Abstract
Development of Leishmania infantum/Leishmania major hybrids was studied in two sand fly species. In Phlebotomus papatasi, which supported development of L. major but not L. infantum, the hybrids produced heavy late-stage infections with high numbers of metacyclic promastigotes. In the permissive vector Lutzomyia longipalpis, all Leishmania strains included in this study developed well. Hybrids were found to express L. major lipophosphoglycan, apparently enabling them to survive in P. papatasi midgut. The genetic exchange of the hybrids thus appeared to have enhanced their transmission potential and fitness. A potentially serious consequence is the future spread of the hybrids using this peridomestic and antropophilic vector.
Keywords: Leishmania transmission, Parasite-vector interaction, Emerging diseases
Recently, Ravel et al. (2006) described natural genetic hybrids between Leishmania infantum and Leishmania major. These two Leishmania species are transmitted by different sand fly vectors to different mammalian reservoir hosts. Human Leishmania infantum infection is a zoonosis with dogs acting as the main reservoir. It causes potentially fatal visceral disease and in Southern Europe it has been associated with human immunodeficiency virus (HIV)-positive patients (for review see Gramiccia and Gradoni, 2005). Throughout the Mediterranean, it is transmitted by about a dozen sand fly species of the subgenus Larroussius (Killick-Kendrick, 1999). In contrast, Leishmania major circulates in arid and semi-arid areas between rodents, causing self-healing cutaneous lesions in humans (for review see Gramiccia and Gradoni, 2005). In the Middle East and Magreb area it is transmitted by Phlebotomus (Phlebotomus) papatasi (Killick-Kendrick, 1999). The discovery of natural hybrids between these very divergent Leishmania species raised many questions about the genetic exchange processes of Leishmania and possible circulation of hybrids under natural conditions (Ravel et al., 2006). This led to the current study on experimental infections of L. infantum/major hybrids in sand flies.
Laboratory studies examining the development of different Leishmania strains in a range of sand fly species showed that these vectors fall into two groups. Few sand fly species are specific vectors as they display remarkable specificity for the Leishmania they transmit. For example, P. papatasi supports the development of L. major but not other parasite species tested (Killick-Kendrick et al., 1994; Pimenta et al., 1994). In contrast, most of the other sand fly species examined to date support the development of a broad range of Leishmania species and fall into the second group called permissive vectors (Volf and Myskova, 2007; Myskova et al., 2007). These include Phlebotomus species transmitting parasites of the Leishmania donovani complex (Pimenta et al., 1994; Myskova et al., in press) and Lutzomyia longipalpis, the New World vector of Leishmania infantum (Walters et al., 1993). We have chosen one member of each sand fly group to compare their susceptibility to infection by the hybrids and their presumed parental species, to evaluate their differences in fitness.
Differences were found between hybrids and parental Leishmania species in their development in P. papatasi. This led us to study lipophosphoglycan (LPG), the major surface molecule of the parasite, which is critical for L. major to establish infection in P. papatasi (Pimenta et al., 1992). Terminal galactosyl residues of L. major LPG are recognized by the P. papatasi midgut receptor PpGalec (Kamhawi et al., 2004). The specific LPG-dependent attachment of Leishmania promastigotes to the P. papatasi midgut receptor enables the parasite to avoid expulsion when the sand fly defecates (Kamhawi et al., 2004). We found that the hybrids were recognized by the mAbs specific to β1,3-galactosyl-residues unique for LPG and secreted proteophosphoglycan of L. major (Kelleher et al., 1994; Ilg et al., 1996).
Four Leishmania strains were used: L. major LV561 (MHOM/IL/67/LRC-L137 Jericho II), L. infantum OG-VL (MHOM/TR/2000/OG-VL) and two hybrid strains isolated in the Leishmanioses Unit, Lisbon, from HIV-infected patients: LEM4833 (MHOM/PT/94/IMT208) and LEM4891 (MHOM/PT/2004/IMT367). Parasites were maintained on 199 medium (Sigma) supplemented with 20% FCS (Gibco) and gentamycin (50 μg/ml). Laboratory colonies of P. papatasi (originating from Sanliurfa, Turkey) and Lutzomyia longipalpis (Jacobina, Brazil) were maintained at 25-26 °C under a photoperiod of 14 h light/10 h dark. Female sand flies were fed through a chick-skin membrane with 5-day-old promastigotes (density of 106 cells per ml) in heat-inactivated rabbit blood. Blood-engorged females were separated, maintained at a constant temperature of 25 °C and sacrificed on days 2, 7 and 10 after the blood meal. The location and number of promastigotes in the midgut was estimated under a light microscope. Parasite density was graded according to accepted criteria (Cihakova and Volf, 1997), i.e. < 100, 100-1,000 and > 1,000 parasites/gut were graded as low, medium and heavy infection, respectively. Data were statistically evaluated using the Statgraphics 4.2 programme (Manugistics, Rockville, MD). Numbers of sand flies from four experiments were pooled and infection rates were statistically compared using a X2 test. In addition, P. papatasi midgut smears were fixed with methanol, stained with Giemsa and microscopically examined as described by Cihakova and Volf (1997). Briefly, the position of kinetoplast in relation to the nucleus was examined and body length, body width and flagellar length were measured. Parasite stages were classified according to descriptions given by Walters (1993). Metacyclic promastigotes were distinguished by flagellum at least 2.0 times the body length.
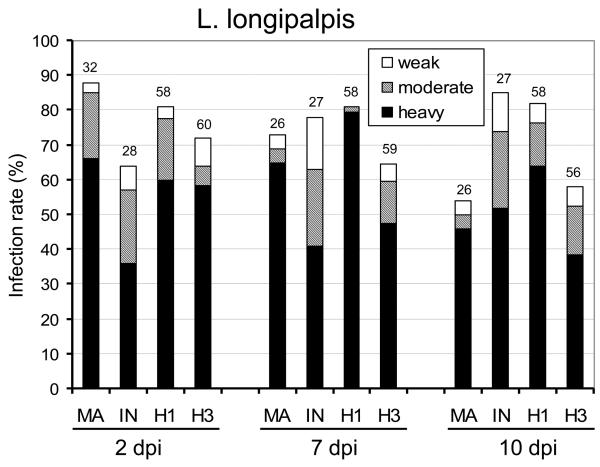
In L. longipalpis all parasite strains used developed well by producing high infection rates and a high percentage of heavy infections in all intervals studied (Fig. 1A). Infection rates of hybrids varied from 58 to 82% on days 2, 7 and 10 p.i. In general, both hybrid development in L. longipalpis was similar to both parental controls, i.e. L. infantum and L. major. On day 10, infection rates of hybrid H3 resembled those of L. major while hybrid H1 produced infection rates similar to L. infantum (Fig. 1A). Colonization of the stomodeal valve was observed in the majority of infected females in all Leishmania strains used.
Fig. 1.
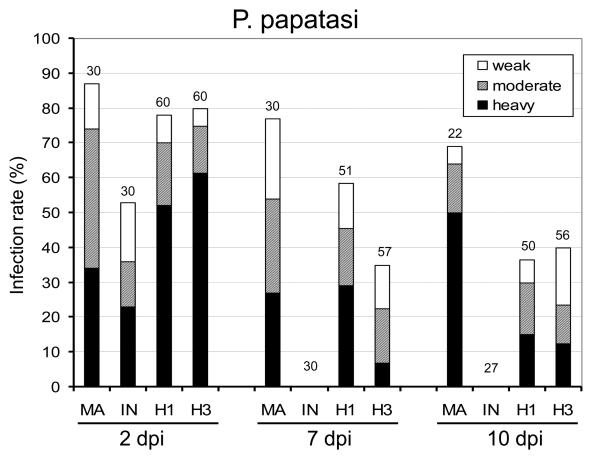
Development of Leishmania hybrids in Lutzomyia longipalpis and Phlebotomus papatasi. Infection rates and density of Leishmania major (MA), Leishmania infantum (IN), hybrid LEM4891 (H1) and hybrid LEM4833 (H3) in sand fly midgut on days 2, 7 and 10 p.i. Infections were classified into three categories: heavy (more than 1,000 promastigotes per gut) - black bars, moderate (100-1,000) - grey bars, light (1-100) - white bars. Numbers above the bars indicate the number of dissected females. A) Development in L. longipalpis: the infection rate and the intensity of infection did not differ between Leishmania strains studied. B) Development in P. papatasi: on days 7 and 10 p.i., the infection rate and the intensity of infection significantly differed between L. major, hybrids and L. infantum.
In P. papatasi (Fig. 1B) the highest infection rates were observed with L. major. In this positive control, the infection rate was about 70% and heavy infection 50% on day 10 p.i. On day 10 the colonization of the stomodeal valve was observed in 91% of infected females and metacyclic promastigotes represented 21% of morphological stages found in smears from late-stage infections. In contrast, L. infantum parasites were only detectable on day 2 and were absent in P. papatasi females examined on days 7 and 10.
Hybrid strains produced late-stage infections (on days 7 and 10 p.i.) in 35-58% of P. papatasi females, reaching heavy infections in about 15% on day 10 (Fig. 1B). The two different hybrids did not differ significantly in their infection rates until day 7 (P < 0.001). Importantly, both hybrids developed significantly better than L. infantum (P < 0.001 on every recorded day), although less efficiently than L. major (P < 0.05 on day 2, P < 0.001 on days 7 and 10) in P. papatasi (Fig. 1B). By day 10, hybrids had reached the stomodeal valve in half of infected females (50% and 59% for LEM4891 and LEM4833, respectively) heavily colonizing this part of the gut on some occasions (38% for LEM4891 and 18% for LEM4833). Metacyclic promastigotes represented about 11% of morphological stages found in smears from late-stage infections (9.3% for LEM4891 and 13.3% for LEM4833).
Mouse mAb WIC 79.3 (Kelleher et al., 1994) was used for detection of L. major LPG by indirect immunofluorescent microscopy and by direct agglutination. For immunofluorescent studies, promastigotes from 4-day-old culture (log phase) were washed in PBS (150 mM NaCl, pH 7.4) and adjusted to 107 cells per ml. Drops (10 μl) were air-dried on slides and fixed in methanol. The slides were washed in PBS and incubated for 30 min at room temperature with ascites fluid from hybridoma-bearing mice diluted to 1:500 in PBS. The promastigote-bound antibodies were detected with Alexa Fluor 488 goat anti-mouse IgG (Molecular Probes) diluted to 1:500 in PBS. Parasites were then mounted in Vectashield mounting medium with propidium iodide (Vector Laboratories) and checked under an Olympus BX51 fluorescent microscope. In negative controls the diluted ascites fluid was replaced with PBS.
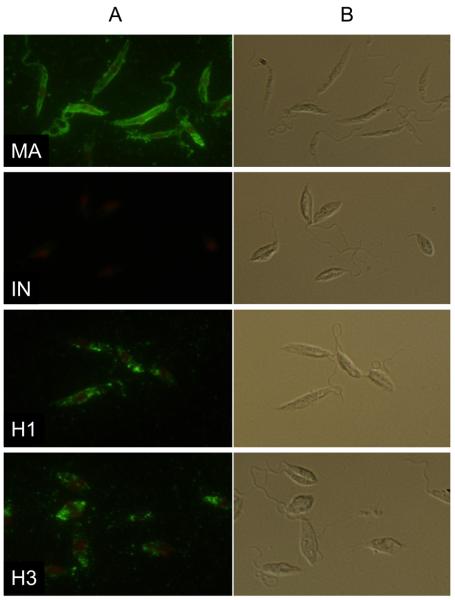
Indirect fluorescence (Fig. 2) revealed themAbs had a high reactivity with promastigotes of L. major (positive control) and no reaction with those of L. infantum (negative control). Intermediate fluorescence intensity was observed in hybrids. Their reaction with WIC 79.3 antibodies was weaker than that seen in the positive control. In most cases, a “patchy” reaction was observed in the hybrids (Fig. 2). No reaction was observed in negative controls where ascites fluid was replaced with PBS (data not shown).
Fig. 2.
Detection of Leishmania major lipophosphoglycan using specific mAbs. A) Indirect immunofluorescence of L. major (MA), Leishmania infantum (IN), hybrid LEM4891 (H1) and hybrid LEM4833 (H3) with WIC 79.3 antibodies. Note the intermediate phenotype of hybrids. B) Same by differential interference contrast (Nomarski).
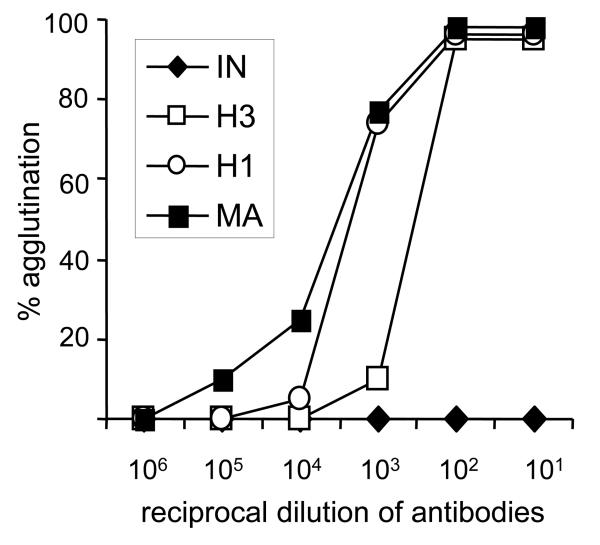
In agglutination assays, suspension of promastigotes (2 × 107 cells per ml) was mixed with a equal volume of WIC 79.3 antibodies in PBS containing 5% FCS and incubated at room temperature for 1 h. After gentle mixing, the number of single, unagglutinated promastigotes was determined in a hemocytometer. Monoclonal antibodies agglutinated promastigotes of both hybrid strains. Agglutination profiles confirmed that the amount of surface LPG expressed by hybrids was lower than in the control L. major strain. In contrast, L. infantum promastigotes were not agglutinated by a high concentration of mAbs (Fig. 3).
Fig. 3.
Agglutination profiles of Leishmania hybrids with mAbs. Leishmania major (MA), Leishmania infantum (IN), hybrid LEM4891 (H1) and hybrid LEM4833 (H3) were incubated with WIC 79.3 to detect the localization of L. major lipophosphoglycan on parasite surface.
Experimental infection of different sand fly species with hybrids and their presumed parental parasites revealed striking differences. While all Leishmania strains tested developed well in the permissive vector L. longipalpis, not all were able to do so in P. papatasi. In this specific vector, L. major and both hybrid strains produced late-stage infections (7 and 10 days p.i.) and colonized the stomodeal valve, while L. infantum was defecated from the midgut with blood meal remains and did not develop further. Results obtained with the L. infantum-P. papatasi pair confirmed that the specificity of parasite-vector interaction is confined to late-stage development when parasites must attach to the midgut epithelium of the vector (review by Kamhawi, 2006). Our study further supports the hypothesis that LPG has a crucial role in the attachment of L. major to P. papatasi midgut. Both hybrid strains posses L. major LPG, as shown by indirect immunofluorescence and agglutination with WIC 79.3 antibodies. The presence of this LPG appears to enable them to attach to the P. papatasi midgut, apparently via PpGalec receptors.
During their life cycle, Leishmania parasites adapt themselves to varied and heterogeneous environments; those of insect vectors and vertebrate hosts differ in temperature, pH and many other parameters. Sexual recombination is conventionally believed to play a major role in organismal adaptive evolution. Therefore, one might expect such an event to occur in parasites, such as Leishmania spp., to ensure their fitness for survival in varying environments. In Leishmania, however, details of sexuality and genetic exchange remain elusive. Genetic exchange seems to be extremely rare under natural conditions (Tibayrenc and Ayala, 1999) and it was not possible to obtain hybrids by experimental crossings. As reviewed by Victoir and Dujardin (2002), Leishmania strains have developed non-sexual mechanisms for generating the large repertoire of genotypes and these asexual mechanisms contribute efficiently to parasite fitness. This postulation was made in light of the paradigm that parasites are more successful without sex (Ayala, 1998; Victoir and Dujardin, 2002). However, our study showed that fitness of L. major/L. infantum hybrids was increased when compared with L. infantum. Genetic exchange appears to have conferred on the latter a certain level of L. major LPG that enables the hybrid parasites to survive in a specific vector, P. papatasi. This means that sex may have increased the transmission potential of hybrids and positively affected parasite fitness.
The finding of Leishmania hybrids' ability to develop in P. papatasi may have important epidemiological implications. Phlebotomus papatasi is wide-spread in Europe, Africa and Asia, particularly in those areas that lie between 20 and 45 degrees in the northern latitudes. It breeds as far west as Portugal, throughout the Mediterranean littoral, the Adriatic region, the Balkans, the Middle East, North Africa, Central Asia and India. It is a peridomestic and antropophilic sand fly, reaching high densities in many places. In addition to its well-known aggressive behaviour in biting humans, P. papatasi transmits phleboviruses and zoonotic cutaneous leishmaniases caused by L. major (review by Lewis and Ward, 1987; Lane, 1993). On the other hand, this sand fly is not a vector of visceral leishmaniases because it is refractory to infection by L. infantum and L. donovani (Pimenta et al., 1994; Myskova et al., in press). Our findings revealed that L.infantum/L. major hybrids causing visceral disease in HIV-positive patients develop heavy late-stage infections in P. papatasi. This suggests that in nature, and particularly in Europe, hybrid strains may circulate by using this sand fly vector, thereby increasing the risk of their spreading into new foci throughout the broad range of P. papatasi distribution.
Acknowledgements
We would like to thank Anna Svarovska for help during the experimental work and K.P. Chang for the L. infantum isolate and critical reading of the manuscript. We also acknowledge M. Svobodova and S. Turco, who kindly provided us with the antibodies. This work was supported by the Ministry of Education of the Czech Republic (VZ 21620828, LC06009), Czech Science Foundation (206/06/0967), Wellcome Trust (PV and JP) and INCO-CT-2004-509086.
References
- Ayala F. Is sex better? Parasites say “no”. Proc. Natl. Acad. Sci. USA. 1998;95:3346–3348. doi: 10.1073/pnas.95.7.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cihakova J, Volf P. Development of different Leishmania major strains in vector sandflies Phlebotomus papatasi and P. duboscqi. Ann. Trop. Med. Parasitol. 1997;91:267–279. doi: 10.1080/00034989761120. [DOI] [PubMed] [Google Scholar]
- Gramiccia M, Gradoni L. The current status of zoonotic leishmaniases and approaches to disease control. Int. J. Parasitol. 2005;35:1169–1180. doi: 10.1016/j.ijpara.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Ilg T, Stierhof YD, Craik D, Simpson R, Handman E, Bacic A. Purification and structural characterization of a filamentous mucin-like proteophosphoglycan secreted by Leishmania parasites. J. Biol. Chem. 1996;271:21583–21596. doi: 10.1074/jbc.271.35.21583. [DOI] [PubMed] [Google Scholar]
- Kamhawi S, Ramalho-Ortigao M, Pham VM, Kumar S, Lawyer PG, Turco SJ, Barillas-Mury C, Sacks DL, Valenzuela JG. A role for insect galectins in parasite survival. Cell. 2004;119:329–341. doi: 10.1016/j.cell.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Kamhawi S. Phlebotominae sand flies and Leishmania parasites: friends or foes? Trends Parasitol. 2006;22:439–445. doi: 10.1016/j.pt.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Kelleher M, Curtis JM, Sacks DL, Handman E, Bacic A. Epitope mapping of monoclonal antibodies directed against lipophosphoglycan of Leishmania major promastigotes. Mol. Biochem. Parasitol. 1994;66:187–200. doi: 10.1016/0166-6851(94)90146-5. [DOI] [PubMed] [Google Scholar]
- Killick-Kendrick R, Killick-Kendrick M, Tang Y. Anthroponotic cutaneous leishmaniasis in Kabul, Afghanistan: the low susceptibility of Phlebotomus papatasi to Leishmania tropica. Trans. R. Soc. Trop. Med. Hyg. 1994;88:252–253. doi: 10.1016/0035-9203(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Killick-Kendrick R. The biology and control of phlebotomine sand flies. Clin. Dermatol. 1999;17:279–289. doi: 10.1016/s0738-081x(99)00046-2. [DOI] [PubMed] [Google Scholar]
- R.P. Lane. Sandflies (Phlebotominae) In: Lane RP, Crosskey RW, editors. Medical Insects and Arachnids. Chapman and Hall; London: 1993. pp. 78–119. [Google Scholar]
- Lewis DJ, Ward RD. Transmission and vectors. In: Peters W, Killick-Kendrick R, editors. The Leishmaniases in Biology and Medicine. Vol. 1. Academic Press; London: 1987. pp. 235–262. [Google Scholar]
- Myskova J, Svobodova M, Beverley SM, Volf P. A lipophosphoglycan - independent development of Leishmania in permissive sand flies. Microb. Infect. 2007;9 doi: 10.1016/j.micinf.2006.12.010. doi: 10.1016/j.micinf.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimenta PF, Saraiva EMB, Rowton E, Modi GB, Garraway LA, Beverley SM, Turco SJ, Sacks DL. Evidence that the vectorial competence of phlebotomine sand flies for different species of Leishmania is controlled by structural polymorphisms in the surface lipophosphoglycan. Proc. Natl. Acad. Sci. USA. 1994;91:9155–9159. doi: 10.1073/pnas.91.19.9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimenta PF, Turco SJ, Mcconville MJ, Lawyer PG, Perkins PV, Sacks DL. Stage-specific adhesion of Leishmania promastigotes to the sandfly midgut. Science. 1992;256:1812–1815. doi: 10.1126/science.1615326. [DOI] [PubMed] [Google Scholar]
- Ravel C, Cortes S, Pratlong F, Morio F, Dedet JP, Campino L. First report of genetic hybrids between two very divergent Leishmania species: Leishmania infantum and Leishmania major. Int. J. Parasitol. 2006;36:1383–1388. doi: 10.1016/j.ijpara.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Tibayrenc M, Ayala FJ. Evolutionary genetics of Trypanosoma and Leishmania. Microb Infect. 1999;1:465–472. doi: 10.1016/s1286-4579(99)80050-1. [DOI] [PubMed] [Google Scholar]
- Victoir K, Dujardin JC. How to succeed in parasitic life without sex. Asking Leishmania. Trends Parasitol. 2002;18:81–85. doi: 10.1016/s1471-4922(01)02199-7. [DOI] [PubMed] [Google Scholar]
- Volf P, Myskova J. Sand flies and Leishmania: specific versus permissive vectors. Trends Parasitol. 2007;23 doi: 10.1016/j.pt.2006.12.010. doi:10.1016/j.pt.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters LI. Leishmania differentiation in natural and unnatural sand fly hosts. J. Eukaryot. Microbiol. 1993;40:196–206. doi: 10.1111/j.1550-7408.1993.tb04904.x. [DOI] [PubMed] [Google Scholar]
- Walters LI, Irons KP, Chaplin G, Tesh RB. Life cycle of Leishmania major (Kinetoplastida: Trypanosomatidae) in the neotropical sand fly Lutzomyia longipalpis (Diptera: Psychodidae) J. Med. Entomol. 1993;30:699–718. doi: 10.1093/jmedent/30.4.699. [DOI] [PubMed] [Google Scholar]