Abstract
The goal of this research project was to quantitatively assess whether transcutaneous triceps stimulation can overcome the expression of abnormal torque patterns in the paretic upper limb of subjects with hemiparetic stroke. Abnormal torque patterns consist of strong coupling between shoulder abduction (SAB) and elbow flexion (EF) or between elbow extension (EE) and shoulder adduction (SAD) torques. Both patterns reduce the active range of motion during arm movements. Eight chronic stroke subjects with moderate to severe (Fugl-Meyer assessment scores of 21/66–36/66) upper limb motor impairment participated in this study. Shoulder and elbow joint torques were measured with a 6-degrees-of-freedom load cell under isometric conditions, while the triceps muscle was stimulated to generate EE torques. At the same time the subjects were asked to lift up their arm to generate different SAB torque levels. The obtained isometric results showed that electrical stimulation can overcome abnormal torque patterns in chronic stroke subjects while generating SAB. This is likely to have potential benefits to increase the reaching workspace of the paretic arm.
Keywords: Functional electrical stimulation, Triceps stimulation, Upper extremities, Joint torque
Sensorimotor deficits and restricted mobility are among the more common problems following stroke. Clinically recognized motor deficits following stroke are weakness or paralysis and spasticity or hyperactive stretch reflexes. However, another more disabling deficit following stroke is a significant disturbance in movement coordination. Abnormal coordination is expressed in the form of abnormal muscle synergies and results in limited and stereotypic movement patterns, which are functionally disabling (1,2). Abnormal coupling between shoulder abduction and elbow flexion (SAB/EF) and shoulder adduction and elbow extension (SAD/EE) torques were quantitatively characterized (3,4) and are estimated to be one of the main factors related to reaching deficits following stroke (5,6). Transcutaneous functional electrical stimulation (FES) of the upper limb could provide the means to overcome abnormal torque coupling. Up to now FES in the upper extremities has been mainly applied to improve or partially restore grasp function (7,8). In addition to hand function a few laboratories investigated the control of elbow extension movement in spinal cord injured (SCI) subjects using FES (9,10). However, to date nobody has attempted to employ FES in hemiparetic stroke to reduce the effect of abnormal muscle and torque coupling between the elbow and shoulder. The present study was designed to investigate whether selective FES stimulation of elbow extensors can reduce the expression of the flexion synergy (i.e., SAB/EF coupling) thus potentially enhancing the paretic arm’s workspace during free reaching.
MATERIALS AND METHODS
Subjects
Eight subjects (5 male and 3 female; age 44–73) with a unilateral brain injury resulting from a stroke at least two years prior to participation in this study were selected. All subjects showed a moderate to severe upper limb motor impairment (Fugl-Meyer assessment scores of 21/66–36/66). Selection criteria for the hemiparetic subjects were the following: (1) Paresis confined to one side, with significant motor impairment of the upper limb; (2) Absence of muscle tone abnormalities and motor or sensory deficits in the unimpaired arm. (3) Absence of severe wasting or contracture of the impaired upper limb; (4) Absence of significant sensory deficits in the impaired upper limb; (5) Absence of severe cognitive or affective dysfunction; (6) Absence of severe concurrent medical problems; and (7) Absence of brainstem lesions as determined from clinical or radiological reports. All subjects signed informed consent, which was reviewed and approved by the Institutional Review Board of Northwestern University.
Setup
Subjects were seated in a Biodex chair with shoulder and waist strapped to restrain trunk and shoulder girdle movement during testing. The forearm, wrist, and hand were attached to a 6-degrees-of-freedom (6-DOF) load cell (JR3 Inc., Woodland, CA, U.S.A., Model #45E15A) using fiberglass casting and a Delrin ring mounted at the wrist. The attached arm was positioned with 75° SAB and 90° EF angles such that the middle finger was aligned to the medial sagittal plane of the body. Isometric shoulder and elbow torques were measured and the SAB torque was displayed in real-time on a computer monitor placed in front of the subject. The triceps brachii muscle was stimulated with a Compex Motion (11) transcutaneous electric stimulator using trapezoidal stimulation patterns with 1 s amplitude ramps and 5–12 s constant stimulation depending on the task. A stimulation frequency of 25 Hz was used in all trials. The stimulation amplitude was adjusted to generate the strongest EE torque without causing discomfort. The pulse duration that caused the highest EE torque was chosen from four durations, i.e., 150, 200, 300, and 500 μs.
Experiment
In the first part of the experiment the stimulation amplitude and pulse duration that generated the highest EE torque were determined. This was achieved by using the 1 s ramps to determine the maximum stimulation amplitude for a given pulse duration. Increasing the stimulation amplitude didn’t always result in a higher EE torque because antagonistic muscles were activated at higher amplitudes (see Fig. 1). The highest stimulation amplitude that did not generate cocontraction of antagonistic muscles was determined for each of the four pulse durations and the pulse duration/amplitude pair that produced the highest EE torque was then chosen for the rest of the experiment.
FIG. 1.
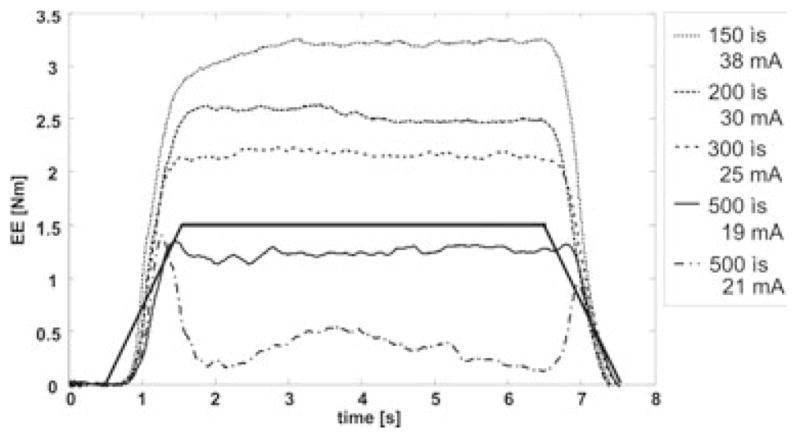
In most subjects shorter pulse durations generated higher EE torques. The EE torque decreased when reaching greater stimulation amplitudes presumably due to activation of antagonistic muscles (compare lowest plot [dashed, 500 μs and 21 mA] with 2nd plot from below [500 μs and 19 mA]).
In the second part of the experiment the subjects were asked to lift their arms with different SAB torque levels while the triceps muscle was stimulated to produce an EE torque. Subjects could see on a dial on the screen the actual SAB torque. They were asked to adjust and hold SAB torque for 12 s within a range of ±10%. For each trial the required SAB torque was randomly changed. During the holding phase the triceps muscle was stimulated for a duration of 7 s. In the third part of the experiment the subjects had to perform different levels of SAB as in the previous paradigm, but in addition the subjects were instructed to generate voluntary EE torques during triceps stimulation.
RESULTS
On average, without the subjects’ volitional intervention a maximum EE torque of 6.3 (± 4.2) Nm or 25.8 (± 14)% of maximum voluntary contraction could be generated by triceps stimulation (n = 8). It was generated with an average stimulation amplitude of 51 (± 15) mA and an average pulse duration of 181.25 (± 53) μs.
The maximum EE torque was calculated as the difference of the mean torque between 2 s and 5 s after stimulation onset and the mean torque between 500 ms and 0 ms prior to triceps stimulation.
When subjects were asked to lift up their arms in order to generate a SAB torque an abnormal coupling with EF was generated (as shown in the upper plot of Fig. 2). In six subjects the abnormal torque coupling could be reversed by electrical stimulation (Fig. 3).
FIG. 2.
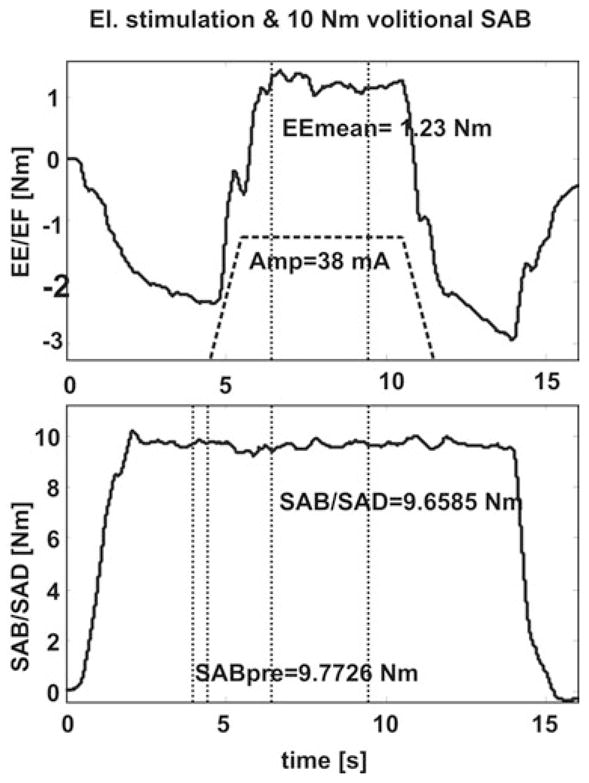
The upper plot shows how the abnormal EF torque coupling generated by voluntary SAB of 10 Nm (lower plot) can be reversed by applying triceps stimulation.
FIG. 3.
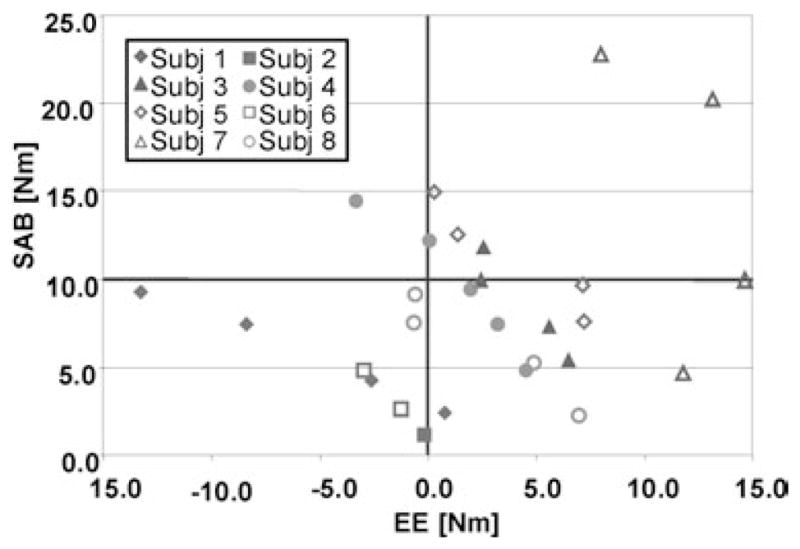
The stimulated elbow torques of 8 subjects are plotted against the SAB torques the subjects were asked to generate voluntarily. Negative elbow torques are EF torques, occurring from abnormal torque coupling that could not be reversed by electrical stimulation.
On average, while stimulating the triceps muscle subjects could achieve a SAB torque of 11.7 (± 7.2) Nm without generating concurrent EF torques. EF torque could be eliminated by generating FES-induced average EE torques of 6.7 (± 3.8) Nm. Four subjects could lift up their paretic arms against gravity (SAB torque of ~10 Nm) and have a net FES-induced EE torque at the same time. In two subjects the abnormal EF torque coupling could not be eliminated during SAB when electrically stimulating the triceps. When the subjects were instructed to volitionally assist triceps FES a SAB of 13.7 (± 7.0) Nm could be achieved while not generating abnormal EF torques (n = 6, Figs. 4 and 5). During stimulation of the triceps a net EE torque of 6.6 (± 4.2) Nm was generated. In the same two subjects no reversal from EF to EE was obtained during triceps stimulation, even if these subjects tried to volitionally extend their elbows during SAB. They were not able to lift up the arm against gravity and hold a constant torque level either.
FIG. 4.
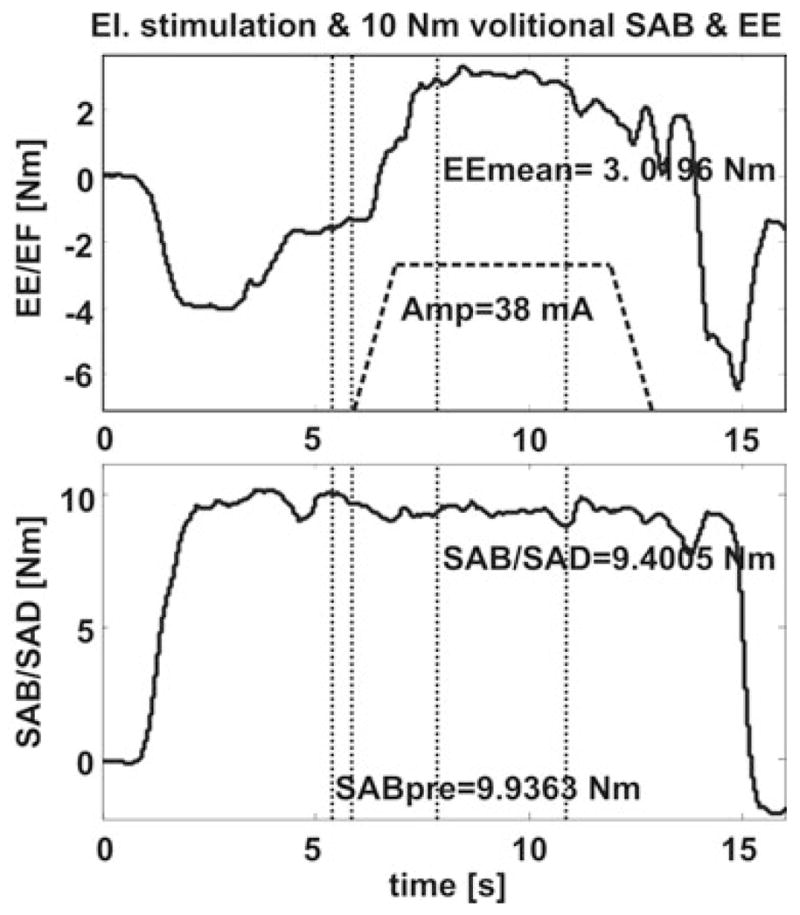
In the third part of the experiment the subjects were asked to generate submaximal voluntary EE contraction during SAB. After EE was generated by the subject for about 2 s triceps stimulation was added to the voluntary contraction.
FIG. 5.
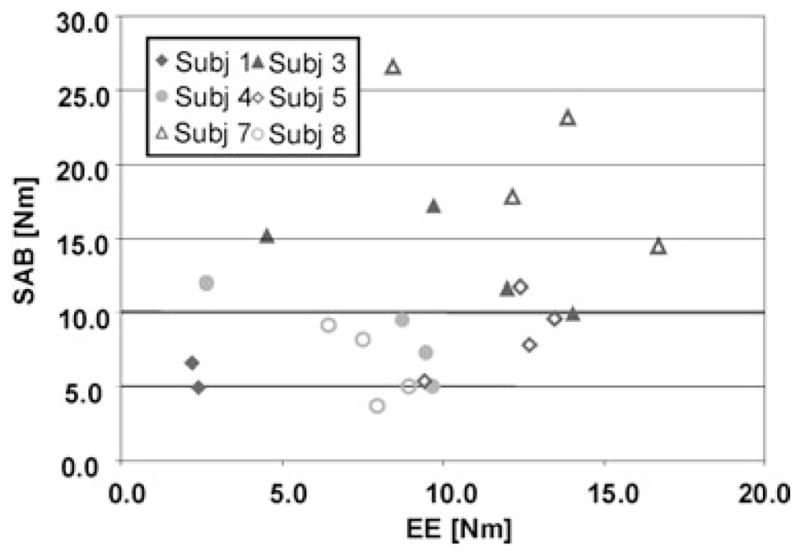
Six subjects could lift up the arm and produce together with triceps stimulation a net EE torque, which can be interpreted as a reaching-out movement. Four subjects could perform SAB torques high enough to lift up the arm against gravity, while maintaining an EE net torque during stimulation.
DISCUSSION
In the present study the possibility of using triceps stimulation to overcome abnormal torque patterns in chronic stroke subjects was investigated under isometric conditions. In six out of eight subjects the results showed that FES of the triceps muscle could generate enough torque to overcome abnormal EF coupling during SAB. In four subjects triceps stimulation could overcome abnormal EF torques when the subjects were generating SAB torques higher than 10 Nm. Such SAB torques are necessary to lift up the arm against gravity. The measured net EE torque during SAB that were higher than 10 Nm indicate that the subjects would be able to lift the arm and generate a net extension torque due to triceps stimulation. However, two subjects could not generate the SAB torques necessary to lift up the arm against gravity. Furthermore, in these subjects triceps stimulation could not overcome their abnormal elbow/shoulder torque coupling.
The results showed that the torques generated by triceps stimulation added to the voluntarily generated EE torques and therefore may have beneficial effects during dynamic reaching (5,6). The EE torque increase was possible, because the volitionally generated EE torques were submaximal. The subject at the same time had to generate EE torques and keep the SAB torque constant. Such dual tasks could only be performed with submaximal voluntary contractions and require training (12).
In the present study we only performed isometric measurements. Dynamic experiments that directly show an increase of the active workspace generated by triceps stimulation will be investigated in future experiments.
A future goal of our work will be to develop a suitable control strategy that enables FES-assisted arm movements and takes into account voluntary arm movement. A system that enables controlled FES-assisted arm movements may help subjects suffering from stroke to enlarge the active workspace. It could be used to train the subjects to overcome their abnormal torque synergies.
Acknowledgments
This work was supported by the grant no. 823B-067648 from the Swiss National Science Foundation and the US National Institutes of Health (RO1 HD39343).
Footnotes
Presented in part at the 8th Vienna International Workshop on Functional Electrical Stimulation, held September 10–13, 2004, in Vienna, Austria.
References
- 1.Brunnstrom S. Movement Therapy in Hemi-Plegia. New York: Harper & Row; 1970. [Google Scholar]
- 2.Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118:495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- 3.Dewald JP, Beer RF. Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve. 1995;24:273–83. doi: 10.1002/1097-4598(200102)24:2<273::aid-mus130>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Beer RF, Given JD, Dewald JP. Task-dependent weakness at the elbow in patients with hemiparesis. Arch Phys Med Rehabil. 2001;80:766–72. doi: 10.1016/s0003-9993(99)90225-3. [DOI] [PubMed] [Google Scholar]
- 5.Dewald JP, Sheshadri V, Dawson ML, Beer RF. Upper-limb discoordination in hemiparetic stroke: implications for neurorehabilitation. Top Stroke Rehabil. 2001;8:1–12. doi: 10.1310/WA7K-NGDF-NHKK-JAGD. [DOI] [PubMed] [Google Scholar]
- 6.Beer RF, Dewald JP, Dawson ML, Rymer WZ. Task-dependent effects of support condition on planar arm movements in chronic hemiparesis. Exp Brain Res. 2004;156:458–70. doi: 10.1007/s00221-003-1807-8. [DOI] [PubMed] [Google Scholar]
- 7.Peckham PH, Keith MW, Kilgore KL, et al. Efficacy of an implanted neuroprosthesis for restoring hand grasp in tetraplegia: a multicenter study. Arch Phys Med Rehabil. 2001;82:1380–8. doi: 10.1053/apmr.2001.25910. [DOI] [PubMed] [Google Scholar]
- 8.Popovic MR, Popovic DB, Keller T. Neuroprostheses for grasping. Neurol Res. 2001;24:443–52. doi: 10.1179/016164102101200311. [DOI] [PubMed] [Google Scholar]
- 9.Crago PE, Memberg WD, Usey MK, et al. An elbow extension neuroprosthesis for individuals with tetraplegia. IEEE T Rehabil Eng. 2002;6:1–6. doi: 10.1109/86.662614. [DOI] [PubMed] [Google Scholar]
- 10.Popovic D, Popovic M. Tuning of a non-analytical hierarchical control system for reaching with FES. IEEE T Bio-Med Eng. 1998;45:203–12. doi: 10.1109/10.661268. [DOI] [PubMed] [Google Scholar]
- 11.Keller T, Popovic MR, Pappas IP, Muller PY. Transcutaneous functional electrical stimulator “Complex Motion. Artif Organs. 1998;26:219–23. doi: 10.1046/j.1525-1594.2002.06934.x. [DOI] [PubMed] [Google Scholar]
- 12.Ellis MD, Holubar B, Acosta A, Dewald JP. Reduced Constraints in Elbow/Shoulder Joint Torque Patterns in Hemiparetic Stroke Using a Multi-DOF Isometric Strengthening Protocol. New Orleans, LA: Society for Neuroscience; 2002. [Google Scholar]


