Abstract
The severity of allergic asthma is dependent, in part, on the intensity of peribronchial inflammation. P-selectin is known to play a role in the development of allergen-induced peribronchial inflammation and airway hyperreactivity. Selective inhibitors of P-selectin-mediated leukocyte endothelial-cell interactions may therefore attenuate the inflammatory processes associated with allergic airway disease. Novel P-selectin inhibitors were created using a polyvalent polymer nanoparticle capable of displaying multiple synthetic, low molecular weight ligands. By assembling a particle that presents an array of groups, which as monomers interact with only low affinity, we created a construct that binds extremely efficiently to P-selectin. The ligands acted as mimetics of the key binding elements responsible for the high-avidity adhesion of P-selectin to the physiologic ligand, PSGL-1. The inhibitors were initially evaluated using an in vitro shear assay system in which interactions between circulating cells and P-selectin-coated capillary tubes were measured. The nanoparticles were shown to preferentially bind to selectins expressed on activated endothelial cells. We subsequently demonstrated that nanoparticles displaying P-selectin blocking arrays were functionally active in vivo, significantly reducing allergen-induced airway hyperreactivity and peribronchial eosinophilic inflammation in a murine model of asthma.
Keywords: selectin inhibitor, asthma, eosinophil, lung
The incidence of asthma worldwide has increased dramatically in recent years, and it is now considered to be the most common chronic childhood illness (1, 2). A disproportionate increase in asthma incidence has been identified in inner-city children, and this may be partly due to cockroach allergen sensitivity, which affects a significant proportion of urban asthmatics (3, 4). In comparison, the incidence of asthma is significantly lower in rural communities where sensitivity to the allergen is almost nonexistent (5).
The widespread belief that selectin inhibition will deliver therapeutic benefits in a variety of inflammatory diseases is based on the observation that leukocyte rolling mediated by selectins represents a first step in leukocyte recruitment to sites of inflammation (6, 7). The severity of allergic airway disease is primarily dependent on the extent of the inflammatory response and attenuation of leukocyte infiltration in the lung is frequently correlated with clinical improvement (8, 9). The current preferred treatment for severe asthma is the use of corticosteroids, which although effective, are associated with significant systemic side effects, including growth suppression in children (10).
Recent research has focused on more specific methods of inhibiting eosinophil and lymphocyte recruitment into the lungs. Several studies have demonstrated a role for adhesion molecules in the attenuation of allergic airway inflammation. ICAM-1/LFA-1, E-, L- and P-selectins have all been shown to play a role in the development of peribronchial inflammation and airway hyperreactivity associated with allergic inflammation (11–15). Among the selectins, P-selectin may be a particularly useful target in allergic inflammation because expression of this adhesion molecule is up-regulated by physiological levels of Th2 cytokines, including IL-4 and IL-13 (16). In addition, eosinophils have been shown to express more PSGL-1 than neutrophils, and as a result, eosinophils bind preferentially to the P-selectin found at low concentrations on airway endothelium during allergen challenge (17, 18).
Numerous studies have attempted to identify the structural features required for high-affinity selectin binding in order to generate specific polyvalent antagonists (19), although some low molecular weight, noncarbohydrate, nonpeptide inhibitors of P-selectin have also been identified (20–22). The novel inhibitors described in this report are based on polymerized liposome nanoparticles (PLNP) that display multiple copies of ligand mimetics simulating PSGL-1. In our efforts to simplify the mimetic to only the elements in PSGL-1 responsible for the adhesion to P-selectin, we removed from the sialyl Lewis X (sLex) carbohydrate all but the key carbohydrate unit fucose. This carbohydrate was displayed on the PLNP along with polyvalent sulfate ester groups and emulates the sLex/sulfated tyrosine super ligand motif shown to be critical for P-selectin binding (23). An essential component in any successful drug discovery/development program is the use of in vitro assays that recreate the conditions present in vivo. To optimize the design of P-selectin inhibitors, which block the interaction of circulating leukocytes to blood vessel endothelial cells, the screening assay must duplicate the forces encountered in the blood. In this study, we used an in vitro recirculating loop assay (ProteoFlow, LigoCyte Pharmaceuticals, Bozeman, MT) that rolls PSGL-1-expressing human leukocytes (U-937 cells) over P-selectin-coated capillary surfaces at physiological shear rates to identify potent selectin antagonists. Potent selectin PLNP were identified in vitro, and their ability to bind preferentially to selectins on activated endothelial cells was confirmed in vivo. We subsequently demonstrated that the selectin-targeted nanoparticles are functionally active in a murine model of allergic airway disease, attenuating both peribronchial inflammation and airway hyperreactivity induced by allergen challenge.
Materials and Methods
Animals
Balb/c and C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME) and maintained under specific pathogen-free conditions in the University of Michigan animal facility. E/P-selectin-deficient mice were a gift from Daniel Bullard at the University of Alabama.
Preparation of PLNP
The detailed chemical synthesis of the fucose-containing and polyethyline glycol (PEG)-containing lipid monomers will be reported elsewhere. In brief, the α-C-allyl-glycoside of l-fucose was prepared according to procedures in the literature. This molecule was elaborated into an amine-terminated glycoside by the sequential addition of 3-mercaptoproprionic acid followed by ethylene diamine. The amino group of this fucose glycoside was condensed with the carboxylic acid of 10,12-pentacosyadiynoic acid to yield the amide-linked fucosylated lipid. The PEG-containing lipid was likewise synthesized by condensing amine-terminated and MeO (methoxyl)-terminated PEG5000 with the carboxylic acid of 10,12-pentacosadiynoic acid to yield the amide-linked PEGylated lipid. The other lipids and the PLNP were prepared as described previously (24). In brief, liposomes were prepared from a solution containing 5 mol% of the α-C-fucose terminated lipid, 25 mol% of the sulfate ester lipid, 69 mol% of the matrix lipid (ethanolamide of pentacosadiynoic acid: EAPDA), and 1 mol% polyethylene glycol (PEG) terminated lipid by the probe sonication method (25). After cooling, the PLNP were polymerized by UV irradiation (254 nm) and passed through a 0.2 μm cellulose acetate filter to remove trace large aggregates. Particle size analysis showed the remaining material to have an effective diameter of 73 nm (with a particle size range from ∼30 to 140 nm). Negative control PLNP was also prepared in which PEG lipid was increased from 1 to 15 mol%, and matrix lipid decreased from 69 to 55 mol%.
In vitro Proteoflow shear assays
A closed loop, recirculating, in vitro shear assay system (ProteoFlow) was used to evaluate the ability of PLNP to block tethering and rolling of the human promonocytic cell line, U937, on E- and P-selectin as described previously (26). Mouse P- or E-selectin/human IgM chimera (27) was bound to glass capillary tubes (100 μl “microcaps,” Drummond Scientific Company, Broomall, PA). U937 cells (ATCC) (4 × 106 in 3 ml RPMI containing 10% fetal calf serum [FCS]) were infused into the ProteoFlow system under high flow rate (4–5 dynes/cm2). After 1 min, the flow rate was reduced to physiologically relevant 2 dynes/cm2 (28, 29), and video recording was initiated. Cells tether and roll on P- or E-selectin 1–2 min after reduction of flow rate and plateau 6–7 min later. Leukocyte P- or E-selectin interaction was established 8 min before injection of PLNP, and the interaction was recorded for an additional 8 min. Video recordings were analyzed by freezing frames and counting numbers of cells interacting at 30-s intervals. Data are representative of results obtained in 3–4 independent experiments.
PLNP binding to lung vessels of mice in LPS-induced inflammation
Mice were injected i.v. with 2 mg/kg LPS (serotype 0111:B4) 2 h before PLNP injection. Selectin PLNP were diluted in FCS or phosphate-buffered saline (PBS) (5 μl of a 12 mg/ml suspension) and injected i.v. in a final volume of 200 μl. Mice were killed 3 h later, and lung samples were collected.
Sensitization and induction of the allergic airway response
Normal mice were sensitized and challenged with cockroach antigen (CRA) to induce a Th2-type response (30, 31). Mice were injected i.v. with 5 μl of a 12 mg/ml suspension of either selectin PLNP or control PLNP in PBS 2 h after CRA challenge on day 21 of the sensitization protocol. Airway hyperreactivity was assessed in mice 24 h after the final i.t. CRA challenge. Airway hyperreactivity (AHR) was measured in ventilated, anaesthetized mice using a Buxco (Troy, NY) mouse plethysmograph as described previously (32). Baseline and methacholine-induced peak airway resistance (optimal i.v methacholine dose, 312.5 μg/kg) were determined, and the change in AHR was calculated by subtracting baseline readings from the peak methacholine-induced airway resistance. Data are means ± SE change in airway resistance (cm H2O/ml/sec) for 4–5 animals per group.
Histological analysis of lung inflammation
Whole lungs were fixed by inflation with formalin and processed into paraffin by using standard histological techniques. Tissue sections (5 μm) were stained with hematoxylin and eosin for analysis of peribronchial eosinophil accumulation (×200) or remained unstained for examination of the distribution of the autofluorescent PLNP by fluorescence microscopy (×400). Peribronchial eosinophil counts were analyzed by counting the number of eosinophils surrounding the airways in 50 high power fields (×1000).
Statistical analysis
Results are expressed as mean ± SE. Statistical significance was calculated by ANOVA or unpaired Student's t test to calculate the two-tailed P value. Significance was determined as values of P < 0.05.
Results
Blockade of P-selectin-dependent rolling in vitro
Potent selectin inhibitors were identified following in vitro testing of formulations in which the ratio of fucose, sulfate and PEG groups were varied. The total polymerizable lipid content was kept constant by the addition of neutral matrix lipid. The optimal ratios of the four lipids were found to be fucose:sulfate:PEG:matrix 5:25:1:69. A schematic diagram of the P-selectin blocking PLNP is shown in Figure 1. To assess formulation changes, we used the ProteoFlow assay system in which glass capillary surfaces were directly coated with chimeric selectin proteins. PSGL-1-expressing U-937 cells were introduced to the closed system, and interactions with the adhesion proteins were monitored. In the absence of PLNP, the number of U-937 cells interacting with the coated capillary tubes gradually increased with time to 100–200 interactions/field after 6–7 min of flow. Administration of PLNP, after establishing the leukocyte-selectin rolling interaction, reversed the existing rolling completely inhibiting new cell attachment, as evidenced by the return of U-937 cells to circulation (Fig. 2A). By increasing the PEG lipid level from 1 to 15%, selectin inhibitory activity of the PLNP was abolished (Fig. 2A), providing a nanoparticle that could be used as a negative control PLNP in further experiments. The leukocyte/P-selectin inhibition activity of the PLNP showed a dose-dependent decrease (Fig. 2B), with no effect on leukocyte/E-selectin interactions (Fig. 2C).
Figure 1.
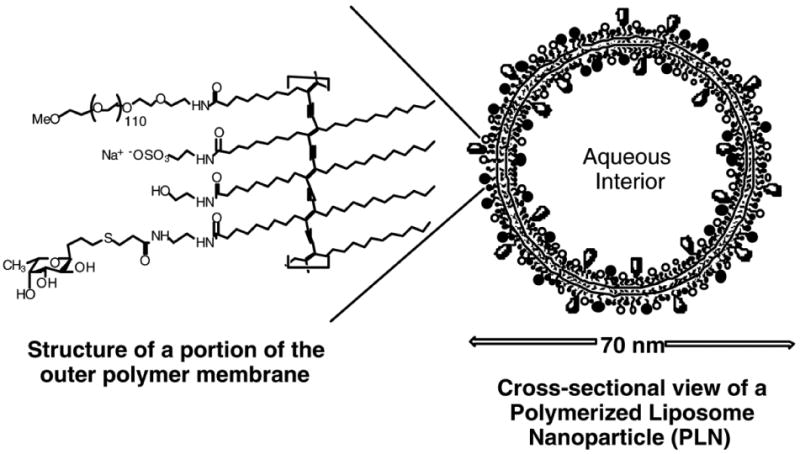
Interior and exterior surfaces of the polymerized lipid nanoparticle displaying the polyvalent ligands (fucose and sulfate ester groups) that mimic the physiological P-selectin super ligand: PSGL-1.
Figure 2. Inhibition of P-selectin-mediated, but not E-selectin-mediated, leukocyte cell tethering/rolling by PLNP under shear in vitro.
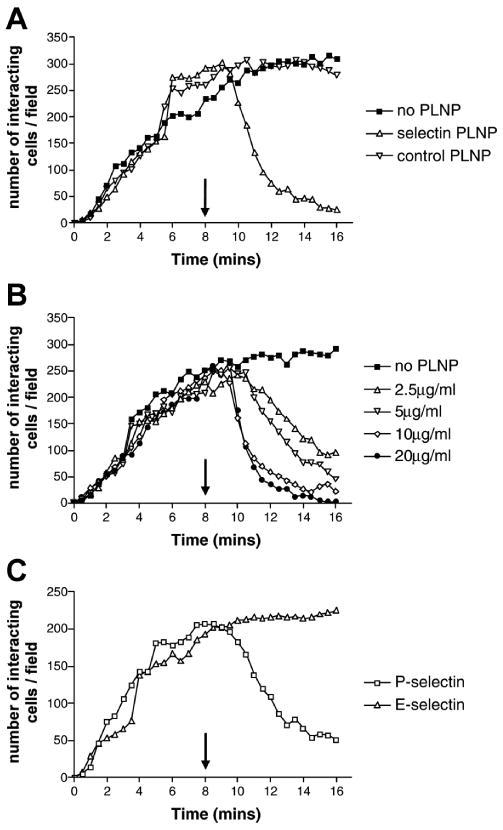
A) Selectin-blocking PLNP (1% PEG) or negative control PLNP (15% PEG) were administered after U-937 cell rolling was established on P-selectin-coated capillary tubes, and the number of cells interacting with the wall of the capillary tube was determined. B) Rolling of U937 cells was established on P-selectin chimera-coated capillary tubes, and the dose-dependent effect of P-selectin blocking PLNP was determined. C) Comparison of the inhibitory effect of selectin-blocking PLNP on U-937 interactions with P- or E-selectin chimera-coated capillary tubes. All data are representative of three to four independent experiments showing similar results.
Binding of nanoparticles in LPS-induced inflammation
To evaluate PLNP binding in lung tissue and establish the pattern of PLNP distribution within the lungs following i.v. administration (Fig. 3), we used an endotoxin model of systemic activation in which E- and P-selectin expression is up-regulated (33). Wild-type (WT) C57Bl6 mice and mice deficient in E- and P-selectin (E/P−/−) expression were injected with i.v. LPS and then received PLNP by i.v. injection 2 h later. Tissue samples were collected for histological analysis 3 h after PLNP injection. PLNP have a bright fluorescence in the rodamine channel, a unique property specific to this type of polymer backbone, making the particles easy to visualize in tissue sections. In the absence of LPS, very few of the PLNP were found in contact with the endothelium of blood vessels in the lungs of WT mice (data not shown). Following i.v. LPS treatment for 2 h, extensive PLNP binding was visible mainly on the endothelial cells within the lung vasculature (Fig. 3A). In E/P−/− mice stimulated with LPS, there appeared to be little or no direct binding of the PLNP to the endothelial cells, although some PLNP did appear to be associated with a small number of cells near the wall of blood vessels in the peribronchial regions of the lungs (Fig. 3B). The cells were identified as leukocytes by analysis of hematoxylin and eosin-stained serial histological sections of the lung.
Figure 3. Selectin-specific PLNP binding to endothelium in the lungs following LPS treatment.
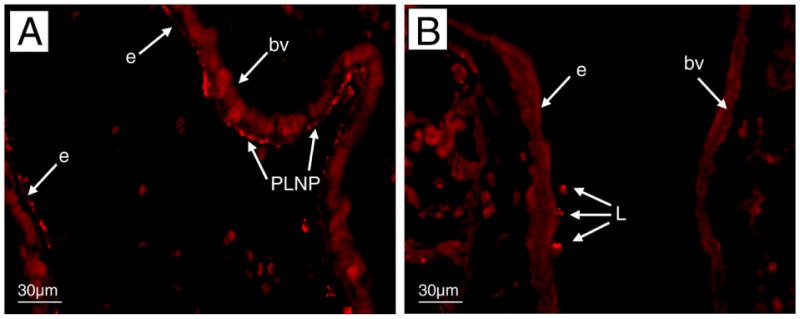
WT C57/BL6 (A) or E/P−/− (B) mice received selectin PLNP intravenously 2 h after LPS administration, and lung tissues were collected 3 h later. The pattern of binding of fluorescent PLNP was examined by fluorescence microscopy (×400). Data is representative of three independent experiments. PLNP, nanoparticles; e, endothelium; bv, blood vessel; L, leukocyte.
Attenuation of allergic inflammation by selectin PLNP
To evaluate PLNP activity in allergic asthma, we examined the pattern of PLNP binding in the murine model in which animals are sensitized with cockroach allergen (CRA) (30, 31). Nanoparticles were infused i.v. (5 μl of 12 mg/ml PLNP suspension in 200 μl of PBS) 2 h after the final airway CRA challenge and distribution of P-selectin-specific PLNP or control PLNP was assessed in lungs collected 24 h later. Negative control PLNP were not detected in the lungs either in association with leukocytes or endothelial cells within the vasculature (Fig. 4A). In contrast, the P-selectin-specific PLNP were visible on the endothelial cell surface of several different sized blood vessels in peribronchial regions of the lungs almost 24 h after administration, indicating the continued presence of selectin expression in this model (Fig. 4B). These PLNP were also seen in association with the alveolar walls but were not present in large airways.
Figure 4. Binding of selectin PLNP in lungs following allergen challenge.
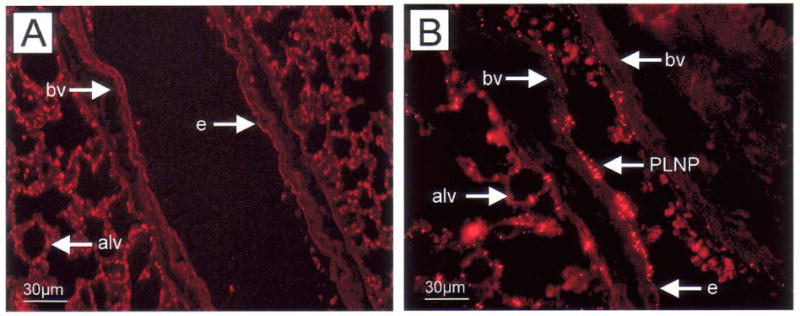
Negative control PLNP (a) or selectin-specific PLNP (b) were administered i.v. to Balb/c mice 2 h after the final intratracheal CRA challenge (day 20). The presence of PLNP in lungs collected 24 h later was established by fluorescence microscopy (×400). PLN, nanoparticles; e, endothelium; bv, blood vessel.
We next examined whether a single administration of the P-selectin-specific PLNP was effective in altering the responses in CRA-induced lung inflammation. Balb/c mice received PLNP by i.v. administration 2 h after the final i.t. CRA challenge. AHR was assessed 24 h later. There was no significant difference in the level of AHR detected in mice receiving negative control PLNP or CRA in the absence of PLNP (Fig. 5). Methacholine-induced AHR was significantly decreased in mice treated with P-selectin-specific PLNP compared with those treated with CRA alone or control PLNP. The reduction in AHR was accompanied by a significant reduction in peribronchial inflammation in PLNP-treated mice (Fig. 6B) compared with control mice (Fig. 6A). Assessment of peribronchial eosinophilia showed a significant decrease in the recruitment of these cells around the airway following selectin PLNP treatment (Fig. 7). Thus, selectin PLNP administration attenuates the level of both peribronchial inflammation and pulmonary hyperresponsiveness associated with allergen challenge.
Figure 5. Attenuation of cockroach allergen-induced AHR by selectin PLNP.
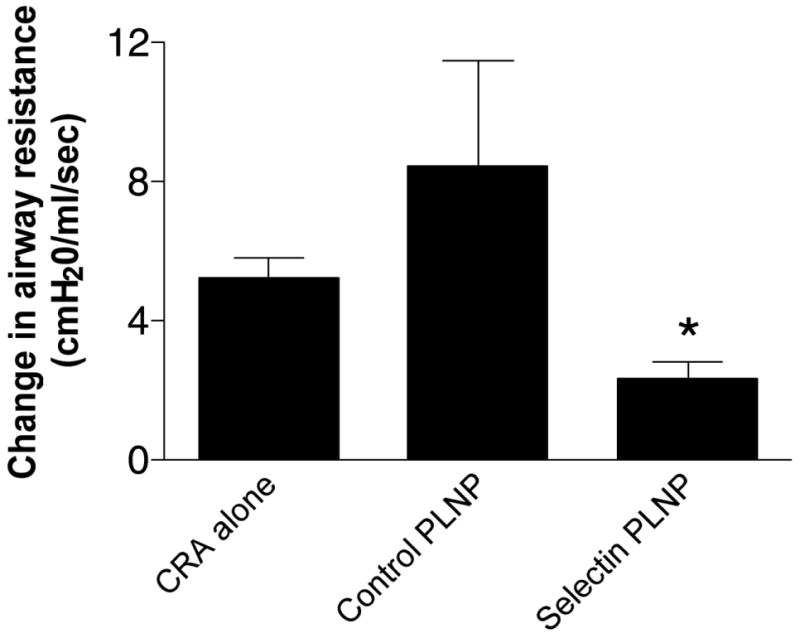
Negative control PLNP or selectin-specific PLNP were administered i.v. to Balb/c mice, 2 h after the final intratracheal CRA challenge (day 20). The level of airway resistance was determined following methacholine challenge, and the effect of administration of selectin PLNP or control PLNP compared with mice receiving CRA alone. Data is representative of three independent experiments and expressed as mean ±SE (n=5–6 mice per group).
Figure 6. Reduction of cockroach allergen-induced peribronchial inflammation by selectin PLNP.
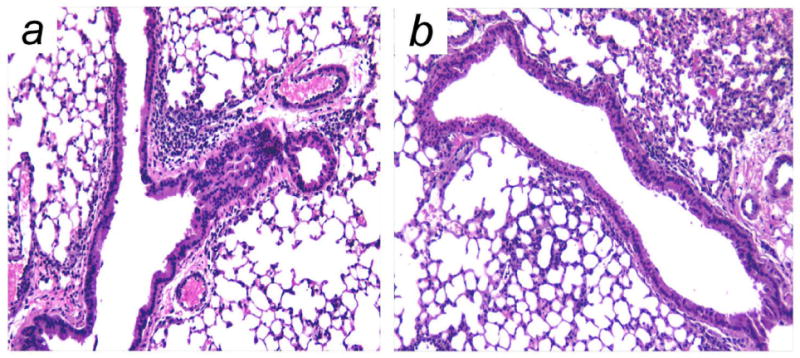
Peribronchial inflammation was assessed in hematoxylin and eosin-stained lung sections collected 24 h after administration of (A) CRA alone or (B) CRA with selectin PLNP (×200). Data are representative of images obtained in three independent experiments.
Figure 7. Inhibition of peribronchial eosinophil recruitment by selectin PLNP.
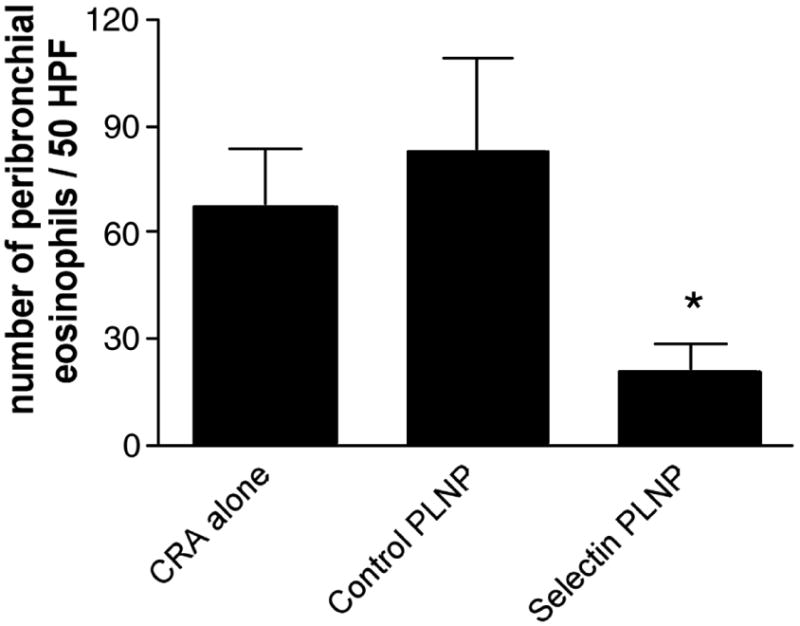
The number of peribronchial eosinophils was determined in 50 high power fields from each hematoxylin and eosin-stained lung section (×1000). Data are representative of three independent experiments and expressed as mean ±SE (n=5–6 mice per group).
Discussion
Several studies have identified selectins as potential targets for the design of novel anti-inflammatory agents, including selectin antagonists (19–22, 24, 34–42) and selectin-targeted immunoliposomes designed for local delivery of compounds to the endothelium (43–46). Research has focused on identifying the structural features required for high selectin binding affinity, and based on these studies, some low molecular weight, noncarbohydrate, nonpeptide inhibitors of P-selectin have been identified (34–37, 40). Therapeutically viable P-selectin inhibitors have proven difficult to realize due to the weak binding of the monovalent ligands and extreme cost associated with the production of physiological ligand-based inhibitors such as sLex or PSGL-1. Advances in this area have also been hampered by the lack of high-throughput physiologic assays that rigorously measure the potency of potential inhibitors under conditions that truly mimic blood flow. Many reported selectin inhibitors have typically been assayed under static conditions. Although this may give a starting point from which to design inhibitors, the inhibition measured under static conditions with this type of adhesion protein can be completely irrelevant when subjected to shear forces (20). Antiquated in vitro assays primarily measure adhesion in static assays, but we now know that interactions must be measured under shear forces that approximate those defined in vivo to gain a full appreciation of an interaction that takes place in the blood.
To simulate the microenvironment a drug and leukocyte would experience in vivo, we used an in vitro system in which the luminal surfaces of capillary tubes were coated with P-selectin proteins. The “vessels” are integrated into a loop system in which fluid can be recirculated via a peristaltic pump. PSGL-1-expressing U-937 cells were injected into the system, and their interaction with the protein monolayer was monitored by videomicroscopy. Potential P-selectin inhibitors are infused into the assay, and their effect on leukocyte-protein interactions are readily measured.
Identification of critical interactions that occur between P-selectin and the physiologic ligand PSGL-1 served as a starting point for the design of our inhibitor. In examining the crystal structure of this complex, fucose (in the sLex tetrasaccharide) makes several critical hydrogen bonds to the calcium and protein backbone in the binding site of the lectin domain (23). In addition, strong ionic interactions are facilitated by sulfated tyrosine residues neighboring the glycosylation site on the N terminus of PSGL-1 with the P-selectin His and Arg groups. Although fucose as the unmodified monosaccharide has no reported P-selectin blocking activity, several sulfated carbohydrates (47, 48) and sulfated fucooligosaccharides (40) do show inhibition. Therefore, if fucose and sulfate could be presented to P-selectin in the proper orientation on a synthetic carrier, a relatively simple inhibitor could be designed. Several studies have shown that multimerizing selectin ligands increases the potency of the displayed ligand orders of magnitude over the monovalent form (20–22, 24, 38, 39, 49). In this methodology, the optimal distance between the binding sites need not be accurately known. With a random distribution of ligands on a polyvalent surface, the selectin protein can find an orientation that binds with high affinity.
We and others have found that polyvalent display of fucose or fucosylated oligosaccharides in proximity to multiple sulfate ester groups creates a potent inhibitor for P-selectin-mediated adhesion (21, 22, 24, 49). Our PLNP display fucose and sulfate groups on a macromolecular structure created by mixing lipids functionalized with these molecules to direct self-assembly into liposome bilayers. The liposomes are stabilized by photochemically cross-linking the lipid monomers into polydiacetylene polymers maintaining the size and shape as the precursor liposome, increasing stability (50). The resulting PLNP have a size and composition that can be easily controlled, and, as a result of polymerization, they are less likely to fuse with themselves or other lipid membranes. The nonspecific effect of nanoparticle administration on peripheral blood granulocyte and mononuclear cell counts was examined in control untreated mice. Intravenous administration of negative control PLNP, selectin-specific PLNP, or vehicle alone (PBS) had no significant effect on the number of leukocytes in the circulation either 8 or 24 h later (data not shown). We have demonstrated that the PLNP are targeted primarily to P-selectin on the endothelium and preferentially inhibit established P-selectin-dependent, but not E-selectin-dependent, leukocyte rolling/attachment in vitro. The inhibition of cell rolling is dose-dependent, with a total particle weight IC50 of ∼2 μg/ml. This represents a carbohydrate concentration of 34 ng/ml.
The severity of allergic airway disease is primarily dependent on the extent of the inflammatory response, and attenuation of leukocyte infiltration in the lung is frequently correlated with clinical improvement (8, 9). Several studies have demonstrated a role for adhesion molecules in the attenuation of allergic airway inflammation. ICAM-1/LFA-1 as well as E- and P-selectins have all been shown to play a role in the development of peribronchial inflammation and airway hyperreactivity (11, 12, 14). When administered 2 h after an intratracheal CRA challenge, the P-selectin-specific PLNP significantly attenuated both methacholine-induced airway hyperresponsiveness and peribronchial eosinophilia measured 24 h later. At this time point, PLNP could still be identified in association with the endothelial cell surface of blood vessels found throughout the lung, although we concentrated primarily on the vessels in peribronchial regions of the lung. Because 24 h was the only time point at which nanoparticle binding was assessed, the maximum length of time the PLNP remained attached to selectins on the endothelium has yet to be determined. Although P-selectin expression is generally considered to be a transient feature of acute inflammation, prolonged expression of this adhesion molecule in the lung vasculature has previously been demonstrated in lung injury models (51, 52). It is possible that a single administration of PLNP may inhibit leukocyte recruitment for significantly longer than 24 h.
Our studies indicate that binding of nanoparticles to the endothelium is selectin-specific in both LPS- and CRA-induced inflammation. In the absence of an inflammatory stimulus, very few nanoparticles could be identified in direct contact with the endothelium. Furthermore, in the same lung inflammation models in E/P selectin−/− mice, PLNP binding to the endothelium was not observed. Some nanoparticles were found in association with leukocytes either in contact with the endothelium or in the vessel lumen in these mice. It was not possible to distinguish whether the PLNP were binding to the surface of the marginated leukocytes or had been phagocytosed. Additional studies are underway to examine this latter aspect.
In summary, these studies demonstrate the discovery of a P-selectin inhibitor with potent in vivo activity by using a physiologically relevant in vitro shear assay system. The use of novel glycomimetics to specifically deliver sterically inhibitory P-selectin binding nanoparticles to the endothelium may have therapeutic potential for allergies and asthma, as well as other inflammatory diseases.
Acknowledgments
This research was funded by NIH SBIR grant R44 AI43789-03. We thank the following people for preparing PLNP and conducting biological screening assays and for technical assistance in the preparation of this manuscript: Evelyn E. Benson, Sarah Warwood, Kelli Buckingham-Meyer, Gayle M. Watts, Virginia Perry, and James Grenzebach. We also thank Mark Jutila for critical reading of the manuscript.
References
- 1.International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema. Lancet. 1998;351:1225–1232. [PubMed] [Google Scholar]
- 2.International Study of Asthma and Allergies in Childhood (ISAAC) Worldwide variations in the prevalence of asthma symptoms. Eur Respir J. 1998;12:315–335. doi: 10.1183/09031936.98.12020315. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro JM. Childhood asthma in the United States: urban issues. Pediatr Pulmonol. 2002;33:47–55. doi: 10.1002/ppul.10029. [DOI] [PubMed] [Google Scholar]
- 4.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, Mitchell H, McNiff-Mortimer K, Lynn H, Ownby D, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 5.Mielck A, Reitmeir P, Wjst M. Severity of childhood asthma by socioeconomic status. Int J Epidemiol. 1996;25:388–393. doi: 10.1093/ije/25.2.388. [DOI] [PubMed] [Google Scholar]
- 6.Tedder TF, Steeber DA, Chen A, Engel P. The selectins: vascular adhesion molecules. FASEB J. 1995;9:866–873. [PubMed] [Google Scholar]
- 7.Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- 8.Hartert TV, Peebles RS., Jr Epidemiology of asthma: the year in review. Curr Opin Pulm Med. 2000;6:4–9. doi: 10.1097/00063198-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Busse WW, Banks-Schlegel S, Wenzel SE. Pathophysiology of severe asthma. J Allergy Clin Immunol. 2000;106:1033–1042. doi: 10.1067/mai.2000.111307. [DOI] [PubMed] [Google Scholar]
- 10.Skoner DP. Balancing safety and efficacy in pediatric asthma management. Pediatrics. 2002;109:381–392. [PubMed] [Google Scholar]
- 11.Broide DH, Sullivan S, Gifford T, Sriramarao P. Inhibition of pulmonary eosinophilia in P-selectin- and ICAM-1-deficient mice. Am J Respir Cell Mol Biol. 1998;18:218–225. doi: 10.1165/ajrcmb.18.2.2829. [DOI] [PubMed] [Google Scholar]
- 12.De Sanctis GT, Wolyniec WW, Green FH, Qin S, Jiao A, Finn PW, Noonan T, Joetham AA, Gelfand E, Doerschuk CM, et al. Reduction of allergic airway responses in P-selectin-deficient mice. J Appl Physiol. 1997;83:681–687. doi: 10.1152/jappl.1997.83.3.681. [DOI] [PubMed] [Google Scholar]
- 13.Tang ML, Fiscus LC. Important roles for L-selectin and ICAM-1 in the development of allergic airway inflammation in asthma. Pulm Pharmacol Ther. 2001;14:203–210. doi: 10.1006/pupt.2001.0293. [DOI] [PubMed] [Google Scholar]
- 14.Lukacs NW, John A, Belin A, Bullard DC, Knibbs RN, Stoolman LM. E- and P- selectins are essential for the development of cockroach allergen-induced airway responses. J Immunol. 2002;169:2120–2125. doi: 10.4049/jimmunol.169.4.2120. [DOI] [PubMed] [Google Scholar]
- 15.Romano SJ, Slee DH. Targeting selectins for the treatment of respiratory diseases. Curr Opin Investig Drugs. 2001;2:907–913. [PubMed] [Google Scholar]
- 16.Woltmann G, McNulty CA, Dewson G, Symon FA, Wardlaw AJ. Interleukin-13 induces PSGL-1/P-selectin-dependent adhesion of eosinophils, but not neutrophils, to human umbilical vein endothelial cells under flow. Blood. 2000;95:3146–3152. [PubMed] [Google Scholar]
- 17.Symon FA, Lawrence MB, Williamson ML, Walsh GM, Watson SR, Wardlaw AJ. Functional and structural characterization of the eosinophil P-selectin ligand. J Immunol. 1996;157:1711–1719. [PubMed] [Google Scholar]
- 18.Edwards BS, Curry MS, Tsuji H, Brown D, Larson RS, Sklar LA. Expression of P-selectin at low site density promotes selective attachment of eosinophils over neutrophils. J Immunol. 2000;165:404–410. doi: 10.4049/jimmunol.165.1.404. [DOI] [PubMed] [Google Scholar]
- 19.Marinier A, Martel A, Bachand C, Plamondon S, Turmel B, Daris JP, Banville J, Lapointe P, Ouellet C, Dextraze P, et al. Novel mimics of sialyl Lewis X: design, synthesis and biological activity of a series of 2- and 3-malonate substituted galactoconjugates. Bioorg Med Chem. 2001;9:1395–1427. doi: 10.1016/s0968-0896(01)00015-3. [DOI] [PubMed] [Google Scholar]
- 20.Sanders WJ, Gordon EJ, Dwir O, Beck PJ, Alon R, Kiessling LL. Inhibition of L-selectin-mediated leukocyte rolling by synthetic glycoprotein mimics. J Biol Chem. 1999;274:5271–5278. doi: 10.1074/jbc.274.9.5271. [DOI] [PubMed] [Google Scholar]
- 21.Pochechueva TV, Galanina OE, Bird MI, Nifantiev NE, Bovin NV. Assembly of p-selectin ligands on a polymeric template. Chem Biol. 2002;9:757–762. doi: 10.1016/s1074-5521(02)00157-6. [DOI] [PubMed] [Google Scholar]
- 22.Nishida Y, Uzawa H, Toba T, Sasaki K, Kondo H, Kobayashi K. A facile synthetic approach to L- and P-selectin blockers via copolymerization of vinyl monomers constructing the key carbohydrate modules of sialyl LewisX mimics. Biomacromolecules. 2000;1:68–74. doi: 10.1021/bm990011o. [DOI] [PubMed] [Google Scholar]
- 23.Somers WS, Tang J, Shaw GD, Camphausen RT. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1. Cell. 2000;103:467–479. doi: 10.1016/s0092-8674(00)00138-0. [DOI] [PubMed] [Google Scholar]
- 24.Bruehl RE, Dasgupta F, Katsumoto TR, Tan JH, Bertozzi CR, Spevak W, Ahn DJ, Rosen SD, Nagy JO. Polymerized liposome assemblies: bifunctional macromolecular selectin inhibitors mimicking physiological selectin ligands. Biochemistry. 2001;40:5964–5974. doi: 10.1021/bi002921s. [DOI] [PubMed] [Google Scholar]
- 25.New RRC. In: Liposomes: A Practical Approach. New RRC, editor. Oxford University Press; Oxford: 1990. pp. 33–104. [Google Scholar]
- 26.Bargatze RF, Kurk S, Watts G, Kishimoto TK, Speer CA, Jutila MA. In vivo and in vitro functional examination of a conserved epitope of L- and E-selectin crucial for leukocyte-endothelial cell interactions. J Immunol. 1994;152:5814–5825. [PubMed] [Google Scholar]
- 27.Knibbs RN, Craig RA, Maly P, Smith PL, Wolber FM, Faulkner NE, Lowe JB, Stoolman LM. Alpha(1,3)-fucosyltransferase VII-dependent synthesis of P- and E-selectin ligands on cultured T lymphoblasts. J Immunol. 1998;161:6305–6315. [PubMed] [Google Scholar]
- 28.Ley K, Cerrito M, Arfors KE. Sulfated polysaccharides inhibit leukocyte rolling in rabbit mesentery venules. Am J Physiol. 1991;260:H1667–H1673. doi: 10.1152/ajpheart.1991.260.5.H1667. [DOI] [PubMed] [Google Scholar]
- 29.Perry MA, Granger DN. Role of CD11/CD18 in shear rate-dependent leukocyte-endothelial cell interactions in cat mesenteric venules. J Clin Invest. 1991;87:1798–1804. doi: 10.1172/JCI115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chensue SW, Lukacs NW, Yang TY, Shang X, Frait KA, Kunkel SL, Kung T, Wiekowski MT, Hedrick JA, Cook DN, et al. Aberrant in vivo T helper type 2 cell response and impaired eosinophil recruitment in CC chemokine receptor 8 knockout mice. J Exp Med. 2001;193:573–584. doi: 10.1084/jem.193.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell EM, Kunkel SL, Strieter RM, Lukacs NW. Temporal role of chemokines in a murine model of cockroach allergen- induced airway hyperreactivity and eosinophilia. J Immunol. 1998;161:7047–7053. [PubMed] [Google Scholar]
- 32.Lukacs NW, Strieter RM, Warmington K, Lincoln P, Chensue SW, Kunkel SL. Differential recruitment of leukocyte populations and alteration of airway hyperreactivity by C-C family chemokines in allergic airway inflammation. J Immunol. 1997;158:4398–4404. [PubMed] [Google Scholar]
- 33.Eppihimer MJ, Wolitzky B, Anderson DC, Labow MA, Granger DN. Heterogeneity of expression of E- and P-selectins in vivo. Circ Res. 1996;79:560–569. doi: 10.1161/01.res.79.3.560. [DOI] [PubMed] [Google Scholar]
- 34.Ohta S, Inujima Y, Abe M, Uosaki Y, Sato S, Miki I. Inhibition of P-selectin specific cell adhesion by a low molecular weight, non-carbohydrate compound, KF38789. Inflamm Res. 2001;50:544–551. doi: 10.1007/PL00000232. [DOI] [PubMed] [Google Scholar]
- 35.Slee DH, Romano SJ, Yu J, Nguyen TN, John JK, Raheja NK, Axe FU, Jones TK, Ripka WC. Development of potent non-carbohydrate imidazole-based small molecule selectin inhibitors with antiinflammatory activity. J Med Chem. 2001;44:2094–2107. doi: 10.1021/jm000508c. [DOI] [PubMed] [Google Scholar]
- 36.Kaila N, Xu GY, Camphausen RT, Xiang Y. Identification and structural determination of a potent P-selectin inhibitor. Bioorg Med Chem. 2001;9:801–806. doi: 10.1016/s0968-0896(00)00298-4. [DOI] [PubMed] [Google Scholar]
- 37.Schon MP, Krahn T, Schon M, Rodriguez ML, Antonicek H, Schultz JE, Ludwig RJ, Zollner TM, Bischoff E, Bremm KD, et al. Efomycine M, a new specific inhibitor of selectin, impairs leukocyte adhesion and alleviates cutaneous inflammation. Nat Med. 2002;8:366–372. doi: 10.1038/nm0402-366. [DOI] [PubMed] [Google Scholar]
- 38.Simanek EE, GJ M, Jablonowski JA, Wong CH. Selectin-carbohydrate interactions: From natural ligands to designed mimics. Angew Chem Int Ed. 1998;38:2300–2324. doi: 10.1021/cr940226i. [DOI] [PubMed] [Google Scholar]
- 39.Sears P, Wong CH. Carbohydrate Mimetics: A New Strategy for Tackling the Problem of Carbohydrate-Mediated Biological Recognition. Angew Chem Int Ed Engl. 1999;38:2300–2324. [PubMed] [Google Scholar]
- 40.Kaila N, Thomas BET. Design and synthesis of sialyl Lewis(x) mimics as E- and P-selectin inhibitors. Med Res Rev. 2002;22:566–601. doi: 10.1002/med.10018. [DOI] [PubMed] [Google Scholar]
- 41.Hicks AE, Leppanen A, Cummings RD, McEver RP, Hellewell PG, Norman KE. Glycosulfopeptides modeled on P-selectin glycoprotein ligand 1 inhibit P-selectin-dependent leukocyte rolling in vivo. FASEB J. 2002;16:1461–1462. doi: 10.1096/fj.02-0075fje. [DOI] [PubMed] [Google Scholar]
- 42.Kurokawa K, Kumihara H, Kondo H. A solid-phase synthesis for beta-turn mimetics of sialyl Lewis X. Bioorg Med Chem Lett. 2000;10:1827–1830. doi: 10.1016/s0960-894x(00)00358-9. [DOI] [PubMed] [Google Scholar]
- 43.Spragg DD, Alford DR, Greferath R, Larsen CE, Lee KD, Gurtner GC, Cybulsky MI, Tosi PF, Nicolau C, Gimbrone MA., Jr Immunotargeting of liposomes to activated vascular endothelial cells: a strategy for site-selective delivery in the cardiovascular system. Proc Natl Acad Sci USA. 1997;94:8795–8800. doi: 10.1073/pnas.94.16.8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stahn R, Grittner C, Zeisig R, Karsten U, Felix SB, Wenzel K. Sialyl Lewis(x)-liposomes as vehicles for site-directed, E-selectin-mediated drug transfer into activated endothelial cells. Cell Mol Life Sci. 2001;58:141–147. doi: 10.1007/PL00000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bendas G, Krause A, Schmidt R, Vogel J, Rothe U. Selectins as new targets for immunoliposome-mediated drug delivery. A potential way of anti-inflammatory therapy. Pharm Acta Helv. 1998;73:19–26. doi: 10.1016/s0031-6865(97)00043-5. [DOI] [PubMed] [Google Scholar]
- 46.Kessner S, Krause A, Rothe U, Bendas G. Investigation of the cellular uptake of E-Selectin-targeted immunoliposomes by activated human endothelial cells. Biochim Biophys Acta. 2001;1514:177–190. doi: 10.1016/s0005-2736(01)00368-6. [DOI] [PubMed] [Google Scholar]
- 47.Koenig A, Norgard-Sumnicht K, Linhardt R, Varki A. Differential interactions of heparin and heparan sulfate glycosaminoglycans with the selectins. Implications for the use of unfractionated and low molecular weight heparins as therapeutic agents. J Clin Invest. 1998;101:877–889. doi: 10.1172/JCI1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ikami T, Tsuruta N, Inagaki H, Kakigami T, Matsumoto Y, Tomiya N, Jomori T, Usui T, Suzuki Y, Tanaka H, et al. Synthetic studies on selectin ligands/inhibitors. Synthesis and biological evaluation of sulfated and phosphorylated beta-D-galacto- and lactopyranosides containing fatty-alkyl residues of different carbon chain lengths. Chem Pharm Bull (Tokyo) 1998;46:797–806. doi: 10.1248/cpb.46.797. [DOI] [PubMed] [Google Scholar]
- 49.Galustian C, Childs RA, Stoll M, Ishida H, Kiso M, Feizi T. Synergistic interactions of the two classes of ligand, sialyl-Lewis(a/x) fuco-oligosaccharides and short sulpho-motifs, with the P- and L-selectins: implications for therapeutic inhibitor designs. Immunology. 2002;105:350–359. doi: 10.1046/j.1365-2567.2002.01369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ringsdorf H, Schlarb B, Venzmer J. Molecular architecture and function of polymeric oriented systems: models for the study of organization, surface recognition and dynamics of biomembranes. Agnew Chem Int Ed. 1988;27:114–158. [Google Scholar]
- 51.Bless NM, Tojo SJ, Kawarai H, Natsume Y, Lentsch AB, Padgaonkar VA, Czermak BJ, Schmal H, Friedl HP, Ward PA. Differing patterns of P-selectin expression in lung injury. Am J Pathol. 1998;153:1113–1122. doi: 10.1016/S0002-9440(10)65655-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolber FM, Curtis JL, Milik AM, Fields T, Seitzman GD, Kim K, Kim S, Sonstein J, Stoolman LM. Lymphocyte recruitment and the kinetics of adhesion receptor expression during the pulmonary immune response to particulate antigen. Am J Pathol. 1997;151:1715–1727. [PMC free article] [PubMed] [Google Scholar]


