Abstract
Stable Isotope Standards and Capture by Anti-Peptide Antibodies (SISCAPA) utilizes antibodies to enrich peptides from complex matrices for quantitation by stable isotope dilution mass spectrometry. SISCAPA improves sensitivity and limits the sample handling required for plasma-based analysis. Thus far, SISCAPA assays have been performed using polyclonal antibodies, yet monoclonal antibodies are an attractive alternative since they provide exquisite specificity, a renewable resource, and the potential for isolation of clones with very high affinities (10-9 M or better). The selection of a good monoclonal antibody out of hundreds-to-thousands of clones presents a challenge, since the screening assay should ideally be in the format of the final SISCAPA assay, but performing the assays manually is labor- and time-intensive. In this manuscript, we demonstrate that monoclonal antibodies can be used in SISCAPA assays, and we describe an automated high-throughput SISCAPA method that makes screening of large numbers of hybridomas feasible while conserving time and resources.
Keywords: SISCAPA, anti-peptide antibody, monoclonal antibody screening, selected reaction monitoring-mass spectrometry, automation, ELISA
1. Introduction
Tremendous time and effort have been invested in protein biomarker discovery and verification since biomarkers hold the promise of early detection and better diagnosis, prognosis, and treatment of disease. Verification, which requires the development of a quantitative assay for each candidate biomarker, remains a major bottleneck in the biomarker pipeline (Rifai et al., 2006; Paulovich et al., 2008) since “gold standard” technologies such as an ELISA are costly and have lengthy development lead times.
As an alternative and more specific verification assay technology, Anderson et al. (Anderson et al., 2004) introduced Stable Isotope Standards and Capture by Anti-Peptide Antibodies (SISCAPA), which uses antibodies to capture and enrich proteotypic peptides from proteolyzed biospecimens such as plasma or tissue lysates, resulting in 102-103 fold enrichment of the target peptide. The enriched analyte is then quantified by stable isotope dilution selected reaction monitoring-mass spectrometry (SRM-MS) using a spiked-in isotopically labeled synthetic peptide. The technique has been adapted to a magnetic bead format (Whiteaker et al., 2007a; Whiteaker et al., 2007b) and used to configure assays for several potential and known biomarkers (Whiteaker et al., 2007a; Hoofnagle et al., 2008; Kuhn et al., 2009).
To date, SISCAPA has been performed with affinity-purified polyclonal antibodies (PcAbs). However, for some applications a monoclonal antibody (McAb) would be advantageous. For example, if an assay is to be performed many times, then a standardized renewable affinity reagent will be required. Additionally, it is theoretically possible that by screening large numbers of hybridoma supernatants, very high affinity monoclonal antibodies might be identified, producing the most sensitive SISCAPA assays possible. (Many therapeutic McAbs have affinities of ∼10-9 M or better (Wark and Hudson, 2006).) Identifying the McAb with the highest affinity for a peptide target involves screening 100s-1000s of hybridoma supernatants, depending on the antigen. A major hurdle to the development of monoclonal antibodies for SISCAPA assays is that manually screening hybridoma supernatants in the current assay format is labor-intensive and low-throughput. In addition, screening exclusively by traditional ELISA is undesirable because ELISA results do not correlate perfectly with SISCAPA assay activity. Therefore, there is a strong need to develop a protocol to screen through the hundreds-to-thousands of hybridoma supernatants necessary to identify high quality monoclonal antibodies for SISCAPA assays.
In this study, we demonstrate that monoclonal antibodies can be used to configure SISCAPA assays. Additionally, we develop an automated, high-throughput method for screening hybridoma supernatants directly in the SISCAPA assay format. The techniques described herein will allow several hundred supernatants to be screened per week per operator, thereby facilitating the development of high affinity monoclonal antibodies for SISCAPA assays.
2. Materials and Methods
2.1 Peptide and antibody reagents
The two peptides used in these studies (identifiers ADAM17(VDN) and CRP(APL)) had the sequences VDNEELLPK (molecular weight 1055.6 Da) and APLTKPLK (molecular weight 866.6 Da), respectively. The ADAM17(VDN) and CRP(APL) peptides are derived from the human proteins tumor necrosis factor (TNF)-alpha converting enzyme (ADAM 17, molecular weight 93 021 Da) and C-reactive protein (CRP, molecular weight 25 039 Da), respectively. Synthetic peptides were obtained from Sigma (St. Louis, MO) (for ADAM17(VDN)) and from the Massachusetts Institute of Technology (Cambridge, MA) (for CRP(APL)). For the stable isotope-labeled versions of the peptides, the C-terminal lysine of ADAM17(VDN) was 13C and 15N labeled (molecular weight 1063.5 Da), and the C-terminal lysine of CRP(APL) was 13C labeled (molecular weight 872.6 Da).
The mouse monoclonal antibodies for ADAM17(VDN) and CRP(APL) were generated at Epitomics (Burlingame, CA). For each target, six mice were immunized with a peptide-GSGC-SMCC-KLH (succinimidyl-4-(N-maleimidomethyl)-cyclohexane-1-carboxylate-keyhole lympet hemocyanin) conjugate. The cysteine in the -GSGC- linker sequence provided a thiol group for covalently linking the peptide to the carrier protein, while the -GSG- sequence served as a spacer with fairly low antigenicity (Anderson et al., 2004). The titers of the mice were tested with peptide-GSGC-SMCC-BSA (bovine serum albumin) ELISA, and the spleens of the two mice with the highest titers were harvested. Fusions were performed with the lymphocytes of the two best mice and twenty 96-well plates containing hybridomas were prepared for each mouse. The hybridomas were screened with peptide-GSGC-SMCC-BSA ELISA and positive hybridomas were expanded to 24-well plates. The expanded hybridomas were screened by peptide-GSGC-SMCC-BSA ELISA once more and the supernatants of hybridomas that were confirmed in the second screen were subsequently tested with peptide-glutaraldehyde-BSA ELISA and free peptide ELISA (see below). For ADAM17(VDN), ELISA-positive primary clones were screened with manual SISCAPA and the five best-performing primary clones were subcloned. The subclones were in turn tested with manual SISCAPA and the best-performing subclone was chosen for production and subsequent purification by Protein-A affinity chromatography. For CRP(APL), monoclonal antibody screening with ELISA and SISCAPA has thus far been performed up to the primary clone stage.
2.2 ELISAs
2.2.1 Peptide-glutaraldehyde-BSA screening ELISA
ELISA plates were purchased from Greiner Bio-One (650061, Monroe, NC), and 10× tris-buffered saline (TBS) was from Cellgro (46-012-CM, Mediatech, Manassas, VA). Alkaline phosphatase conjugated goat-anti-mouse 2° antibody (31326), diethanolamine substrate buffer (34064), and p-nitrophenyl phosphate (pNPP) substrate (34045) were obtained from Pierce Biotechnology (Rockford, IL).
The ADAM17(VDN) or CRP(APL) peptide-glutaraldehyde-BSA screening antigen was diluted to 1 μg/mL in carbonate-bicarbonate coating buffer. A 50 μL aliquot was added to each well of an ELISA plate, and the plate was sealed with seal film and kept at 4°C overnight. The plate was washed once with 1× TBS-0.05% Tween-20 (TBST) and 60 μL of a 1% BSA in 1× TBS blocking solution was added. The plate was shaken at room temperature (RT) for 1 hour, after which the blocking solution was emptied from the plate and a 50 μL aliquot of each primary clone supernatant was added to separate wells. The plate was shaken at RT for 1.5 hours and then washed once with TBST. A 50 μL aliquot of goat-anti-mouse 2° antibody, diluted 1:2,500 with 1% BSA in 1× TBS, was added and the plate was shaken at RT for 1 hour. The plate was subsequently washed twice with TBST and 50 μL of a 5% diethanolamine substrate buffer-1% pNPP substrate solution in Millipore water was added and allowed to react for 15 minutes at RT. 50 μL of 3 N NaOH was added immediately after the 15 minutes to stop the reaction. The absorbance in each well was read at 405 nm. ELISA results were deemed positive if their absorbance optical density (OD) values were greater than half of the OD value of the positive control.
2.2.2 Free peptide screening ELISA
Dimethyl sulfoxide (DMSO) was purchased from Sigma (D2650, St. Louis, MO) and powdered milk was from EMD Chemicals (EM-1.15363.0500, San Diego, CA). The other materials were the same as used in the peptide-glutaraldehyde-BSA ELISA above.
Free light ADAM17(VDN) or CRP(APL) peptide in powder form was dissolved in DMSO and distilled water to produce a concentration of 1 mg/mL peptide in 1% DMSO concentration (DMSO was added first to dissolve the peptide completely, then the water was added). The peptide solution was further diluted to 4 μg/mL using distilled water. A 50 μL aliquot of this peptide solution was added to each well of an ELISA 96-well plate (0.2 μg of peptide per well) and the plate was dried at 37°C overnight. A 200 μL aliquot of 3% (w/v) powdered milk in 1× TBS was added to each well for blocking, the plate was sealed with seal film and incubated for 1 hour at 37°C. The plate was then washed three times with TBST and 50 μL of each primary clone supernatant was added to separate wells. The plate was incubated at 37°C for 1 hour and then washed twice with TBST. 50 μL of 1:2,500 diluted 2° antibody (with 1% BSA in 1× TBS) was added to each well and the plate was incubated for 1 hour at RT on a shaker. The plate was washed three times with TBST and 50 μL of a 5% diethanolamine substrate buffer-1% pNPP substrate solution in Millipore water was added to each well. The reaction was allowed to proceed for 15 minutes, after which 50 μL of 3 N NaOH was added to stop the reaction. The plate was read at 405 nm. ELISA results were deemed positive if their absorbance optical density (OD) values were greater than twice the average background OD values.
2.2.3 Quantitative mouse IgG ELISA
Corning Costar EIA/RIA polystyrene ELISA 96-well plates (3369), phosphate buffered saline (PBS, BP39920), and Tween-20 (BP337-500) were bought from Fisher Scientific (Waltham, MA). Thermal Seal Films for PCR (12-168) were from Genesee Scientific (San Diego, CA). Carbonate-bicarbonate coating buffer (C3041-100CAP), 3, 3′, 5, 5′-tetramethyl benzidine (TMB) liquid substrate system for ELISA (T0440-1L), and hydrochloric acid were obtained from Sigma. Nonfat dry milk (9999) was obtained from Cell Signaling Technology (Danvers, MA). Standard mouse IgG protein (ChromPure Mouse IgG, whole molecule, 015-000-003), goat-anti-mouse 1° antibody (115-005-072), and horseradish peroxidase enzyme-conjugated 2° antibody (115-035-164) were from Jackson ImmunoResearch Laboratories (West Grove, PA).
A 100 μL aliquot of 1 μg/mL of the 1° antibody in carbonate-bicarbonate buffer (1 capsule of carbonate-bicarbonate salt dissolved in 100 mL H2O) was added to each well of an ELISA plate, the plate was covered with seal film and incubated at 4°C overnight. The buffer was discarded, and 250 μL of 3% (w/v) nonfat dry milk in 1× PBS-0.05% Tween-20, pH 7.4, was added to each well and the plate was left covered at room temperature (RT) for 1 hour. A 20 ng/mL standard mouse IgG solution was prepared in 1% (w/v) nonfat dry milk in 1× PBS-0.05% Tween-20. In addition, the mouse primary clone or subclone supernatants were diluted 1:500 with 1% (w/v) nonfat dry milk in 1× PBS-0.05% Tween-20. The plate was washed three times with 1% (w/v) nonfat dry milk in 1× PBS-0.05% Tween-20 and 100 μL of 1% (w/v) nonfat dry milk in 1× PBS-0.05% Tween-20 was added to each well except to row A. To row A, 150 μL of the 20 ng/mL standard mouse IgG solution and 150 μL of each of the diluted supernatants were added in duplicate (five supernatants could be tested in one plate). A series of 1:3 serial dilutions using 50 μL of the IgG and supernatant solutions in row A were carried out down the plate; the plate was covered and left at RT for 1 hour. The plate was again washed three times and 100 μL of 1:5,000 diluted (with 1% (w/v) nonfat dry milk in 1× PBS-0.05% Tween-20) enzyme-conjugated 2° antibody was added to each well and incubated for 1 hour. The plate was washed three times and 100 μL of the TBM ELISA substrate (solution at RT) was added to each well. The color development was stopped after ∼5 minutes by addition of 50 μL of 0.4 N HCl. The plate was shaken for 30 seconds and allowed to equilibrate for 15 minutes. The absorbance was recorded at 405 nm and a calibration curve was generated based on the standard protein IgG absorbances. Dilution points of the supernatant samples that fell into the linear range of the standard IgG curve were chosen to calculate the supernatants' antibody concentrations.
2.3 Human plasma trypsin digestion
Human plasma (P9523-5mL) was obtained from Sigma. The lyophilized plasma was resuspended in 5 mL of distilled water and the plasma proteins were reduced by adding urea (8 M), Tris (50 mM, pH 8), and dithiothreitol (DTT) (10 mM) and by shaking at 60°C for 1 hour (the concentrations are final concentrations). The proteins were subsequently alkylated for 30 minutes at room temperature in the dark after adding freshly-made iodoacetamide (IAM) to a final concentration of 15 mM. After alkylation, 20 mM Tris, pH 8, was added to decrease the urea concentration to 1 M. The proteins were then digested by adding trypsin (T1426-250MG, Sigma) in a 1:20 w/w enzyme:protein ratio and by shaking the solution at 37°C overnight. The digest was desalted using a Supelco Discovery DSC-18 SPE column (52607-U, Sigma) as follows: the column was conditioned with 2× 5 mL of 0.1% formic acid-80% acetonitrile and 2× 5 mL of 0.1% formic acid-5% acetonitrile. The plasma digest was added to the column and the digest was washed with 2× 5 mL of 0.1% formic acid-5% acetonitrile. 2× 5 mL of 0.1% formic acid-80% acetonitrile were added to the column to elute the peptides. The sample was then dried down using a SpeedVac and resuspended in 5 mL of 1× PBS.
2.4 Manual SISCAPA experiments to determine monoclonal antibody recovery efficiency
2.4.1 Coupling antibodies to magnetic beads
Sheep-anti-mouse IgG magnetic beads (Dynabeads M-280, 112-01D) were obtained from Invitrogen (Carlsbad, CA). Monoclonal antibodies were coupled in a ratio of 0.03 μg antibody per μL of sheep-anti-mouse beads, which is within the recommended ratios of the manufacturer. Typically, 55 μL of the bead suspension (enough for 2 SISCAPA repeat experiments of 25 μL each) were transferred to 1.6 mL Eppendorf tubes, the tubes were placed on a magnetic holder for 2 minutes, and the suspension liquid was discarded. The beads were washed twice with 200 μL of 1× PBS and, where possible, an appropriate volume of a hybridoma's supernatant was added that contained 1.65 μg of antibody based on the quantitative mouse IgG ELISA. For supernatants with low antibody concentrations (and of which more than 330 μL would be needed to yield 1.65 μg), 330 μL supernatant was used for 55 μL of beads to be able to repeat the coupling if necessary and have enough for other experiments (only ∼1 mL of supernatant was available per hybridoma). The tubes were tumbled for 2 hours at RT and then centrifuged and put back onto the magnet. The supernatant liquid was discarded and the beads were washed twice with 200 μL of 1× PBS. 55 μL of 1× PBS were added to the tubes and the antibody-coupled beads were kept at 4°C until use.
2.4.2 Recovery efficiency experiments using manual SISCAPA
The SISCAPA experiments were performed with 1:100 diluted (with 1× PBS) trypsin-digested human plasma. Lima bean trypsin inhibitor (LS002829, Worthington Biochemical Corporation, Lakewood, NJ) had been added to undiluted, trypsin-digested plasma in a 1:2 trypsin inhibitor:trypsin ratio (w/w) to avoid digestion of the antibodies during capture. Separate SISCAPA experiments were performed for each monoclonal antibody; that is, antibody-coupled beads from different supernatants were not mixed together and multiplexed.
For the SISCAPA experiments, 100 fmol/μL, 50 fmol/μL, and 100 fmol/μL light peptide stocks in 1:100 diluted plasma digest were prepared for ADAM17(VDN) primary clone, ADAM17(VDN) subclone, and CRP(APL) primary clone samples, respectively. For each capture, 25 μL of antibody coupled magnetic beads was added to a 1.6 mL Eppendorf tube and the 1× PBS liquid was discarded. 100 μL of 1:100 diluted plasma digest containing 500 fmol or 250 fmol light ADAM17(VDN) peptide (for primary clone or subclone experiments, respectively), or 500 fmol light CRP(APL) peptide was added and the suspension was mixed and tumbled overnight at 4°C. The plasma solution was discarded and the beads were washed with 1 mL of 1× PBS (for the ADAM17(VDN) experiments) or 500 μL of 1/10× PBS by tumbling for 5 minutes at RT. The PBS was discarded and 5% acetic acid was mixed with the beads and the suspension was incubated for 8 minutes at 98°C to elute the peptides from the antibodies. A 500 fmol or 250 fmol aliquot of heavy ADAM17(VDN) peptide (for primary clone or subclone experiments, respectively), or 500 fmol heavy CRP(APL) peptide was added to the eluates. Pre-capture samples were prepared containing equal amounts of light and heavy peptides in 5% acetic acid (same amounts of peptides as used for the capture samples).
2.5 SISCAPA recovery efficiency experiments using the KingFisher platform
The 96-well plate KingFisher magnetic particle processor (Thermo Scientific, Waltham, MA) has a round, rotating platform that operates akin to a lazy Susan. Magnetic beads are transferred between plates by having an inert, plastic comb inserted into the wells and in turn having a magnetic comb inserted into the hollow fingers of the plastic comb. The beads adhere to the tips of the plastic comb and after the plastic and magnetic combs together are lifted out of one well plate, another well plate is rotated into position under the combs. The combs are lowered back into the wells and the magnetic comb is retracted upwards which releases the beads into the solutions in the wells. Rapid up- and downward movement of the plastic comb accomplishes mixing of the beads with the solutions.
Eight plates can be put in slots on the platform. Plates 1-6 and plate 8 were wide-bottomed 96-well plates (97002540, Fisher Scientific) and plate 7 was a PCR plate (HSP9601, BioRad). A 96 tip plastic comb (97002514, Fisher Scientific) was used with the KingFisher's PCR magnetic comb (head). For these experiments, the wells in Plate 1 contained 20 μL of sheep-anti-mouse magnetic beads (Dynabeads M-280, 112-01D, Invitrogen) plus 80 μL of 1× PBS. The PBS was added to increase the volume to enhance mixing in the wells and to act as a wash of the beads. Plate 2 contained varying volumes of primary clone supernatants depending on the quantitative ELISA results. For one experiment, 0.6 μg of antibody was coupled to 20 μL of beads. Appropriate volumes of 1× PBS were added to arrive at 200 μL total in each well of Plate 2. A lesser than calculated volume of the mouse CRP(APL)-5 (mCRP(APL)-5) primary clone supernatant had to be used due to its low antibody concentration. We chose to use 100 μL of the mCRP(APL)-5 supernatant (plus 100 μL of 1× PBS). Plate 3 contained 500 fmol light CRP(APL) peptide in 100 μL of 1:100 diluted plasma digest. Plates 4 and 5 contained 200 μL of 1× PBS + 0.03% CHAPS (28300, Thermo Scientific), and Plate 6 contained 200 μL of 1/10× PBS + 0.03% CHAPS for washing the beads after peptide capture; the CHAPS reduced bead loss during the transfers between wash plates. Plate 7 contained 500 fmol heavy CRP(APL) peptide in 25 μL of 5% acetic acid for eluting the light peptide from the antibodies. Plate 8 was reserved for the plastic comb at the beginning and at the end of a KingFisher method.
The sequence of the KingFisher procedure (see also Figure 1 and the Supplemental Method File 1) was for the robot to: 1) pick up the plastic comb from Plate 8; 2) insert the comb into Plate 1 containing the beads and mix for 5 minutes, 3) pick up the beads, transfer them to Plate 2 and mix them with the primary clone supernatants for 1 hour; 4) transfer the beads to Plate 3 and mix them with the peptide-plasma solutions for 2 hours; 5) transfer the beads to Plates 4, 5, and then 6 and mix the beads for 1 minute with the wash buffer in each plate; 6) transfer the beads to Plate 7 and mix with the heavy peptide and acid solution for 8 minutes; 7) transfer the beads back to Plate 1 and then return the plastic comb to Plate 8. Due to acetic acid's volatility, Plate 7 was not prepared and inserted into the KingFisher until ∼10 minutes before the beads were transferred to it.
Figure 1.
KingFisher plate set-up and processing steps for monoclonal antibody screening SISCAPA assay. The magnetic beads are picked up by the magnetic PCR head (not shown) and transferred sequentially from one plate to the next, starting at Plate 1. Peptides are eluted from the antibodies in Plate 7 (with subsequent return of the beads to Plate 1), and the eluates are transferred manually to an autosampler 96-well plate for LC-SRM-MS analysis.
Plate 7 was subsequently put onto a 96-well plate magnet and the eluates were transferred to a separate PCR plate if small amounts of beads were left in Plate 7 that had not been completely transferred out of the wells by the KingFisher. A pre-capture sample was prepared containing 500 fmol of each, light and heavy CRP(APL) peptide, in 25 μL of 5% acetic acid.
In a second set of KingFisher experiments, the primary clone supernatant volumes were not adjusted based on antibody concentration, but rather a fixed volume of 30 μL of the supernatants was used. A 170 μL aliquot of 1× PBS was added to the supernatants to enhance the mixing with the beads.
2.6 SISCAPA response curve experiments with Protein-A affinity purified monoclonal antibody against ADAM17(VDN)
To avoid interference at the low concentration end of the response curve from endogenous VDNEELLPK (ADAM17(VDN)) peptide in plasma (from protein ADAM 17), a response curve was generated for which the heavy peptide concentration was varied and the light peptide served as the internal standard. The curve consisted of 10 points, starting with 1000 fmol of heavy peptide per capture and decreasing by 1:4 serial dilutions; a 0 fmol/capture sample (blank) was included in the 10 points. The specific amounts of heavy peptide captured were 1000, 250, 62.5, 15.6, 3.9, 0.98, 0.24, 0.06, 0.02, and 0 fmol. The amount of light peptide in each capture was 100 fmol. Three SISCAPA captures were performed for each point.
Each capture was performed with a 100 μL total volume containing the light and heavy peptides, 10 μL diluted human plasma digest (including trypsin inhibitor) (final dilution of plasma was 1:10), 2 μg of Protein-A affinity purified ADAM17(VDN) monoclonal antibody, and 1× PBS + 0.03% CHAPS. The capture samples were prepared in a wide-bottomed 96-well plate (97002540, Fisher Scientific) that was subsequently sealed and incubated overnight at 4°C. The subsequent steps were carried out on the KingFisher platform (see Supplemental Method File 2) using a slightly different method than used for monoclonal antibody screening, although the same types of well plates were used in each position. Plate 8 again seated the plastic comb. The comb was initially inserted into Plate 1 which contained 5 μL of Dynal magnetic protein G beads (100-04D, Invitrogen, Carlsbad, CA) and 245 μL of 1× PBS + 0.03% CHAPS per well. The beads were mixed in Plate 1 for 5 minutes and then washed for 5 minutes in Plate 2 with 250 μL of 1× PBS + 0.03% CHAPS. The beads were transferred to Plate 3, which contained the peptide-antibody-plasma solution that had been incubated overnight, and the beads were mixed in this plate for 2 hours at RT to allow for antibody coupling to the beads. The beads were subsequently washed in Plates 4, 5, and 6 for 1 minute at a time (Plates 4 and 5 contained 250 μL of 1× PBS + 0.03% CHAPS and Plate 6 contained 1/10× PBS + 0.03% CHAPS). The peptides were eluted for 8 minutes at RT in Plate 7, which contained 13 μL of 5% acetic acid + 0.03% CHAPS in each well. The eluates were then manually transferred by pipetting to a 96-well PCR plate that was used in the autosampler. Ten μL of each eluate was injected onto the LC-SRM-MS system.
2.7 Nanoliquid chromatography-selected reaction monitoring-mass spectrometry (LC-SRM-MS)
The LC system was an Eksigent NanoLC 2D with a 10 port switching valve and an Eksigent nano autosampler (Eksigent Technologies, Dublin, CA). Mobile phases A and B in the high-flow channel (channel 1) consisted of 2% acetonitrile + 0.1% formic acid in water. Mobile phase A in the low-flow channel (channel 2) was 0.1% formic acid in water, and mobile phase B was 0.1% formic acid in 90% acetonitrile in water. A 300 m ID × 5 mm LC Packings trap column (Acclaim PepMap 100 C18, 5 μm 100 Å, 160454, in a precolumn holder, 160431, Dionex, Sunnyvale, CA) was connected at the 10 port valve, along with a 75 μm ID, 360 μm OD transfer capillary that in turn was connected to the analytical column via a microtee (P-875, Upchurch Scientific, Oak Harbor, WA). The analytical column consisted of either a 75 m ID, 360 m OD IntegraFrit column (IF360-75-50-N-5) or a 10 μm tip ID, 75 μm capillary ID, 360 μm OD PicoFrit column (PF360-75-10-N-5, New Objective, Woburn, MA) packed to 10.0 cm with ReproSil-Pur C18-AQ, 3 μm (Dr. Maisch GmbH, Ammerbuch, Germany). In the case of the IntegraFrit column, a 10 μm tip ID uncoated Pico Tip emitter (FS360-20-10-N-20-C12, New Objective) served as the electrospray tip. The electrospray voltage was applied at the microtee via a platinum wire. Samples were loaded onto the trap and washed at 3.0 μL/minute for 5 minutes using 2% acetonitrile + 0.1% formic acid (channel 1) in water before gradient elution. A 10 minute linear gradient was then developed using the low-flow channel from 5-40% B at 0.3 μL/minute, after which mobile phase B was increased to 90% over 2 minutes and then held at 90% for 3 minutes (at 0.3 μL/minute). Mobile phase B was subsequently returned to 5% over 1 minute and held at 5% for 15 minutes (at 0.3 μL/minute) to equilibrate the column. One run from injection to injection lasted approximately 40 minutes. A blank run was inserted between the recovery efficiency sample runs to avoid carry-over.
The mass spectrometer used for these experiments was a 4000 QTRAP (Applied Biosystems/MDS Sciex, Foster City, CA) used in the positive ion mode. Preliminary infusion experiments of the ADAM17(VDN) and CRP(APL) peptides were performed to select the three most intense parent ion-fragment ion transitions (ADAM17(VDN) light peptide: 528.8 → 599.4 m/z (y5), 528.8 → 842.5 m/z (y7), 528.8 → 957.5 m/z (y8); ADAM17(VDN) heavy peptide: 532.8 → 607.4 m/z (y5), 532.8 → 850.5 m/z (y7), 532.8 → 965.5 m/z(y8); CRP(APL) light peptide: 434.3 → 357.2 m/z (y3), 434.3 → 586.4 m/z (y5), 434.3 → 699.5 m/z (y6); CRP(APL) heavy peptide: 437.3 → 363.2 m/z (y3), 437.3 → 592.4 m/z (y5), 437.3 → 705.5 m/z (y6)) and to optimize the SRM-MS voltages (collision energy, declustering potential, entrance potential, and collision cell exit potential) for the transitions (see Supplemental Table 1). The applied electrospray voltage was 2300 V, the collision gas pressure was set to medium, the curtain gas and ion source gas 1 (GS1) were set to 15.0, and the interface heater was set to 150°C. The dwell time for each transition was 30 milliseconds and quadrupole 1 and quadrupole 3 were set to unit resolution. Separate SRM-MS acquisition methods were created for the ADAM17(VDN) and CRP(APL) peptides.
2.8 Data analysis
The MS data were analyzed using Applied Biosystems' MultiQuant v.1.0 and v.1.1 software using a smoothing width of 3 points. For recovery efficiency experiments, the peaks of all three transitions of each peptide were manually integrated and used for data analysis. For the ADAM17(VDN) response curve experiments, only the most intense transition was integrated and used for quantitative analysis. The ADAM17(VDN) most intense light and heavy peptide transitions were 528.8 → 842.5 m/z and 532.8 → 850.5 m/z, respectively. Following peak integration, further data analysis and calculations were performed in Excel. For the response curve data, a linear regression was performed on the log-transformed heavy-to-light peptide peak area ratios versus the log-transformed actual protein concentration (calculated based on the spiked-in heavy peptide amounts). The limit of detection (LOD) was determined based on the three blank samples (the three SISCAPA process replicates without heavy peptide spiked in); the LOD was calculated as the average signal in the heavy peptide m/z channel plus three times the standard deviation of that signal (at the same retention time at which the light peptide signal was detected).
3. Results and Discussion
Monoclonal antibodies would be beneficial for SISCAPA because they provide a renewable resource and the potential for developing very high sensitivity SISCAPA assays. We sought to develop a monoclonal antibody-based SISCAPA assay for two target peptides, VDNEELLPK (identifier ADAM17(VDN)) and APLTKPLK (identifier CRP(APL)). Although it is always preferable to use the desired end assay format for screening monoclonals, throughput of SISCAPA is more limited compared to other assays, such as the commonly used enzyme-linked immunosorbent assay (ELISA). Therefore, we investigated two questions in this study: first, how well do ELISA-based screening assays perform in screening hybridoma supernatants for assessing the eventual performance of monoclonal antibodies in the SISCAPA end assay, and second, can we develop a high-throughput, automated protocol for screening hybridoma supernatants directly using the SISCAPA assay format. The latter would be beneficial since the sheer number of monoclonal antibody hybridomas that are generated makes it impossible to test all of them by currently available (manual) SISCAPA protocols.(Whiteaker et al., 2007a; Whiteaker et al., 2007b; Kuhn et al., 2009) Finally, we characterize the performance of a purified monoclonal antibody in the SISCAPA assay.
3.1 Screening monoclonal antibody supernatants by ELISA vs. SISCAPA
3.1.1 Monoclonal antibody against peptide target VDNEELLPK (ADAM17(VDN))
To generate hybridomas against the ADAM17(VDN) peptide, six mice were immunized with a peptide-GSGC-SMCC-KLH (succinimidyl-4-(N-maleimidomethyl)-cyclohexane-1-carboxylate-keyhole lympet hemocyanin) conjugate (see Methods section). Spleens were harvested from the two mice with the highest titers (determined by peptide-GSGC-SMCC-BSA (bovine serum albumin)-based ELISA), and fusions were performed. After expansion to 24-well plates, 158 primary clone hybridomas tested positive in the peptide-GSGC-SMCC-BSA ELISA assay. Subsequent testing of top ELISA-positive hybridomas with manual SISCAPA revealed poor correlation between the ELISA and SISCAPA results (data not shown), probably due to immunoreactivity of the clones to the SMCC chemical linker in the ELISA antigen that resulted in false ELISA positives.
Based on these results, the hybridomas were re-screened by ELISA using two separate forms of the target peptide; the first ELISA screen used a peptide-glutaraldehyde-BSA (pep-glu-BSA) conjugate, whereas the second ELISA screen used the free VDNEELLPK peptide (unconjugated). Note that both the chemical linker (glutaraldehyde) and conjugate (BSA) used in the former ELISA screen were different from those used in the initial immunogen to avoid false positives resulting from the antibodies' binding to these entities. The pep-glu-BSA conjugate could theoretically perform better than the free peptide due to better steric accessibility of the peptide for antibody binding during the ELISA assay. The 90 best primary clone hybridomas of the original 158 (based on peptide-GSGC-SMCC-BSA ELISA results) had been frozen and retained, and these 90 were tested in the re-screens. Of the 90 clones, only 29 were positive in the pep-glu-BSA ELISA, see Supplemental Table 2, column A, consistent with our hypothesis that there had been significant reactivity to the SMCC chemical linker in the peptide-GSGC-SMCC-BSA ELISA conjugate. Further, only two of the 90 clones were positive in the free peptide-based ELISA (these two were also positive in the pep-glu-BSA ELISA) (Supplemental Table 2, column B). The discrepancy between the pep-glu-BSA and the free peptide ELISA might be due to the increased steric accessibility of the peptide in the pep-glu-BSA ELISA, as hypothesized above. Some of the peptide molecules in the free peptide ELISA might lie flat on the plates and hence would be less accessible to antibody binding. The discrepancy also raised the question of how many false positives and negatives were included in these ELISA results when compared with the actual SISCAPA end assay. Therefore, the next step was to determine the degree of correlation between the ELISA results and the activity of the monoclonals in the SISCAPA assay format.
To this end, we measured SISCAPA recovery efficiencies (defined below) for 22 primary clones (see Table 1, a condensed version of Supplemental Table 2), 19 of which had been positive in the pep-glu-BSA ELISA assay (including the two free peptide ELISA positives), and 3 of which had been negative in the pep-glu-BSA ELISA. Primary clone supernatants that were ELISA positive but had low antibody concentrations (5 μg/mL or below, see Supplemental Table 2, column C) were eliminated from the study because the ultimate antibody production yield would be poor, rendering these hybridomas less desirable. (The antibody concentrations in the supernatants were determined by quantitative mouse IgG ELISA, see Methods section 2.2.3.) For the 22 primary clones that were tested by SISCAPA, equal amounts of antibodies from the supernatants were coupled to the sheep-anti-mouse beads, see Methods section 2.4.1.
Table 1. Peptide ELISA screening, quantitative mouse IgG ELISA, and manual SISCAPA results for ADAM17(VDN) mouse primary clone antibodies that were tested by manual SISCAPA.
Grey shaded fields indicate positive ELISA results (see Methods section).
| Primary clone ID (mADAM17(VDN)-X) | A | B | C | D | E |
|---|---|---|---|---|---|
| Peptide-glutaraldehyde-BSA ELISA optical density (OD) | Free peptide ELISA OD | Supernatant antibody concentration (μg/mL) (n=2)a | Manual SISCAPA Volume of supernatant containing 1.65 mg antibody for coupling to 55 μL of beads (μL) | Manual SISCAPA Recovery efficiency (%) (n=1) | |
| 2 | 0.66 | 0.10 | 19.0 +/- 0.2 | 87 | 1 |
| 3 | 0.19 | 0.08 | 26.4 +/- 0.3 | 63 | 0 |
| 5 | 0.78 | 0.27 | 5.2 +/- 0.9* | 351 | 0 |
| 6 | 0.70 | 0.12 | 38.9 +/- 2.3 | 42 | 8 |
| 23 | 0.53 | 0.09 | 22.2 +/- 0.4 | 74 | 1 |
| 29 | 0.52 | 0.07 | 19.3 +/- 0.5 | 85 | 0 |
| 38 | 0.37 | 0.09 | 24.8 +/- 0.3 | 67 | 0 |
| 39 | 0.69 | 0.09 | 22.0 +/- 2.3 | 75 | 7 |
| 41 | 0.32 | 0.07 | 42.8 +/- 0.1 | 39 | 1 |
| 48 | 0.07 | 0.09 | 31.9 +/- 0.6 | 52 | 0 |
| 49 | 0.06 | 0.07 | 19.8 +/- 1.1 | 83 | 0 |
| 54 | 1.23 | 0.21 | 45.7 +/- 3.9* | 36 | 2 |
| 55 | 0.17 | 0.09 | 8.9 +/- 0.1 | 185 | 0 |
| 57 | 0.51 | 0.07 | 12.1 +/- 0.1 | 136 | 1 |
| 61 | 0.75 | 0.09 | 30.3 +/- 4.9* | 54 | 1 |
| 67 | 1.07 | 0.10 | 15.5 +/- 0.8* | 106 | 3 |
| 71 | 0.50 | 0.09 | 57.8 +/- 1.0 | 29 | 1 |
| 72 | 0.52 | 0.09 | 13.1 +/- 1.0 | 126 | 1 |
| 83 | 0.95 | 0.10 | 43.8 +/- 3.5* | 38 | 18 |
| 85 | 0.75 | 0.09 | 30.7 +/- 2.5 | 54 | 4 |
| 88 | 1.22 | 0.10 | 12.0 +/- 1.3* | 138 | 12 |
| 90 | 0.49 | 0.08 | 21.0 +/- 0.2 | 79 | 1 |
n=2 for most primary clone supernatants and the uncertainty given indicates the range of values. However, for the primary clone supernatants marked with an *, n=4 and the uncertainty given represents +/- one standard deviation.
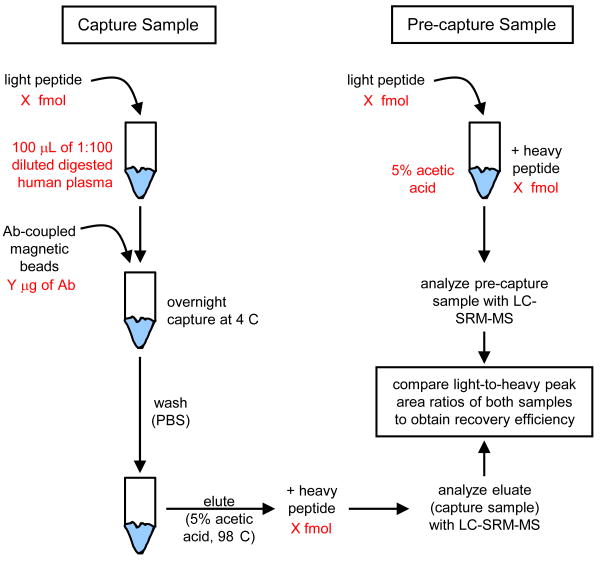
The recovery efficiency of the SISCAPA process provides a metric for assessment of an antibody's ability to capture the specific peptide against which the antibody was raised. The recovery efficiency is defined as the percentage of an initial peptide amount that is bound by the antibody and detected with liquid chromatography-selected reaction monitoring-mass spectrometry (LC-SRM-MS) upon elution of the peptide from the antibody. A schematic illustrating the assay is shown in Figure 2.
Figure 2.
Workflow of the recovery efficiency SISCAPA assay. Antibody performance is assessed by determining an antibody's peptide recovery (the percentage of a peptide amount captured by the antibody and detected by LC-SRM-MS upon elution of the peptide from the antibody). A capture sample is prepared by adding a known amount of light peptide to an aliquot of diluted, digested human plasma, and subsequently adding antibody (previously coupled to magnetic beads). After capture, light peptide bound to the antibody is eluted and a known amount of heavy peptide (equivalent to the light peptide amount initially used) is added to the eluate. A pre-capture sample is prepared using equal amounts of light and heavy peptide (equivalent to the amounts used for the capture sample). Both samples are analyzed by LC-SRM-MS and the light-to-heavy peptide peak area ratios are compared to arrive at the recovery efficiency (the peak area ratio of the capture sample is divided by the ratio of the pre-capture sample).
The results of the manual SISCAPA recovery efficiency experiments for the 22 mouse ADAM17(VDN) (mADAM17(VDN)) primary clones are given in Table 1, column E. Of the 22, 7 showed no capture (0% recovery efficiency), 8 showed recovery efficiencies of 1%, and only 7 had recovery efficiencies above 1% (up to 18%). Significantly, the top six SISCAPA positive primary clones had been negative in the free peptide ELISA assay (mADAM17(VDN)-6, -39, -67, -83, -85, -88), and therefore these would have been missed based on free peptide ELISA screening (that is, they were false ELISA negatives). Overall, the free peptide ELISA results included 14 false negatives (70% of the 20 free peptide ELISA negative clones tested by SISCAPA). One of the free peptide ELISA positives was the seventh-best SISCAPA positive primary clone (mADAM17(VDN)-54, recovery efficiency 2%). The second free peptide ELISA positive primary clone was a false positive since it was negative in SISCAPA (mADAM17(VDN)-5). There were no false pep-glu-BSA ELISA negatives of the 3 analyzed by SISCAPA; however, there were 4 pep-glu-BSA ELISA false positives (21%) of the 19 pep-glu-BSA ELISA positives tested by SISCAPA. We conclude that the pep-glu-BSA ELISA screening resulted in false positives, whereas the free peptide ELISA screening resulted in a false positive and false negatives (with respect to SISCAPA capture activity), highlighting the advantage of using the SISCAPA assay format for the hybridoma screening process.
Based on the manual SISCAPA results, the best five primary clones were chosen for subcloning (mADAM17(VDN)-6, -39, -83, -85, and -88) to obtain monoclonal antibodies. Between 7 and 21 subclones were generated for each primary clone, and subclones that were positive by pep-glu-BSA ELISA were tested with quantitative mouse IgG ELISA (to determine the supernatants' antibody concentrations) and screened by manual SISCAPA, see Supplemental Table 3 for results. Overall, there were no false pep-glu-BSA ELISA positives with the exception of the mADAM17(VDN)-85-X subclones (the mADAM17(VDN)-85 primary clone had a low recovery efficiency to start with (4%), yet it is unclear why the subclones showed hardly any recovery efficiency). Interestingly, the SISCAPA recovery efficiency variation within each primary clone's subclones was relatively small, suggesting that the primary clones consisted mostly of single, or at least of only a few, hybridomas. This was also supported by the relatively uniform pep-glu-BSA ELISA results of positive subclones (Supplemental Table 3, column A) when compared to the spread of primary clone pep-glu-BSA ELISA ODs (Table 1, column A). Subclone mADAM17(VDN)-83-17 was chosen for antibody production and Protein-A purification based on the SISCAPA results; subclones mADAM17(VDN)-83-18, mADAM17(VDN)-6-3, and mADAM17(VDN)-6-1 were frozen as reserves.
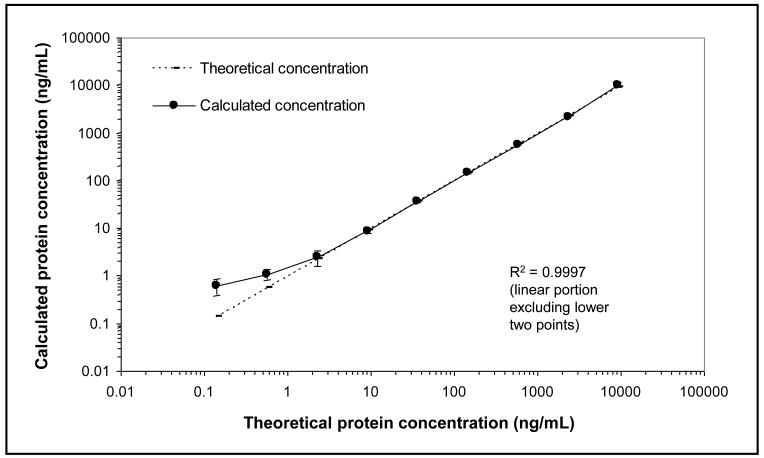
We subsequently characterized the performance of the purified mADAM17(VDN)-83-17 monoclonal antibody by generating a response curve using 1:10 diluted, digested human plasma as the sample matrix, see Figure 3. The response curve showed a linear range of response of over 3 orders of magnitude and excellent linearity (R2 = 0.9997). The reproducibility of the assay was excellent, as the average percent coefficient of variation (%CV) of the calculated protein concentrations was 8.7%. We found a limit of detection of 0.03 ng/mL at the peptide level (in original, undiluted plasma), corresponding to approximately 300 attomoles of peptide injected onto the mass spectrometer. This corresponds to a protein concentration limit of detection of 3 ng/mL in original plasma. (Note that these back-calculated concentrations do not take into account analyte losses during the digestion and desalting processes, as would be encountered when measuring peptides released from endogenous full length proteins.) These performance characteristics are consistent with those reported in polyclonal antibody-based SISCAPA assays,(Berna et al., 2006; Whiteaker et al., 2007a; Hoofnagle et al., 2008; Kuhn et al., 2009) demonstrate the feasibility of using a monoclonal antibody for SISCAPA, and raise the possibility of identifying very high affinity McAbs if enough hybridomas can be screened.
Figure 3.
Response curve for the mouse ADAM17(VDN)-83-17 monoclonal antibody SISCAPA assay. Varying amounts of heavy peptide and a constant amount of light peptide were spiked into 10 μL of human plasma digest, the sample was diluted to 100 μL, and the peptides were captured, eluted, and detected using SISCAPA LC-SRM-MS. Linear regression analysis was performed on log-transformed peak area ratios of the heavy-to-light peptide signals versus the theoretical protein concentrations (back-calculated to undiluted plasma). Calculated protein concentrations were obtained with the resulting regression coefficients.
3.1.2 Monoclonal antibody against peptide target APLTKPLK (CRP(APL))
The other peptide target for which we sought to generate a monoclonal antibody-based SISCAPA assay was APLTKPLK (identifier CRP(APL)). As was done for the mADAM17(VDN) monoclonal antibodies, six mice were immunized with an APLTKPLK-GSGC-SMCC-KLH conjugate for CRP(APL) and fusions were performed with spleen cells from the two mice with the highest titers. As before, initial hybridoma screening was performed with pep-GSGC-SMCC-BSA ELISA, and 82 primary clones (primary clone IDs mCRP(APL)-X) were pep-GSGC-SMCC-BSA ELISA positive. Sixty-eight of the 82 were also re-screened with pep-glu-BSA and free peptide ELISA. The results are given in Supplemental Table 4. Our experience with the CRP(APL) antigen was comparable to the ADAM17(VDN) target. Specifically, of the 68 clones positive by pep-GSGC-SMCC-BSA ELISA, only seven were positive in the pep-glu-BSA-based ELISA, again pointing to significant reactivity to the SMCC chemical linker used in the immunogen, as was the case for ADAM17(VDN). Only three of the original 68 positive clones were positive in the free peptide-based ELISA (these three were also positive in the pep-glu-BSA-based ELISA).
We next determined the SISCAPA recovery efficiency (using manual SISCAPA) of 11 primary clones, 6 of which had tested positive in the pep-glu-BSA ELISA screen (including two free peptide ELISA positives), as well as five primary clones that had been negative in the ELISA screens. One hybridoma that had been positive in both ELISA screens (mCRP(APL)-3) was not tested with SISCAPA since its supernatant antibody concentration was 0 μg/mL (based on quantitative (mouse IgG) ELISA results). Equal amounts of antibodies from each supernatant were coupled to sheep-anti-mouse beads (unless the antibody concentration was very low and more supernatant volume than available would have been needed for coupling). The SISCAPA results are summarized in Table 2 (a condensed version of Supplemental Table 4). Four primary clones were positive with manual SISCAPA, and their recovery efficiencies ranged from 7 to 91% with the inter-day variability (between +/- 0.1 and +/- 5.1%) being on the levels of what we have observed in previous manual SISCAPA experiments performed in our lab (∼+/- 5%, unpublished results). As was the case for the mADAM17(VDN) clones, the SISCAPA results identified false negatives in the free peptide ELISA data (2 of 9 (22%) free peptide ELISA negative primary clones were positive by SISCAPA) and false positives in the pep-glu-BSA ELISA data (2 of 6 (33%) pep-glu-BSA ELISA positive primary clones were negative by SISCAPA).
Table 2. Peptide ELISA screening, quantitative mouse IgG ELISA, and manual SISCAPA results for CRP(APL) mouse primary clone antibodies that were tested by manual SISCAPA.
Grey shaded fields indicate positive ELISA results (see Methods section).
| Primary clone ID (mCRP(APL)-X) | A | B | C | D | E |
|---|---|---|---|---|---|
| Peptide-glutaraldehyde-BSA ELISA optical density (OD) | Free peptide ELISA OD | Supernatant antibody concentration (μg/mL) (n=2)a | Manual SISCAPA Volume of supernatant containing 1.65 mg antibody for coupling to 55 μL of beads (μL)b | Manual SISCAPA Recovery efficiency (%) (n=2)c | |
| 5 | 2.48 | 1.86 | 1.4 +/- 0.1 | 1179 | 41 +/- 5.1 |
| 8 | 2.55 | 0.29 | 0.4 +/- 0.0 | 4125 | 0 |
| 12 | 0.16 | 0.20 | 0.3 +/- 0.0 | 5500 | 0 |
| 29 | 0.20 | 0.18 | 39.5 +/- 0.3 | 42 | 0 +/- 0.1 |
| 34 | 2.07 | 0.19 | 24.9 +/- 0.5 | 66 | 91 +/- 0.4 |
| 36 | 2.07 | 0.24 | 18.3 +/- 0.1 | 90 | 7 +/- 0.4 |
| 44 | 0.17 | 0.16 | 54.5 +/- 0.2 | 30 | 0 |
| 46 | 1.34 | 0.24 | 0.1 +/- 0.0 | 16500 | 0 |
| 52 | 0.16 | 0.20 | 0.3 +/- 0.0 | 5500 | 0 |
| 59 | 0.16 | 0.12 | 5.9 +/- 0.3 | 280 | 0 |
| 78 | 2.35 | 0.70 | 30.4 +/- 0.3 | 54 | 79 +/- 4.2 |
duplicate measurements on the same ELISA plate.
for primary clone supernatants with low antibody concentrations, 330 μL of supernatant was used for coupling to 55 μL of beads since only a limited amount (∼1 mL) of supernatant was available from each clone.
n=2 for the SISCAPA positive primary clones and for mCRP(APL)-29, which served as a negative control in subsequent KingFisher experiments. Repeats were performed on two different days.
Overall, consistent for both peptide targets, the screening process using ELISAs and manual SISCAPA was severely limiting for two reasons: a) there was imperfect correlation between the screening ELISA results and the ultimate performance of a given antibody in the SISCAPA assay format, and b) it was not possible to screen all available hybridomas using the low-throughput, labor-intensive, manual SISCAPA assay format. Given this experience, we sought to develop a high-throughput implementation of the SISCAPA assay that would allow us to screen large numbers of hybridomas reliably and as early as possible using the SISCAPA end assay format.
3.2 Development of a high-throughput automated SISCAPA assay using the KingFisher magnetic bead processing platform
The earliest point at which high-throughput screening with SISCAPA is practical is when the hybridoma numbers have been reduced from thousands to hundreds (considering, for example, the resources (such as magnetic beads) that would be needed to screen thousands of hybridomas). Therefore, even though the correlation between the pep-glu-BSA ELISA and SISCAPA results as determined in the previous section was not perfect, no false negatives were observed for the pep-glu-BSA ELISA assays and hence this ELISA screen can still be used as a fast initial screen when thousands of hybridomas need to be tested after fusion. In addition, we accept the false positive rate of the pep-glu-ELISA screen so that the thousands of hybridomas can quickly be reduced to hundreds. Our goal for the development of a high-throughput SISCAPA assay was thus to be able to screen hundreds of hybridoma supernatants by SISCAPA that were positive by pep-glu-BSA ELISA, so that the best SISCAPA-performing clone could be identified.
A platform that was specifically designed for robotic handling of magnetic beads is the 96-well plate KingFisher magnetic particle processor. The KingFisher has been demonstrated as a reliable device for automating magnetic bead handling of proteomic samples.(Jimenez et al., 2007; Ficarro et al., 2009) We created a KingFisher method that would execute the same sample processing steps as used for manual SISCAPA, but in an automated fashion, allowing for more samples to be processed in parallel (see Methods section 2.5).
To assess the performance and reliability of the KingFisher SISCAPA assay when compared with manual SISCAPA, we tested the four mCRP(APL) primary clones that had been positive by manual SISCAPA and used mCRP(APL)-29 as a negative control (see Table 2). The KingFisher SISCAPA LC-SRM-MS experiments were performed twice, each time on a different day, and the recovery efficiencies are given in Table 3, column B (the recovery efficiencies obtained with the manual SISCAPA experiments (Table 2, column E) are reproduced in Table 3, column C for ease of comparison). Equal amounts of antibodies were again used for each primary clone (except for mCRP(APL)-5, see Table 3, column A). The four primary clones that were positive in manual SISCAPA were also positive in the KingFisher SISCAPA experiments, and the negative control again showed no capture. The KingFisher recovery efficiencies ranged from 14 to 69%, slightly lower than the efficiencies obtained using manual SISCAPA, yet this might be due to the shorter peptide-to-antibody capture time in the KingFisher experiment (2 hours vs. overnight). The inter-day variances were also very comparable to the ones obtained with manual SISCAPA. In addition, the ranking of primary clone performance (highest to lowest recovery efficiency) was the same for both types of SISCAPA experiments. This means that the same best-performing primary clones would be chosen for subcloning based on either SISCAPA method, and that we, therefore, had successfully automated SISCAPA screening using the KingFisher platform.
Table 3. SISCAPA recovery efficiency results for manual SISCAPA vs. KingFisher SISCAPA using CRP(APL) primary clone antibodies.
All experiments were performed in duplicate on separate days.
| Primary clone ID (mCRP(APL)-X) | A | B | C | D |
|---|---|---|---|---|
| KingFisher SISCAPA Volume of supernatant containing 0.6 μg antibody for coupling to 20 μL beads (μL) | KingFisher SISCAPA (adjusted supernatant volumes) Recovery efficiency (%) | Manual SISCAPA Recovery efficiency (%) | KingFisher SISCAPA (30 μL fixed supernatant volumes) Recovery efficiency (%) | |
| 5 | 429a | 29 +/- 2.0 | 41 +/- 5.1 | 6 +/- 3.9 |
| 29 | 15 | 0 +/- 0.1 | 0 +/- 0.1 | 0 +/- 0.2 |
| 34 | 24 | 69 +/- 0.5 | 91 +/- 0.4 | 72 +/- 1.8 |
| 36 | 33 | 14 +/- 0.8 | 7 +/- 0.4 | 7 +/- 2.2 |
| 78 | 20 | 64 +/- 4.2 | 79 +/- 4.2 | 58 +/- 9.2 |
Due to limited supernatant volume, only 100 μL of the mCRP(APL)-5 supernatant was used for the KingFisher SISCAPA experiments with adjusted supernatant volumes.
The KingFisher experiments performed so far used adjusted supernatant volumes to ensure equal loading of McAb across the hybridoma clones. Since the quantitative mouse IgG ELISA assays are quite time-consuming (15-20 primary clone supernatants can be tested per day), the advantage of automation that the KingFisher platform provides would be diminished if quantitative ELISA assays had to be performed on all primary clones. Hence, we performed further experiments to determine whether the KingFisher assay would still yield reliable results (with respect to identifying SISCAPA negatives and positives, but not with respect to rank-ordering the highest affinity clones) even when the supernatant volumes were not adjusted. If so, then quantitative ELISA assays would only need to be performed on SISCAPA-positive primary clones. Performing the screening in this way would save enormous time by having to determine the antibody concentration of only a subset of primary clone supernatants.
To test how reliable the KingFisher SISCAPA results would be using constant supernatant volumes, we performed experiments using 30 μL of supernatant. The same primary clones were tested here as in the previous KingFisher experiments. The 30 μL volume exceeded the previous volumes used for some supernatants, but for mCRP(APL)-5, this was ∼14× less than the volume needed to couple 0.6 μg of Ab to 20 μL of beads (see Table 3, column A). The recovery efficiency results for these experiments are listed in Table 3, column D. The recovery efficiency for mCRP(APL)-5 suffered compared with the previous KingFisher results (Table 3, column B) due to the decreased amount of antibody coupled to the beads. However, its recovery efficiency was still greater than 0% so that it would not have become a false negative in this screen. (It is conceivable that supernatants that would be positive in SISCAPA might be missed in this constant-volume screen because of very low antibody concentrations (ng/mL), yet these hybridomas would not be desirable anyway since their production yield would be very low.) The recovery efficiency results of the other four primary clones (Table 3, column D) were very comparable to the ones obtained with the KingFisher experiments for which adjusted supernatant volumes were used (Table 3, column B). Hence, primary clones can undergo a preliminary screen with KingFisher SISCAPA experiments using fixed supernatant volumes to determine which primary clones are SISCAPA positive and negative. In this way, quantitative ELISA (and possibly adjusted-volume KingFisher SISCAPA experiments) need to be performed only on SISCAPA positive primary clones (in some cases, such as for mCRP(APL)-34, -36, and -78 for example, no further SISCAPA experiments would even be needed since the differences in recovery efficiencies are quite large and the antibody concentration differences are relatively small).
Our success with the KingFisher SISCAPA assay prompted us to test the remaining 55 pep-glu-BSA ELISA negative mCRP(APL) primary clones using 30 μL of each supernatant and the KingFisher SISCAPA assay format. All 55 were negative in the SISCAPA assay as well (0% capture; mCRP(APL)-78 was used as a positive control); that is, the pep-glu-BSA ELISA results had included no false negatives.
With the establishment of the reliability of the KingFisher SISCAPA assay when compared to the manual SISCAPA assay, our goal of increasing the throughput of screening hybridoma supernatants by SISCAPA has been attained. The total sample processing time has been shortened from ∼22 hours to ∼4 hours, see Supplemental Table 5. The times given in the table are based on 15 samples, which is the number of samples that can practically be processed in one day when performing manual SISCAPA (done in a serial fashion using Eppendorf tubes). In contrast, the KingFisher platform features parallel sample processing in 96-well plates, and up to 96 samples can be processed at one time. Therefore, the sample processing is sped up by another factor of about six for a total sample preparation time savings of ∼108 hours (18 hours difference × ∼6) for 96 samples. Screening hundreds of supernatants by KingFisher SISCAPA has thus become efficient and practical.
As an additional improvement to the SISCAPA LC-SRM-MS process, we have shortened the LC run times from injection-to-injection from ∼40 minutes to ∼14 minutes (this 14 minute method was used to test the 55 pep-glu-BSA ELISA negative mCRP(APL) primary clones mentioned above). This improvement makes it theoretically feasible to run 96 samples from one plate in one day per operator, KingFisher processor, and MS instrument.
One further step that could be taken to speed up monoclonal antibody screening would be applicable when hybridomas had been generated against multiple peptides. In that case, KingFisher SISCAPA recovery efficiency experiments could include an equal number of primary clones for the different peptides, and eluates from single primary clones to the different peptides could be combined prior to LC-SRM-MS analysis. The LC-SRM-MS acquisition methods can be modified to monitor multiple peptide transitions' mass-to-charge values and hence the analysis could easily be multiplexed.
3.3 Proposed pipeline for screening monoclonal antibodies for SISCAPA assays
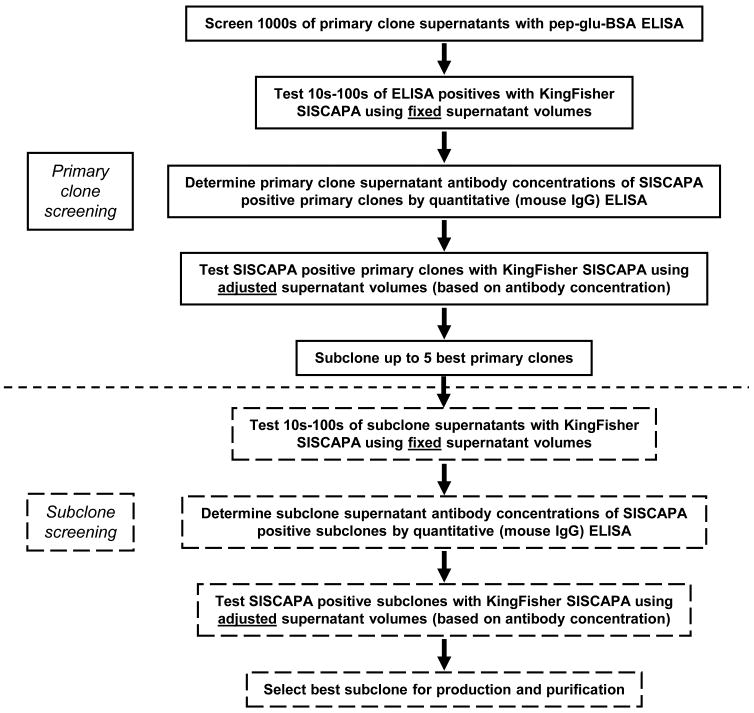
Based on our experience with pep-glu-BSA ELISA and KingFisher SISCAPA LC-SRM-MS screening, we have developed a pipeline for identifying monoclonal antibodies that perform best in SISCAPA assays from thousands of hybridomas, see Figure 4. As stated earlier, the false positives observed with the pep-glu-BSA ELISA do not negate its use in hybridoma screening, given also that no pep-glu-BSA ELISA false negatives were determined for mADAM17(VDN) and mCRP(APL). Indeed, the pep-glu-BSA ELISA screen can be efficiently used when screening the initial twenty 96-well plates of hybridomas and then confirming the primary clones after expansion into 24-well plates since the pep-glu-BSA ELISA assay is still faster than the KingFisher SISCAPA assay for screening these initial, large numbers of hybridomas. False ELISA positives can be eliminated with the KingFisher SISCAPA assay once the number of primary clones has been reduced to tens or hundreds from thousands of clones. KingFisher experiments with primary clones can initially be performed using constant supernatant volumes and quantitative mouse IgG ELISA assays can then determine the antibody concentrations in the SISCAPA positive supernatants. Depending on the range of recovery efficiencies and antibody concentrations, confirmatory KingFisher SISCAPA experiments might be performed using adjusted supernatant volumes to obtain reliable ranking of the clones. The best primary clones (usually not more than five) can be subcloned, and the same strategy of ‘fixed supernatant volume KingFisher SISCAPA—quantitative ELISA—adjusted supernatant volume KingFisher SISCAPA’ can be followed for the subclone screening. The best-performing subclone can then be chosen for production and purification with the assurance that no well-performing clones were missed.
Figure 4.
Pipeline for hybridoma screening using peptide-glutaraldehyde-BSA ELISA and KingFisher SISCAPA LC-SRM-MS assays. An initial first-pass screen using pep-glu-BSA ELISA will eliminate a majority of primary clone hybridomas that would not be effective in SISCAPA assays. This will reduce the number of available hybridomas from thousands to tens to hundreds. Some hybridomas are expected to pass the ELISA screen that will subsequently be negative in SISCAPA (false ELISA positives). These false positives will be identified by KingFisher SISCAPA screening using fixed supernatant volumes and will hence be eliminated from further consideration. Quantitative mouse IgG ELISA assays will be performed with SISCAPA positive supernatants, and KingFisher SISCAPA experiments using adjusted supernatant volumes can then be performed to enable rank-ordering of the primary clones. Up to five best-performing primary clones can be subcloned, and the KingFisher SISCAPA assay strategy of ‘fixed supernatant volume KingFisher SISCAPA—quantitative ELISA—adjusted supernatant volume KingFisher SISCAPA’ can be repeated to select the best subclone for production and purification.
The method we have developed here is also suitable for evaluating antibodies for iMALDI-based verification studies.(Jiang et al., 2007) The iMALDI technique uses anti-peptide antibodies as well, and the same procedure that is used for SISCAPA-based assays would also work for iMALDI-based assays, providing that the steps to elute the heavy and light peptides from the beads were omitted, and direct MALDI analysis of the beads was used for the analysis instead of elution and LC-electrospray ionization (ESI)-SRM-MS. In addition, once the best monoclonal antibody has been selected, the automated KingFisher SISCAPA protocol given here (or a protocol similar to it) could be used for performing automated high-throughput SISCAPA-LC-SRM-MS or iMALDI analyses of large numbers of samples. We have recently developed such a method that enables automated and multiplexed quantification of protein biomarkers using peptide-based KingFisher SISCAPA-LC-multiple reaction monitoring (MRM)-MS.(Whiteaker et al., in press)
In conclusion, the data obtained with the KingFisher SISCAPA assays coupled with LC-SRM-MS analysis showed much better correlation with manual SISCAPA data than the pep-glu-BSA and free peptide ELISA screening data. The main drawback of the free peptide ELISA was that it resulted in false negatives and it even missed the best-performing primary clones in these particular datasets. The false positives obtained with pep-glu-BSA ELISA constituted a less severe drawback and indeed the pep-glu-BSA ELISA should be used for eliminating hybridoma negatives early in the screening process when thousands of hybridomas need to be screened. The KingFisher SISCAPA LC-SRM-MS assay, however, is preferable once hybridoma numbers have been reduced to tens to hundreds of primary clones and provides automated, comprehensive, and reliable screening of monoclonal antibody supernatants.
Supplementary Material
Acknowledgments
The authors would like to thank Mary Trute for help with sample preparation. The authors wish to thank Terry Pearson at the University of Victoria, British Columbia, for sharing his protocol for the free peptide ELISA. This work was supported by grants from the Entertainment Industry Foundation (EIF) and the EIF Women's Cancer Research Fund to the Breast Cancer Biomarker Discovery Consortium, the National Cancer Institute (U24 CA126476) Clinical Proteomic Technology Assessment for Cancer (CPTAC) team as part of the NCI Clinical Proteomic Technologies for Cancer (http://proteomics.cancer.gov) initiative, and generous gifts from the Keck Foundation, the Canary Foundation, and the Paul G. Allen Family Foundation.
Abbreviations
- ADAM17(VDN)
identifier for peptide VDNEELLPK from tumor necrosis factor (TNF)-alpha converting enzyme (ADAM 17)
- BSA
bovine serum albumin
- CRP(APL)
identifier for peptide APLTKPLK from C-reactive protein (CRP)
- DMSO
dimethyl sulfoxide
- ELISA
enzyme-linked immunosorbent assay
- KLH
keyhole limpet hemocyanin
- LC
liquid chromatography
- McAb
monoclonal antibody
- OD
optical density
- PBS
phosphate buffered saline
- PcAb
polyclonal antibody
- pep-glu-BSA
peptide-glutaraldehyde-BSA
- pNPP
p-nitrophenyl phosphate
- RT
room temperature
- SISCAPA
Stable Isotope Standards and Capture by Anti-Peptide Antibodies
- SMCC
succinimidyl-4-(N-maleimidomethyl)-cyclohexane-1-carboxylate
- SRM-MS
selected reaction monitoring-mass spectrometry
- TBS
tris-buffered saline
- TBST
1× tris-buffered saline-0.05% Tween-20
- TMB
3, 3′, 5, 5′-tetramethyl benzidine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson NL, Anderson NG, Haines LR, Hardie DB, Olafson RW, Pearson TW. Mass spectrometric quantitation of peptides and proteins using Stable Isotope Standards and Capture by Anti-Peptide Antibodies (SISCAPA) J Proteome Res. 2004;3:235–244. doi: 10.1021/pr034086h. [DOI] [PubMed] [Google Scholar]
- Berna M, Schmalz C, Duffin K, Mitchell P, Chambers M, Ackermann B. Online immunoaffinity liquid chromatography/tandem mass spectrometry determination of a type II collagen peptide biomarker in rat urine: Investigation of the impact of collision-induced dissociation fluctuation on peptide quantitation. Anal Biochem. 2006;356:235–243. doi: 10.1016/j.ab.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Ficarro SB, Adelmant G, Tomar MN, Zhang Y, Cheng VJ, Marto JA. Magnetic bead processor for rapid evaluation and optimization of parameters for phosphopeptide enrichment. Anal Chem. 2009;81:4566–4575. doi: 10.1021/ac9004452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoofnagle AN, Becker JO, Wener MH, Heinecke JW. Quantification of thyroglobulin, a low-abundance serum protein, by immunoaffinity peptide enrichment and tandem mass spectrometry. Clin Chem. 2008;54:1796–1804. doi: 10.1373/clinchem.2008.109652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Parker CE, Hoadley KA, Perou CM, Boysen G, Borchers CH. Development of an immuno tandem mass spectrometry (iMALDI) assay for EGFR diagnosis. Proteomics Clin Appl. 2007;1:1651–1659. doi: 10.1002/prca.200700009. [DOI] [PubMed] [Google Scholar]
- Jimenez CR, Filali ZE, Knol JC, Hoekman K, Kruyt FAE, Giaccone G, Smit AB, Li KW. Automated serum peptide profiling using novel magnetic C18 beads off-line coupled to MALDI-TOF-MS. Proteomics Clin Appl. 2007;1:598–604. doi: 10.1002/prca.200600483. [DOI] [PubMed] [Google Scholar]
- Kuhn E, Addona T, Keshishian H, Burgess M, Mani DR, Lee RT, Sabatine MS, Gerszten RE, Carr SA. Developing Multiplexed Assays for Troponin I and Interleukin-33 in Plasma by Peptide Immunoaffinity Enrichment and Targeted Mass Spectrometry. Clin Chem. 2009;55:1108–1117. doi: 10.1373/clinchem.2009.123935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulovich AG, Whiteaker JR, Hoofnagle AN, Wang P. The interface between biomarker discovery and clinical validation: The tar pit of the protein biomarker pipeline. Proteomics Clin Appl. 2008;2:1386–1402. doi: 10.1002/prca.200780174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifai N, Gillette MA, Carr SA. Protein biomarker discovery and validation: the long and uncertain path to clinical utility. Nat Biotechnol. 2006;24:971–983. doi: 10.1038/nbt1235. [DOI] [PubMed] [Google Scholar]
- Wark KL, Hudson PJ. Latest technologies for the enhancement of antibody affinity. Adv Drug Deliv Rev. 2006;58:657–670. doi: 10.1016/j.addr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Whiteaker JR, Zhang H, Zhao L, Wang P, Kelly-Spratt KS, Ivey RG, Piening BD, Feng LC, Kasarda E, Gurley KE, Eng JK, Chodosh LA, Kemp CJ, McIntosh MW, Paulovich AG. Integrated pipeline for mass spectrometry-based discovery and confirmation of biomarkers demonstrated in a mouse model of breast cancer. J Proteome Res. 2007a;6:3962–3975. doi: 10.1021/pr070202v. [DOI] [PubMed] [Google Scholar]
- Whiteaker JR, Zhao L, Zhang HY, Feng LC, Piening BD, Anderson L, Paulovich AG. Antibody-based enrichment of peptides on magnetic beads for mass-spectrometry-based quantification of serum biomarkers. Anal Biochem. 2007b;362:44–54. doi: 10.1016/j.ab.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteaker JR, Zhao L, Anderson L, Paulovich AG. An automated and multiplexed method for high throughput peptide immunoaffinity enrichment and multiple reaction monitoring mass spectrometry-based quantification of protein biomarkers. Mol Cell Proteomics. doi: 10.1074/mcp.M900254-MCP200. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.