Scholar]
- 17.Hosmer DW, Lemeshow S. Applied Logistic Regression. Wiley; New York, NY: 1989. [Google Scholar]
- 18.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 19.Parkinson Study Group Effect of deprenyl on the progression of disability in early Parkinson’s disease. N Engl J Med. 1989;321(20):1364–1371. doi: 10.1056/NEJM198911163212004. [DOI] [PubMed] [Google Scholar]
- 20.McDermott MP, Jankovic J, Carter J, et al. The Parkinson Study Group Factors predictive of the need for levodopa therapy in early, untreated Parkinson’s disease. Arch Neurol. 1995;52(6):565–570. doi: 10.1001/archneur.1995.00540300037010. [DOI] [PubMed] [Google Scholar]
- 21.Schrag A, Hovris A, Morley D, Quinn N, Jahanshahi M. Young- versus older-onset Parkinson’s disease: impact of disease and psychosocial consequences. Mov Disord. 2003;18(11):1250–1256. doi: 10.1002/mds.10527. [DOI] [PubMed] [Google Scholar]
- 22.Ravina B, Camicioli R, Como PG, et al. The impact of depressive symptoms in early Parkinson disease. Neurology. 2007;69(4):342–347. doi: 10.1212/01.wnl.0000268695.63392.10. [DOI] [PMC free article] [PubMed] [Google Scholar]