Abstract
Photoreceptor cells maintain a roughly constant length by continuously generating new outer segments from their base while simultaneously releasing mature outer segments engulfed by the retinal pigment epithelium (RPE). Thus post-mitotic RPE cells phagocytose an immense amount of material over a lifetime, disposing of photoreceptor cell waste while retaining useful content. This review focuses on current knowledge of outer segment phagocytosis, discussing the steps involved along with their critical participants as well as how various perturbations in outer segment (OS) disposal can lead to retinopathies.
Diseases of the retina rob millions of their eyesight each year with macular degeneration (AMD) representing the leading cause of age-related blindness in the developed world. Retinal health requires important interactions between photoreceptor cells, responsible for collecting and processing visual stimuli, and the retinal pigment epithelium (RPE), a monolayer of cells lying at the boundary between the eye and the bloodstream. This review will examine current progress made in understanding this important interaction.
The Vertebrate Eye
The vertebrate eye represents such a sublime example of evolution that even Darwin had difficulty positing its emergence. Exhaustive studies of vision across a wide range of species has shown that the modern vertebrate eye is strikingly similar to that present in the last common ancestor of all jawed vertebrates ~400 million years ago. This conservation is also evident at the molecular level where PAX6 appears to act as a global regulator of eye development (34, 74), reinforcing the great importance of vision as a selective advantage.
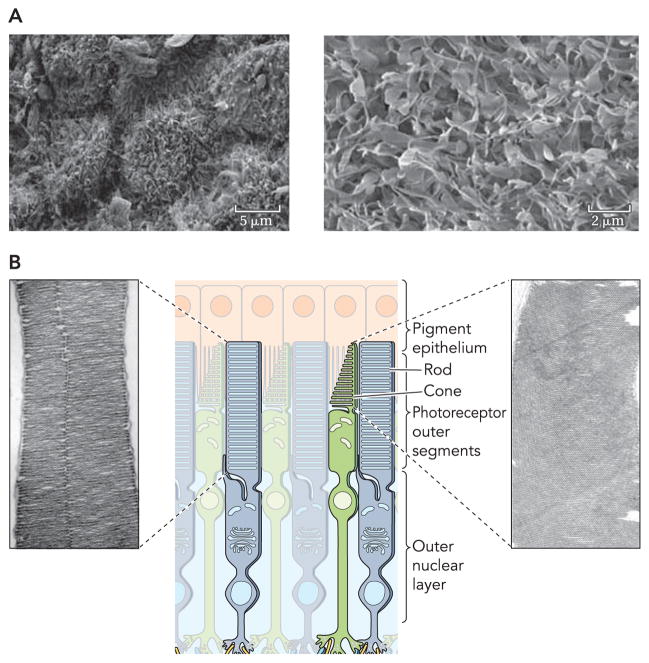
The process of vision begins with collection of light by photoreceptor cells in the retina. Photoreceptor cells are comprised of two types: rods that govern vision in low-light settings and cones that collect photons in ambient light and discern color differences. Rod cells are exquisitely sensitive to light but have a low photon saturation threshold, whereas cones have an extremely high saturation threshold but a relatively low sensitivity. Each rod and cone cell consists of four distinct components: a synaptic terminal, an OS, an inner segment, and a cilium connecting the outer and inner segments (FIGURE 1) (60). The OS envelops the photopigments responsible for photon absorption. Rod OS (ROS) are made up of a stack of individualized discs unconnected to the ciliary plasma membrane, whereas cone OS (COS) differ by having a series of invaginations continuously connected to the cilium plasma membrane (FIGURE 1) (60). Light perception within the OS is achieved by pigments, opsins, which are G protein-coupled receptors that require a bound chromophore to absorb photons (71). The chromophore, 11-cis-retinal, is a derivative of β-carotene, a vitamin A analog entirely derived from the animal’s diet.
FIGURE 1. Detailed representation of interactions between photoreceptor outer segments and RPE cells.
A: high-resolution electron micrograph of RPE cells (left) with a higher resolution image of microvilli on the apical RPE surface (right). Scale bars represent 5 and 2 μm in the low- and high-resolution images. B: graphical representation of rod and cone structures accompanied by EM images of rod and cone outer segments that display the bands and invaginations of these photoreceptors (adapted from Ref. 60).
RPE
The RPE is a monolayer of cells that performs many functions vital for retinal preservation (8, 59, 81). The apical membrane of the RPE lies adjacent to the OS of photoreceptor cells, whereas the basolateral membrane contacts Bruch’s membrane (57).
The retina is exposed to high levels of light throughout the day, which, if unabated, can lead to damaging photo-oxidative reactions and ultimately to retinal degeneration. The RPE has evolved several strategies to prevent these noxious effects. Light filtration by RPE pigments functions as a preventative mechanism against photo-oxidation, high levels of antioxidants expedite either enzymatic or non-enzymatic removal of resulting chemically reactive species, and RPE cells also can repair considerable damage to DNA, proteins, and lipids.
In addition to its role as a protective barrier, the RPE transports nutrients and ions between the retina and the choriocapillaris. The RPE conveys new retinoids from the bloodstream and is absolutely required for the regeneration of 11-cis-retinal in a process referred to as the “visual cycle” (78, 83). Upon absorption of a photon, 11-cis-retinal, bound as a chromophore to a light-absorbing pigment, is converted to all-trans-retinal, changing the pigment to an “active” state (61, 69). Through a number of spatially distinct steps, all-trans-retinal is then converted back to 11-cis-retinal for use in another transduction cycle (84).
Proper functioning of photoreceptor cells requires a delicate balance of proteins, lipids, and metabolites, which, if disturbed, can lead to retinal degeneration. To prevent the toxic effects of accumulated photo-oxidative products, photoreceptor cells undergo a daily renewal process wherein ~10% of their volume is shed and subsequently phagocytosed by adjacent RPE cells. To maintain a relatively constant length, the photoreceptor OS basally regenerates roughly the same volume of cellular material each day. It has been estimated that each RPE cell phagocytoses hundreds of thousands of OS discs over a human lifetime. Because RPE cells are postmitotic, they must efficiently dispose of this material daily or suffer the damaging effects of OS component build-up. This review presents our present understanding of how the RPE functions as one of the most active phagocytic cell groups in the body and how perturbations in this important function can result in degenerative eye disease.
Photoreceptor Renewal
Species and environmental control of photoreceptor cell renewal
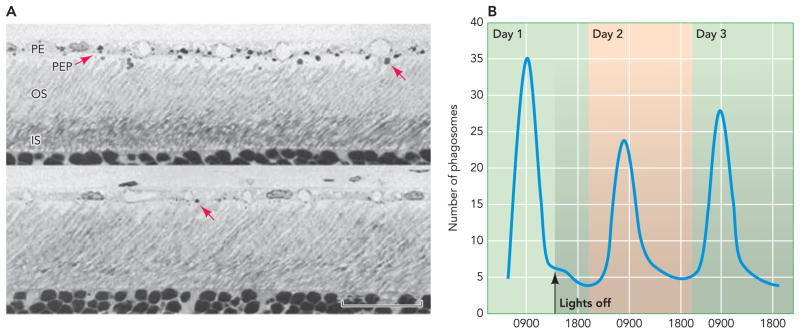
Although daily renewal of OS is required for proper retinal function in all species studied, differences do occur as species have evolved and adjusted to their photopic environments. Elegant experiments performed by Young and colleagues in the late 1960s presented the first evidence of ROS renewal (90, 92). Pulse-chase experiments with radioactive amino acids showed a discrete band of radioactive material that moved over time from the basal end of the OS and eventually was shed from the distal end (FIGURE 2). These experiments suggested that the densely packed discs within the ROS are constantly being produced and shed from the distal end of photoreceptors (91). Studies in mammals, fish, and amphibians demonstrated a highly conserved mechanism for ROS renewal (27, 51, 68, 87, 88). Temperature and light were shown to modulate the rate at which OS shedding occurred, with higher temperatures and light intensities resulting in increased shedding (5, 6). These findings led to hypotheses about possible circadian regulation of OS shedding. LaVail demonstrated that albino rats entrained to a 12-h day/night schedule displayed a burst of OS shedding starting shortly after the onset of light. This cycling continued after the animals were moved to complete darkness (FIGURE 3) (53). With only slight differences, this circadian control has been demonstrated in several other species with the exception of some species of frogs (3).
FIGURE 2. Photoreceptor renewal.
A: radioautographs of photoreceptor cells showing progression of a radioactive protein band during renewal. Numbered panels display time after injections: 0.5 h (2), 2 days (3), 3 days (4), 4 days (5), 1 wk (6), and 9 days (7). Arrow in panel 7 is pointing to a shed packet of outer segment. B: representation of radioactive band progression during photoreceptor renewal (adopted from Ref. 90).
FIGURE 3. Circadian shedding of photoreceptor outer segments.
A: light micrographs of rat retinas taken at different times of the day: a. 1.75 h after light onset (a) and 9 h after light onset (b). Arrows in a and b point to phagosomes stained with toluidine blue. B: counts of phagosomes throughout a day and for two days after lights were turned off. Shaded area represents time when lights are off (adapted from Ref. 52). PE, pigmented epithelium; PEP, pigmented epithelial cell processes; OS, outer segment; IS, inner segment.
Initial pulse-chase experiments showed a distinct difference between rods and cones. Administration of a radioactive amino acid failed to create a discrete band of radioactive material in cones, instead producing diffuse labeling throughout the COS (89). This finding was interpreted as evidence that cones did not shed OS as rods do. Ultrastructural studies of COS demonstrated that disks remain in contact with the plasma membrane throughout the entire OS. Thus COS are morphologically distinct from ROS with disks that are separated from the plasma membrane and from each other, providing an explanation for the diffuse pattern of radioactive labeling seen in COS (9). Discovery of a cone-rich diurnal squirrel species presented an excellent opportunity to determine the shedding capability of COS (1, 55). COS shedding occurred similarly to ROS shedding except that the burst of shedding occurred when lights were turned off (55). Subsequently, it was shown that COS were shed in a number of other species by what is generally believed to be a conserved mechanism (2, 80).
OS phagocytosis
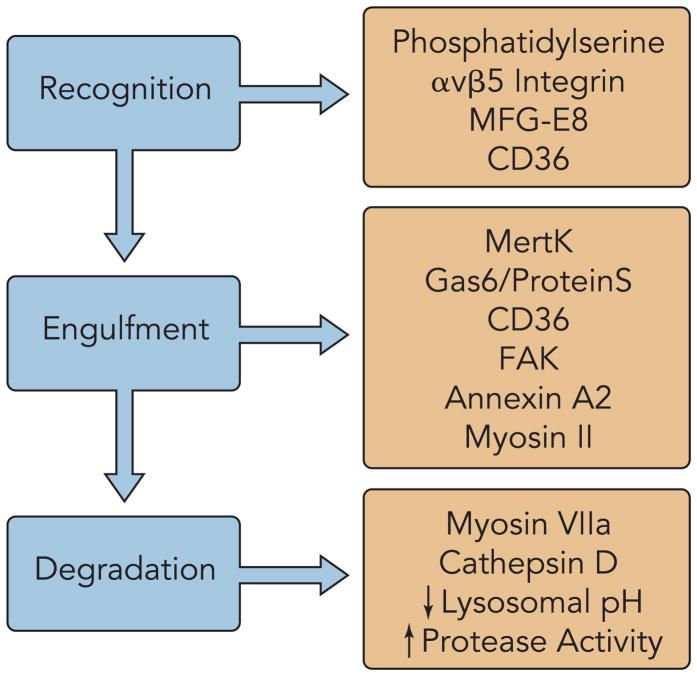
Phagocytosis of bacterial or apoptotic cells by professional macrophages occurs by a complex mechanism that requires a diverse set of components (28) (FIGURE 4). This process is dictated by a number of temporally distinct steps, including recognition, binding, intracellular signaling, internalization, and ultimate digestion. Similarly, phagocytosis of shed OS by RPE cells requires its own set of machinery, although it borrows some of the components involved in general phagocytosis. With the development of modern molecular and biochemical techniques, including polarized RPE cell cultures, many of the important players in OS phagocytosis have been identified.
FIGURE 4. Photoreceptor phagocytosis components.
Components are categorized by their involvement in photoreceptor recognition, engulfment, and degradation separated by their involvement in different stages of the renewal process.
OS recognition and binding
A diffusible substance that facilitates communication between OS and RPE cells to regulate phagocytosis of shed OS has not been definitively identified. That said, our understanding of how professional macrophages recognize possible substrates allows some insights into how RPE cells may recognize OS. In most cells, components of the plasma membrane lipid bilayer have an asymmetric distribution, with phospholipids containing terminal primary amino groups, such as phosphatidylserine (PS) and phosphatidylethanolamine (PE), localized to the cytoplasmic surface (20, 86). During apoptosis, this asymmetry is disrupted, and PS, exposed extracellularly in patches, is thought to act as an “eat-me” signal (19). Analysis of the lipid content of OS plasma membranes indicated relatively high levels of the anionic phospholipids, PS, and phosphatidylcholine (PC) (7). PS-containing liposomes can bind to fresh RPE cells, whereas PC- and PE-containing liposomes cannot (77). These findings suggest that RPE cell recognition of shed OS may occur by a mechanism similar to that involved in apoptotic phagocytosis.
Once a macrophage recognizes a substrate, it binds the latter, often through a receptor-based mechanism. Significant work involving general macrophages has defined a role for the vitronectin receptors, αvβ3 and αvβ5, and the scavenger receptor CD36 in substrate-binding by macrophages. Work by Finnemann and colleagues definitively showed that αvβ5 is required for OS binding to the RPE (24, 26, 65). Using αvβ5 antibodies, these investigators demonstrated that inhibition of αvβ5 function led to decreased binding of shed OS but had no effect on phagocytosis of OS already bound to the RPE (24). Mice without the β5 subunit were shown to lack the synchronized phagocytosis exhibited by wild-type mice while still retaining basal uptake levels. Loss of β5 ultimately resulted in age-related blindness due to inefficient phagocytosis and accumulation of autofluorescent storage bodies in the RPE (65). Indeed, αvβ5 has also been shown to complex with the tetraspanin CD81, an interaction that promotes particle binding (13).
Although αvβ3 and αvβ5 have been demonstrated to be instrumental for substrate binding, they do not bind substrate directly. Rather they bind an opsonin that recognizes the eat-me signal on their phagocytic targets. Using a fishing technique to identify proteins involved in macrophage recognition, milk fat globule-EGF-factor 8 (MFG-E8) was found to be capable of binding both PS and αvβ3 in cell cultures (41). MFG-E8 is a heavily glycosylated protein that contains an NH2-terminal signal peptide, two EGF-like repeat domains, with the second including an RGD-integrin binding motif, followed by two discoidin domains likely to be involved in binding PS. Although αvβ5 had already been shown to be important for OS binding, its cognate ligand in the RPE had not been identified. Proteomic analysis of RPE cells confirmed the presence of MFG-E8 and revealed that its diurnal expression peaked shortly after light onset. Mice lacking MFG-E8 appeared to resemble β5−/− mice, with loss of the synchronized burst of phagocytosis upon light exposure while retaining a basal level of phagocytosis (41, 64). This work confirms an essential role for MFG-E8 as a ligand for αvβ5 during RPE phagocytosis.
CD36 is a class B scavenger receptor that participates in a number of phagocytic mechanisms and recognizes a diverse set of ligands (21). In the search for receptors mediating recognition of OS, it was shown that CD36 is expressed in both human and rat RPE cells (77). Inhibition of receptor function by CD36 antibodies resulted in decreased ROS binding and internalization by RPE cells in culture. One of the many substrates demonstrated for CD36 binding is PS, which CD36 binds directly (32). Antibody-mediated inhibition of CD36 resulted in decreased binding to PS-containing liposomes, providing evidence that CD36 recognition of OS could result from direct binding to exposed PS (76). Although a number of important players in OS particle recognition and binding have been identified, other proteins regulating this activity probably exist as well.
RPE signaling leading to engulfment
Recognition and binding represent the first obligatory steps before phagocytosis, but binding per se is not sufficient to stimulate phagocytosis of shed OS. This concept was confirmed by studying the causative agent for the retinal dystrophy found in the Royal College of Surgeons (RCS) rat line. Adult retinas of this line show complete atrophy of the RPE and photoreceptor layers. Their RPE cells could bind shed OS at wild-type levels but were unable to internalize the bound particles (12). Interestingly, RCS RPE cells were fully capable of internalizing nonspecific particles, suggesting that at least two distinct phagocytitic pathways exist within the RPE. D’Cruz et al. used a positional cloning strategy to identify the gene responsible for the RCS phenotype and isolated the receptor tyrosine kinase MertK (15). To further emphasize the role of this receptor, three mutations in human MertK were shown to result in retinal dystrophies (29). Transfer of MertK to RCS RPE cells by a recombinant adenovirus resulted in complete rescue of the RCS phenotype, providing biochemical evidence to support its role in RPE phagocytosis (22). As with avβ5, it is not believed that MertK recognizes its target directly but uses a bridge protein ligand instead. Ligands for MertK are Gas6 and Protein S, which are homologous members of a family of K+-dependent proteins characterized by posttranslational γ-carboxylation of glutamic acid residues (38, 40, 62, 73). These γ-carboxylated residues have been shown to bind PS and are required for MertK activation of particle engulfment (39, 50, 63). Currently, there is a debate as to whether Gas6 is required for MertK-induced engulfment of shed OS. A gas6−/− mouse line showed no evidence of RPE phagocytosis disruption even though biochemical data indicate it can induce a MertK-mediated engulfment (38, 73).
A shared role for all receptors discussed so far is to recognize the presence of OS at the apical membrane of the RPE. Because the receptors themselves do not physically move OS into the RPE, they must be involved in a signaling pathway that results in the reorganization of the RPE plasma membrane and engulfment of OS. Phosphorylation is a common first step in a myriad of signaling pathways. Focal adhesion kinase (FAK) is a non-receptor tyrosine kinase that resides at sites of integrin clustering known as focal adhesions. FAK also is essential for the engulfment of OS during RPE phagocytosis (23). OS binding to the avβ5 receptor mobilizes a pool of FAK to the apical RPE and causes an autophosphorylation event. This activation of FAK ultimately results in phosphorylation of MertK, confirmed by probing RPE proteins with phosphotyrosine MertK antibodies after challenging RPE with OS (23). Activation of MertK leads to an increase in InsP3, which does not occur in RPE cells lacking MertK. Although phagocytosis of OS increased InsP3 production, its role in regulating engulfment is poorly understood (46, 75). Exposure of RPE cells to OS resulted in an increased number of phosphorylated proteins, a response that was aberrant in RCS rats (47). In addition to the kinases MertK and FAK, phosphorylation of the tyrosine kinase Src also increased in response to OS challenge (54). Pharmacological increases in intracellular free Ca2+ and protein kinase C resulted in a reduced ingestion of OS, but how these players directly affect this process is ill defined (37). The second messenger cAMP acts as an important modulator of phagocytotic activity downstream of initial signaling by InsP3/Ca2+ (18, 36). An increase in intracellular cAMP reduces phagocytotic activity; cAMP shuts off OS phagocytosis by stimulating both β-adrenergic and A2 adenosine receptors (33, 36). Regulation of both InsP3/Ca2+ and cAMP by serotonin through PKC has been demonstrated in cultured rat RPE cells (66, 67, 70). Signaling between such receptors and the cytoskeleton remains a challenging area requiring substantial investigation.
OS internalization
The signaling discussed above is needed for internalization of OS, but engulfment also requires significant cytoskeletal reorganization. This reorganization results in actin-mediated extension of pseudopods that ultimately engulf the particle, a process extensively studied in macrophage phagocytosis, but many of its mechanisms remain unknown. The best studied Fcγ receptor (FcγR) phagocytosis system is briefly discussed here (16). Recognition of substrates by the macrophage results in recruitment of FcγR to the site of contact, resulting in the initiation of a phosphorylation cascade involving a number of kinases (16, 30). In the case of FcγR, actin polymerization is driven by the GTPase, Rac1 and/or Rac2, as well as by cell division control protein 42 (Cdc42) (11). Identity of the guanine nucleotide exchange factor (GEF) required for Rac1 GTPase and Cdc42 activity is currently being debated (35, 72). Downstream of Rac1, the effectors Wiskott-Aldrich syndrome protein and actin-related protein 2/3 (Arp2/3) are clearly involved in actin polymerization during FcγR-mediated phagocytosis (14, 58). Information available regarding RPE phagocytosis of OS is relatively sparce, but recently there have been some potentially important findings. Annexins (Anxs) are a family of Ca2+- and phospholipid-binding proteins known to be involved in a number of cellular processes including exo- and endocytosis, apoptosis, membrane trafficking, and anti-inflammatory responses. Anx A2 is a potent regulator of actin dynamics recently shown to nucleate actin polymerization in vitro on beads coated with phosphatidylinositol-4,5-bisphosphate (42, 43). Anx A2 and A4 were just found to be enriched in phagocytically competent RPE cells as well. Although the function of A4 is unclear, A2 was detected in newly formed phagosomes in the RPE, and siRNA-mediated downregulation resulted in an inability of these cells to internalize OS (54). Additionally, anx2−/− mice displayed aberrant activation of the signaling proteins FAK and c-Src (54).
Formation of a pseudopod during particle engulfment requires significant reorganization of the actin cytoskeleton, whereas the force needed to produce a pseudopod demands the action of a motor protein, namely a myosin. Although several myosin classes have been implicated in phagocytosis, myosin II is the only one found to be involved in internalization of OS by the RPE (82). Recently, it was found that myosin II interacts with Mertk upon challenge with OS. Primary rat RPE cells showed a significant redistribution of myosin II-A and II-B in response to OS challenge, both myosins co-localizing with engulfed OS over time. Strikingly, RPE cells harvested from RCS rats showed no redistribution of myosin in response to OS, suggesting an important role for Mertk in the localization of myosin to the sites of engulfment (82).
While encircling a particle, one pseudopod must fuse with another advancing from the opposite side. This process requires a number of proteins interacting in concert to exert the necessary force needed to fuse the two membranes together (52). Cadherins are a class of proteins that employ a Ca2+-dependent mechanism to bind cadherins on adjacent cells, forming adherens junctions. Cadherins require a class of proteins, catenins, that associate with the cytoplasmic domain of cadherins to assemble a protein complex that can associate with the actin cytoskeleton. β-Catenin complexes with membrane-bound cadherin during initiation of adherin junctions, referred to as puncta. These complexes bind additional proteins, i.e., α-catenin, formin, and vasodilator-stimulated phosphoprotein (VASP). The presence of these protein complexes results in formation of bundles of radial actin cables that assemble on each side of the puncta and provide anchors to the underlying cortical actin ring. Actin reorganization subsequently results in formation of mature adherens junctions and fusion of the two membranes. Although this process has been studied in a number of cell types, it is not yet known whether it applies to RPE cells. However, it is likely that a similar progression of events occurs to expedite fusion of advancing pseudopods.
Phagosomal degradation of OS
Once internalized, phagosomes proceed through a maturation process that ultimately leads to degradation of their contained proteins and lipids. As with other aspects of OS phagocytosis, mechanisms that control this degradation are not completely understood, but what is known about phagocytosis in general again allows the proposal of some plausible hypotheses (28). It is generally understood that phagosomal maturation proceeds through a number of fusion stages that include fusion with early endosomes, late endosomes, and lysosomes. The final structure formed after fusion with lysosomes is known as the phagolysosome. Each step is characterized by changes in the presence of proteins on the membrane and in the lumen, as well as by progressive acidification of the lumen and increased production of reactive oxygen species.
Because RPE cells are polarized and fixed, phagocytosis always occurs on their apical membranes facing photoreceptor cells. And once internalized, phagosomes are moved from the apical membrane toward the basal membrane where they are degraded (31, 44, 45). A defect in this process results in the retinal disease associated with Usher Syndrome 1B. The genetic mutation causing this disease lies within the motor protein myosin VIIa responsible for transport of phagosomes from the apical to the basal membrane of the RPE (31). Mutant myosin VIIa cells are unable to move phagosomes away from the apical membrane, resulting in blockage of RPE phagocytosis and subsequent photoreceptor degeneration.
Studies of RPE phagosomes have documented the presence of lytic enzymes and acidification of the OS phagosomal lumen. Cathepsin D is an acidic lysosomal protease present in all cells (93). Analysis of RPE cells in whole rat eyes revealed that opsin was detectable by immunolabeling in ROS phagosomes that did not contain cathepsin D, but once cathepsin D appeared, opsin levels decreased rapidly. Phagosomal pH has been shown to decrease after fusion with lysosomes, and this acidification is blocked by addition of the H+-ATPase inhibitor bafilomycin A (17). Direct phagosome-lysosome interactions have been visualized by electron microscopy in a number of species (10, 17, 44). In contrast, unspent chromophore 11-cis-retinal is re-used and sent back to OS (4).
Oxidation products and retinal diseases
Accumulation of photo-oxidized products in the RPE occurs as an organism ages, and this has been linked to a number of retinal diseases. Lipofuscin is an autofluorescent cellular waste product that cannot be degraded or removed from cells. Lipofuscin in the retina, formed from the condensation of two molecules of all-trans-retinal and one molecule of phoshatidylethanolamine, is believed to accumulate because of incomplete digestion of OS in the RPE (79, 85). Once in the RPE, lipofuscin is converted to the photo-inducible free-radical generator A2E, a pyridinium bisretinoid toxic to RPE cells. Because RPE cells are postmitotic, they cannot dilute A2E by simple cellular division, so a progressive buildup of A2E occurs within the cell. Indeed, one damaging effect of A2E is inhibition of phagolysosomal degradation of OS by the RPE (25). Additionally, oxidized low-density lipoproteins (oxLDL) inhibit the breakdown of OS in the RPE by delaying the maturation of RPE phagosomes (48, 49). OS renewal is a tightly regulated process, with the RPE challenged daily by a new batch of phagocytosed OS. Breakdown in the ability of this cell layer to efficiently degrade these products would eventually lead to a build-up of undigested lipids and proteins within the RPE, resulting in further production of compounds like A2E. Such excessive accumulation of photo-oxidized products in the RPE is believed to be an underlining cause of degenerative retinal diseases such as age-related macular degeneration. However, an alternative hypothesis is that all-trans-retinal is a toxin, whereas A2E represents a product of detoxification (56).
Daily renewal of photoreceptor OS is of profound importance to the maintenance of retinal health. Although a number of components have been identified since the initial observations by Young, this process remains poorly understood. This review is meant to serve as a platform for further investigations into what is likely to be a future area of intense research.
Acknowledgments
We thank Dr. Leslie T. Webster, Jr. for critical comments on the manuscript.
This research was supported by National Eye Institute Grants EY-09339 and EY-019050. B. M. Kevany was supported by a CWRU Department of Nutrition Metabolic Training Grant postdoctoral fellowship award from National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-007319.
References
- 1.Anderson DH, Fisher SK. Disc shedding in rodlike and cone-like photoreceptors of tree squirrels. Science. 1975;187:953–955. doi: 10.1126/science.1145180. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DH, Fisher SK, Steinberg RH. Mammalian cones: disc shedding, phagocytosis, renewal. Invest Ophthalmol Vis Sci. 1978;17:117–133. [PubMed] [Google Scholar]
- 3.Basinger S, Hoffman R, Matthes M. Photoreceptor shedding is initiated by light in the frog retina. Science. 1976;194:1074–1076. doi: 10.1126/science.1086510. [DOI] [PubMed] [Google Scholar]
- 4.Batten ML, Imanishi Y, Tu DC, Doan T, Zhu L, Pang J, Glushakova L, Moise AR, Baehr W, Van Gelder RN, Hauswirth WW, Rieke F, Palczewski K. Pharmacological and rAAV gene therapy rescue of visual functions in a blind mouse model of Leber congenital amaurosis. PLoS Med. 2005;2:e333. doi: 10.1371/journal.pmed.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besharse JC, Hollyfield JG. Turnover of mouse photoreceptor outer segments in constant light and darkness. Invest Ophthalmol Vis Sci. 1979;18:1019–1024. [PubMed] [Google Scholar]
- 6.Besharse JC, Hollyfield JG, Rayborn ME. Photoreceptor outer segments: accelerated membrane renewal in rods after exposure to light. Science. 1977;196:536–538. doi: 10.1126/science.300504. [DOI] [PubMed] [Google Scholar]
- 7.Boesze-Battaglia K, Albert AD. Phospholipid distribution among bovine rod outer segment plasma membrane and disk membranes. Exp Eye Res. 1992;54:821–823. doi: 10.1016/0014-4835(92)90040-y. [DOI] [PubMed] [Google Scholar]
- 8.Bok D. The retinal pigment epithelium: a versatile partner in vision. J Cell Sci Suppl. 1993;17:189–195. doi: 10.1242/jcs.1993.supplement_17.27. [DOI] [PubMed] [Google Scholar]
- 9.Bok D, Young RW. The renewal of diffusely distributed protein in the outer segments of rods and cones. Vision Res. 1972;12:161–168. doi: 10.1016/0042-6989(72)90108-3. [DOI] [PubMed] [Google Scholar]
- 10.Bosch E, Horwitz J, Bok D. Phagocytosis of outer segments by retinal pigment epithelium: phagosome-lysosome interaction. J Histochem Cytochem. 1993;41:253–263. doi: 10.1177/41.2.8419462. [DOI] [PubMed] [Google Scholar]
- 11.Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282:1717–1721. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- 12.Chaitin MH, Hall MO. Defective ingestion of rod outer segments by cultured dystrophic rat pigment epithelial cells. Invest Ophthalmol Vis Sci. 1983;24:812–820. [PubMed] [Google Scholar]
- 13.Chang Y, Finnemann SC. Tetraspanin CD81 is required for the alpha v beta5-integrin-dependent particle-binding step of RPE phagocytosis. J Cell Sci. 2007;120:3053–3063. doi: 10.1242/jcs.006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coppolino MG, Krause M, Hagendorff P, Monner DA, Trimble W, Grinstein S, Wehland J, Sechi AS. Evidence for a molecular complex consisting of Fyb/SLAP, SLP-76, Nck, VASP and WASP that links the actin cytoskeleton to Fcgamma receptor signalling during phagocytosis. J Cell Sci. 2001;114:4307–4318. doi: 10.1242/jcs.114.23.4307. [DOI] [PubMed] [Google Scholar]
- 15.D’Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, Vollrath D. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet. 2000;9:645–651. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- 16.Daeron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 17.Deguchi J, Yamamoto A, Yoshimori T, Sugasawa K, Moriyama Y, Futai M, Suzuki T, Kato K, Uyama M, Tashiro Y. Acidification of phagosomes and degradation of rod outer segments in rat retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1994;35:568–579. [PubMed] [Google Scholar]
- 18.Edwards RB, Flaherty PM. Association of changes in intracellular cyclic AMP with changes in phagocytosis in cultured rat pigment epithelium. Curr Eye Res. 1986;5:19–26. doi: 10.3109/02713688608995161. [DOI] [PubMed] [Google Scholar]
- 19.Fadeel B, Quinn P, Xue D, Kagan V. Fat(al) attraction: oxidized lipids act as “eat-me” signals. HFSP J. 2007;1:225–229. doi: 10.2976/1.2800110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fadok VA, de Cathelineau A, Daleke DL, Henson PM, Bratton DL. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J Biol Chem. 2001;276:1071–1077. doi: 10.1074/jbc.M003649200. [DOI] [PubMed] [Google Scholar]
- 21.Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001;108:785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng W, Yasumura D, Matthes MT, LaVail MM, Vollrath D. Mertk triggers uptake of photoreceptor outer segments during phagocytosis by cultured retinal pigment epithelial cells. J Biol Chem. 2002;277:17016–17022. doi: 10.1074/jbc.M107876200. [DOI] [PubMed] [Google Scholar]
- 23.Finnemann SC. Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. EMBO J. 2003;22:4143–4154. doi: 10.1093/emboj/cdg416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finnemann SC, Bonilha VL, Marmorstein AD, Rodriguez-Boulan E. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires alpha(v)beta5 integrin for binding but not for internalization. Proc Natl Acad Sci USA. 1997;94:12932–12937. doi: 10.1073/pnas.94.24.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finnemann SC, Leung LW, Rodriguez-Boulan E. The lipofuscin component A2E selectively inhibits phagolysosomal degradation of photoreceptor phospholipid by the retinal pigment epithelium. Proc Natl Acad Sci USA. 2002;99:3842–3847. doi: 10.1073/pnas.052025899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finnemann SC, Silverstein RL. Differential roles of CD36 and alphavbeta5 integrin in photoreceptor phagocytosis by the retinal pigment epithelium. J Exp Med. 2001;194:1289–1298. doi: 10.1084/jem.194.9.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher SK, Pfeffer BA, Anderson DH. Both rod and cone disc shedding are related to light onset in the cat. Invest Ophthalmol Vis Sci. 1983;24:844–856. [PubMed] [Google Scholar]
- 28.Flannagan RS, Cosio G, Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol. 2009;7:355–366. doi: 10.1038/nrmicro2128. [DOI] [PubMed] [Google Scholar]
- 29.Gal A, Li Y, Thompson DA, Weir J, Orth U, Jacobson SG, Apfelstedt-Sylla E, Vollrath D. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat Genet. 2000;26:270–271. doi: 10.1038/81555. [DOI] [PubMed] [Google Scholar]
- 30.Ghazizadeh S, Bolen JB, Fleit HB. Physical and functional association of Src-related protein tyrosine kinases with Fc gamma RII in monocytic THP-1 cells. J Biol Chem. 1994;269:8878–8884. [PubMed] [Google Scholar]
- 31.Gibbs D, Kitamoto J, Williams DS. Abnormal phagocytosis by retinal pigmented epithelium that lacks myosin VIIa, the Usher syndrome 1B protein. Proc Natl Acad Sci USA. 2003;100:6481–6486. doi: 10.1073/pnas.1130432100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med. 2006;203:2613–2625. doi: 10.1084/jem.20060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gregory CY, Abrams TA, Hall MO. Stimulation of A2 adenosine receptors inhibits the ingestion of photoreceptor outer segments by retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1994;35:819–825. [PubMed] [Google Scholar]
- 34.Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science. 1995;267:1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- 35.Hall AB, Gakidis MA, Glogauer M, Wilsbacher JL, Gao S, Swat W, Brugge JS. Requirements for Vav guanine nucleotide exchange factors and Rho GTPases in FcgammaR- and complement-mediated phagocytosis. Immunity. 2006;24:305–316. doi: 10.1016/j.immuni.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Hall MO, Abrams TA, Mittag TW. The phagocytosis of rod outer segments is inhibited by drugs linked to cyclic adenosine monophosphate production. Invest Ophthalmol Vis Sci. 1993;34:2392–2401. [PubMed] [Google Scholar]
- 37.Hall MO, Abrams TA, Mittag TW. ROS ingestion by RPE cells is turned off by increased protein kinase C activity and by increased calcium. Exp Eye Res. 1991;52:591–598. doi: 10.1016/0014-4835(91)90061-i. [DOI] [PubMed] [Google Scholar]
- 38.Hall MO, Obin MS, Heeb MJ, Burgess BL, Abrams TA. Both protein S and Gas6 stimulate outer segment phagocytosis by cultured rat retinal pigment epithelial cells. Exp Eye Res. 2005;81:581–591. doi: 10.1016/j.exer.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 39.Hall MO, Obin MS, Prieto AL, Burgess BL, Abrams TA. Gas6 binding to photoreceptor outer segments requires gamma-carboxyglutamic acid (Gla) and Ca2+ and is required for OS phagocytosis by RPE cells in vitro. Exp Eye Res. 2002;75:391–400. [PubMed] [Google Scholar]
- 40.Hall MO, Prieto AL, Obin MS, Abrams TA, Burgess BL, Heeb MJ, Agnew BJ. Outer segment phagocytosis by cultured retinal pigment epithelial cells requires Gas6. Exp Eye Res. 2001;73:509–520. doi: 10.1006/exer.2001.1062. [DOI] [PubMed] [Google Scholar]
- 41.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 42.Hayes MJ, Shao D, Bailly M, Moss SE. Regulation of actin dynamics by annexin 2. EMBO J. 2006;25:1816–1826. doi: 10.1038/sj.emboj.7601078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayes MJ, Shao DM, Grieve A, Levine T, Bailly M, Moss SE. Annexin A2 at the interface between F-actin and membranes enriched in phosphatidylinositol 4,5,-bisphosphate. Biochim Biophys Acta. 2009;1793:1086–1095. doi: 10.1016/j.bbamcr.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herman KG, Steinberg RH. Phagosome degradation in the tapetal retinal pigment epithelium of the opossum. Invest Ophthalmol Vis Sci. 1982;23:291–304. [PubMed] [Google Scholar]
- 45.Herman KG, Steinberg RH. Phagosome movement and the diurnal pattern of phagocytosis in the tapetal retinal pigment epithelium of the opossum. Invest Ophthalmol Vis Sci. 1982;23:277–290. [PubMed] [Google Scholar]
- 46.Heth CA, Marescalchi PA. Inositol triphosphate generation in cultured rat retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1994;35:409–416. [PubMed] [Google Scholar]
- 47.Heth CA, Schmidt SY. Protein phosphorylation in retinal pigment epithelium of Long-Evans and Royal College of Surgeons rats. Invest Ophthalmol Vis Sci. 1992;33:2839–2847. [PubMed] [Google Scholar]
- 48.Hoppe G, Marmorstein AD, Pennock EA, Hoff HF. Oxidized low density lipoprotein-induced inhibition of processing of photoreceptor outer segments by RPE. Invest Ophthalmol Vis Sci. 2001;42:2714–2720. [PubMed] [Google Scholar]
- 49.Hoppe G, O’Neil J, Hoff HF, Sears J. Accumulation of oxidized lipid-protein complexes alters phagosome maturation in retinal pigment epithelium. Cell Mol Life Sci. 2004;61:1664–1674. doi: 10.1007/s00018-004-4080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishimoto Y, Ohashi K, Mizuno K, Nakano T. Promotion of the uptake of PS liposomes and apoptotic cells by a product of growth arrest-specific gene, gas6. J Biochem. 2000;127:411–417. doi: 10.1093/oxfordjournals.jbchem.a022622. [DOI] [PubMed] [Google Scholar]
- 51.Johnson NF. Phagocytosis in the normal and ischaemic retinal pigment epithelium of the rabbit. Exp Eye Res. 1975;20:97–107. doi: 10.1016/0014-4835(75)90147-5. [DOI] [PubMed] [Google Scholar]
- 52.Kobielak A, Fuchs E. Alpha-catenin: at the junction of intercellular adhesion and actin dynamics. Nat Rev Mol Cell Biol. 2004;5:614–625. doi: 10.1038/nrm1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.LaVail MM. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science. 1976;194:1071–1074. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- 54.Law AL, Ling Q, Hajjar KA, Futter CE, Greenwood J, Adamson P, Wavre-Shapton ST, Moss SE, Hayes MJ. Annexin A2 regulates phagocytosis of photoreceptor outer segments in the mouse retina. Mol Biol Cell. 2009;20:3896–3904. doi: 10.1091/mbc.E08-12-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Long KO, Fisher SK, Fariss RN, Anderson DH. Disc shedding and autophagy in the cone-dominant ground squirrel retina. Exp Eye Res. 1986;43:193–205. doi: 10.1016/s0014-4835(86)80087-2. [DOI] [PubMed] [Google Scholar]
- 56.Maeda A, Maeda T, Golczak M, Chou S, Desai A, Hoppel CL, Matsuyama S, Palczewski K. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J Biol Chem. 2009;284:15173–15183. doi: 10.1074/jbc.M900322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marmorstein AD. The polarity of the retinal pigment epithelium. Traffic. 2001;2:867–872. doi: 10.1034/j.1600-0854.2001.21202.x. [DOI] [PubMed] [Google Scholar]
- 58.May RC, Caron E, Hall A, Machesky LM. Involvement of the Arp2/3 complex in phagocytosis mediated by FcgammaR or CR3. Nat Cell Biol. 2000;2:246–248. doi: 10.1038/35008673. [DOI] [PubMed] [Google Scholar]
- 59.McBee JK, Palczewski K, Baehr W, Pepperberg DR. Confronting complexity: the interlink of phototransduction and retinoid metabolism in the vertebrate retina. Prog Retinal Eye Res. 2001;20:469–529. doi: 10.1016/s1350-9462(01)00002-7. [DOI] [PubMed] [Google Scholar]
- 60.Mustafi D, Engel AH, Palczewski K. Structure of cone photoreceptors. Prog Retinal Eye Res. 2009;28:289–302. doi: 10.1016/j.preteyeres.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mustafi D, Palczewski K. Topology of class A G protein-coupled receptors: insights gained from crystal structures of rhodopsins, adrenergic and adenosine receptors. Mol Pharmacol. 2009;75:1–12. doi: 10.1124/mol.108.051938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nagata K, Ohashi K, Nakano T, Arita H, Zong C, Hanafusa H, Mizuno K. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem. 1996;271:30022–30027. doi: 10.1074/jbc.271.47.30022. [DOI] [PubMed] [Google Scholar]
- 63.Nakano T, Ishimoto Y, Kishino J, Umeda M, Inoue K, Nagata K, Ohashi K, Mizuno K, Arita H. Cell adhesion to phosphatidylserine mediated by a product of growth arrest-specific gene 6. J Biol Chem. 1997;272:29411–29414. doi: 10.1074/jbc.272.47.29411. [DOI] [PubMed] [Google Scholar]
- 64.Nandrot EF, Anand M, Almeida D, Atabai K, Sheppard D, Finnemann SC. Essential role for MFG-E8 as ligand for alphavbeta5 integrin in diurnal retinal phagocytosis. Proc Natl Acad Sci USA. 2007;104:12005–12010. doi: 10.1073/pnas.0704756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nandrot EF, Kim Y, Brodie SE, Huang X, Sheppard D, Finnemann SC. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking alphavbeta5 integrin. J Exp Med. 2004;200:1539–1545. doi: 10.1084/jem.20041447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nash MS, Osborne NN. Agonist-induced effects on cyclic AMP metabolism are affected in pigment epithelial cells of the Royal College of Surgeons rat. Neurochem Int. 1995;27:253–262. doi: 10.1016/0197-0186(95)00040-f. [DOI] [PubMed] [Google Scholar]
- 67.Nash MS, Wood JP, Osborne NN. Protein kinase C activation by serotonin potentiates agonist-induced stimulation of cAMP production in cultured rat retinal pigment epithelial cells. Exp Eye Res. 1997;64:249–255. doi: 10.1006/exer.1996.0214. [DOI] [PubMed] [Google Scholar]
- 68.O’Day WT, Young RW. Rhythmic daily shedding of outer-segment membranes by visual cells in the goldfish. J Cell Biol. 1978;76:593–604. doi: 10.1083/jcb.76.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Okada T, Ernst OP, Palczewski K, Hofmann KP. Activation of rhodopsin: new insights from structural and biochemical studies. Trends Biochem Sci. 2001;26:318–324. doi: 10.1016/s0968-0004(01)01799-6. [DOI] [PubMed] [Google Scholar]
- 70.Osborne NN, Fitzgibbon F, Nash M, Liu NP, Leslie R, Cholewinski A. Serotonergic, 5-HT2, receptor-mediated phosphoinositide turnover and mobilization of calcium in cultured rat retinal pigment epithelium cells. Vision Res. 1993;33:2171–2179. doi: 10.1016/0042-6989(93)90097-g. [DOI] [PubMed] [Google Scholar]
- 71.Palczewski K. G protein-coupled receptor rhodopsin. Annu Rev Biochem. 2006;75:743–767. doi: 10.1146/annurev.biochem.75.103004.142743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Patel JC, Hall A, Caron E. Vav regulates activation of Rac but not Cdc42 during FcgammaR-mediated phagocytosis. Mol Biol Cell. 2002;13:1215–1226. doi: 10.1091/mbc.02-01-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Prasad D, Rothlin CV, Burrola P, Burstyn-Cohen T, Lu Q, Garcia de Frutos P, Lemke G. TAM receptor function in the retinal pigment epithelium. Mol Cell Neurosci. 2006;33:96–108. doi: 10.1016/j.mcn.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 74.Quiring R, Walldorf U, Kloter U, Gehring WJ. Homology of the eyeless gene of Drosophila to the small eye gene in mice and Aniridia in humans. Science. 1994;265:785–789. doi: 10.1126/science.7914031. [DOI] [PubMed] [Google Scholar]
- 75.Rodriguez de Turco EB, Gordon WC, Bazan NG. Light stimulates in vivo inositol lipid turnover in frog retinal pigment epithelial cells at the onset of shedding and phagocytosis of photoreceptor membranes. Exp Eye Res. 1992;55:719–725. doi: 10.1016/0014-4835(92)90176-s. [DOI] [PubMed] [Google Scholar]
- 76.Ryeom SW, Silverstein RL, Scotto A, Sparrow JR. Binding of anionic phospholipids to retinal pigment epithelium may be mediated by the scavenger receptor CD36. J Biol Chem. 1996;271:20536–20539. doi: 10.1074/jbc.271.34.20536. [DOI] [PubMed] [Google Scholar]
- 77.Ryeom SW, Sparrow JR, Silverstein RL. CD36 participates in the phagocytosis of rod outer segments by retinal pigment epithelium. J Cell Sci. 1996;109:387–395. doi: 10.1242/jcs.109.2.387. [DOI] [PubMed] [Google Scholar]
- 78.Saari JC. Biochemistry of visual pigment regeneration: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2000;41:337–348. [PubMed] [Google Scholar]
- 79.Sparrow JR, Wu Y, Kim CY, Zhou J. Phospholipid meets all-trans-retinal: the making of RPE bis-retinoids. J Lipid Res. doi: 10.1194/jlr.R000687. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Steinberg RH. Phagocytosis by pigment epithelium of human retinal cones. Nature. 1974;252:305–307. doi: 10.1038/252305a0. [DOI] [PubMed] [Google Scholar]
- 81.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 82.Strick DJ, Feng W, Vollrath D. Mertk drives myosin II redistribution during retinal pigment epithelial phagocytosis. Invest Ophthalmol Vis Sci. 2009;50:2427–2435. doi: 10.1167/iovs.08-3058. [DOI] [PubMed] [Google Scholar]
- 83.Takimoto N, Kusakabe T, Tsuda M. Origin of the vertebrate visual cycle. Photochem Photobiol. 2007;83:242–247. doi: 10.1562/2006-06-30-IR-957. [DOI] [PubMed] [Google Scholar]
- 84.Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wolf G. Lipofuscin and macular degeneration. Nutr Rev. 2003;61:342–346. doi: 10.1301/nr.2003.oct.342-346. [DOI] [PubMed] [Google Scholar]
- 86.Wu Y, Tibrewal N, Birge RB. Phosphatidylserine recognition by phagocytes: a view to a kill. Trends Cell Biol. 2006;16:189–197. doi: 10.1016/j.tcb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 87.Young RW. The daily rhythm of shedding and degradation of cone outer segment membranes in the lizard retina. J Ultrastruct Res. 1977;61:172–185. doi: 10.1016/s0022-5320(77)80084-1. [DOI] [PubMed] [Google Scholar]
- 88.Young RW. The daily rhythm of shedding and degradation of rod and cone outer segment membranes in the chick retina. Invest Ophthalmol Vis Sci. 1978;17:105–116. [PubMed] [Google Scholar]
- 89.Young RW. A difference between rods and cones in the renewal of outer segment protein. Invest Ophthalmol. 1969;8:222–231. [PubMed] [Google Scholar]
- 90.Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967;33:61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 1969;42:392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Young RW, Droz B. The renewal of protein in retinal rods and cones. J Cell Biol. 1968;39:169–184. doi: 10.1083/jcb.39.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zaidi N, Maurer A, Nieke S, Kalbacher H. Cathepsin D: a cellular roadmap. Biochem Biophys Res Commun. 2008;376:5–9. doi: 10.1016/j.bbrc.2008.08.099. [DOI] [PubMed] [Google Scholar]