Abstract
The breast cancer treatment drug tamoxifen has been widely administered for more than three decades. This small molecule competes with 17β-estradiol for binding to estrogen receptor, a hormone receptor upregulated in a majority of breast cancers, subsequently initiating programmed cell death. We have synthesized a thiol--PEGylated tamoxifen derivative that can be used to selectively target and deliver plasmonic gold nanoparticles to estrogen receptor positive breast cancer cells with up to 2.7-fold enhanced drug potency in vitro. Optical microscopy/spectroscopy, time-dependent dose-response data, and estrogen competition studies indicate that augmented activity is due to increased rates of intracellular tamoxifen transport by nanoparticle endocytosis, rather than by passive diffusion of the free drug. Both ligand- and receptor- dependent intracellular delivery of gold nanoparticles suggest that plasma membrane localized estrogen receptor alpha may facilitate selective uptake/retention of this and other therapeutic nanoparticle conjugates. Combined targeting selectivity and enhanced potency provides opportunities for both multimodal endocrine treatment strategies and adjunctive laser photothermal therapy.
Introduction
Binding of the steroidal hormone 17β-estradiol (E2) to estrogen receptor (ER)¶ is a process essential to normal cell proliferation and differentiation in women. E2 binding induces a conformational change in ER which allows it to recruit cofactors necessary for the transcription of various genes commonly upregulated in malignant cells (1) (e.g. transforming growth factor alpha (2), c-myc (3), and cathepsin D (4). Accordingly, hormone receptors such as ER or progesterone receptor are overexpressed in 75–80% of all breast cancers (5). Antiestrogen compounds, such as the small molecule breast cancer treatment drug tamoxifen (TAM) compete with E2 for binding to ER, conformationally preventing adoption of associated transcription cofactors and subsequently initiating programmed cell death (6-9).
Diagnostic and therapeutic applications of functionalized nanoparticles are highly attractive due to the inherently multivalent nature of their surfaces (10-14). Like divalent antibodies, the binding affinity of a nanoparticle conjugate is enhanced proportional to the density of its binding sites. Receptor-mediated therapeutic response (i.e. potency) is similarly increased as a function of local ligand concentration and in cases where intracellular drug transport relies on passive diffusion, uptake of nanoparticle conjugates can greatly increase delivery rates (15, 16). Enhanced permeability and retention (EPR) of nanosized drug conjugates can also lead to augmented and preferential accumulation at tumor sites in vivo (17, 18). Due to their biocompatibility (19, 20), stability (21), and potential use in phothermal laser treatments (18, 22-26), gold nanoparticles are excellent candidates for such ligand-receptor targeting strategies of cancer treatment.
Selective targeting and delivery of gold nanoparticles functionalized with ligands of cell surface receptors overexpressed by malignant cells has been well documented. Huang et al. have shown that oral cancer cells upregulating human epidermal growth factor receptor (EGFR, HER1, ErbB1) can be selectively labeled and photothermally destroyed by gold nanospheres and nanorods targeted with IgG antibodies (24, 27). ScFv fragments of anti-EGFR have also been used to selectively target and accumulate gold nanoparticles at tumor sites in vivo (28). Folate receptor has been employed to selectively deliver gold nanospheres to malignant cells in vitro (29), while Wei and coworkers have similarly demonstrated selective uptake and photothermal therapy of cancer cells using gold nanorods functionalized with a thiol-poly(ethylene glycol) (PEG-SH) folate derivative (25, 30).
Like several members of the hormone receptor family, ER isoforms are located both intracellularly and on the cell membrane (31-33). Gold nanoparticle analogs of the commercial pharmaceutical tamoxifen could therefore act not only as, selective targeting agents, but also as increasingly potent endocrine treatments for malignancies which overexpress ER (e.g. breast cancer). To this aim, a thiol-polyethylene glycol tamoxifen derivative was synthesized for subsequent gold nanoparticle (AuNP) conjugation (Scheme 1). A biocompatible (18, 34) PEG-SH linker was employed (i) to enable covalent attachment to the AuNP surface (Au-S 126 kJ*mol-1) (35, 36), (ii) to minimize opsonin binding and reticulo-endothelial system uptake (37), (iii) to suppress non-specific cell binding/uptake (38) and protein adsorption (18, 21), and (iv) to afford stability (21) over a wide range of temperature, ionic strength, and pH.
Scheme 1.
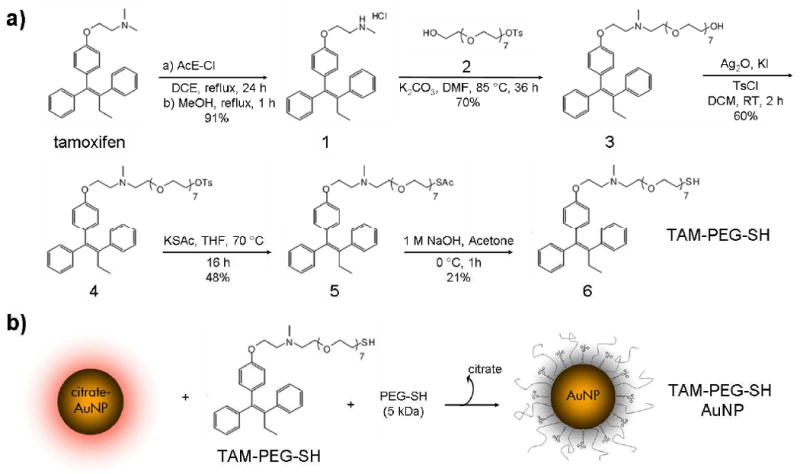
Synthesis of thiol-pegylated tamoxifen (TAM-PEG-SH) (a) and covalent attachment to 25 nm gold nanoparticles (AuNPs) (b).
Our results have shown enhanced potency and selective intracellular delivery of tamoxifen-targeted gold nanoparticles to ER(+) breast cancer cells in vitro. Particle uptake was observed in both a receptor- and ligand- dependent fashion with up to 2.7-fold enhanced drug potency versus the free drug. Both delivery and therapeutic response were shown to be suppressed by estrogen competition. Optical microscopy/spectroscopy and cell viability indicate that augmented growth inhibition versus the free drug can be attributed to increased rates of intracellular TAM transport by cellular uptake of the nanoparticle conjugate. Receptor- and ligand- dependent nanoparticle delivery suggests that the plasma membrane localized estrogen receptor alpha may facilitate selective particle uptake and presents future opportunities for coadministration of laser photothermal therapy (18, 22-26).
Experimental Procedures
Synthesis of thiol-pegylated tamoxifen (TAM-PEG-SH)
Octaethylene glycol (OEG), Tamoxifen, and all chemicals used in the synthesis were purchased from Sigma Aldrich. Anhydrous solvents and other reagents were purchased and used without further purification. Analtech silica gel plates (60 F254) were used for analytical TLC, and Analtech preparative TLC plates (UV 254, 2000 μm) were used for purification. UV light was used to examine the spots. 200-400 Mesh silica gel was used in column chromatography. NMR spectra were recorded on a Varian-Gemini 400 magnetic resonance spectrometer. 1H and 13C NMR spectra (Figures S1-8) were recorded in parts per million (ppm) relative to the peaks of CDCl3, (7.24 and 77.0 ppm, respectively). Mass spectra were recorded at the Georgia Institute of Technology mass spectrometry facility in Atlanta, GA.
Synthesis of N-Desmethyl tamoxifen (1)
The synthetic procedure was adapted from Olofson et al. (39). Briefly, tamoxifen (0.53 g, 1.43 mmol) was dissolved in anhydrous CH2Cl2 (15 ml) at 0 °C followed by addition of α-chloroethyl chloroformate (0.17 ml, 1.49 mmol). After 15 min at 0 °C, the reaction was refluxed for 24 h. The solvent was evaporated off to obtain a yellowish oil, to which methanol (10 ml) was added and refluxed for approximately 3 h. The solvent was evaporated off, and purification performed by gel filtration using CH2Cl2, then 10:1 CH2Cl2/CH3OH to obtain 0.52 g (91 %) of N-desmethyl tamoxifen 2. 1H NMR (DMSO, 400 MHz) δ 0.89 (3H, t, J = 7.2 Hz), 2.43 (2H, q, J = 14.8, 7.6 Hz), 2.56 (3H, s), 3.12 (2H, t, J = 4.0 Hz), 4.08 (2H, t, J = 4.8 Hz), 6.57 (2H, d, J = 8.8 Hz), 6.76 (2H, d, J = 8.8 Hz), 7.03 – 7.31 (10H, m), 9.56 (1H, br); HRMS [FAB, mnba] (C25H27NO)+ calcd, 358.2171; found, 358.2198.
Synthesis of Tosyl octaethylene glycol (2)
The synthetic procedure was adapted from Bouzide and Sauvé (40). Briefly, octaethylene glycol (0.50 g, 1.35 mmol) was dissolved in anhydrous CH2Cl2 (7 ml) at 0 °C, followed by addition of freshly prepared Ag2O (0.47 g, 2.02 mmol), KI (0.09 g, 0.50 mmol), and then TsCl (0.26 g, 1.35 mmol). The reaction mixture was left to stir at 0 °C, under argon for 30 min, after which TLC deemed the reaction complete. Ag2O was filtered off over a pad of celite cake washing with 12:1 CH2Cl2/CH3OH. The filtrate was concentrated and purified on a silica column using 3:2, then gradually 1:4 CH2Cl2/acetone to yield the title compound as a colorless oil (0.55 g, 78 %). 1H NMR (CDCl3, 400 MHz) δ 2.44 (3H, s), 2.81 (1H, br), 3.58 – 3.70 (30H, m), 4.15 (2H, t, J = 4.8 Hz), 7.35 (2H, d, J = 8.4 Hz), 7.80 (2H, d, J = 8.4 Hz); HRMS [ESI] (C23H40O11S + H)+ calcd, 525.2364; found, 525.2377.
Synthesis of Tamoxifen-OEG-OH (3)
N-Desmethyl tamoxifen (1) (0.27 g, 0.68 mmol) and tosyl octaethylene glycol (2) (0.54 g, 1.05 mmol) were dissolved in anhydrous DMF (10 ml), followed by addition of K2CO3 (0.95 g, 6.85 mmol), and stirred under argon at ∼ 85 °C for 24 h. DMF was evaporated off. Ethyl acetate was added to the residue and the resulting suspension was filtered off to remove excess K2CO3. Solvent was evaporated from the filtrate and the crude was purified by preparatory TLC using 12:1:0.1 CH2Cl2/CH3OH/NH4OH to obtain 0.342 g (70 %) of compound 3 as an oil. 1H NMR (CDCl3, 400 MHz) δ 0.90 (3H, t, J = 7.2 Hz), 1.85 (1H, br), 2.31 (3H, s), 2.44 (2H, q, J = 14.0, 7.2 Hz), 2.64 (2H, t, J = 6.0 Hz), 2.76 (2H, t, J = 6.0 Hz), 3.53 – 3.72 (30H, m), 3.91 (2H, t, J = 6.4 Hz), 6.54 (2H, m), 6.76 (2H, m), 7.10 – 7.40 (10H, m); 13C NMR (CDCl3, 100 MHz) δ 13.8, 29.2, 43.3, 50.7, 56.6, 57.2, 61.7, 65.6, 69.1, 70.3, 70.5, 70.6, 70.7, 70.8, 73.0, 113.6, 126.2, 126.7, 128.0, 128.3, 129.7, 129.9, 132.1, 135.8, 138.4, 141.5, 142.6, 144.0, 156.8; HRMS [ESI] (C41H59NO9 + H)+ calcd, 710.4262; found, 710.4253.
Synthesis of Tamoxifen-OEG-Tosylate (4)
Tamoxifen-OEG-OH (3) (0.33 g, 0.46 mmol) was dissolved in anhydrous CH2Cl2 (10 ml) at 0 °C, followed by addition of Ag2O (0.16 g, 0.69 mmol), KI (0.03 g, 0.18 mmol), and then TsCl (0.096 g, 0.5 mmol). Stirring was continued for 2 h at 0 °C, then at room temperature, overnight. Ag2O was filtered off through a pad of celite cake washing with ethyl acetate. Purification was performed on silica column eluting with 12:1 CH2Cl2/CH3OH yielding the title compound as oil (0.24 g, 60 %). 1H NMR (CDCl3, 400 MHz) δ 0.91 (3H, t, J = 7.2 Hz), 2.31 – 2.46 (8H, m), 2.71 (2H, br), 2.83 (2H, br), 3.57 – 3.70 (28H, m), 3.95 (2H, br), 4.15 (2H, t, J = 4.4 Hz), 6.53 (2H, d, J = 8.4 Hz), 6.76 (2H, d, J = 8.8 Hz), 7.10 – 7.34 (12H, m), 7.80 (2H, d, J = 8.4 Hz); LRMS [ESI] (C48H65NO11S + H)+ calcd, 864.1; found, 864.5.
Synthesis of Tamoxifen-OEG-SAc (5)
KSAc (0.079 g, 0.69 mmol) was added to tamoxifen-OEG-Tosylate (4) (0.12 g, 0.14 mmol) dissolved in anhydrous THF and refluxed under argon at ∼ 75 °C for 16 h. TLC analysis indicated a substantial consumption of the starting material. THF was evaporated off, and the crude product dissolved in ethyl acetate. Decolorizing carbon was added and then filtered. Solvent was evaporated from the filtrate and the crude was purified by preparatory TLC using 12:1 CH2Cl2/CH3OH to obtain 50 mg (48 %) of 5 as reddish oil. 1H NMR (CDCl3, 400 MHz) δ 1.03 (3H, t, J = 7.2 Hz), 2.45 (3H, s), 2.47 (3H, s), 2.56 (2H, q, J = 15.2, 7.6 Hz), 2.80 (2H, t, J = 6.0 Hz), 2.91 (2H, t, J = 5.6 Hz), 3.21 (2H, t, J = 6.0 Hz), 3.70 – 3.80 (28H, m), 4.05 (2H, t, J = 6.0 Hz), 6.66 (2H, d, J = 8.0 Hz), 6.88 (2H, d, J = 8.0 Hz), 7.22 – 7.46 (10H, m); HRMS [ESI] (C43H61NO9S + H)+ calcd, 768.4139; found, 768.4118.
Synthesis of Thiol-peglated tamoxifen (6)
Tamoxifen-OEG-SAc (5) (0.05 g, 0.065 mmol) was dissolved in acetone (1.5 ml) at 0 °C, followed by addition of 1M NaOH (1.5 ml) and stirring continued at 0 °C for 7 h. The reaction mixture was quenched with water (20 ml), and then extracted with 20 % CH3OH in CH2Cl2 (4 × 15 mL). The organic layers were combined, dried under sodium sulfate, evaporated and purified on preparatory TLC using 11:1 CH2Cl2/CH3OH to give 10 mg (21 %) of the title compound as reddish semi-solid. 1H NMR (CDCl3, 400 MHz) δ 0.90 (3H, t, J = 7.2 Hz), 2.32 (3H, s), 2.44 (2H, q, J = 8.0,7.6 Hz), 2.65 (2H, t, J = 6.0 Hz), 2.76 (2H, t, J = 6.4 Hz), 2.85 (2H, t, J = 6.8 Hz), 3.53 – 3.72 (28H, m), 3.91 (2H, t, J = 6.0 Hz), 6.52 (2H, d, J = 8.8 Hz), 6.74 (2H, d, J = 8.8 Hz), 7.08 – 7.32 (10H, m); 13C NMR (CDCl3, 100 MHz) δ 13.5, 28.9, 29.6, 38.3, 43.3, 56.4, 57.0, 65.6, 69.2, 69.6, 70.3, 70.4, 70.5, 70.6, 113.3, 125.9, 126.4, 127.8, 128.0, 129.4, 129.6, 131.8, 135.4, 138.1, 141.2, 142.3, 143.7, 156.6; HRMS [ESI] (C41H59NO8S]+ calcd, 725.3961; found, 725.4011.
Gold nanoparticle synthesis and TAM-PEG-SH conjugation
Gold nanoparticles (25 nm dia) were synthesized by Turkevich reduction of chloroauric acid (41). Briefly, 20 mL of 3.5 mg/mL aqueous sodium citrate was added to 200 mL of 1.0 mM aqueous HAuCl4 under reflux, with stirring. The solution was refluxed for 15 min, then removed from the heat and stirred for an additional 30 min. Excess sodium citrate was removed from the crude AuNP solution by centrifugation (13,000 × g).
TAM-PEG-SH (5 mg) was solubilized in 100 μL ethanol and diluted to 0.5 mM in deionized water. 0.5 mM PEG-SH (5 kDa, Lysan Bio) was solubilized in deioninized water and PEG-SH or a 1:1 ratio TAM-PEG-SH and PEG-SH were added at a 1.4 × 104-fold molar excess to a concentrated solution of citrate-capped AuNPs followed by overnight sonication. Particle concentration was estimated using the molar extinction coefficient for 23 nm citrate-capped gold nanospheres determined by Orendorff and Murphy (42) (1.3 × 109 M-1 cm-1). TAM-PEG-SH AuNP conjugates were dispersed in DMEM growth media supplanted with 10% v/v heat-inactivated fetal bovine serum, penicillin (100 U/ml), streptomycin (100 μg/ml), 4.5 g/L glucose, 4.5 g/L sodium pyruvate, without L-glutamine and phenol red to final ligand concentrations of 0.1, 0.5, 1, 2, 5, 10, and 20 μM and used immediately.
Gold Nanoparticle and Bioconjugate Characterization
Gold nanoparticles were analyzed by diffraction-contrast Transmission Electron Microscopy (TEM, JEOL 100CX II) and UV-Vis absorption spectroscopy (Ocean Optics, HR4000CG-UV-NIR). Absorption of TAM-PEG-SH at 280 nm was used to quantify the number of bound TAM-PEG-SH ligands per nanoparticle. An aqueous solution of gold nanospheres was incubated with a 1.4 × 104-fold molar excess of both TAM-PEG-SH and PEG-SH overnight with sonication. Nanoparticle-conjugates were removed from solution by centrifugation (45 min, 13,000 × g) and the observed change in UV absorption (280 nm) before and after nanoparticle conjugation was used to approximate the number of bound ligands. No contribution to absorption by PEG-SH was observed at these wavelengths and it assumed to occupy the majority of the remaining surface sites.
Zeta potential of the gold nanoparticles and conjugates was measured using a NanoZS Zetasizer particle analyzer (Malvern) equipped with a 633 nm laser.
Cell culture and nanoparticle incubation
ERα(-) MDA-MB-231 and ERα(+) MCF-7 breast cancer cells (human adenocarcinoma, ATCC) or ERα(+) human squamous cell carcinoma (43-45) (HSC-3) cells were cultured to 105 cells/cm2 in DMEM growth media supplanted with 10% v/v heat-inactivated fetal bovine serum, penicillin (100 U/ml), streptomycin (100 μg/ml), 4.5 g/L glucose, 4.5 g/L sodium pyruvate, without L-glutamine and phenol red at 37 °C in a 5% CO2 humidified atmosphere. Growth media was removed from the cell cultures and replaced with identical media containing gold nanoparticle conjugates heated to 37 °C at time = 0 h.
Cell viability assay
Following incubation, growth media containing gold nanoparticle conjugates was removed and cells were rinsed twice in sterile Dulbecco's phosphate buffered saline (DPBS). Mitochondrial dehydrogenase activity was assessed by MTT or XTT spectrophotometric assay (Sigma TOX1, TOX2) following the manufacturer's instructions. The assay was performed using a SpectraMax Plus 384 microplate reader and statistical analysis was performed by t-test.
Selected-area absorption microspectrometry and dark-field scattering microscopy
Collagen-coated growth substrates were prepared by immersion of 18 mm dia glass coverslips in ethanol, followed by 30 min UV sterilization. Coverslips were immersed in a 0.22 μm filtered 0.04 mg/mL collagen (Roche) solution -prepared by solubilization in 5 mL 1% v/v aqueous acetic acid and dilution in 250 mL sterile DPBS, for 6 h at 37 °C in a 5% CO2 humidified atmosphere. The coated substrates were rinsed in sterile DPBS and placed in 12-well plates immediately prior to cell passage. Following incubation with gold nanoparticle conjugates, substrates were twice rinsed in sterile DPBS buffer and cells were fixed in cold 4 % wt/wt paraformaldehyde in DPBS buffer for 15 min. Coverslips were coated in glycerol, then mounted and sealed onto glass slides.
Dark-field microscopy was performed using an inverted objective Olympus IX70 microscope fitted with a dark-field condenser (U-DCW), 100×/1.35 oil Iris objective (UPLANAPO), tungsten lamp, and a Nikon D200 digital SLR camera. Optical extinction spectra were obtained in a transmission configuration using a SEE110 absorption microspectrometer fitted with a pinhole aperture, fiber optic-coupled CCD array detector, 50 × objective, and tungsten lamp. Periodic oscillations observed in some spectra are the result of interference between adjacent surfaces of the glass slides.
Results and Discussion
The crude AuNP colloid (ca. 3 nM) was found by TEM to be predominantly comprised of 25 nm gold spheres exhibiting an extinction maximum at 530 nm (Figure S9). Based on the change in UV absorption (280 nm) of TAM solutions following nanoparticle conjugation and removal, we estimate 12,000 TAM-PEG-SH ligands per particle, 41 % of the maximum theoretical surface coverage for a 25 nm dia Au (111) surface. A change in zeta potential from -38.4 mV to -5.79 mV was also observed following TAM-PEG-SH functionalization. To preserve aqueous stability, TAM-PEG-SH AuNPs were not centrifuged prior to in vitro experiments, leaving 13 % of free drug in solution. For comparison with the free drug, concentrations for the nanoparticle conjugate are reported as total ligand concentration (i.e. TAM-PEG-SH) throughout.
Dark-field scattering microscopy was performed to assess intracellular nanoparticle uptake. Figure 1 illustrates representative images of ERα(+) [MCF-7, top] and ERα(-) [MDA-MB-231, bottom] breast cancer cells incubated for 24 h with 1 μM TAM-PEG-SH AuNPs and PEG-SH AuNPs. ERα(+) breast cancer cells displayed a high degree of intracellular and perinuclear localization of TAM-PEG-SH AuNPs, while ER(-) breast cancer cells showed no such labeling. These findings are consistent with both reported expression levels and cellular localization (46) of ERα in MCF-7 (47-49) and MDA-MB-231 (48, 50) cell lines. As anticipated (38), AuNPs labeled only with PEG-SH exhibited no apparent cellular labeling or uptake for either ERα(+) or ERα(-) breast cancer cells. Uptake of TAM-PEG-SH AuNPs by ERα(+) breast cells was observed to be time-dependent, with marginal cell surface labeling at 2-6 h and a high degree of perinuclear and cytoplasmic localization at 24 h (Figure S10). To further demonstrate ER expression-dependent targeting, ERα(+) human squamous [HSC-3] oral cancer cells were incubated for 24 h in the presence of 1 μM TAM-PEG-SH AuNPs and PEG-SH AuNPs. Dark-field scattering images from HSC-3 cells show selective uptake of the TAM-PEG-SH AuNPs in a manner similar to that obtained from MCF-7 breast cancer cells (Figure S11). Selected-area optical extinction spectra obtained from the ERα(+) and ERα(-) breast cells exhibited AuNP surface plasmon extinction exclusively from perinuclear regions of ERα(+) cells incubated with TAM-PEG-SH AuNPs (s/n∼10) (Figure S12). Extinction from PEG-SH AuNPs was not observed from either cell line.
Figure 1.
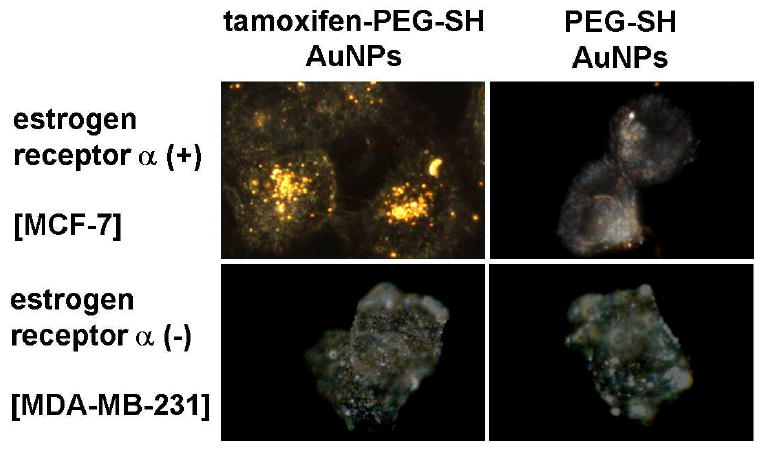
Dark-field scattering microscopy showing ligand- and receptor- dependent intracellular targeting of breast cancer cells by gold nanoparticle conjugates. Representative dark-field scattering images of ERα(+) [MCF-7, top] and ERα(-) [MDA-MB-231, bottom] human adenocarcinoma cells incubated for 24 h with 1 μM TAM-PEG-SH AuNP (left) and PEG-SH AuNP (right) conjugates (ca. 1.2 × 104 TAM-PEG-SH per AuNP).
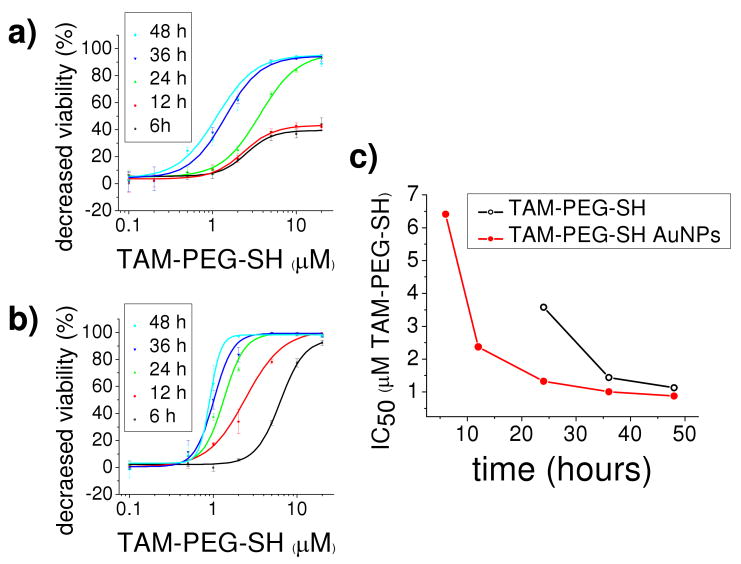
Figures 2a and b illustrate time-dependent dose-response curves for cell viability of ERα(+) MCF-7 breast cancer cells incubated with equivalent concentrations of TAM-PEG-SH as the free drug and the nanoparticle conjugate, respectively. A comparison of the time-dependent IC50 (50% inhibitory concentration) values obtained for the free drug and its AuNP conjugate indicate 1.3 - 2.7 fold enhanced potency (Figure 2c) for TAM-PEG-SH AuNPs. While IC50 values for TAM-PEG-SH alone are comparable to or better than those previously reported for MCF-7 breast cancer cells treated with both tamoxifen (43) and its active metabolite (51), a much more dramatic improvement is observed upon nanoparticle ligation, in contrast to the free drug, with significant growth inhibition observed for TAM-PEG-SH AuNPs at both 6 and 12 h incubation (6.4 and 2.4 μM IC50, respectively). In accordance with previous studies (19), no cytotoxic effects were observed in MCF-7 cells treated with PEG-SH AuNPs at the highest concentrations and incubation times used in the present study (P>0.75). Moreover, cytotoxic effects were not observed in ERα(-) MDA-MB-231 breast cancer cells incubated with TAM-PEG-SH alone, PEG-SH AuNPs, or TAM-PEG-SH AuNPs at the highest concentrations and incubation times used in the present study (P>0.28, 0.33, and 0.11, respectively). Although differences in sensitivity to and rates of particle/drug uptake and metabolism for ERα(+) and (-) cell lines may contribute to variation in apparent cytotoxicity, the observed ligand-dependency correlates well with levels of cellular ER expression, particularly under the conditions of extended incubation time and excess concentration used. In addition, cell viability following incubation with TAM-PEG-SH AuNPs in the absence of free drug was found statistically indistinguishable in difference from that observed in its presence. Here, AuNPs functionalized with TAM-PEG-SH equivalent to that present at the 24 h IC50 of the conjugate in the presence of free drug subsequently exhibited 57 ± 14 % cell viability (P>0.6). The lack of significant growth inhibition by the free drug at short incubation times, together with an observed decrease in the disparity between IC50 values of the free drug and the AuNP conjugate over time, and the apparent ligand-dependent response indicate increased rates of TAM-PEG-SH transport by the AuNP conjugate.
Figure 2.
Time-dependent dose-response curves for cell viability of estrogen receptor alpha positive [MCF-7] breast cancer cells incubated with equivalent concentrations of TAM-PEG-SH as a free drug (a) and as a gold nanoparticle conjugate (b). Time-dependent IC50 (50% inhibitory concentration) values showing 1.3 – 2.7 times enhanced potency from the nanoparticle conjugate versus the free drug. Error bars represent standard deviation. 3.6, 1.4, 1.1 μM TAM-PEG-SH IC50 (24, 36, 48 h, respectively) versus 6.4, 2.4, 1.3, 1.0, 0.88 μM TAM-PEG-SH AuNP IC50 (6, 12, 24, 36, 48 h, respectively).
Although our results with the HSC-3 cell line, an ERα(+) oral cancer cell line, further attested to the role of ERα in nanoparticle uptake, it is however conceivable that particle lipophilicity could also contribute to differences in cellular uptake and cytotoxicity. In light of this possibility, blocking experiments were performed using ERα's endogenous ligand 17β-estradiol (estrogen) to further confirm receptor-dependent targeting and therapeutic response. ERα(+) MCF-7 breast cells were incubated overnight with increasing concentrations of estrogen, followed by 24 h incubation with 10 μM TAM-PEG-SH AuNPs. Image overlays from bright-field transmission and dark-field scattering microscopy of these cells (Figure 3) indicate near complete suppression of TAM-PEG-SH AuNP intracellular localization at estrogen concentrations as low as 20 nM. Decreased cell surface labeling was also observed with increasing estrogen concentration. Such competitive effects are in agreement with previous reports indicating 1-2 orders of magnitude greater ERα binding affinity for 17β-estradiol versus TAM (52). Cell viability experiments with ERα(+) breast cells incubated for 24 h with 10 μM TAM-PEG-SH AuNPs and previously blocked overnight with equimolar concentrations of estrogen were also performed (Figure 4). As in previous studies with the free drug (8), the cytotoxic activity of TAM-labeled AuNPs was near completely suppressed following pre-exposure of the cells to estrogen (P>0.87), while they retained optimal potency in the absence of estrogen (P<0.0001). These findings correlate ERα binding with both TAM-PEG-SH AuNP intracellular localization and subsequent cell death.
Figure 3.
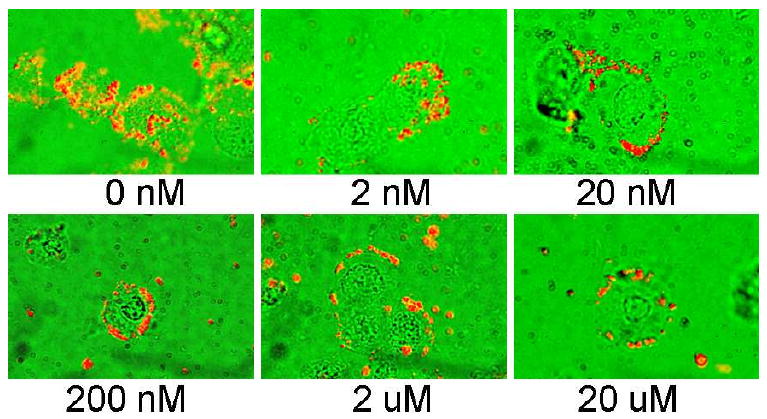
Representative dark-field scattering (red) and bright-field transmission (green) image overlays of TAM-PEG-SH AuNP competitive binding following 24 h incubation with 17β-estradiol. ERα(+) breast cancer cells [MCF-7] were incubated overnight with increasing concentrations of estrogen, followed by 24 h incubation with 10 μM tamoxifen-gold nanoparticle conjugates.
Figure 4.
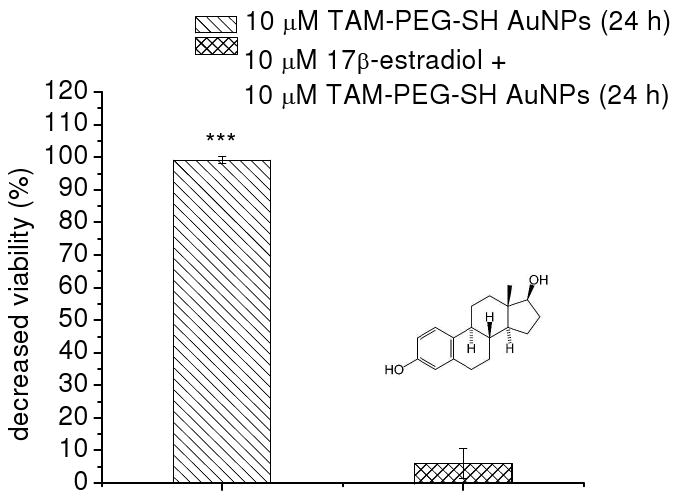
Suppression of TAM-PEG-SH AuNP activity by estrogen competition in ERα(+) breast cancer cells. Growth inhibition of MCF-7 cells incubated for 24 h with 10 μM TAM-PEG-SH AuNPs when previously untreated (left) and treated overnight with 10 μM 17β-estradiol (right).
The ERα expression-dependent uptake observed here also suggests that the cell membrane-associated receptor may facilitate intracellular nanoparticle transport. Indeed, plasma membrane localized ERα is well documented, as is its recognition of both antibody epitopes for the nuclear receptor and 17β-estradiol in mammalian cells (31, 33). The functions of membrane ERα beyond classical gene transcription, and more recently membrane-initiated signaling, are however less understood (53). Comprehensive studies by Levin and coworkers indicate intracellular transport and caveolar localization of ERα in the plasma membrane of MCF-7 cells in vitro (via caveolin-1 and -2 association) (49). In order to determine whether plasma membrane localized ERα could contribute to receptor-mediated endocytosis of TAM-PEG-SH AuNP conjugates, cytotoxicity was examined under conditions of negligible endocytotic activity. MCF-7 cell viability was shown to increase by 87 ± 2 % following incubation with 20 μM TAM-PEG-SH AuNPs for 6 h at 4 °C versus 37 °C (P<0.04), indicating that endocytosis – in addition to ERα binding and intracellular particle delivery – is required for therapeutic response from tamoxifen-labeled AuNP conjugates.
Conclusions
In summary, tamoxifen-gold nanoparticle conjugates were shown to selectively target estrogen receptor alpha in human breast cancer cells with up to 2.7-times enhanced potency in vitro. Optical microscopy and spectroscopy indicate a high degree of perinuclear and cytoplasmic localization of the targeted particles, while neither localization nor cytotoxic effects were observed from the untargeted nanoparticles. Time-dependent dose-response studies show that augmented potency results from increased rates of drug transport by nanoparticle uptake versus passive diffusion of the free drug. Receptor-selective and estrogen-competitive cytotoxicity/uptake of the nanoparticle conjugates indicates no additive effects associated with the gold particles themselves and suggests that plasma membrane-localized ERα may facilitate selective endocytotic transport of these and other therapeutic nanoparticle conjugates. Increased potency and selective intracellular delivery of tamoxifen-gold nanoparticle conjugates provides opportunities for further enhancement by co-functionalization or adjunctive laser photothermal therapy.
Supplementary Material
Acknowledgments
The authors wish to acknowledge generous support from Merck, the National Cancer Institute (CCNE Award U54CA119338), the US Deptartment of Energy (NO:DE-FG02-97 ER14799), the Center for Nanostructured Materials Technology' under '21st Century Frontier R&D Programs' of the Korean Ministry of Education, Science and Technology (Grant No. 08K1501-01910), the Georgia Institute of Technology, and the Blanchard Foundation. Cell subculture by Omar Dellanoy-Bruno, electron microscopy by Steven W. Hayden, and the facilities of the Center for Nanostructure Fabrication and Characterization (CNCF) is also acknowledged.
Footnotes
Abbreviations: estrogen receptor (ER), 17β-estradiol (E2), tamoxifen (TAM), enhanced permeability and retention (EPR), polyethylene glycol (PEG), epidermal growth factor receptor (EGFR), thiol-polyethylene glycol (PEG-SH), gold nanoparticle (AuNP), thiol-polyethylene glycol tamoxifen (TAM-PEG-SH), Dulbecco's phosphate buffered saline (DPBS).
Supporting Information Available: 13C and 1H NMR spectra, TEM and UV-Vis analysis, time-dependent uptake images, oral cancer cell targeting images, selected-area microspectrometry, imaging/spectroscopy of conjugate stability, and estrogen competition images. This material is available free of charge via the Internet at http://pubs.acs.org.
Literature Cited
- 1.Obrero M, Yu DV, Shapiro DJ. Estrogen receptor-dependent and estrogen receptor-independent pathways for tamoxifen and 4-hydroxytamoxifen-induced programmed cell death. J Biol Chem. 2002;277:45695–45703. doi: 10.1074/jbc.M208092200. [DOI] [PubMed] [Google Scholar]
- 2.Bates SE, Davidson NE, Valverius EM, Freter CE, Dickson RB, Tam JP, Kudlow JE, Lippman ME, Salomon DS. Expression of Transforming Growth Factor-Alpha and Its Messenger Ribonucleic-Acid in Human-Breast Cancer - Its Regulation by Estrogen and Its Possible Functional-Significance. Mol Endocrinol. 1988;2:543–555. doi: 10.1210/mend-2-6-543. [DOI] [PubMed] [Google Scholar]
- 3.Dubik D, Dembinski TC, Shiu RPC. Stimulation of c-Myc Oncogene Expression Associated With Estrogen-Induced Proliferation of Human-Breast Cancer-Cells. Cancer Res. 1987;47:6517–6521. [PubMed] [Google Scholar]
- 4.Krishnan V, Wang XH, Safe S. Estrogen Receptor-SP1 Complexes Mediate Estrogen-Induced Cathepsin-D Gene-Expression in MCF-7 Human Breast-Cancer Cells. J Biol Chem. 1994;269:15912–15917. [PubMed] [Google Scholar]
- 5.Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007;8:235–244. doi: 10.1016/S1470-2045(07)70074-8. [DOI] [PubMed] [Google Scholar]
- 6.Jordan VC. Chemoprevention of breast cancer with selective oestrogen-receptor modulators. Nat Rev Cancer. 2007;7:46–53. doi: 10.1038/nrc2048. [DOI] [PubMed] [Google Scholar]
- 7.Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95:927–937. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- 8.Coezy E, Borgna JL, Rochefort H. Tamoxifen and Metabolites in MCF7 Cells: Correlation between Binding to Estrogen Receptor and Inhibition of Cell Growth. Cancer Res. 1982;42:317–323. [PubMed] [Google Scholar]
- 9.Jordan VC. Tamoxifen: A most unlikely pioneering medicine. Nat Rev Drug Discovery. 2003;2:205–213. doi: 10.1038/nrd1031. [DOI] [PubMed] [Google Scholar]
- 10.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat Biotechnol. 2005;23:1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 11.Montet X, Funovics M, Montet-Abou K, Weissleder R, Josephson L. Multivalent Effects of RGD Peptides Obtained by Nanoparticle Display. J Med Chem. 2006;49:6087–6093. doi: 10.1021/jm060515m. [DOI] [PubMed] [Google Scholar]
- 12.Gestwicki JE, Cairo CW, Strong LE, Oetjen KA, Kiessling LL. Influencing Receptor-Ligand Binding Mechanisms with Multivalent Ligand Architecture. J Am Chem Soc. 2002;124:14922–14933. doi: 10.1021/ja027184x. [DOI] [PubMed] [Google Scholar]
- 13.Goodman CM, Rotello VM. Biomacromolecule surface recognition using nanoparticles. Mini-Rev Org Chem. 2004;1:103–114. [Google Scholar]
- 14.Gibson JD, Khanal BP, Zubarev ER. Paclitaxel-functionalized gold nanoparticles. J Am Chem Soc. 2007;129:11653–11661. doi: 10.1021/ja075181k. [DOI] [PubMed] [Google Scholar]
- 15.Cho K, Wang X, Nie S, Chen Z, Shin DM. Therapeutic Nanoparticles for Drug Delivery in Cancer. Clin Cancer Res. 2008;14:1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 16.Chawla JS, Amiji MM. Biodegradable poly([var epsilon]-caprolactone) nanoparticles for tumor-targeted delivery of tamoxifen. Int J Pharm. 2002;249:127–138. doi: 10.1016/s0378-5173(02)00483-0. [DOI] [PubMed] [Google Scholar]
- 17.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv Enzyme Reg. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 18.von Maltzahn G, Park JH, Agrawal A, Bandaru NK, Das SK, Sailor MJ, Bhatia SN. Computationally Guided Photothermal Tumor Therapy Using Long-Circulating Gold Nanorod Antennas. Cancer Res. 2009;69:3892–3900. doi: 10.1158/0008-5472.CAN-08-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 20.Khan JA, Pillai B, Das TK, Singh Y, Maiti S. Molecular effects of uptake of gold nanoparticles in HeLa cells. Chembiochem. 2007;8:1237–1240. doi: 10.1002/cbic.200700165. [DOI] [PubMed] [Google Scholar]
- 21.Zhang F, Skoda MWA, Jacobs RMJ, Zorn S, Martin RA, Martin CM, Clark GF, Goerigk G, Schreiber F. Gold nanoparticles decorated with oligo(ethylene glycol) thiols: Protein resistance and colloidal stability. J Phys Chem A. 2007;111:12229–12237. doi: 10.1021/jp074293v. [DOI] [PubMed] [Google Scholar]
- 22.Dickerson EB, Dreaden EC, Huang X, El-Sayed IH, Chu H, Pushpanketh S, McDonald JF, El-Sayed MA. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Lett. 2008;269:57–66. doi: 10.1016/j.canlet.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Photothermal Tumor Ablation in mice using near infrared absorbing nanoshells. Cancer Lett. 2004;209:171–176. doi: 10.1016/j.canlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Huang X, El-Sayed IH, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc. 2006;128:2115–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 25.Huff TB, Tong L, Zhao Y, Hansen MN, Cheng JX, Wei A. Hyperthermic effects of gold nanorods on tumor cells. Nanomedicine. 2007;2:125–132. doi: 10.2217/17435889.2.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, Hazle JD, Halas NJ, West JL. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci USA. 2003;100:13549–13554. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Sayed IH, X H, El-Sayed MA. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006;239:129–135. doi: 10.1016/j.canlet.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 28.Qian XM, Peng XH, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, Yang L, Young AN, Wang MD, Nie SM. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nature Biotechnol. 2008;26:83–90. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- 29.Dixit V, Van den Bossche J, Sherman DM, Thompson DH, Andres RP. Synthesis and Grafting of Thioctic Acid-PEG-Folate Conjugates onto Au Nanoparticles for Selective Targeting of Folate Receptor-Positive Tumor Cells. Bioconjugate Chem. 2006;17:603–609. doi: 10.1021/bc050335b. [DOI] [PubMed] [Google Scholar]
- 30.Tong L, Zhao Y, Huff TB, Hansen MN, Wei A, Cheng JX. Gold Nanorods Mediate Tumor Cell Death by Compromising Membrane Integrity. Adv Mater. 2007;19:3136–3141. doi: 10.1002/adma.200701974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levin ER. Integration of the Extranuclear and Nuclear Actions of Estrogen. Mol Endocrinol. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell CH, Bulayeva N, Brown DB, Gametchu B, Watson CS. Regulation of the membrane estrogen receptor-alpha: role of cell density, serum, cell passage number, and estradiol. FASEB J. 2002;16:1917–1927. doi: 10.1096/fj.02-0182com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zivadinovic D, Gametchu B, Watson CS. Membrane estrogen receptor-alpha levels in MCF-7 breast cancer cells predict cAMP and proliferation responses. Breast Cancer Res. 2005;7:R101–R112. doi: 10.1186/bcr958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niidome T, Yamagata M, Okamoto Y, Akiyama Y, Takahashi H, Kawano T, Katayama Y, Niidome Y. PEG-modified gold nanorods with a stealth character for in vivo applications. J Controlled Release. 2006;114:343–347. doi: 10.1016/j.jconrel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Jang SS, Jang YH, Kim YH, Goddard WA, Flood AH, Laursen BW, Tseng HR, Stoddart JF, Jeppesen JO, Choi JW, Steuerman DW, DeIonno E, Heath JR. Structures and Properties of Self-Assembled Monolayers of Bistable [2]Rotaxanes on Au (111) Surfaces from Molecular Dynamics Simulations Validated with Experiment. J Am Chem Soc. 2005;127:1563–1575. doi: 10.1021/ja044530x. [DOI] [PubMed] [Google Scholar]
- 36.Lavrich DJ, Wetterer SM, Bernasek SL, Scoles G. Physisorption and Chemisorption of Alkanethiols and Alkyl Sulfides on Au(111) J Phys Chem B. 1998;102:3456–3465. [Google Scholar]
- 37.Gref R, Domb A, Quellec P, Blunk T, Muller RH, Verbavatz JM, Langer R. The Controlled Intravenous Delivery of Drugs Using PEG-Coated Sterically Stabilized Nanospheres. Adv Drug Delivery Rev. 1995;16:215–233. doi: 10.1016/0169-409X(95)00026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nativo P, Prior IA, Brust M. Uptake and Intracellular Fate of Surface-Modified Gold Nanoparticles. ACS Nano. 2008;2:1639–1644. doi: 10.1021/nn800330a. [DOI] [PubMed] [Google Scholar]
- 39.Olofson RA, Martz JT, Senet JP, Piteau M, Malfroot T. A New Reagent for the Selective, High-Yield N-Dealkylation of Tertiary-Amines - Improved Syntheses of Naltrexone and Nalbuphine. J Org Chem. 1984;49:2081–2082. [Google Scholar]
- 40.Bouzide A, Sauve G. Silver(I) oxide mediated highly selective monotosylation of symmetrical diols. Application to the synthesis of polysubstituted cyclic ethers. Org Lett. 2002;4:2329–2332. doi: 10.1021/ol020071y. [DOI] [PubMed] [Google Scholar]
- 41.Turkevich J, Stevenson PC, Hillier J. A Study of the Nucleation and Growth Processes in the Synthesis of Colloidal Gold. Discuss Faraday Soc. 1951:55–75. [Google Scholar]
- 42.Orendorff CJ, Murphy CJ. Quantitation of Metal Content in the Silver-Assisted Growth of Gold Nanorods. J Phys Chem B. 2006;110:3990–3994. doi: 10.1021/jp0570972. [DOI] [PubMed] [Google Scholar]
- 43.Guthrie N, Gapor A, Chambers AF, Carroll KK. Inhibition of Proliferation of Estrogen Receptor-Negative MDA-MB-435 and -Positive MCF-7 Human Breast Cancer Cells by Palm Oil Tocotrienols and Tamoxifen, Alone and in Combination. J Nutr. 1997;127:544S–548S. doi: 10.1093/jn/127.3.544S. [DOI] [PubMed] [Google Scholar]
- 44.Jordan VC. New insights into the metabolism of tamoxifen and its role in the treatment and prevention of breast cancer. Steroids. 2007;72:829–842. doi: 10.1016/j.steroids.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nozoe T, Oyama T, Takenoyama M, Hanagiri T, Sugio K, Yasumoto K. Significance of immunohistochemical expression of estrogen receptors alpha and beta in squamous cell carcinoma of the esophagus. Clin Cancer Res. 2007;13:4046–4050. doi: 10.1158/1078-0432.CCR-07-0449. [DOI] [PubMed] [Google Scholar]
- 46.Htun H, Holth LT, Walker D, Davie JR, Hager GL. Direct visualization of the human estrogen receptor alpha reveals a role for ligand in the nuclear distribution of the receptor. Mol Biol Cell. 1999;10:471–486. doi: 10.1091/mbc.10.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brooks SC, Locke ER, Soule HD. Estrogen Receptor in a Human Cell Line (MCF-7) from Breast Carcinoma. J Biol Chem. 1973;248:6251–6253. [PubMed] [Google Scholar]
- 48.Horwitz KB, Zava DT, Thilagar AK, Jensen EM, McGuire WL. Steroid Receptor Analyses of Nine Human Breast Cancer Cell Lines. Cancer Res. 1978;38:2434–2437. [PubMed] [Google Scholar]
- 49.Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER. ERs associate with and regulate the production of caveolin: Implications for signaling and cellular actions. Mol Endocrinol. 2002;16:100–115. doi: 10.1210/mend.16.1.0757. [DOI] [PubMed] [Google Scholar]
- 50.Chen XM, Danes C, Lowe M, Herliczek TW, Keyomarsi K. Activation of the estrogen-signaling pathway by p21(WAF1/CIP1) in estrogen receptor-negative breast cancer cells. J Natl Cancer Inst. 2000;92:1403–1413. doi: 10.1093/jnci/92.17.1403. [DOI] [PubMed] [Google Scholar]
- 51.Seeger H, Diesing D, Gückel B, Wallwiener D, Mueck AO, Huober J. Effect of tamoxifen and 2-methoxyestradiol alone and in combination on human breast cancer cell proliferation. J Steroid Biochem Mol Biol. 2003;84:255–257. doi: 10.1016/s0960-0760(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 52.Rich RL, Hoth LR, Geoghegan KF, Brown TA, LeMotte PK, Simons SP, Hensley P, Myszka DG. Kinetic analysis of estrogen receptor/ligand interactions. Proc Natl Acad Sci USA. 2002;99:8562–8567. doi: 10.1073/pnas.142288199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qing L, Pallas DC, Surks HK, Baur WE, Mendelsohn ME, Karas RH. Striatin assembles a membrane signaling complex necessary for rapid, nongenomic activation of endothelial NO synthase by estrogen receptor alpha. Proc Natl Acad Sci USA. 2004;101:17126–17131. doi: 10.1073/pnas.0407492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.