Abstract
Myofibroblasts play an important role in morphogenesis, inflammation, and fibrosis in most tissues. The amniotic membrane stroma can maintain keratocytes in cultures and prevent them from differentiating into myofibroblasts. However, it is unknown whether the AM stroma can also reverse differentiated myofibroblasts. In this study, we found that amniotic membrane stromal cells (AMSCs), which adopted fibroblastic phenotype in vivo, quickly and completely differentiated into myofibroblasts during ex vivo culture in DMEM/FBS on plastic within 2 passages. When cultured on type I collagen, the myofibroblasts maintained their phenotype, however, when these myofibroblasts were re-seeded onto a cryopreserved amniotic membrane stromal surface, they reversed to the fibroblast phenotype. Moreover, we found that the amniotic membrane stromal extract not only helps maintain primary AMSCs fibroblastic phenotype in vitro, but also can reverse differentiated myofibroblasts back to fibroblasts. This reversal was not coupled with cell proliferation. We concluded that the amniotic membrane stroma contains soluble factors that can regulate the mesenchymal cell differentiation. Further investigation into the identity of these factors and the control mechanisms may unravel a new scar-reversing strategy.
Myofibroblasts are a unique group of cells phenotypically intermediate between smooth muscle cells and fibroblasts. Through the secretion of inflammatory and anti-inflammatory cytokines, chemokines, growth factors, lipid and gaseous inflammatory mediators, extracellular matrix proteins and proteases, myofibroblasts play an important role in morphogenesis, oncogenesis, inflammation, wound healing, and fibrosis in most organs and tissues [for review, see Powell et al. (1999)]. During normal wound healing, fibroblasts migrate to the wound area and differentiate into myofibroblasts under the influence of growth factors such as TGF-β1 and the mechanical stress developed within a given tissue [for review, see (Gabbiani, 2003)]. Although myofibroblasts gradually disappear by apoptosis in the resolution phase (Desmouliere et al., 1995), they persist in many pathological situations, and continue to remodel the extracellular matrix, resulting in connective-tissue contracture and hypertrophic scar formation [for review, see (Tomasek et al., 2002)]. Therefore, it is important to control myofibroblast differentiation in order to attenuate the “dark side” of the pathological wound healing. Currently, no attempts have been made to reverse the scar created by myofibroblasts.
The Amniotic membrane (AM) is the innermost layer of the fetal membrane. It consists of a simple epithelium, a thick basement membrane and a subjacent avascular stroma. Clinical transplantation of the AM is effective in reducing scar formation during ocular surface reconstruction in ophthalmology [for reviews see (Sippel et al., 2001; Tseng, 2002; Bouchard and John, 2004; Dua et al., 2004)]. Previous work from this laboratory showed that transcript expression of TGF-β2, β3, and TGF-β receptor II by human corneal and limbal fibroblasts (Tseng et al., 1999) as well as TGF-β2, β3, TGF-β receptor I and receptor II by human conjunctival and pterygium fibroblasts (Lee et al., 2000) were downregulated when these fibroblasts were cultured on the stromal surface of AM. As a result, transcript expression of downstream genes such as α-smooth muscle actin (α-SMA), EDA-containing fibronectin and α5β1 integrins were also downregulated. This suggests that the AM stromal matrix can prevent fibroblast differentiation into myofibroblasts. Subsequent studies further showed that human (Espana et al., 2003, 2004) and murine (Kawakita et al., 2005) keratocyte phenotype as judged by their characteristic dendritic morphology as well as expression of corneal stroma-specific keratocan, can maintain their phenotype without differentiation into α-SMA-expressing myofibroblasts when cultured on the AM stromal surface even when TGF-β is added in a serum-containing medium because of downregulation of the Smad signaling pathway (Kawakita et al., 2005). Collectively, these results indicated that the AM stromal matrix is not only pathologically significant in preventing myofibroblast differentiation, but also physiologically important in maintaining neural crest-derived corneal keratocytes. However, it remains unclear whether the AM stromal matrix can reverse pre-existing differentiated myofibroblasts.
To address this question, we isolated mesenchymal cells from human AM stromal matrix, termed amniotic membrane stromal cells (AMSCs), and noted that they quickly differentiated into α-SMA expressing myofibroblasts when cultured on plastic in a serum-containing medium. However, both the AM stromal matrix and the soluble AM stromal extracts (ASE) prevented myofibroblast differentiation of HASCs, as well as reverted their already-differentiated myofibrolasts to a fibroblast phenotype when cultured back on the AM stromal surface or by adding ASE to the above plastic cultures. The significance of these findings is further discussed.
Materials and Methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM), Hank’s balanced salt solution (HBSS), amphotericin B, gentamicin, fetal bovine serum (FBS), 0.25% trypsin/0.53 mM EDTA, Live and Dead cell viability assay reagent, and FITC conjugated phalloidin were purchased from Invitrogen (Carlsbad, CA). Bovine serum albumin (BSA), insulin-transferrin-sodium selenite media supplement, formaldehyde, protease inhibitor cocktail, mouse anti-desmin antibody, FITC conjugated anti-mouse, goat, and rat IgG, propidium iodide, and Hoechst-33342 dye were from Sigma (St. Louis, MO). Transwell inserts were from Corning Incorporated (Corning, NY). Type I collagen was from BD Biosciences (Bedford, MA). BCA™ protein assay kit was from Pierce (Rockford, IL). Dispase II and collagenase were from Roche (Penzberg, Germany). Mouse anti-αSMA and Ki67 antibodies were from DakoCytomation (Carpinteria, CA). Rabbit anti-vimentin antibody was from Abcam (Cambridge, MA). Mouse anti-EDA fibronectin antibody was from Chemicon (Temecula, CA). HRP conjugated anti-mouse IgG was from BioRad (Hercules, CA). Anti-fade mounting solution was from Vector Laboratories (Burlingame, CA). Cryopreserved human AM was obtained from Bio-Tissue (Miami, FL).
Cell cultures
Human tissue was handled according to the Declaration of Helsinki. The fresh human placenta was obtained from Baptist Hospital (Miami, FL) following cesarean section. Informed consent was obtained under an IRB-approved protocol. After two rinses with PBS (with gentamicin and amphotericin B added), the AM was mechanically peeled from the chorion, cut into pieces (~30 mm in diameter), and digested with 10 mg/ml Dispase II in DMEM with 10% FBS at 37°C for 20 min. After that, the amniotic epithelium was removed by surgical peeling under a dissecting microscope, and the remaining stroma was further digested by 2 mg/ml collagenase in DMEM with 10% FBS at 37°C for 14 h. Cells were collected by centrifuge at 800g for 5 min, and resuspended and cultured in DMEM with 10% FBS under a humidified atmosphere of 5% CO2 in air at 37°C, the culture medium was changed every 2 days. Prior to enzyme digestion, the AM was also embedded in O.C.T for cryosectioning. Human corneoscleral tissues were obtained from the Florida Lions Eye Bank (Miami, FL), from which corneal fibroblasts were harvested as previously reported (Espana et al., 2003), and cultured in DMEM containing 10% FBS. Passage 1 corneal fibroblasts were used in our experiments.
Preparation of water-soluble AM stromal extract and AM inserts
Using aseptic techniques, the cryopreserved human AM was briefly washed 2–3 times with HBSS to remove the storage medium. The AM stroma was scraped off by a spatula, frozen in the air phase of liquid nitrogen, and grounded to fine particles with a BioPulverizer (Biospec Products, Inc., Bartlesville, OK). The particles were then homogenized on ice with a Tissue Tearor (Biospec Products, Inc., Dremel, WI) in PBS, pH 7.4, for 1 min. The homogenate was mixed by rotation for 1 h and centrifuged at 14,000g for 30 min at 4°C. The supernatant in PBS was then collected, and stored in aliquots at −80°C. This water-soluble protein extract was designated as the amniotic stromal extract (ASE). The protein concentration of the extract was quantified with a BCA assay. For the preparation of the AM inserts, the AM was thawed immediately before use, washed three times with HBSS, cut into pieces approximately 2.5 × 2.5 cm in size, and fastened onto a culture insert with the stromal matrix side facing up as previously reported (Meller and Tseng, 1999).
Immunostaining
Cryostat sections (4-µm) of the AM were fixed in acetone for 10 min at −20°C; cultured AMSCs and AM whole mounts with AMSCs were fixed in 4% paraformaldehyde for 30 min at 4°C. The Sections or cultured cells were rinsed three times for 5 min each with PBS, and then incubated in 0.2% Triton X-100 for 10 min. After three rinses with PBS for 5 min each and preincubation with 2% BSA to block nonspecific staining, the sections or cells were incubated with anti-αSMA (1:200), anti-desmin (1:200), and anti-vimentin (1:200) antibodies for 1 h. After three washes with PBS for 15 min, they were incubated with an FITC or Texas Red conjugated secondary antibodies for 45 min. For labeling of F-actin, cells were further stained with FITC conjugated phalloidin at the concentration of 200 U/mL for 15 min. After three additional PBS washes for 15 min each, the nuclei were stained with PI (1:2,000) for 1 min or Hoechst-33342 (10 µg/ml) for 15 min, then analyzed with a fluorescence microscope. For immunohistochemical staining of Ki67, endogenous peroxidase activity was blocked by 0.6% hydrogen peroxide for 10 min. Nonspecific staining was blocked by 1% normal goat serum for 30 min. Cells were then incubated with anti-Ki67 antibody (1:50) for 1 h. After three washes with PBS for 15 min each, cells were incubated with biotinylated rabbit anti-mouse IgG (1:100) for 30 min, followed by incubation with ABC reagent for 30 min. The reaction product was developed with DAB for 5 min, and examined under a light microscope.
Western blot analysis
Cultured AMSCs or myofibroblasts from plastic, collagen, or AM surface were collected and extracted in cold RIPA buffer [50 mM Tris–Cl, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and protease inhibitor cocktail]. Equal amounts of proteins extracted from lysates were separated on 4–15% sodium dodecyl sulphate–polyacrylamide gels (SDS–PAGE), and then electrophorectically transferred to nitrocellulose membranes. After 1 h of blocking in 5% nonfat milk, the blots were incubated with primary antibodies to α-SMA and ED-A fibronectin using β-actin as a loading control. The specific binding was detected by anti-mouse or anti-rabbit horseradish peroxidase (HRP)-conjugated antibodies, and visualized by enhanced chemiluminescence method.
Statistical analysis
All experiments described above were repeated three times, each in triplicate or more. Group means were compared using the appropriate version of Student’s unpaired t-test. Test results were reported as two-tailed P values, where P < 0.05 was considered statistically significant. Summary data are reported as means ± SD.
Results
The in vivo phenotype of AMSCs
After removal of the amniotic epithelial cells by Dispase II, AMSCs in the AM could be observed in situ. Through a phase contrast microscope, they exhibited a dendritic morphology and maintained intercellular contacts via thin processes (Fig. 1A). Cell viability, dendritic morphology and intercellular contacts were better visualized by staining with Live and Dead assay (Fig. 1B). Immunostaining of the AM cross-sections showed that AMSCs did not express α-SMA (Fig. 1C) or desmin (Fig. 1D). In contrast, as a positive control, umbilical cord mesenchymal cells showed strong staining to both α-SMA and desmin (see the inset of Fig. 1C,D, respectively), indicating that they are smooth muscle cells. All AMSCs expressed vimentin (Fig. 1E). These data collectively indicate that AMSCs are of a fibroblast phenotype in vivo.
Fig 1.
In vivo AMSC phenotype. AMSCs were dendritic in shape and maintained intercellular contacts in situ (A). Cell viability, dendritic morphology and intercellular contacts were better visualized by staining with the Live and Dead assay (B). AMSCs did not express α-SMA (C) or desmin (D). In contrast, as a positive control, umbilical cord mesenchymal cells showed strong staining to both α-SMA and desmin (inset of C and D, respectively). However, all AMSCs expressed vimentin (E). Dot lines separate AM epithelium from AM stroma. Nuclear counterstaining was performed by DAPI (C,D) and PI (E), respectively. Bar represents 50 µm.
Rapid myofibroblast differentiation of AMSCs in vitro
To investigate the differentiation of AMSCs in vitro, collagenase-isolated AMSCs were plated on plastic dishes and cultured in DMEM with 10% FBS at a density of 200 cells/mm2. Within 4–5 days, the cells adopted a typical fibroblast cell shape (Fig. 2A) and remained α-SMA negative (Fig. 2E). However, some cells started to increase in cell size, change shape (Fig. 2B), and express α-SMA (Fig. 2F) at the end of 1 week of culturing. After subcultured to another plastic dish in the same medium for 1 week at Passage 1, the majority of cells exhibited a typical myofibroblastic cell shape (Fig. 2C), and eventually almost all cells turned into a myofibroblastic shape and had prominent microfilaments after 1 week cultivation at Passage 2 (Fig. 2D). Accordingly, α-SMA-positive myofibroblasts dramatically increased from 71.9 ± 3.7% at the primary culture to 93.9 ± 4.1% at Passage one and to 98.5 ± 1.7% at Passage 2 (Fig. 2I). During this time, the expression of desmin continued to be negative (data not shown). Western blot analysis (Fig. 2J) revealed that AMSCs weakly expressed ED-A fibronectin in vivo but did not express α-SMA. However, expression of α-SMA and ED-A fibronectin dramatically increased at the end of the primary culture and continued to be so at Passage 2. These results indicate that AMSCs rapidly differentiate into myofibroblasts on plastic in this serum-containing medium.
Fig 2.
Rapid myofibroblast differentiation of AMSCs in vitro. AMSCs cultured on plastic in DMEM with 10% FBS exhibited a typical fibroblast cell shape (A) and remained α-SMA negative (E) at Day 4 of the primary culture (P0). However, some cells started to increase the cell size, changed shape (B), and expressed α-SMA (F) at 1 week primary culture. After subculturing, the majority of cells turned into a typical myofibroblast cell shape (C) and expressed α-SMA (G) after 1 week at Passage 1. Nearly all cells turned into myofibroblastic-like shape (D) and showed strong expression of α-SMA (H) after 1 week at Passage 2. Accordingly, α-SMA-positive myofibroblasts dramatically increased from 71.9 ± 3.7% at 1 week primary culture to 93.9 ± 4.1% at Passage 1 and 98.5 ± 1.7% at Passage 2 (I). Western blot analysis confirmed the dramatic increase of protein expression of α-SMA and ED-A fibronetin (Fn). (J) Bar represents 100 µm.
Differentiated myofibroblasts from AMSCs could be reversed if subcultured on AM stromal matrix
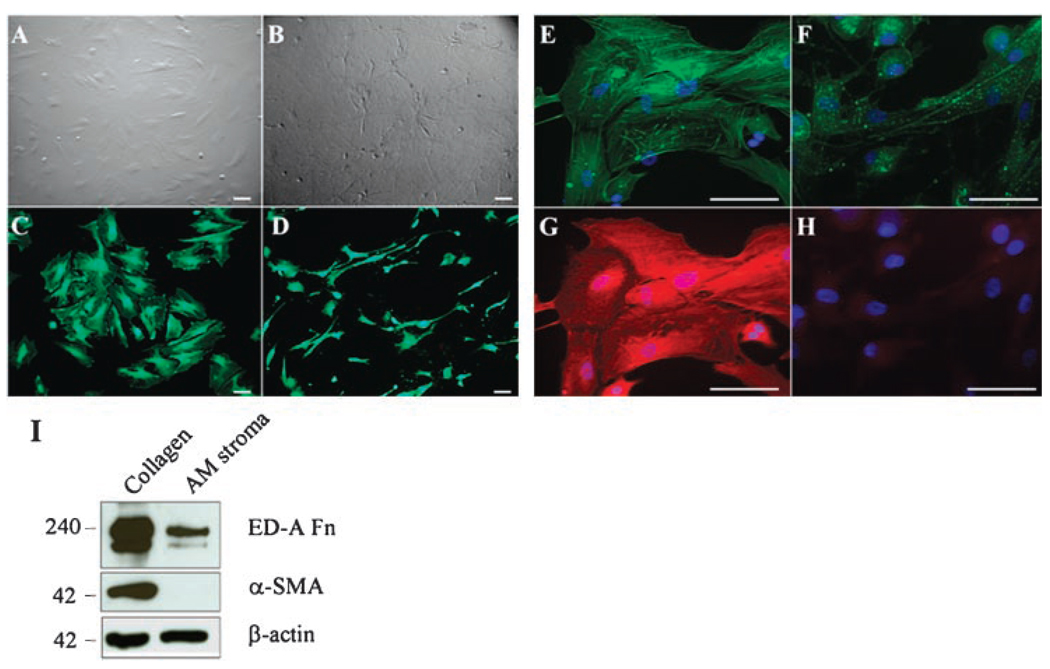
Our previous study showed that the AM can inhibit myofibroblast differentiation of human (Espana et al., 2003, 2004) or mouse (Kawakita et al., 2005) keratocytes when cultured on the stromal matrix of the AM from the primary culture. To further investigate whether the AM stromal matrix was also effective in reversing the phenotype of differentiated myofibroblasts from AMSCs at Passage 2, we subcultured the cells onto the AM stromal matrix or a collagen I-coated dish. After 7 days of cultivation in DMEM with 10% FBS, cells on collagen I maintained a myofibroblastic shape (Fig. 3A). In contrast, cells seeded on the AM stromal matrix exhibited a mixture of round, spindle, elongated, and dendritic shapes (Fig. 3B). Live and Dead assay confirmed that cells on both collagen I (Fig. 3C) and AM matrix (Fig. 3D) remained 100% viability, but revealed a remarkable difference in the cell shape. Phalloidin staining showed vivid stress fibers (Fig. 3E), which also contained strong α-SMA expression (Fig. 3G) in myofibroblasts seeded on collagen I. In contrast, the phalloidin staining became weak and spotty (Fig. 3F), and α-SMA staining became obscured in cells seeded on AM stromal matrix (Fig. 3H). Western blot analysis confirmed that myofibroblasts derived from AMSCs continuously expressed abundant amounts of ED-A fibronectin and α-SMA when seeded on type I collagen (Fig. 3I). In contrast, expression of ED-A fibronectin was decreased and α-SMA became undetectable when seeded on AM stromal matrix (Fig. 3I). These results collectively indicate that the myofibroblasts differentiated from AMSCs could be reversed to a fibroblast phenotype when subcultured on the AM stromal matrix.
Fig 3.
Reversal of differentiated myofibroblasts to fibroblasts when AMSCs were cultured on AM stromal matrix. Myofibroblasts derived from AMSCs at P2 were subcultured on type I collagen (A,C,E,G) or AM stromal matrix (B,D,F,H) in DMEM with 10% FBS for 7 days. Live and Dead assay showed cells on both collagen (C) and AM stromal matrix (D) remained 100% viability, but exhibited a different cell shape. Phalloidin and α-SMA double staining showed vivid stress fibers (E) and strong α-SMA expression (G) in myofibroblasts on collagen cultures. In contrast, phalloidin staining became weak and spotty (F), and α-SMA became obscured in cells subcultured on AM stromal matrix (H). Western blot analysis showed decreased expression of ED-A fibronectin (Fn) and undetectable expression of α-SMA by AMSCs seeded on AM stromal matrix as compared to those seeded on type I collagen (I). Bars represent 100 µm.
Amniotic stromal extract prevented myofibroblast differentiation of AMSCs and reverse differentiated myofibroblasts
To further investigate whether the aforementioned reversal activity by the AM stromal matrix was retained in the water-soluble AM stromal extracts (ASE), primary cultures of AMSCs (P0) were cultured on plastic in DMEM containing 10% FBS with or without 100 µg/ml ASE. After 4 days of cultivation, AMSCs maintained a spindle fibroblastic shape regardless addition of the ASE (Fig. 4A,B, respectively). However, at that time, cells already expressed α-SMA without ASE (Fig. 4E), but remained devoid of α-SMA expression when ASE was added (Fig. 4F). When cultures were extended to 10 days (similar to what was shown in Fig. 2), AMSCs adopted an enlarged cell shape, and vividly expressed phalloidin-positive stress fibers (Fig. 4C) and α-SMA (Fig. 4G) in the absence of ASE. In contrast, AMSCs aggregated into spheres of varying sizes with a smaller nucleus in the presence of ASE (Fig. 4D). These cells in the sphere remained viable based on the Live and Dead assay (not shown). Some spheres were detached from the plastic dish, but could still reattach to a new plastic dish to generate growth of myofibroblasts when switched back to DMEM/10%FBS (not shown). Phalloidin staining did not show any stress fibers (Fig. 4D), and α-SMA expression in the sphere was weak (Fig. 4H). These results indicated that ASE indeed could prevent myofibroblast differentiation of AMSCs if added in the beginning of cultivation.
Fig 4.
AM stromal extracts (ASE) prevented myofibroblast differentiation of AMSCs. Phalloidin staining (upper part) and α-SMA staining (lower part) were performed in the primary culture of the AMSCs. Cells maintained as pindle fibroblastic shape after 4 days cultivation on plastic in DMEM/10% FBS without (A) or with (B) ASE. However, at that time, cells already started to express α-SMA without ASE (E), but did not express α-SMA when ASE was added (F). When cultures were extended for 10 days, cells became enlarged and exhibited prominent stress fibers (C), and strong expression of α-SMA (G) without ASE. In contrast, AMSCs aggregated into spheres of varying sizes with addition of ASE. These spheres did not express any stress fibers (D), but expressed weak α-SMA staining (H). Bar represents 100 µm.
To examine whether the aforementioned activity of ASE could still be observed on already-differentiated myofibroblasts, we added 100 µg/ml ASE to Passage 2 AMSCs plastic cultures containing DMEM with 10% FBS for 1 week. As described earlier (Fig. 2), by this time nearly all cells turned into myofibroblasts with prominent stress fibers and strong expression of α-SMA. Addition of ASE allowed cells to revert to an elongated or spindle shape (Fig. 5A) with a notable decrease of α-SMA expression (Fig. 5B). Western blot analysis further confirmed a decreased expression of EDA fibronectin and α-SMA after the addition of ASE (Fig. 5C). These results indicate that the AM stroma contains soluble factor(s) that could suppress myofibroblast differentiation of AMSCs if added early, and also reverse differentiated myofibroblasts to fibroblasts if added later of the culture.
Fig 5.
Amniotic stromal extract (ASE) reversed differentiated myofibroblasts. Myofibroblasts differentiated from AMSCs on plastic in DMEM/10% FBS at Passage 2 (Fig. 2) were cultured with addition of ASE for 1 week. Cells reverted from a squamous shape to an elongated or spindle shape (A), while α-SMA staining became notably decreased (B). Western blot analysis showed expression of ED-A fibronectin and α-SMA were both reduced compared to that of the control without ASE (C). Bar represents 100 µm.
Reversal of myofibroblasts was not associated with cell proliferation
To further determine whether the aforementioned phenotypic reversal of AMSCs from myofibroblasts to fibroblasts by ASE was accompanied by cellular proliferation or not, we switched the medium from DMEM plus 10% FBS to serum-free DMEM/ITS in Passage 3 cultures. The passage 3 culture cells cultured in serum-free DMEM/ITS showed that nearly all the cells turned into myofibroblasts (Fig. 2). During a course of 6 days of observation, cells in the control culture maintained the same myofibroblast morphology with prominent stress fibers in the cytoplasm (Fig. 6A–D). However, cells in the cultures with additional ASE gradually changed shape from large and flattened on Day 0 (Fig. 4E) to spindle shaped and elongated on Days 2 and 4 (Fig. 6F,G, respectively). Finally some cells shrank to a small size on Day 6 (Fig. 6H). Such a dramatic morphological change caused by addition of ASE was accompanied by the loss of α-SMA-expressing stress fibers (Fig. 6I–L). Ki67 staining confirmed that myofibroblasts in DMEM/ITS of P3 cultures did not exhibit any cellular proliferation regardless of whether ASE was added or not (Fig. 6M,N, respectively). As a control, Passage 1AMSCs showed occasional Ki67-positive nuclei when cultured on plastic in DMEM/10%FBS (Fig. 6O), while many human corneal fibroblasts cultured on plastic in DMEM/10%FBS showed Ki67-positive nuclei (Fig. 6P). These results strongly support the notion that ASE reversed the differentiated myofibroblasts of AMSCs to fibroblasts without affecting their cellular proliferation.
Fig 6.
Reversal of myofibroblasts by ASE was not associated with cell proliferation. Passage 3 AMSCs were cultured in DMEM/ITS without (A–D) or with ASE (E–H) for different times. α-SMA-expressing stress fibers were gradually decreased from Days 0 to 6 after addition of ASE (I–L), and such a change correlated well with morphological changes (E–H). Ki67 staining showed that there was no cellular proliferation no matter if ASE was added or not (M, N, respectively). AMSCs in P1 cultures showed occasional Ki67-positive nuclei (O), while many human corneal fibroblasts cultured in DMEM/10% FBS showed Ki67-positive nuclei (P). Bars represent 100 µm.
Discussion
Previous studies have shown that myofibroblast differentiation from fibroblasts is facilitated by the addition of FBS or TGF-β. However, only <25% of standard fibroblast cultures under these conditions actually differentiate into myofibroblasts (Fini and Girard, 1990; Jester et al., 1996; Masur et al., 1996). Other studies showed that >60% of rabbit keratocytes differentiate into myofibroblasts when 0.25–1 ng/ml exogeneous TGF-β was added (Maltseva et al., 2001). Masur et al. (1996) reported that 80% of human corneal keratocytes can turn into myofibroblast when plated at a low density (5 cells per mm2) in DMEM/F12 medium with 10% FBS, while only 5–10% of fibroblasts seeded at a high density (500 cells per mm2) become myofibroblasts. Hepatic stellate cells cultured on plastic in a serum-containing medium can also spontaneously transdifferentiate to a myofibroblast-like cell type (Sato et al., 2003). Our study demonstrated that another type of mesenchymal cell can spontaneously differentiate to myofibroblast in vitro culture without exogeneous addition of TGF-β even when seeded at a high density (200 cells per mm2). In view of the abundance of human placental tissue and the rapid and complete transition into myofibroblasts in serum-containing medium, we believe that this culturing system is an ideal model for investigating myofibroblast differentiation.
Previously, it has been shown that TGFβ1 together with a mechanical stress plays an important role in promoting myofibroblast differentiation. Several factors have been identified to inhibit myofibroblast activity and may thus potentially suppress fibrosis. Among them, Interferon-γ can inhibit TGFβ1-induced myofibroblast differentiation of palatal fibroblasts (Yokozeki et al., 1999) and hepatic stellate cells (Weng et al., 2007). The α-SMA N-terminal peptide AcEEED can reduce force generation by the myofibroblast and exerts antifibrotic activity (Hinz et al., 2002). Differentiation of myofibroblasts from fibroblasts of human Tenon’s capsule under the stimulation of TGF-β in 10% FBS can be prevented by SB203580, a p38 inhibitor (Meyer-Ter-Vehn et al., 2006). Our laboratory has demonstrated that the AM is another potent substrate that may inhibit myofibroblasts differentiation of keratocytes through downregulation of TGF-β signaling pathway (Espana et al., 2003). Herein, we reported that such an action could also be extended to another cell type, that is, AMSCs. Furthermore, for the first time, we demonstrated that such an anti-fibrotic action was preserved in the soluble fraction of the AM stromal extract (Fig. 4). The notion that the AM stroma contains soluble factor(s) capable of inhibiting the pivotal TGF-β signaling pathway was supported by the finding that the TGF-β1 promoter activity was largely downregulated by ASE in ex vivo cultured human corneal fibroblasts (manuscript in preparation). Downregulation of TGF-β signaling is not only crucial to prevent myofibroblast differentiation (hence scar formation) of normal corneal, limbal, and conjunctival fibroblasts (Tseng et al., 1999; Lee et al., 2000) and abnormal pterygium fibroblasts (Lee et al., 2000), but also necessary to preserve the normal phenotype of corneal stromal keratocytes from differentiation into fibroblasts (Espana et al., 2003, 2004; Kawakita et al., 2005, 2006). Further study is under way to delineate the component(s) responsible for such an action.
Besides prevention of myofibroblast differentiation and preservation of normal keratocytes, herein we demonstrated that differentiated myofibroblasts from the AMSCs in a serum-containing medium can revert to a fibroblast phenotype when cultured back to the stromal side of AM (Fig. 3) or by addition of ASE (Fig. 5). At the present time, we do not know whether this reversal action is also mediated by the aforementioned putative factor(s) that downregulates TGF-β signaling. Previously, myofibroblast-like cells derived from hepatic stellate cells can revert to α-SMA-negative quiescent cells when cultured on a basement membrane like substrate (Matrigel™; Sohara et al., 2002). Cultured myofibroblasts differentiated from rabbit keratocytes in DMEM-F12 with TGF-β1 and 1%FBS can revert to a fibroblastic phenotype when treated with FGF-1 or -2, heparin, and 10% FBS (Maltseva et al., 2001). Moreover, they found that FGF and heparin could not reverse the myofibroblast phenotype when the majority of myofibroblasts were in G0, raising the argument that FGF may selectively induce proliferation of a subpopulation of fibroblasts, resulting in myofibroblast reversal. However, our study showed that ASE could reverse myofibroblast in serum-free medium without proliferation as evidenced by negative Ki67 staining (Fig. 6).
Intriguingly, it has been shown that the cellular microenvironment provided by the extracellular matrix may alter the response of myofibroblasts to the growth factors. In this context, the mechanical stress generated by the matrix is one prerequisite for the transition of fibroblasts to myofibroblasts (Hinz and Gabbiani, 2003). Therefore, one may wonder whether myofibroblasts reversed to fibroblasts phenotype when seeded back to the stromal side of AM could be due to the loss of mechanical stress. However, our finding that the same phenomenon could still be reproduced by addition of ASE on plastic cultures (Fig. 5) suggested the presence of soluble factors other than mechanical stress in reversion of myofibroblasts to fibroblasts.
Interestingly, primary cultures of AMSCs in a serum-containing medium for 10 days formed spheres of cell aggregates in the presence of ASE (Fig. 4). Previously, several studies have shown that sphere cultures help maintain the non-differentiated status of several cell types (Svendsen et al., 1998; Du et al., 2005; Yokoo et al., 2005; Gonzalez et al., 2006). AMSCs spheres in our culture did not show stress fiber formation or obvious expression of α-SMA, but remained viable and capable of differentiating into myofibroblasts when subcultured in serum-containing medium. These findings suggest that ASE helps AMSCs maintain their progenitor status, a notion resembling what has been reported that amniotic membrane can help preserve and expand limbal epithelial progenitor cells [for review, see (Grueterich et al., 2003)]. Future studies may help unravel whether the soluble fraction from the AM stroma contains the effective components which may be used in anti-scaring or anti-fibrosis therapy, as well as facilitate stem cell preservation and expansion.
Acknowledgments
The project described was supported by grants EY 06819, EY 15735 and EY017497 (to SCGT), from National Eye Institute, National Institutes of Health, Bethesda, Maryland, a research grant from TissueTech, Inc., and an unrestricted grant from Ocular Surface Research & Education Foundation, Miami, FL. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contract grant sponsor: National Eye Institute, National Institutes of Health, Bethesda, Maryland;
Contract grant numbers: EY 06819, EY 15735, EY 017497.
Contract grant sponsor: TissueTech, Inc.
Footnotes
Proprietary Interests: SCGT and his family are more than 5% shareholders of TissueTech, Inc., which owns US Patents Nos. 6,152,142 and 6,326,019 on the method of preparation and clinical uses of human amniotic membrane distributed by Bio-Tissue, Inc.
Literature Cited
- Bouchard CS, John T. Amniotic membrane transplantation in the management of severe ocular surface disease: Indications and outcomes. Ocul Surf. 2004;2:201–211. doi: 10.1016/s1542-0124(12)70062-9. [DOI] [PubMed] [Google Scholar]
- Desmouliere A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- Du Y, Funderburgh ML, Mann MM, SundarRaj N, Funderburgh JL. Multipotent stem cells in human corneal stroma. Stem Cells. 2005;23:1266–1275. doi: 10.1634/stemcells.2004-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dua HS, Gomes JA, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49:51–77. doi: 10.1016/j.survophthal.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Espana EM, He H, Kawakita T, Di Pascuale MA, Raju VK, Liu CY, Tseng SCG. Human keratocytes cultured on amniotic membrane stroma preserve morphology and express keratocan. Invest Ophthalmol Vis Sci. 2003;44:5136–5141. doi: 10.1167/iovs.03-0484. [DOI] [PubMed] [Google Scholar]
- Espana EM, Kawakita T, Liu CY, Tseng SCG. CD-34 expression by cultured human keratocytes is downregulated during myofibroblast differentiation induced by TGF-beta1. Invest Ophthalmol Vis Sci. 2004;45:2985–2991. doi: 10.1167/iovs.04-0201. [DOI] [PubMed] [Google Scholar]
- Fini ME, Girard MT. The pattern of metalloproteinase expression by corneal fibroblasts is altered by passage in cell culture. J Cell Sci. 1990;97:373–383. doi: 10.1242/jcs.97.2.373. [DOI] [PubMed] [Google Scholar]
- Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- Gonzalez P, Epstein DL, Luna C, Liton PB. Characterization of free-floating spheres from human trabecular meshwork (HTM) cell culture in vitro. Exp Eye Res. 2006;82:959–967. doi: 10.1016/j.exer.2005.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueterich M, Espana EM, Tseng SCG. Ex vivo expansion of limbal epithelial stem cells: Amniotic membrane serving as a stem cell niche. Surv Ophthalmol. 2003;48:631–646. doi: 10.1016/j.survophthal.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Hinz B, Gabbiani G. Mechanisms of force generation and transmission by myofibroblasts. Curr Opin Biotechnol. 2003;14:538–546. doi: 10.1016/j.copbio.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Hinz B, Gabbiani G, Chaponnier C. The NH2-terminal peptide of alpha-smooth muscle actin inhibits force generation by the myofibroblast in vitro and in vivo. J Cell Biol. 2002;157:657–663. doi: 10.1083/jcb.200201049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Barry-Lane PA, Cavanagh HD, Petroll WM. Induction of alpha-smooth muscle actin expression and myofibroblast transformation in cultured corneal keratocytes. Cornea. 1996;15:505–516. [PubMed] [Google Scholar]
- Kawakita T, Espana EM, He H, Hornia A, Yeh LK, Ouang J, Liu CY, Tseng SC. Keratocan expression of murine keratocytes is maintained on amniotic membrane by downregulating TGF-beta signaling. J Biol Chem. 2005;280:27085–27092. doi: 10.1074/jbc.M409567200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakita T, Espana EM, He H, Parel J-M, Liu CY, Tseng SCG. Preservation and expansion of primate keratocyte phenotype by downregulating TGF-B signaling in a low calcium serum-free medium. Invest Ophthalmol Vis Sci. 2006;47:1918–1927. doi: 10.1167/iovs.05-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-B, Li D-Q, Tan DTH, Meller D, Tseng SCG. Suppression of TGF-β signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res. 2000;20:325–334. [PubMed] [Google Scholar]
- Maltseva O, Folger P, Zekaria D, Petridou S, Masur SK. Fibroblast growth factor reversal of the corneal myofibroblast phenotype. Invest Ophthalmol Vis Sci. 2001;42:2490–2495. [PubMed] [Google Scholar]
- Masur SK, Dewal HS, Dinh TT, Erenburg I, Petridou S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc Natl Acad Sci USA. 1996;93:4219–4223. doi: 10.1073/pnas.93.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller D, Tseng SCG. Conjunctival epithelial cell differentiation on amniotic membrane. Invest Ophthalmol Vis Sci. 1999;40:878–886. [PubMed] [Google Scholar]
- Meyer-Ter-Vehn T, Gebhardt S, Sebald W, Buttmann M, Grehn F, Schlunck G, Knaus P. p38 inhibitors prevent TGF-beta-induced myofibroblast transdifferentiation in human tenon fibroblasts. Invest Ophthalmol Vis Sci. 2006;47:1500–1509. doi: 10.1167/iovs.05-0361. [DOI] [PubMed] [Google Scholar]
- Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol. 1999;277:C1–C9. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- Sato M, Suzuki S, Senoo H. Hepatic stellate cells: Unique characteristics in cell biology and phenotype. Cell Struct Funct. 2003;28:105–112. doi: 10.1247/csf.28.105. [DOI] [PubMed] [Google Scholar]
- Sippel KC, Ma JJK, Foster CS. Amniotic membrane surgery. Curr Opin Ophthalmol. 2001;12:269–281. doi: 10.1097/00055735-200108000-00006. [DOI] [PubMed] [Google Scholar]
- Sohara N, Znoyko I, Levy MT, Trojanowska M, Reuben A. Reversal of activation of human myofibroblast-like cells by culture on a basement membrane-like substrate. J Hepatol. 2002;37:214–221. doi: 10.1016/s0168-8278(02)00103-4. [DOI] [PubMed] [Google Scholar]
- Svendsen CN, ter Borg MG, Armstrong RJ, Rosser AE, Chandran S, Ostenfeld T, Caldwell MA. A new method for the rapid and long term growth of human neural precursor cells. J Neurosci Methods. 1998;85:141–152. doi: 10.1016/s0165-0270(98)00126-5. [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Tseng SCG. Amniotic membrane transplantation for ocular surface reconstruction. Biosci Rep. 2002;21:481–489. doi: 10.1023/a:1017995810755. [DOI] [PubMed] [Google Scholar]
- Tseng SC, Li DQ, Ma X. Suppression of transforming growth factor-beta isoforms, TGF-beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol. 1999;179:325–335. doi: 10.1002/(SICI)1097-4652(199906)179:3<325::AID-JCP10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Weng H, Mertens PR, Gressner AM, Dooley S. IFN-gamma abrogates profibrogenic TGF-beta signaling in liver by targeting expression of inhibitory and receptor Smads. J Hepatol. 2007;46:295–303. doi: 10.1016/j.jhep.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Yokoo S, Yamagami S, Yanagi Y, Uchida S, Mimura T, Usui T, Amano S. Human corneal endothelial cell precursors isolated by sphere-forming assay. Invest Ophthalmol Vis Sci. 2005;46:1626–1631. doi: 10.1167/iovs.04-1263. [DOI] [PubMed] [Google Scholar]
- Yokozeki M, Baba Y, Shimokawa H, Moriyama K, Kuroda T. Interferon-gamma inhibits the myofibroblastic phenotype of rat palatal fibroblasts induced by transforming growth factor-beta1 in vitro. FEBS Lett. 1999;442:61–64. doi: 10.1016/s0014-5793(98)01626-3. [DOI] [PubMed] [Google Scholar]