Abstract
Leishmaniasis is a vector-borne disease transmitted to human and other mammalian hosts by sand fly bite. Here we show that immunization with Leishmania mexicana promastigote secretory gel (PSG) or a chemically defined synthetic glycovaccine containing the glycans found in L. mexicana PSG can both provide significant protection against challenge by the bite of infected sand flies. Only the glycan from L. mexicana was protective, those found in other species did not protect against L. mexicana infection. Further, neither PSG nor the glycovaccine protected against artificial needle challenge, which is traditionally used in antileishmanial vaccine development. Conversely, an antigen preparation that was effective against needle challenge offered no protection against sand fly bite. These findings provide a new target for Leishmania vaccine development and demonstrate the critical role of the vector in the evaluation of candidate vaccines for leishmaniasis and other vector-borne diseases.
Keywords: leishmaniasis, vaccine, sand fly, promastigote secretory gel
INTRODUCTION
Prophylactic vaccines are currently unavailable against the important human parasitic diseases that plague the tropics and are a major challenge for the biomedical research community. Vector-borne diseases are particularly difficult to tackle, as protection must be effective against natural challenge via their arthropod vectors that frequently enhance transmission of parasites. The leishmaniases are a group of vector-borne diseases transmitted by the bites of blood feeding sand flies and are a relatively attractive target disease for vaccine development because there is convincing evidence of natural immunity to infection against many Leishmania species [1]. However, to date, vaccine development has proved more difficult than expected, partly due to various complexities in the immune response to leishmaniasis [2-4]. Therefore, in addition to the development of known antigens for vaccination [5], new targets are urgently required. In that regard, recent work has shown that sand flies with mature transmissible Leishmania infections have their midgut blocked by promastigote secretory gel (PSG)[6, 7], causing the fly to regurgitate both parasites and PSG during blood feeding [8]. PSG is mainly composed of a high molecular weight glycoprotein called filamentous proteophosphoglycan (fPPG)[9], and the glycan part of fPPG was shown to be responsible for significant disease exacerbation. Interestingly, the carbohydrate antigens of several bacterial pathogens have yielded effective vaccines [10, 11]. Therefore, we have investigated whether PSG or an analogue could be used to vaccinate mice against cutaneous leishmaniasis caused by L. mexicana.
Conventional models used in Leishmania vaccine development invariably use needle challenge in mice, as this is both convenient and reproducible [12]. Mice are a reasonable choice of model for human infection since rodents are naturally infected by Leishmania, and are important reservoirs of human disease in some cases [13]. The most commonly used mouse strain is the BALB/c because this strain is highly susceptible to Leishmania infection [12], and, therefore, any protection obtained is significant. Infection of BALB/c mice with L. mexicana results in aggressive non-healing cutaneous lesions. Mice are inoculated with parasites either via subcutaneous injection into the rump or footpad or intradermally into the ear [14], seeking to mimic deposition of parasites into the skin by sand flies. Considerable variations exist in both the size and quality of infective inocula used. Typically 105-106 culture-derived parasites are injected, much higher than the numbers injected by sand flies, ~103 per bite in the L. mexicana/Lutzomyia longipalpis parasite-vector combination [8]. Sometimes the correct life cycle stage is used, metacyclic promastigotes, which are known to be specifically pre-adapted for survival in a mammalian host [15]. However, whether these various factors and the use of infected sand flies are important or not in Leishmania vaccine development has not been addressed to date. To our knowledge, the only previous vaccine study in which infected sand flies were used was the elegant work by Kamhawi et al. on sand fly salivary antigens [16], although no direct comparison with needle challenge was made in that study. Our previous work indicated that this could be an important issue, because fly bite infections led to more severe disease and higher parasite burdens in naïve mice [8]. Therefore, this comparison was incorporated into the present study. Challenge by the bites of individual infected sand flies has not been used in any previous vaccine study, but most closely replicates infection under natural conditions.
MATERIALS AND METHODS
Parasites and sand flies
Female Lu. longipalpis were infected by feeding on 2×106 L. mexicana lesion amastigotes/ml rabbit blood [7]. Flies were maintained for 7 days to allow mature infections to develop, PSG plugs were isolated by dissection [7]. PSG was separated from parasites by dispersion in PBS (5 μl/plug) and then centrifuged in four 5-min rounds (10,000g), and supernatants were retained and stored at −70°C. Soluble Leishmania antigen (SLA) was prepared from L. mexicana promastigotes as described elsewhere [17].
Immunization and challenge
Mice were vaccinated by subcutaneous injection of 20 μl volumes of PBS containing PSG, synthetic glycovaccines or SLA into the dorsal surface of the left hindfoot of 8-10 week old BALB/c mice. PSG was used at 5 μg/injection/mouse, glycovaccines at 5 μg/injection/mouse and SLA at 25 μg/injection/mouse. Recombinant IL-12 was used at 0.33 μg/mouse and CpG ODN 1826 [18] at 50 μg/mouse. Immunization was performed twice, at 2-week intervals, and was followed by challenge after a further 2-week interval. Challenge was to the right hindfoot either by subcutaneous injection of 1000 cultured metacyclic promastigotes or by allowing individual infected sand flies to bite once [8]. Lesion development was monitored by measuring the swelling of the right foot with Vernier calipers and subtracting the width of the contralateral uninfected foot. At the end of experiments mice were humanely sacrificed, and parasite burdens in the feet determined either by direct counting via haemocytometer or by limiting dilution. All procedures involving animals were approved by a local Animal Welfare Committee and performed in accordance with UK Government (Home Office) and EC regulations.
Glycovaccine synthesis
Synthetic glycans, which are fragments of lipo- and proteophosphoglycans from L. donovani [19], L. mexicana [20] and L. major [21], were synthesised and conjugated to recombinant tetanus toxin fragment C protein [22] using the procedures described [23, 24].
Statistical analysis
Analysis of serial data was performed essentially as described [25]. Sequential measurements for each individual mouse were used to calculate the area under the curve for a plot of lesion thickness against time. The distribution of values in some groups showed evidence of non-normality using the Shapiro-Wilk test and therefore non-parametric analysis was performed. The areas derived for each group of mice and final parasite burdens were compared using the Mann-Whitney U two–sided test in which the null hypothesis is that the two distributions are not significantly different. The null hypothesis was rejected if P<0.05. Statistical analysis was performed using the StatsDirect software package version 2.3.1.
RESULTS
Partial protection against infected sand fly bite, provided by PSG
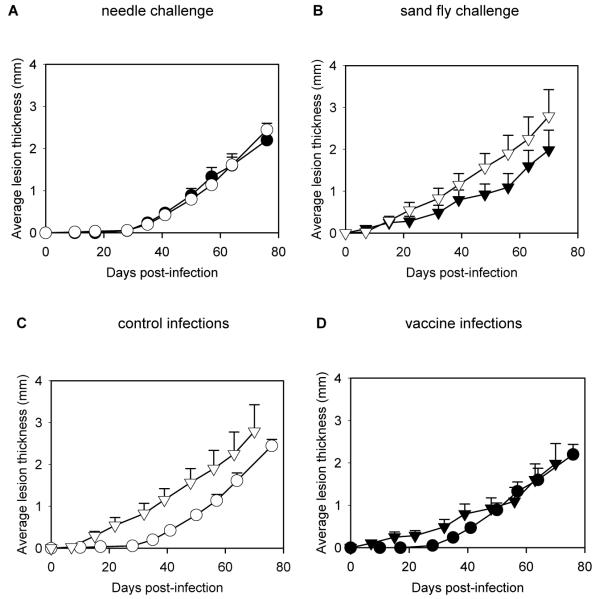
L. mexicana PSG was isolated from infected Lu. longipalpis and used to vaccinate BALB/c mice. The mice were challenged either by needle inoculation or by the single bites of L. mexicana-infected sand flies. For needle inoculation 103 metacyclic promastigotes were used to mimic the average dose given by a sand fly, and for bite challenge each mouse was exposed to the bite of a single infected sand fly. The results showed that PSG did not protect against needle challenge with L. mexicana in BALB/c mice (figure 1A, P > 0.05, Mann-Whitney U 2-sided test). There was no significant difference in the rate of lesion growth between the control and PSG-immunized groups. This was reflected in the final mean (± SE) parasite burdens from the 2 groups of mice: 2.17 × 108 (±1.06) amastigotes/mouse in the control group compared with 2.57 ×108 (±1.2) amastigotes/mouse in the PSG-immunized group (P > 0.05). However, when mice were challenged by sand fly bite, immunization with PSG provided partial protection (figure 1B). The PSG-immunized group showed a reduced rate of lesion growth and 30% reduction in final lesion size, although this was not statistically significant (P > 0.05) because of the high variance within the sand fly bite challenged groups (probably reflecting natural variation in infection intensity and feeding behaviour between individual flies). However, the mean (± SE) parasite burdens were significantly reduced: 1.42 × 108 (±0.48) amastigotes/mouse for the control group, compared with 6.48 × 107 (±2.75) amastigotes/mouse for the PSG-immunized group, a 54% reduction in parasite burden (P = 0.047).
Figure 1.
Immunization of mice with promastigote secretory gel (PSG). Four groups of 9-10 BALB/c mice were either sham-inoculated (○, ▽) or immunized with PSG (●, ▼) and challenged by needle injection with 1000 Leishmania mexicana metacyclic promastigotes (○, ●)(A) or by single bites of L. mexicana infected sand flies (▽,▼)(B). C, Comparison of infections in the control groups, by needle (○) or sand fly bite (▽). D, Comparison of infections in the PSG-immunized groups, infected by needle (●) or sand fly bite (▼). Error bars represent 1 SE.
These results are interesting in several ways. First, it should be noted that any protection in the BALB/c mouse/L. mexicana model is significant, especially without the use of adjuvants, as is the case in the present study. Second, they indicate that the outcomes of needle and fly bite challenge are potentially different in a Leishmania immunization context. In naïve mice fly bite produces a more severe disease due to the co-inoculation of PSG, and, therefore, is a more potent challenge than needle inoculation [8]. This can also be seen in the present study (figure 1C), in which infections in the control groups from the two experiments are compared. However, in our study where PSG itself was used for immunization, this strong exacerbation effect was effectively neutralised, as shown by the comparison of the two vaccinated groups (figure 1D). Thus these results show that the potent disease exacerbatory properties of PSG can be neutralised by prior immunization, even in the highly susceptible BALB/c model. Third, although the protective effect was only partial, it suggests that it may be possible to exploit PSG egestion by sand flies in Leishmania vaccines.
Protection against infected sand fly bite, provided by glycovaccines
There would be a variety of practical problems with using PSG itself as a vaccine in humans, even if protection could be improved over that described above. For example, the isolation of this material from infected sand flies would be impossible to increase to the standard and scale required for good manufacturing practice (GMP). Also, although fPPG is known to be the major component of PSG, there may be other minor components that could be important in the immunization context, and fPPG is itself a very large and complex glycoprotein with many potential epitopes [9]. Competing epitopes may antagonise each other and reduce any protective effect. From our previous work on the transmission mechanism, the disease exacerbation effect of PSG was shown to be specifically associated with the glycan component of fPPG [8]. Therefore, to address these concerns and to improve protective efficacy, we tested whether chemically-defined synthetic glycovaccines based on known Leishmania glycans might be able to confer protection against infection. Phosphoglycans of various structures from L. donovani, L. mexicana and L. major were chemically prepared [17-19]. They were conjugated to the TetC peptide of tetanus toxoid as an adjuvant carrier protein to produce four glycovaccines: TetC-Ldon, TetC-Lmex, TetC-Lmaj and TetC-LmajAra2 (figure 2). Tetanus toxoid has previously been used in the production of anti-bacterial polysaccharide vaccines as unconjugated saccharides are poorly immunogenic [10, 11].
Figure 2.
Chemical structures of the glycovaccines. Recombinant tetanus toxin fragment C (TetC, 33 aa, 53.4 kDa) was conjugated via a (CH2)9 linker to the glycans shown. These are representative of glycan structures found on proteophosphoglycans and lipophosphoglycans of the 3 species indicated. In Leishmania major, the glycan side chains undergo developmental regulation, and the glycan chain of the conjugate TetC-LmajAra2 is found on metacyclic promastigote glycans. The hapten loading (n) per mole of TetC for the four glycovaccines was 4.85 for TetC-Ldon, 4.15 for TetC-Lmex, 3.37 for TetC-Lmaj and 3.80 for TetC-LmajAra2. Ara, arabinose; Gal, galactose; Glc, glucose; Man, mannose.
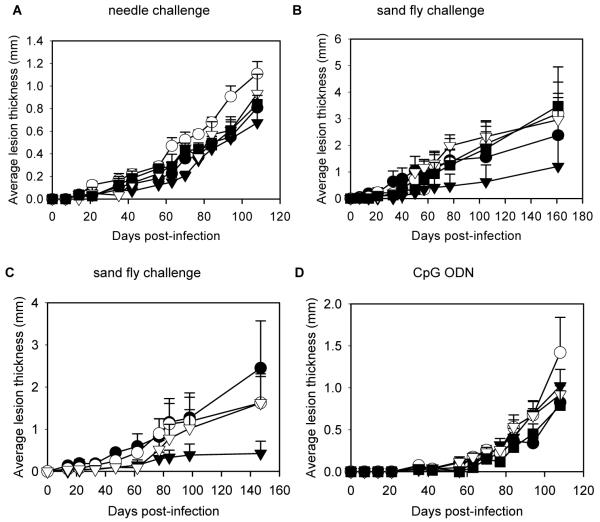
The glycovaccines were used (without any adjuvant) to immunize mice, which were then challenged either by needle inoculation or infected sand fly bite as above. Similar to the results with PSG, none of the synthetic glycovaccines were able to protect against needle inoculation (figure 3A). Similarly, the majority of the glycovaccines were unable to protect against fly bite challenge (figure 3B). However, there was one important exception: the TetC-Lmex glycovaccine conferred significant protection compared with the TetC control vaccine (P = 0.041), resulting in a 50% reduction in final lesion size. The corresponding mean (± SE) parasite burdens were also significantly reduced: 2.60 × 108 (±1.78) amastigotes/mouse for the TetC control group compared with 1.56 ×107 (±1.55) amastigotes/mouse for the TetC-Lmex-immunized group, a 94% reduction in parasite burden (P = 0.023). This is an impressive and intriguing result because the only glycovaccine that gave protection was that containing glycans with structures homologous to the infecting parasite, L. mexicana. Thus it appears that these glycovaccines may induce species- and glycan-specific protection, although we have not tested whether TetC-Lmex is effective against other species of Leishmania. To confirm this important result and also to investigate the role of TetC as a carrier protein a further experiment was performed, in which one group of mice was immunized with TetC-Lmex as described above and another group was immunized with the unconjugated Lmex glycan (figure 3C). This experiment showed that the Lmex glycan alone was unable to provide protection. However, immunization with the TetC-Lmex conjugate again provided a significant reduction in lesion growth (P = 0.036) and resulted in a 74% reduction in lesion size and 24% reduction in mean (± SE) parasite burden: 1.54 × 108 (±1.03) amastigotes/mouse for the TetC control group, compared with 1.17 × 108 (±0.78) amastigotes/mouse for the TetC-Lmex-immunized group (P > 0.05). To confirm the lack of protection against needle challenge a further experiment was performed. In order to boost a potential protective effect the glycovaccines were given together with a CpG oligodeoxynucleotide (ODN) adjuvant that has previously been shown effective in stimulating anti-leishmanial protection in mice [18], followed by needle challenge (figure 3D). However, again no protection was observed. These results demonstrate that PSG or TetC-Lmex gave protection specifically against L. mexicana-infected sand fly bite but were not effective against needle challenge.
Figure 3.
Immunization of mice with glycovaccines. A and B, Immunization of 5 groups of 6-10 BALB/c mice with TetC (●) as a control or with 1 of 4 glycovaccines: TetC-Ldon (○),TetC-Lmex (▼),TetC-Lmaj (▽), and TetC-LmajAra2 (■). Challenge was by needle injection with 1000 Leishmania mexicana metacyclic promastigotes (A) or by single bites of L. mexicana-infected sand flies (B). C, Immunization of 4 groups of 10 mice with PBS (●) or TetC (○) as controls or with TetC-Lmex (▼) or Lmex glycan (▽) followed by challenge by single sand fly bites. D, Immunization of 5 groups of 10 BALB/c mice as in panel A, but including CpG oligodeoxynucleotide (ODN), followed by challenge by injection. Error bars represent 1 SE.
Protection against sand fly bite, not provided by SLA
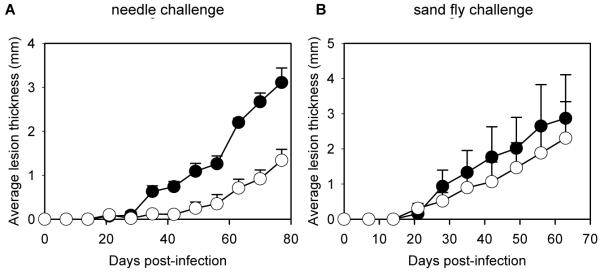
These results raise several issues, including the possibility that a wider range of challenge methods for Leishmania vaccine development should be used. Therefore, we decided to examine the influence that the method of challenge has on protection against sand fly bite with a different Leishmania antigen. For this purpose, we chose SLA, because it has been reported to be effective in protecting mice against L. major infection when the traditional method of needle inoculation is used [17] and similar preparations have been used in human clinical trials [26-29]. BALB/c mice were immunized with SLA and interleukin 12 (IL-12) to drive a protective Th1-type response [30-32], then challenged either by needle inoculation or sand fly bite. As expected, mice challenged by inoculation showed highly significant protection (P = 0.0079) in terms of lesion development (figure 4A), and mean (± SE) parasite burdens were reduced accordingly: 5.08 × 108 (±1.28) amastigotes/mouse for the needle challenge control group, compared with 1.00 × 108 (±0.72) amastigotes/mouse for the needle challenge immunized group, an 80% reduction in parasite burden (P = 0.016). However, SLA plus IL-12 did not give any significant protection against L. mexicana sand fly bite challenge either in terms of lesions (P > 0.05) (figure 4B), or as assessed by mean (± SE) parasite burdens: 5.4 × 108 (±2.5) amastigotes/mouse for the fly bite-challenge control group, compared with 5.66 ×108 (±2.8) amastigotes/mouse for the fly bite-challenge immunized group (P > 0.05). Thus, these results show the converse of those obtained with PSG or TetC-Lmex: SLA plus IL-12 is an experimental vaccine that protects against needle challenge but is ineffective against fly bite.
Figure 4.
Effectiveness of soluble Leishmania antigen (SLA) as protection against needle challenge but not against sand fly bite. Four groups of 5 BALB/c mice were either sham-inoculated with PBS (●) or immunized with SLA plus interleukin-12 (○), then challenged by needle inoculation of 1000 Leishmania mexicana metacyclic promastigotes (A) or by bites of L. mexicana-infected sand flies (B). Error bars represent 1 SE.
DISCUSSION
From the results of the present study, we draw several important conclusions of relevance for vaccines against leishmaniasis and other vector-borne diseases. First, the method of challenge is clearly crucial in vaccine development and evaluation: numbers of organisms, life cycle stage, and route are already known to be important factors that can influence the outcome of challenge – for vector-borne diseases the vector component must also be considered. However, this rarely happens; usually needle-inoculation methods are exclusively used for vaccine research, and, if results are sufficiently encouraging, the jump is made directly into human trials. Specifically with regard to leishmaniasis, the results of the present study indicate that a reevaluation of previous vaccine studies is necessary. Clearly, it is neither practical nor desirable to repeat all of the previous work using challenge by sand fly bite, and our results do not indicate that existing candidate vaccines should be disregarded. However, our results show that a vaccine that is intended to protect people against natural challenge by sand fly bite should at least be experimentally tested for protection against sand fly bite, before more expensive and time-consuming primate and/or human clinical trials are undertaken. Clearly, one would exercise caution before conducting human trials, given results such as those obtained with SLA and L. mexicana in the present study; alternatively an experimental vaccine able to confer protection against sand fly challenge in the laboratory would be very encouraging and could be a useful tool in deciding which of several alternatives might be the best option for human trials, which is a critical step in vaccine development. Given our results, it is not surprising that the SLA-based vaccines were ineffective in humans exposed to natural sand fly challenge [26-29].
Second, an improved understanding of the mechanism of natural Leishmania transmission has led to the discovery of PSG as a new target for Leishmania vaccine development. Often vaccines are developed without a clear understanding of how they work [33], and the precise mechanism behind the protection obtained in the present study remains to be elucidated, however, it has already been shown that Leishmania phosphoglycans can inhibit IL-12 production [34, 35] and are important in various ways in the establishment of infection [36-39]. It should also be noted that PSG has been found in all parasite-vector combinations that have been properly examined [6]. Although the mechanistic details vary, it is also clear for L. mexicana and other species that the initiation of a Th1 response by IL-12 is critical for resistance to leishmaniasis [40]. Further research is now required to carry the present findings forward, improve efficacy and explore protection in other host-parasite systems. Despite these challenges, this approach starts with the distinct advantage of affording protection against natural challenge.
Finally, this study moves parasite glycovaccines a step forward, beyond the neutralising anti-toxin effects reported in malaria models [41], and, to our knowledge, is the first demonstration that a synthetic glycovaccine can have a direct anti-parasite effect in leishmaniasis or any other parasitic disease. Methods for the large scale production of anti-bacterial glycovaccines that meet GMP standards have recently been developed [42], and the unusual carbohydrate structures of parasites are becoming more precisely described and understood [43]. These advances give strong impetus to further work exploring the use of glycovaccines against parasitic and other infectious diseases [44].
Acknowledgements
The technical assistance of Davina Moor and Jon Archer is gratefully acknowledged. Rob Harrison (LSTM) kindly provided the CpG ODN used in the study and Michael Chance (LSTM) provided valuable advice on the statistical analysis of the data. Recombinant tetanus toxin fragment C was a generous gift of Colin Watts (University of Dundee).
This work received financial support from The Wellcome Trust, UK (grant 064945 to M.E. Rogers and P.A. Bates) and The Royal Society (a Travelling Fellowship for O.V. Sizova).
Footnotes
The authors declare that they have no competing financial or other association that might pose a conflict of interest.
Not presented at a meeting.
References
- 1.Handman E. Leishmaniasis: current status of vaccine development. Clin Microbiol Rev. 2001;14:229–43. doi: 10.1128/CMR.14.2.229-243.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMahon-Pratt D, Alexander J. Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniasis or the visceral disease? Immunol Rev. 2004;201:206–24. doi: 10.1111/j.0105-2896.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- 3.Sacks D, Anderson C. Re-examination of the immunosuppressive mechanisms mediating non-cure of Leishmania infection in mice. Immunol Rev. 2004;201:225–38. doi: 10.1111/j.0105-2896.2004.00185.x. [DOI] [PubMed] [Google Scholar]
- 4.Scott P, Artis D, Uzonna J, Zaph C. The development of effector and memory T cells in cutaneous leishmaniasis: the implications for vaccine development. Immunol Rev. 2004;201:318–38. doi: 10.1111/j.0105-2896.2004.00198.x. [DOI] [PubMed] [Google Scholar]
- 5.Coler RN, Reed SG. Second-generation vaccines against leishmaniasis. Trends Parasitol. 2005;21:244–249. doi: 10.1016/j.pt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Stierhof YD, Bates PA, Jacobson RL, Rogers ME, Schlein Y, Handman E, Ilg T. Filamentous proteophosphoglycan secreted by Leishmania promastigotes forms gel-like three-dimensional networks that obstruct the digestive tract of infected sandfly vectors. Eur J Cell Biol. 1999;78:675–89. doi: 10.1016/S0171-9335(99)80036-3. [DOI] [PubMed] [Google Scholar]
- 7.Rogers ME, Chance ML, Bates PA. The role of promastigote secretory gel in the origin and transmission of the infective stage of Leishmania mexicana by the sandfly Lutzomyia longipalpis. Parasitol. 2002;124:495–507. doi: 10.1017/s0031182002001439. [DOI] [PubMed] [Google Scholar]
- 8.Rogers ME, Ilg T, Nikolaev AV, Ferguson MAJ, Bates PA. Transmission of cutaneous leishmaniasis by sand flies is enhanced by regurgitation of fPPG. Nature. 2004;430:463–7. doi: 10.1038/nature02675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ilg T, Stierhof YD, Craik D, Simpson R, Handman E, Bacic A. Purification and structural characterization of a filamentous, mucin-like proteophosphoglycan secreted by Leishmania parasites. J Biol Chem. 1996;271:21583–96. doi: 10.1074/jbc.271.35.21583. [DOI] [PubMed] [Google Scholar]
- 10.Finn A. Bacterial polysaccharide-protein conjugate vaccines. Brit Med Bull. 2004;70:1–14. doi: 10.1093/bmb/ldh021. [DOI] [PubMed] [Google Scholar]
- 11.Jones C. Vaccines based on the cell surface carbohydrates of pathogenic bacteria. Ann Braz Acad Sci. 2005;77:293–324. doi: 10.1590/s0001-37652005000200009. [DOI] [PubMed] [Google Scholar]
- 12.Hommel M, Jaffe CL, Travi B, Milon G. Experimental models for leishmaniasis and for testing anti-leishmanial vaccines. Ann Trop Med Parasitol. 1995;89(Suppl 1):55–73. doi: 10.1080/00034983.1995.11813015. [DOI] [PubMed] [Google Scholar]
- 13.Ashford RW. The leishmaniases as emerging and reemerging zoonoses. Int J Parasitol. 2000;30:1269–81. doi: 10.1016/s0020-7519(00)00136-3. [DOI] [PubMed] [Google Scholar]
- 14.Belkaid Y, Kamhawi S, Modi G, et al. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J Exp Med. 1998;188:1941–53. doi: 10.1084/jem.188.10.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bates PA, Rogers ME. New insights into the developmental biology and transmission mechanisms of Leishmania. Curr Mol Med. 2004;4:601–9. doi: 10.2174/1566524043360285. [DOI] [PubMed] [Google Scholar]
- 16.Kamhawi S, Belkaid Y, Modi G, Rowton E, Sacks DL. Protection against cutaneous leishmaniasis resulting from bites of uninfected sand flies. Science. 2000;290:1351–4. doi: 10.1126/science.290.5495.1351. [DOI] [PubMed] [Google Scholar]
- 17.Scott P, Pearce E, Natovitz P, Sher A. Vaccination against cutaneous leishmaniasis in a murine model. I. Induction of protective immunity with a soluble extract of promastigotes. J Immunol. 1987;139:221–7. [PubMed] [Google Scholar]
- 18.Rhee EG, Mendez S, Shah JA, et al. Vaccination with heat-killed Leishmania antigen or recombinant leishmanial protein and CpG oligodeoxynucleotides induces long-term memory CD4+ and CD8+ T cell responses and protection against Leishmania major infection. J Exp Med. 2002;195:1565–73. doi: 10.1084/jem.20020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikolaev AV, Rutherford TJ, Ferguson MAJ, Brimacombe JS. Chemical synthesis of disaccharide and phosphorylated oligosaccharide fragments of Leishmania donovani antigenic phosphoglycan. J Chem Soc, Perkin Trans. 1995;1:1977–88. [Google Scholar]
- 20.Higson AP, Tsvetkov YE, Ferguson MAJ, Nikolaev AV. Chemical synthesis of a heptaglycosyl triphosphate fragment of Leishmania mexicana lipo- and proteophosphoglycan and of a phosphorylated trisaccharide fragment of Leishmania donovani surface lipophosphoglycan. J Chem Soc, Perkin Trans 1. 1998:2587–95. [Google Scholar]
- 21.Higson AP, Ross AJ, Tsvetkov YE, Routier FH, Sizova OV, Ferguson MAJ, Nikolaev AV. Synthetic fragments of antigenic lipophosphoglycans from Leishmania major and Leishmania mexicana and their use for characterization of the Leishmania elongating α-D-mannopyranosylphosphate transferase. Chem Eur J. 2005;11:2019–30. doi: 10.1002/chem.200400563. [DOI] [PubMed] [Google Scholar]
- 22.Makoff AJ, Ballantine SP, Smallwood AE, Fairweather NF. Expression of tetanus toxin fragment C in E. coli: its purification and potential use as a vaccine. Bio/Technology. 1989;7:1043–6. [Google Scholar]
- 23.Routier FH, Nikolaev AV, Ferguson MAJ. The preparation of neoglycoconjugates containing inter-saccharide phosphodiester linkages as potential anti-Leishmania vaccines. Glycoconj J. 2000;16:773–80. doi: 10.1023/a:1007171613195. [DOI] [PubMed] [Google Scholar]
- 24.Sizova OV, Nikolaev AV, Ferguson MAJ. The preparation of neoglycoconjugates as potential anti-Leishmania vaccines. International Union of Pure and Applied Chemistry; Program and abstracts of 21st International Carbohydrate Symposium; Cairns, Australia. 2002.p. 251. [Google Scholar]
- 25.Matthews JNS, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. Br Med J. 1990;300:230–5. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Momeni AZ, Jalayer T, Emamjomeh M, et al. A randomised, double-blind, controlled trial of a killed L. major vaccine plus BCG against zoonotic cutaneous leishmaniasis in Iran. Vaccine. 1999;17:466–72. doi: 10.1016/s0264-410x(98)00220-5. [DOI] [PubMed] [Google Scholar]
- 27.Khalil EA, El Hassan AM, Zijlstra EE, et al. Autoclaved Leishmania major vaccine for prevention of visceral leishmaniasis: a randomised, double-blind, BCG-controlled trial in Sudan. Lancet. 2000;356:1565–9. doi: 10.1016/s0140-6736(00)03128-7. [DOI] [PubMed] [Google Scholar]
- 28.Armijos RX, Weigel MM, Calvopina M, Hidalgo A, Cevallos W, Correa J. Safety, immunogenicity, and efficacy of an autoclaved Leishmania amazonensis vaccine plus BCG adjuvant against New World cutaneous leishmaniasis. Vaccine. 2004;22:1320–6. doi: 10.1016/j.vaccine.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Velez ID, Gilchrist K, Arbelaez MP, et al. Failure of a killed Leishmania amazonensis vaccine against American cutaneous leishmaniasis in Colombia. Trans R Soc Trop Med Hyg. 2005;99:593–8. doi: 10.1016/j.trstmh.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Afonso LC, Scharton TM, Vieira LQ, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263:235–7. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 31.Cameron P, McGachy A, Anderson M, et al. Inhibition of lipopolysaccharide-induced macrophage IL-12 production by Leishmania mexicana amastigotes: the role of cysteine peptidases and the NF-kappaB signaling pathway. J Immunol. 2004;173:3297–304. doi: 10.4049/jimmunol.173.5.3297. [DOI] [PubMed] [Google Scholar]
- 32.Rosas LE, Keiser T, Barbi J, et al. Genetic background influences immune responses and disease outcome of cutaneous Leishmania mexicana infection in mice. Int Immunol. 2005;17:1347–57. doi: 10.1093/intimm/dxh313. [DOI] [PubMed] [Google Scholar]
- 33.Lambert PH, Liu M, Siegrist CA. Can successful vaccines teach us how to induce efficient protective immune responses? Nature Med. 2005;11:S54–S62. doi: 10.1038/nm1216. [DOI] [PubMed] [Google Scholar]
- 34.Piedrafita D, Proudfoot L, Nikolaev AV, et al. Regulation of macrophage IL-12 synthesis by Leishmania phosphoglycans. Eur J Immunol. 1999;29:235–44. doi: 10.1002/(SICI)1521-4141(199901)29:01<235::AID-IMMU235>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 35.Feng GJ, Goodridge HS, Harnett MM, Wei XQ, Nikolaev AV, Higson AP, Liew FY. Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J Immunol. 1999;163:6403–12. [PubMed] [Google Scholar]
- 36.Ilg T, Demar M, Harbecke D. Phosphoglycan repeat-deficient Leishmania mexicana parasites remain infectious to macrophages and mice. J Biol Chem. 2001;276:4988–97. doi: 10.1074/jbc.M008030200. [DOI] [PubMed] [Google Scholar]
- 37.Spath GF, Garraway LA, Turco SJ, Beverley SM. The role(s) of lipophosphoglycan (LPG) in the establishment of Leishmania major infections in mammalian hosts. Proc Natl Acad Sci USA. 2003;100:9536–41. doi: 10.1073/pnas.1530604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spath GF, Lye LF, Segawa H, Sacks DL, Turco SJ, Beverley SM. Persistence without pathology in phosphoglycan-deficient Leishmania major. Science. 2003;301:1241–3. doi: 10.1126/science.1087499. [DOI] [PubMed] [Google Scholar]
- 39.Aebischer T, Bennett CL, Pelizzola M, et al. A critical role for lipophosphoglycan in proinflammatory responses of dendritic cells to Leishmania mexicana. Eur J Immunol. 2005;35:476–86. doi: 10.1002/eji.200425674. [DOI] [PubMed] [Google Scholar]
- 40.Buxbaum L, Scott P. Interleukin 10- and Fcγ receptor-deficient mice resolve Leishmania mexicana lesions. Infect Immun. 2005;73:2101–8. doi: 10.1128/IAI.73.4.2101-2108.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schofield L, Hewitt MC, Evans K, Siomos MA, Seeberger PH. Synthetic GPI as a candidate anti-toxic vaccine in a model of malaria. Nature. 2002;418:785–9. doi: 10.1038/nature00937. [DOI] [PubMed] [Google Scholar]
- 42.Verez-Bencomo V, Fernandez-Santana V, Hardy E, et al. A synthetic conjugate polysaccharide vaccine against Haemophilus influenzae type b. Science. 2004;305:522–5. doi: 10.1126/science.1095209. [DOI] [PubMed] [Google Scholar]
- 43.Mendonca-Previato L, Todeschini AR, Heise N, Previato JO. Protozoan parasite-specific carbohydrate structures. Curr Opin Struct Biol. 2005;15:499–505. doi: 10.1016/j.sbi.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 44.Dennis C. Sweet revenge. Nature. 2003;423:580–2. doi: 10.1038/423580a. [DOI] [PubMed] [Google Scholar]