Abstract
There is a growing body of data indicating that gene X child maltreatment interactions at Monoamine Oxidase A (MAOA) play a role in vulnerability to symptoms of Antisocial Personality Disorder (ASPD) but not Major Depression (MD). Using a sample of 538 participants from the Iowa Adoption Studies, we introduce a conceptual model that highlights two distinct pathways from child maltreatment to symptoms of MD, suggesting that maltreatment has different effects depending on genotype and highlighting the importance of including the indirect pathway through ASPD. As predicted by the model, high activity alleles predispose to symptoms of MD in the context of child maltreatment whereas low activity alleles predispose to symptoms of ASPD. We conclude that the GxE interplay at this locus (MAOA) contributes to both symptoms of ASPD and MD and that careful specification of child maltreatment may be essential if genetic association research is to produce replicable results.
Keywords: Depression, Child Maltreatment, Gene X Environment, Family, MAOA, ASPD
Symptoms of Antisocial Personality Disorder (ASPD) are a major societal problem, contributing to a range of social ills and costs (Scott, Knapp, Henderson, & Maughan, 2001), not the least of which is reduced life satisfaction and life opportunities for those with ASPD. Likewise, symptoms of Major Depression (MD) exact a considerable annual economic cost (Greenberg et al., 2003) and are associated with substantial personal burden for sufferers and their family members. Symptoms of MD and ASPD commonly co-occur, especially in adolescence and early adulthood, suggesting a link between these conditions (Patterson & Stoolmiller, 1991). In addition, twin studies suggest that a substantial portion of the genetic contribution to risk for symptoms of MD can be accounted for by genetic effects on symptoms of ASPD (Fu, Heath, Bucholz, et al., 2002), indicating a genetic link between ASPD and MD (see also Rowe, Rijsdijk, Maughan, Hosang, & Eley, 2008). Similarly, conduct disorder, a common childhood precursor of antisocial patterns in adulthood is robustly associated with depressive disorders in early adolescence both in clinical samples (Kovacs, Paulauskas, Gatsonis, & Richards, 1988) and in population samples, possibly because conduct disorder leads to rejection and academic failure (Capaldi, 1992), with these outcomes increasing risk for depressive symptoms (MacPhee & Andrews, 2006; Patterson & Stoolmiller, 1991).
The association between ASPD and MD may also be due, in part, to shared environmental causes related to a negative family environment (Ge et al., 1996; Johnson, Cohen, Brown, Smailes, & Bernstein, 1999), which contributes to the development of both symptoms of ASPD and MD. Children who have been exposed to harsh, punitive parenting or who have experienced physical or sexual abuse are at substantially increased risk for developing conduct disorders in childhood and symptoms of antisocial personality disorder later in life (Rutter, Giller, & Hagell, 1998). Similarly child maltreatment has been linked to later episodes of MD (Mullen, Martin, Anderson, Romans, & Herbison, 1996). However, not all persons exposed to child maltreatment develop elevated symptoms of MD or elevated symptoms of ASPD in adulthood. Nor do all persons develop both conditions. Accordingly, some individual difference variables are necessary to account for differential response to child maltreatment.
Genetic variation linked to gene activity also appears important in understanding the development of both symptoms of MD and symptoms of ASPD (Kendler, Prescott, Myers, & Neale, 2003). At the molecular level, genetic variation in the X chromosome linked gene, Monoamine Oxidase A (MAOA), has been studied with respect to both MD and ASPD spectrum behavior. The most commonly analyzed aspect of MAOA is a variable nucleotide repeat (VNTR), which consists of either 2, 3, 3.5, 4 or 5 copies of a 30 base pair repeat that occurs in the promoter region of MAOA (Sabol, Hu, & Hamer, 1998), with the 3 repeat (3R) and 4 repeat (4R) alleles being by far the most common. The number of repeats is associated with the amount of mRNA transcription from this locus with some (Guo, Ou, Roettger, & Shih, 2008), but not all studies (Pai, Chou, & Huang, 2007), showing that more repeats are associated with greater gene transcription.
Initial support for the role of MAOA activity level in the prediction of symptoms of ASPD was provided by Brunner and colleagues who found that a rare point mutation in exon 8 resulted in the truncation of the MAOA protein product and was associated with a set of aggressive behaviors (Brunner, Nelen, Breakefield, Ropers, & van Oost, 1993). Some (Prom-Wormley et al., 2009), but not all (Prichard, Jorm, Mackinnon, & Easteal, 2007), of the subsequent genotype-phenotype association studies examining the direct association of MAOA activity level with ASPD have been positive. In addition, a significant body of literature has developed indicating that GxE interactions at this locus, particularly those involving child maltreatment, may be important in the etiology of antisocial behavior (Haberstick, et al., 2005; Ducci et al., 2008; Frazzetto et al., 2007; Kim-Cohen et al., 2006).
There is substantially less evidence for an effect of variation in MAOA activity level on symptoms of MD. This is surprising because interest in the locus was initially prompted by the early success of the drug class referred to as monoamine oxidase inhibitors as a treatment for MD. Monoamine oxidase reduces the available supply of biogenic amines by degrading them and facilitating their absorption (i.e. MAOA increases catabolism of neurotransmitters such as dopamine, norepinephrine, and serotonin). Because of its effect on catabolism of these monoaminergic transmitters, one would expect increased brain MAOA to be associated with greater symptoms of MD, an expectation that has been corroborated (Meyer et al., 2006). As a consequence, one would expect the risk allele for symptoms of MD to be different than the risk allele for symptoms of ASPD (i.e. higher activity alleles would confer increased risk for symptoms of MD whereas lower activity alleles have been associated with increased risk for symptoms of ASPD). Supporting this expectation, Kersting and colleagues found that the more active 4R allele of MAOA was significantly associated with complicated grief in female patients (Kersting et al., 2007). Similarly, Yu and co-workers found an increased frequency of the 4R allele among female MD patients as well as enhanced response to antidepressant treatment among 3R allele patients (Yu et al., 2005). Recently, Rivera and associates also found the high activity 4R allele to be associated with increased risk for MD in a primary care sample (Rivera et al., 2009).
Unfortunately, in contrast to the well publicized work on interactions between family environment and genetic variation in the etiology of ASPD, there has been less effort directed toward the examination of GxE effects at MAOA with regard to symptoms of MD. One possible reason for the lack of attention is the absence of a plausible model for the way a single GxE interaction (child maltreatment X MAOA) could play a role in two rather different disorders, particularly disorders with such widely varying phenotypes. In addition, there have been at least two high profile failures to find a GxE interaction effect involving MAOA. The first focused on child maltreatment and MAOA (Caspi et al., 2002), finding no significant effect on symptoms of MD and the second focused on negative family events and MAOA, again finding no significant effect on symptoms of depression (Eley et al., 2004). Finally, Cicchetti and colleagues found that the 3R allele of MAOA interacted with child maltreatment to predict increased symptoms of depression (Cicchetti, Rogosch, & Sturge-Apple, 2007).
One possible explanation for the inconsistency in findings linking the maltreatment X genotype interaction to elevated symptoms of MD is that antisocial behavior, and its propensity to elicit negative social environments (e.g. Capaldi, 1992; MacPhee & Andrew, 2006), may partially mask the effect of the higher activity alleles on increased vulnerability to symptoms of MD. Briefly, if low activity alleles have an indirect association with greater symptoms of MD due to their impact on symptoms of ASPD, this could partially mask the direct association of the higher activity alleles with symptoms of MD. Further contributing to previous inconsistent results is analytic inconsistency across studies due to a lack of consensus regarding which alleles should be considered “high” vs. “low” activity. In the current study we address both these issues. We address the issue of which alleles should be combined in contrasts of “high” vs. “low” activity alleles by examining mRNA transcription, thereby providing a biologically informed basis for designating some alleles as “high” activity and other alleles as “low” activity. We address the role of symptoms of ASPD in the prediction of symptoms of MD by including this pathway in our theoretical model.
The theoretical model we test (See Figure 1) specifies two pathways by which a GxE interaction involving child maltreatment and MAOA might affect symptoms of ASPD and symptoms of MD. It is hypothesized that low activity alleles of MAOA (2R and 3R) interact with the presence of childhood abuse experiences to confer elevated risk for symptoms of ASPD. Because low activity alleles are hypothesized to confer greater risk (i.e. lower activity alleles are associated with risk for greater symptoms of ASPD), we indicate this in the model with a (−) symbol. Because greater symptoms of ASPD are associated with greater symptoms of depression, this relationship is indicated in the model with a (+) symbol. This creates an indirect pathway connecting low activity alleles with elevated symptoms of MD through their effect on symptoms of ASPD. Conversely, high activity alleles are hypothesized to interact with child abuse to create increased risk for symptoms of MD. Because the high activity alleles are hypothesized to confer increased risk of symptoms of MD, this is indicated in the model by a (+). Failing to include the pathway to symptoms of MD from symptoms of ASPD should tend to obscure the connection between the high activity alleles of MAOA and symptoms of MD. In addition, the paths from the interaction term to each of the outcomes (symptoms of MD and ASPD) should be opposite in valence and significantly different.
Figure 1.
Theoretical Model Linking the MAOA x Child Abuse Interaction to Depressive and Antisocial Symptoms
Method
The overall methodologies for the Iowa Adoption studies (IAS) have been extensively described elsewhere (Philibert, 2006; Yates, Cadoret, & Troughton, 1998) and all procedures conducted in these studies have been approved by the University of Iowa Institutional Review Board. Biodata were collected in the last round of data collection for the study (2004- present), the reports of child abuse were obtained in the wave of data collected in 1999–2004, when participants were already adults, and were asked about a range of symptoms as well as a wide range of childhood events. During both waves, participants were interviewed with a modified form of the Semi-Structured Assessment for Genetics of Alcoholism, version II, (SSAGA-II; Bucholz et al., 1994). DSM-IV symptom counts were abstracted from each of the interviews as previously described (Philibert et al., 2007). Lifetime symptoms of ASPD were comprehensively assessed at both interviews for all participants whereas lifetime symptoms of depression were comprehensively reviewed only at the initial round of data collection. Accordingly, we use the mean of symptoms reported at both waves as our index of lifetime ASPD symptoms and use symptoms of MD reported at the initial assessment as our index of lifetime symptoms of MD.
Participants
Adoptees and their adoptive parents were recruited from Iowa adoption agencies starting in 1975. Adoptees were separated from their biological parents shortly after birth and raised by their unrelated adoptive parents. Characteristics of biological parents were not used in determining placement, limiting the potential for passive rGE effects in the sample. Overall, the participants were largely White (94%) and had an average age of 46.48 for males and 44.95 for females at the time of the interview.
Measures
Symptoms of MD and ASPD
The Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA-II) was used to assess maximum lifetime symptom levels for MD and ASPD. The SSAGA is a polydiagnostic instrument that assesses depression and antisocial personality disorder, among others, in a manner consistent with DSM-III-R, DSM-IV, and Feighner RDC (Research Diagnostic Criteria). Previous research indicates acceptable reliability for both the MD symptoms scale and the ASPD symptoms scale. Kappa for life time diagnosis of ASPD and MD based on independent interviews using the SSAGA and administered over a two week period was .70 and .65 respectively (Bucholtz, et al., 1994). In the current sample alpha coefficients were .71 and .94 for ASPD and MD lifetime symptom scales respectively. The correlation of lifetime ASPD symptoms between the two assessments was .72, providing the average ASPD symptom scale used in the current research with an effective reliability of .84 (Rosenthal & Rosnow, 1991).
Child Maltreatment
In this investigation, we focused on child maltreatment within the family involving 1) any injury sustained in the context of punishment by a parent 2) any childhood sexual contact with any family member, and 3) consistent use of harsh physical punishment by a parent. All questions were addressed in the context of asking participants to focus on, and think about, their childhood. To assess childhood injury in the context of punishment, adult participants were asked “Did your Mother/Father ever physically punish you so hard that you hurt the next day or had to see a doctor?” To assess childhood sexual contact with family members, participants were asked “Before you were age 16 years old, were there any sexual contacts between you and any family members, like a parent or step-parent, grandparent, uncle, aunt, brother, sister, or cousin? “Was there sexual contact with a parent or grandparent?” They were also asked “What was the usual way in which your Mother/Father punished you”, with harsh physical punishment being one option (in contrast to non-physical, mild physical, and no punishment). The Child Maltreatment Index (CMI) was incremented by one for all affirmative answers, so that larger scores indicated greater evidence of childhood maltreatment. Alpha for males was .62. Alpha for females was .58. Despite similar alphas, examination of the components of the child abuse index indicated that whereas harsh parenting and physical abuse was reported with similar frequency for males (ns = 17, 12 for harsh parenting and physical abuse respectively) and females (ns = 16, 13 respectively) and were associated to a similar degree for males and females (r = .46 for males and r = .45 for females), sexual abuse was reported much less frequently by males (n = 3) than females (n= 31). Accordingly, we examined sexual abuse separately for females in follow-up analyses.
Genetic Assessment
DNA was prepared from lymphoblast cell lines as previously described (Philibert, Zadorozhnyaya, Beach, & Brody, 2008). PCR amplification of MAOA variable nucleotide repeat (VNTR) polymorphism was conducted using the method of Sabol and colleagues (Sabol et al., 1998). The resulting PCR products were electrophoresed on a 6% non-denaturing polyacrylamide gel and imaged using silver staining (Merril & Pratt, 1986). The resulting alleles were compared to internal standards and genotypes called by two individuals blind to affected status. In cases where there was disagreement, the VNTR was re-amplified. Internal technical replicates were determined for approximately 5% of the samples for the IAS. There were no discrepancies among the technical replicates. Furthermore, no males were erroneously called as females, suggesting that the error rate in the sample was low. Semi independent genotype status was determined for approximately half the sample using DNA from lymphoblasts that originated from the same blood draw, but were prepared through independent processes. No discrepancies were detected in genotypes called based on the replication.
Results
Ten percent of males and 16% of females reported one or more indications of child maltreatment. There were more females than males reporting a probable episode of MD, with 135 (46%) of females and 51 (21%) of males reporting 5 or more maximum lifetime symptoms of MD. In addition, 14 females and 39 males had four or more symptoms of ASPD, indicating probable ASPD. There were 86 males with the 3R allele and 148 with the 4R allele. There were also 41 females homozygous for the 3R allele, 125 homozygous for the 4R allele and 114 females who were heterozygous for the 3R and 4R allele. Other alleles were present infrequently (10 for men; 14 for women). It is important in genetic studies to demonstrate “Hardy-Weinberg equilibrium,” which provides mathematical assurance that the allele frequencies for heterozygotes are consistent with the expected frequency based on the observed frequency of homozygotes. Since only females can be heterozygous for MAOA, the analysis at this locus can only be conducted for them. A chi-square analysis of Hardy-Weinberg equilibrium using 3R and 4R allele frequencies was computed for females and the results were non-significant, indicating that allele drop out had not compromised the quality of the data.
Transfection Study Effects
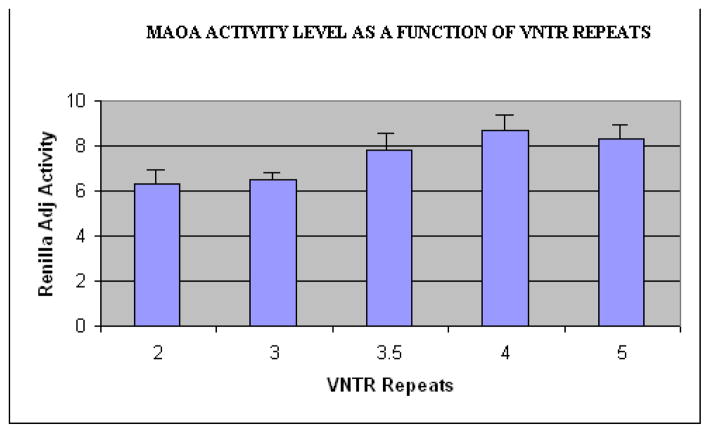
The relationship of MAOA VNTR genotype to mRNA expression was explored using transfection assays (procedural details available on request). A transfection study is one in which DNA is added to a population of eukaryotic cells growing in culture, with the introduced genetic material becoming part of the recipient cells genetic makeup. This allows for controlled examination of the impact of particular alleles on gene expression (i.e. transcription rates) in relevant cells. Briefly, the DNA insert region corresponding to MAOA promoter region for 2R, 3R, 3.5R, 4R, and 5R alleles was cloned, and transcription rates were examined in 4 replications. The results of the transfection experiments for MAOA alleles using the NT2 neuronal cell line and averaged across the four replications are given in Figure 2. As the figure shows, transcription activity increased as a function of VNTR repeats, with the 2R and 3R alleles resulting in lower levels and the 3.5R, 4R and 5R alleles resulting in higher levels. Contrasts between all pairs of alleles indicated that 2R and 3R alleles were non-significantly different in transcription activity, but both were significantly lower in transcription than 3.5R, 4R, and 5R alleles (p < .05 in all cases). The latter three alleles also did not differ significantly from one another. Accordingly, all analyses were conducted contrasting the “low activity” (2R, 3R) alleles with the “high activity” alleles (3.5R, 4R, 5R),2 using the full sample in our analyses (n = 538).
Figure 2.
Results of the Transfection Experiment: MAOA Activity as a Function of 2, 3, 3.5, 4, or 5 VNTRs.
MAOA x Child Maltreatment Effects
MPLUS Analyses
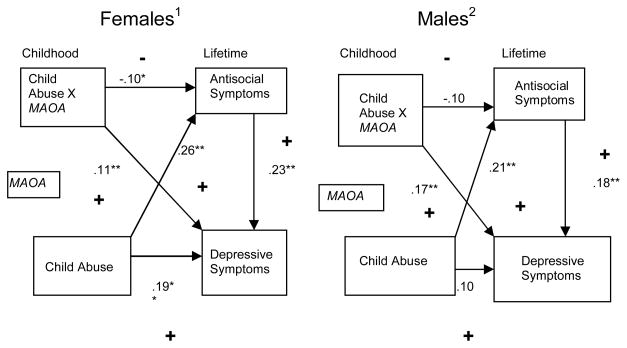
Model fitting was conducted using Mplus version 4 (Muthen & Muthen, 2006) with manifest indicators. The fit function used was maximum likelihood. Based on zero-order relationships in this data set as well as prior research, we began with a model in which the direct effect of “high” vs. “low” activity genotype on outcomes was constrained to be zero. We freely estimated all other paths in the hypothesized model, and standardized parameter estimates and their significance are reported in Figure 3. We examined the fit of the hypothesized model using the overall chi-square test in each case.
Figure 3.
Theoretical Model with Standardized Parameter Estimates for Females and Males.
To directly test the hypothesis that the interaction of MAOA and child maltreatment would have different implications for symptoms of MD vs. symptoms of ASPD (i.e. that they would be opposite in sign and significantly different in magnitude), we used a second set of models in which we constrained the two relevant paths in the hypothesized model to be equal. That is, we constrained the path from the interaction term to symptoms of MD to be the same as the path to symptoms of ASPD. As recommended by Bollen (1989), we examined deterioration of overall model-data fit in terms of chi-square statistics (i.e., ΔΠ2). The change in fit associated with the introduction of the constraint results in a ΔΠ2 with one-degree of freedom. In turn, a significant ΔΠ2 leads to the rejection of the hypothesis that the two paths are equal.1 In all cases we present analyses separately by sex to allow different characterization of the genotype variable as a function of sex. We do so in recognition that for X-linked genes, such as MAOA, it is important to consider the possibility that the gene may function differently for men and women. At a minimum, heterozygous women may function as a mosaic of two genotypes whereas this is not possible for men because they cannot be heterozygous for an x-linked gene. Analytically, this allows females who are heterozygous for low and high activity alleles to be treated as intermediate in analyses, creating three levels of the genetic variable. Conversely, males, who are necessarily hemizygous (i.e. have only one copy) of X-linked genes, have no intermediate value, creating only two levels of the genetic variable.
Simple Correlations
As expected, presence of low vs. high activity variants of MAOA was not significantly associated with symptoms of MD (r = −.02, NS for males and .00, NS for females) or ASPD (r = −.02, NS for males and −.04, NS for females). Consistent with the prior literature, symptoms of MD were positively associated with ASPD symptoms (r =.18, p =.0052 for males and .26, p = .0001for females). However, genotype was not associated with the child maltreatment index in the sample for males (r = −.08, NS) or females (r = .10, NS), failing to support an active rGE effect for child maltreatment in this sample (i.e. there was no evidence that having the risk genotype was associated with the elicitation of the risk environment).
Parameter Estimates
In all cases, consistent with zero-order effects, the direct effect of genotype on outcomes was set to zero. Accordingly, the baseline model had two degrees of freedom. Standardized structural path coefficients are presented in Figure 3 separately for males and females. In keeping with the directional hypotheses for all parameters, exact, one-tailed p-values are reported. As predicted, for both males and females there were positive associations between child maltreatment and symptoms of MD (B = .10, NS for males and B = .18, p = .0012 for females) as well as positive relationships between child maltreatment and symptoms of ASPD (B = .21, p = .00058 for males and B = .26, p = .00001 for females). There were also significant positive relationships between symptoms of ASPD and symptoms of MD (B = .18, p = .0026 for males and B = .23, p = .000032 for females). Accordingly, all hypothesized main effect relationships were in the direction predicted. In addition, for both males and females there was a significant positive relationship between the interaction term (genotype × child maltreatment) and symptoms of MD (B = .17, p = .0044 for males and B = .11, p = .027 for females). However, as hypothesized, there was a negative relationship between the interaction term and symptoms of ASPD (B = −.10, NS for males and B = −.10, p = .048 for females), suggesting different risk alleles for symptoms of MD vs. ASPD. Fit of the hypothesized model was good for males Π2 (2) = .028, NS and females Π2 (2) = 1.30, NS.
The pattern of simple correlations within genotype also was consistent with the hypothesized role of high activity alleles in combination with child maltreatment in the prediction of elevated symptoms of MD for both males and females. Specifically, the correlation between child abuse and symptoms of MD was r = .23, p = .005 for males and .36, p < .001 for females for those with the “high activity” hemi or homozygous genotypes but r = −.06, NS for males and .10, NS for females for the “low activity” hemizygous or homozygous genotypes.
Test of Equality Constraints
To directly examine whether the effect of the interaction term on outcomes differed for symptoms of MD and symptoms of ASPD, we examined whether the two paths differed significantly using nested model comparisons based on chi-square difference tests (Bollen, 1989). We reran the baseline model, imposing an equality constraint on the two relevant pathways (i.e. interaction term to symptoms of depression = interaction term to symptoms of ASPD) and determined the change in chi-square. Because ΔΠ2 for nested chi-square models is itself distributed as chi-square, the resulting value was a Π2 with one degree of freedom. For both males and females, the imposition of an equality constraint on the paths connecting the interaction term to the two outcomes (MD and ASPD) resulted in a significant ΔΠ2. For males the chi-square change was ΔΠ2 (1) = 9.10, p = .003 and for females the chi-square change was ΔΠ2 (1) = 5.68, p = .017, indicating that for both males and females the hypothesized model, which specified that high activity alleles would predispose to symptoms of MD whereas the low activity alleles would predispose to symptoms of ASPD, was a significantly better fit to the data than the alternative model.3
Follow-up Test of Sexual Abuse for Females
Because the child maltreatment index was comprised of three components (Sexual Abuse, Harsh Discipline, and Physical Maltreatment), as noted above, we examined the frequency of subsets of items for males and females. In keeping with prior work, sexual abuse was reported more frequently by females (n = 31) than by males (n = 3). Accordingly we examined the effect of sexual abuse for females only. There was a positive standardized coefficient for the relationship between sexual abuse and symptoms of MD (B = .14, p = .017) as well as a positive relationship between sexual abuse and symptoms of ASPD (B = .33, p =.000001). There was also a significant positive relationship between symptoms of ASPD and symptoms of MD (B = .23, p = .000022). In addition, there was a significant positive relationship between the interaction term (genotype × sexual abuse) and symptoms of MD (B = .19, p = .0013). However, there was a significant negative relationship between the interaction term and symptoms of ASPD (B = −.18, p = .0039). As before, when the paths connecting the interaction term to outcomes were constrained to be equal, there was a significant deterioration in model fit. The chi-square change was ΔΠ2 (1) = 13.94, p = .0002.
Discussion
We have provided support for a theoretical model in which child maltreatment interacts with genetic variability at the MAOA locus, highlighting two distinct pathways linking child maltreatment to symptoms of MD. As the model suggests, in the absence of characterization of child maltreatment, all genotype-symptom associations would have been non-significant, underscoring that failure to characterize key aspects of family environment may be a contributing cause for inconsistencies and failures to replicate in genetic association studies. In addition, merely controlling for the substantial direct, main effect of child maltreatment on symptomatic outcomes for symptoms of MD and ASPD would not have been sufficient to allow the role of genotype to be discerned. It was only by examining child maltreatment – genotype interactions that the role of genotype could be discerned. In addition, there was consistency across gender in the child maltreatment-MAOA interaction effect on symptoms of MD and ASPD. This highlights the importance of continued examination of child maltreatment-genotype interaction effects and the potential value of recent advances in the assessment of family context and particularly of family maltreatment variables (cf. Wamboldt, et al., 2009) for enhancing future research on genetic associations. It should be noted as well that the results were unchanged when analyses were restricted to participants carrying the 3R and 4R alleles only. Results were also unchanged when those reporting childhood onset of MD (age 16 or younger, all female) were excluded.3
Our finding that VNTR copy number corresponded to differences in amount of transcriptional activity is consistent with the prior findings of Deckert, Catalano, & Syagailo (1999) and Guo et al. (2008) and makes more biologically plausible the assertion that there may be an allele specific effect that influences response to child maltreatment. Based on findings that the 2R and 3R alleles produced non-significantly different levels of transcription, but both demonstrated significantly less transcription that the 3.5R, 4R, and 5R alleles, we have provided a biologically informed framework allowing all participants to be included in analyses of variation at the MAOA locus.
There were substantial direct associations of child maltreatment with both symptoms of MD and ASPD. Because the current sample is adopted, these direct associations cannot be attributed to a shared genetic diathesis with the adoptive parents, and so support a model in which some aspects of family environment play a causal role in the production of some symptoms of adult psychopathology. In addition, current results suggest that it is important to examine child maltreatment effects on symptoms of MD jointly with effects on symptoms of ASPD. Controlling for this well documented association may help account for more variance in family models as well as identify sources of differential response to child maltreatment. Because the 3R allele may interact with child maltreatment to produce increased risk for ASPD whereas the 4R allele may interact with child maltreatment to produce increased risk for MD, it is important to examine both outcomes simultaneously to clarify the symptomatic impact of MAOA genotype in connection with child maltreatment.
The current investigation also provides an important replication and extension of the finding that child maltreatment may have a greater effect on ASPD for those with the low activity allele of MAOA. By replicating previous findings in a sample of adoptees, the design rules out passive rGE effects and so more firmly establishes the hypothesis that the results reflect a true GxE effect. Nonetheless, it will be important in future research to identify pathways involving active rGEs which may be implicated in some outcomes. By controlling for ASPD symptoms and their effect on depression, we provide a conceptual model that may prove useful in accounting for previous failures to identify a GxE effect linking MAOA and depression, while underscoring the importance of careful assessment of family context, and particularly child maltreatment, in investigations of genetic contributions to psychiatric symptoms.
The importance of family approaches in the treatment of conduct disorder is well established (Kazdin, 2002), and suggests the potential for remediation of family context to shift developmental trajectories away from antisocial patterns. The two pathway model partially explicated in the current study has potential implications for enhancing our understanding of etiology, prevention, and treatment, positioning the field for more effective secondary prevention efforts and providing additional support for a focus on primary prevention of child maltreatment in the family. For example, although preliminary and in need of replication, our results suggest that the beneficial outcomes of prevention programs may be somewhat different as a function of child genotype, even though there may be benefits extending into adulthood in each case. Likewise, the multiple pathway model suggests the potential for different maintaining variables for symptoms of MD in adulthood, again potentially providing preliminary conceptual tools to guide differential focus for family intervention during treatment. An additional benefit may be enhanced specification of costs of child maltreatment and the cost-benefit ratio of prevention and intervention efforts. By tracking outcomes as a function of vulnerability, eventually a more precise estimate of beneficial effects of prevention and intervention programs may be obtained, suggesting that GxE research may help better inform public policy in this important area.
The current results also suggest the potential to better understand patterns of co-morbidity between symptoms of ASPD and MD. Although tentative, the current pattern of results suggests that comorbidity is driven, in part, by environmental causes that contribute powerfully to both outcomes. Conversely, there was no evidence in the current study that variation at MAOA contributes to the comorbidity of symptoms of ASPD and MD. To the contrary, variation at MAOA was associated with differential reactions to the experience of child maltreatment., Accordingly, the search for genetic factors contributing to comorbidity should continue.
It is also necessary to note that there are several limitations of the current study. Participants were relatively old at the time they reported their experiences of childhood maltreatment (mid-40’s) and these reports were retrospective. In addition, there may have been recency effects in their report of lifetime symptoms of ASPD and MD which were obtained concurrent with the report of child maltreatment. These limitations may have served to artificially strengthen some main effect relationships in the model, particularly those between child maltreatment and symptomatic status. In addition, examination of different types of child maltreatment (e.g. sexual abuse in males, neglect for both males and females) may prove important in refining both GxE and rGE models. At a minimum, in the current sample we found reports of sexual abuse to be much more frequent for females (Ullman & Filipas, 2005), suggesting the potential for somewhat differing effects of child maltreatment by gender (Finkelhor, 1990; Jacobson, Prescott & Kendler, 2002; Thompson, Kingree, & Desai, 2004). Indeed, when we reran our models focusing only on sexual abuse for females, the hypothesized GxE relationships were substantially stronger, suggesting that most of the variance attributable to the GxE effect in the child maltreatment model for females was accounted for by the presence of sexual abuse among maltreated girls. This suggests that, for women, assessment of history of sexual abuse in the family will be important in future examinations of GxE interactions at this locus.
Acknowledgments
This study was supported by a grant to Dr. Philibert (DA015789) as well as support to the first and second authors (DA010923 and DA02173603), and an award to Drs. Brody, Beach, and Philibert (1P30DA027827). We also acknowledge the continuing intellectual contribution of Dr. Remi Cadoret. The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute On Drug Abuse or the National Institutes of Health.
Footnotes
All analyses were also run in a stepwise regression framework. Results are parallel in all respects. In particular, results indicate the same significant main effects for males and females, and indicate that the effect of the interaction of genotype and child abuse is reversed for symptoms of depression vs. ASPD.
All analyses were also conducted using only those participants with common variants of low activity (3R) and high activity (4R) alleles. No changes resulted in the pattern of effects for either males or females. Accordingly results are not reported separately.
There were four participants (all female) who reported an early onset of depressive symptoms. Removing these four participants from the analyses did not change the pattern of observed results for either baseline or constrained models. Of the four participants with early onset, only one reported childhood maltreatment. She reported sexual abuse, physical abuse and harsh punishment.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/fam
Contributor Information
Steven R. H. Beach, Department of Psychology, University of Georgia, Athens, GA
Gene H. Brody, Center for Family Research, University of Georgia, Athens, GA
Tracy D. Gunter, Department of Neurology and Psychiatry, St Louis University School of Medicine, St Louis, MO
Hans Packer, Department of Psychiatry, The University of Iowa, Iowa City, IA.
Pamela Wernett, Department of Psychiatry, The University of Iowa, Iowa City, IA.
Robert A. Philibert, Neuroscience Program and Department of Psychiatry, The University of Iowa, Iowa City, IA
References
- Bollen KA. Structural equations with latent variables. New York: Wiley; 1989. [Google Scholar]
- Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993;262(5133):578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55(2):149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Capaldi D. Co-occurrence of conduct problems and depressive symptoms in early adolescent boys: II. A 2-year follow-up at Grade 8. Development & Psychopathology. 1992;4(1):125–144. doi: 10.1017/s0954579499001959. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Sturge-Apple ML. Interactions of child maltreatment and serotonin transporter and monoamine oxidase A polymorphisms: depressive symptomatology among adolescents from low socioeconomic status backgrounds. Developmental Psychopathology. 2007;19(4):1161–1180. doi: 10.1017/S0954579407000600. [DOI] [PubMed] [Google Scholar]
- Deckert J, Catalano M, Syagailo YV. Excess of high activity monoamine Oxidase A gene promoter alleles in female patients with Panic Disorder. Human Molecular Genetics. 1999;8:621–624. doi: 10.1093/hmg/8.4.621. [DOI] [PubMed] [Google Scholar]
- Ducci F, Enoch MA, Hodgkinson C, Xu K, Catena M, Robin RW, Goldman D. Interaction between a functional MAOA locus and childhood sexual abuse predicts alcoholism and antisocial personality disorder in adult women. Molecular Psychiatry. 2008;13(3):334–347. doi: 10.1038/sj.mp.4002034. [DOI] [PubMed] [Google Scholar]
- Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, Plomin R, Craig IW. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Molecular Psychiatry. 2004;9(10):908–915. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- Finkelhor D. Early and long-term effects of child sexual abuse: An update. Professional Psychology: Research and Practice. 1990;21(5):325–330. [Google Scholar]
- Frazzetto G, Di Lorenzo G, Carola V, Proietti L, Sokolowska E, Siracusano A, Gross C, Troisi A. Early Trauma and Increased Risk for Physical Aggression during Adulthood: The Moderating Role of MAOA Genotype. PLoS ONE. 2007;2(5):e486. doi: 10.1371/journal.pone.0000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Heath AC, Bucholz KK, Nelson E, Goldberg J, Lyons MJ, True WR, Jacob T, Tsuang MT, Eisen SA. Shared genetic risk of Major Depression, Alcohol Dependence, and Marijuana Dependence. Archives of General Psychology. 2002;59:1125–1132. doi: 10.1001/archpsyc.59.12.1125. [DOI] [PubMed] [Google Scholar]
- Ge X, Conger R, Cadoret R, Neiderhiser JM, Yates W, Troughton E, Stewart MA. The Developmental Interface Between Nature and Nurture: A Mutual Influence Model of Child Antisocial Behavior and Parent Behaviors. Developmental Psychology. 1996;32:574–589. [Google Scholar]
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, et al. The economic burden of depression in the United States: how did it change between 1990 and 2000? Journal of Clinical Psychiatry. 2003;64(12):1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- Guo G, Ou XM, Roettger M, Shih JC. The VNTR 2 repeat in MAOA and delinquent behavior in adolescence and young adulthood: associations and MAOA promoter activity. European Journal of Human Genetics. 2008;16(5):626–634. doi: 10.1038/sj.ejhg.5201999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstick BC, Lessem JM, Hopfer CJ, Smolen A, Ehringer MA, Timberlake D, Hewitt JK. Monoamine oxidase A (MAOA) and antisocial behaviors in the presence of childhood and adolescent maltreatment. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2005;135B(1):59–64. doi: 10.1002/ajmg.b.30176. [DOI] [PubMed] [Google Scholar]
- Jacobson KC, Prescott CA, Kendler KS. Sex differences in the genetic and environmental influences on the development of antisocial behavior. Develomental Psychopathology. 2002;14(2):395–416. doi: 10.1017/s0954579402002110. [DOI] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Brown J, Smailes EM, Bernstein DP. Childhood maltreatment increases risk for personality disorders during early adulthood. Archives of General Psychiatry. 1999;56(7):600–606. doi: 10.1001/archpsyc.56.7.600. [DOI] [PubMed] [Google Scholar]
- Kazdin AE. Psychosocial treatments for Conduct Disorder in Children and Adolescents. In: Nathan PE, Gorman JM, editors. Treatments that work. 2. New York: Oxford University Press; 2002. [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60(9):929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kersting A, Kroker K, Horstmann J, Baune BT, Hohoff C, Mortensen LS, Neumann LC, Arolt V, Domschke K. Association of MAO-A variant with complicated grief in major depression. Neuropsychobiology. 2007;56(4):191–196. doi: 10.1159/000120624. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, maltreatment, and gene-environment interaction predicting children’s mental health: new evidence and a meta-analysis. Molecular Psychiatry. 2006;11(10):903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Paulauskas S, Gatsonis C, Richards C. Depressive disorders in childhood. III. A longitudinal study of comorbidity with and risk for conduct disorders. Journal of Affective Disorders. 1988;15(3):205–217. doi: 10.1016/0165-0327(88)90018-3. [DOI] [PubMed] [Google Scholar]
- MacPhee AR, Andrews JJ. Risk factors for depression in early adolescence. Adolescence. 2006;41(163):435–466. [PubMed] [Google Scholar]
- Merril CR, Pratt ME. A silver stain for the rapid quantitative detection of proteins or nucleic acids on membranes or thin layer plates. Analytical Biochemistry. 1986;156(1):96–110. doi: 10.1016/0003-2697(86)90160-0. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Ginovart N, Boovariwala A, Sagrati S, Hussey D, Garcia A, Young T, Praschak-Rieder N, Wilson AA, Houle S. Elevated monoamine oxidase a levels in the brain: an explanation for the monoamine imbalance of major depression. Archives of General Psychiatry. 2006;63(11):1209–1216. doi: 10.1001/archpsyc.63.11.1209. [DOI] [PubMed] [Google Scholar]
- Mullen PE, Martin JL, Anderson JC, Romans SE, Herbison GP. The long-term impact of the physical, emotional, and sexual abuse of children: a community study. Child Abuse and Neglect. 1996;20(1):7–21. doi: 10.1016/0145-2134(95)00112-3. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus user’s guide. Version 4. Los Angeles, CA: Muthen & Muthen; 2006. [Google Scholar]
- Pai CY, Chou SL, Huang F. Assessment of the role of a functional VNTR polymorphism in MAOA gene promoter: a preliminary study. Forensic Science Journal. 2007;6(2):37–43. [Google Scholar]
- Patterson GR, Stoolmiller M. Replications of a dual failure model for boys’ depressed mood. Journal of Consulting and Clinical Psychology. 1991;59(4):491–498. doi: 10.1037//0022-006x.59.4.491. [DOI] [PubMed] [Google Scholar]
- Philibert R. Merging genetic and environmental effects in the Iowa adoption studies: Focus on depression. Annals of Clinical Psychiatry. 2006;18(4):219–222. doi: 10.1080/10401230600948399. [DOI] [PubMed] [Google Scholar]
- Philibert RA, Ryu GY, Yoon JG, Sandhu H, Hollenbeck N, Gunter T, Barkhurst A, Adams W, Madan A. Transcriptional profiling of participants from the Iowa adoption studies. American Journal of Medical Genetics B: Neuropsychiatry Genetics. 2007;144(5):683–690. doi: 10.1002/ajmg.b.30512. [DOI] [PubMed] [Google Scholar]
- Philibert RA, Zadorozhnyaya O, Beach SRH, Brody GH. Comparison of the genotyping results using DNA obtained from blood and saliva. Psychiatric Genetics. 2008;18(6):275–281. doi: 10.1097/YPG.0b013e3283060f81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard ZM, Jorm AF, Mackinnon A, Easteal S. Association analysis of 15 polymorphisms within 10 candidate genes for antisocial behavioural traits. Psychiatric Genetics. 2007;17(5):299–303. doi: 10.1097/YPG.0b013e32816ebc9e. [DOI] [PubMed] [Google Scholar]
- Prom-Wormley EC, Eaves LJ, Foley DL, Gardner CO, Archer KJ, Wormley BK, Maes HH, Riley BP, Silberg JL. Monoamine oxidase A and childhood adversity as risk factors for conduct disorder in females. Psychological Medicine. 2009;39(4):579–590. doi: 10.1017/S0033291708004170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera M, Gutierrez B, Molina E, Torres-Gonzalez F, Bellon JA, Moreno-Kustner B, King M, Nazareth I, Martinez-Gonzalez LJ, Martinez-Espin E, Munoz-Garcia MM, Motrico E, Martinez-Canavate T, Lorente JA, Luna JD, Cervilla JA. High-activity variants of the uMAOA polymorphism increase the risk for depression in a large primary care sample. American Journal of Medical Genetics B: Neuropsychiatry Genetics. 2009;150B(3):395–402. doi: 10.1002/ajmg.b.30829. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Rosnow RL. Essentials of behavioral research: Methods and data analysis. 2. New York: McGraw-Hill; 1991. [Google Scholar]
- Rowe R, Rijsdijk FV, Maughan B, Hosang GM, Eley TC. Heterogeneity in antisocial behaviors and comorbidity with depressed mood: A behavioral genetic approach. The Journal of Child Psychology and Psychiatry. 2008;49:526–534. doi: 10.1111/j.1469-7610.2008.01834.x. [DOI] [PubMed] [Google Scholar]
- Rutter M, Giller H, Hagell A. Antisocial Behavior by Young People. New York: Cambridge University Press; 1998. [Google Scholar]
- Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Human Genetics. 1998;103(3):273–279. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- Scott S, Knapp M, Henderson J, Maughan B. Financial cost of social exclusion: follow up study of antisocial children into adulthood. British Medical Journal. 2001;323(7306):191–194. doi: 10.1136/bmj.323.7306.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MP, Kingree JB, Desai S. Gender Differences in Long-Term Health Consequences of Physical Abuse of Children: Data From a Nationally Representative Survey. American Journal of Public Health. 2004;94:599–604. doi: 10.2105/ajph.94.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman SE, Filipas HH. Gender differences in social reactions to abuse disclosures, post-abuse coping, and PTSD of child sexual abuse survivors. Child Abuse & Neglect. 2005;29:767–782. doi: 10.1016/j.chiabu.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Wamboldt MZ, Beach SRH, Kaslow NJ, Heyman RE, First MB, Reiss D. Describing Relationship Patterns in DSM-V: A Preliminary Proposal. In: Millon T, editor. Contemporary Directions in Psychopathology. 2. New York: Guilford Press; 2009. [Google Scholar]
- Yates W, Cadoret R, Troughton E. The Iowa adoption studies methods and results. In: LaBuda M, Grigorenko E, editors. On the way to individuality: Methodological issues in behavioral genetics. Hauppauge, NY: Nova Science Publishers; 1998. pp. 95–125. [Google Scholar]
- Yu YW, Tsai SJ, Hong CJ, Chen TJ, Chen MC, Yang CW. Association study of a monoamine oxidase a gene promoter polymorphism with major depressive disorder and antidepressant response. Neuropsychopharmacology. 2005;30(9):1719–1723. doi: 10.1038/sj.npp.1300785. [DOI] [PubMed] [Google Scholar]