Abstract
Upon activation, naïve CD4+ T cells differentiate into effector T cells with specific effector functions and cytokine profiles. The Th1/Th2 paradigm has recently been reevaluated to include a third population of T helper cells, producing IL-17 and designated Th17. The differentiation of Th17 cells requires the coordinate and specific action of the proinflammatory cytokine IL-6 and the immunosuppressive cytokine TGF-β. In addition, the IL-12 family member IL-23 is involved in the maintenance of these cells. Analogous to other T helper cell subsets, Th17 commitment is initiated by sequential involvement of STAT molecules, i. e. STAT3 downstream of cytokine receptors, and specific transcription factors, i. e. ROR-γt. Recent data also support the existence of a complex network of cytokines regulating Th17 cells. Clearly, the specific effector functions of Th17 cells expand beyond previously described effects of Th1 and Th2 immunity, with specific roles in host defense against certain pathogens and in organ specific autoimmunity. The potential dynamics of Th17 cell populations and their interplay with other inflammatory cells in the induction of tissue inflammation in host defense and organ-specific autoimmunity are discussed.
Keywords: Th17, regulatory T cells, autoimmunity, inflammation
1. Introduction
CD4+ T cells play an important role in the initiation of immune responses by providing help to other cells. Upon antigenic stimulation, naive CD4+ T cells proliferate and differentiate into different effector subsets characterized by the production of specific cytokines and effector functions. Based on their pioneer work, Mosmann and Coffman proposed 20 years ago that T helper cells could be divided into two distinct subsets, T helper type 1 (Th1) and Th2, characterized by distinct cytokine profiles and effector functions [1]. Th1 cells produce large quantities of interferon (IFN)-γ. These cells elicit delayed type hypersensitivity (DTH) responses, activate macrophages and are highly effective in clearing intracellular pathogens. Th2 cells, on the other hand, produce interleukin 4 (IL-4), IL-5, IL-13 and IL-25. Th2 cells are especially important for IgE production, eosinophilic inflammation, and the clearance of helminth parasitic infections [2] (Table 1). In light of recent data, the Th1/Th2 dichotomy is now being revisited. The discovery of the IL-17 family of cytokines and the analysis of IL-23-mediated effector functions on T cells have suggested the existence of an additional subset of CD4+ T cells that produce IL-17 and were designated Th17 [3,4]. The independence of the Th17 subset with regard to Th1 and Th2 cells was then firmly established by the identification of the cytokines, i. e. the combination of IL-6 and TGF-β [5–7], and the transcription factors, i. e. ROR-γt [8] and STAT3 [9,10] required for the differentiation of Th17 cells. Th17 effector functions are distinct from Th1 and Th2-mediated immunity (Table 1). Th17 cells appear to be critical to enhance host protection against extracellular bacteria and fungi, which are not efficiently cleared by Th1 and Th2 responses. In addition, these cells have emerged as potent inducers of autoimmune diseases.
Table 1.
Effector functions of T helper cell subsets
| Th cell subset | Function |
|---|---|
| Th1 | Host defense against intracellular pathogens |
| Organ-specific autoimmunity | |
| Th2 | Host defense against extracellular pathogens and parasites |
| Allergy | |
| Asthma | |
| Th17 | Host defense against certain pathogens (Klebsiella, Citrobacter, Borrrelia, Fungi) |
| Tissue inflammation | |
| Organ-specific autoimmunity | |
2. The IL-17 family of cytokines
IL-17 is the founding member of the IL-17 family of cytokines, which contains IL-17A (also called IL-17), IL-17B, IL-17C, IL-17D, IL-17E (also called IL-25) and IL-17F [11,12]. While other members of the IL-17 family map to different chromosomes, Il17a and Il17f on mouse chromosome 1 are syntenic to the human genes on chromosome 6. Both IL-17A and IL-17F are produced by a variety of cell types including subsets of CD4+ T cells, CD8+ T cells, γδ-T cells, NK cells and neutrophils. IL-17 production has also been associated with memory CD4+ T cells [13] and recently with the new subset of effector T cells [4], Th17 cells. IL-17A and IL-17F have pro-inflammatory properties [14] and act on a broad range of cell types to induce the expression of cytokines (IL-6, IL-8, GM-CSF, G-CSF), chemokines (CXCL1, CXCL10), and metalloproteinases. IL-17A and IL-17F are also key cytokines for the recruitment, activation and migration of neutrophils [11,15]. On the other hand, IL-17E (or IL-25) is mainly produced by Th2 cells [16]. IL-25 induces the expression of Th2 type cytokines and chemokines such as RANTES and Eotaxin-1 and plays a role in Th2-type allergic responses [16].
Members of the IL-17 family of cytokines signal through receptors of the IL-17R family. This family is composed of IL-17RA, IL-17RB, IL-17RC, IL-17RD and IL-17RE. The ubiquitously expressed IL-17RA binds both IL-17A and IL-17F although IL-17A is bound with higher affinity [15]. IL-17RA appears to exit as preformed multimer prior to ligand binding. Upon binding of IL-17A or IL-17F, the IL-17RA complex undergoes a conformational change, which induces the dissociation of its intracellular domain. To date, it is not clear whether some members of the IL-17R family can associate with other chains from the same or a different family. Similarly, although IL-17 seems to activate NF-κB and MAPK pathways [17,18] and interact with the membrane proximal adaptor Act1 [19,20], the signaling cascade downstream of the IL-17R complexes is not well known.
3. IL-23: a role in adaptive and innate immune responses
The identification of a new member of the IL-12 family of cytokines was seminal in the discovery of Th17 cells. The IL-12 family of cytokines has expanded and in addition to the prototypic IL-12, now includes IL-23 and IL-27. IL-12 is a heterodimeric cytokine composed of p40 and p35 subunits and signals through a receptor complex made of IL-12Rβ1 and IL-12Rβ2 subunits [21]. IL-23 is constituted by the specific p19 subunit and shares the p40 subunit with IL-12. Both IL-23 and IL-12 are produced by activated APC such as DC and macrophages. Accumulating evidence suggests that IL-12 and IL-23 may be produced in response to different stimuli. While microbial products preferentially stimulate IL-12, activation of DCs with PGE2, ATP or anti-CD40 antibody leads to enhanced IL-23 expression [22,23]. Since IL-12 and IL-23 might compete for the p40 subunit and are induced by distinct stimuli, it is conceivable that these two cytokines are not produced simultaneously by the same APCs. Similar to DC1 and DC2, there might be a distinct type of DC specialized in the production of IL-23.
IL-23 sends signals through a heterodimeric receptor complex consisting of IL-12Rβ1 and IL-23R [21]. Based on the similarities of the cytokines and their receptors, IL-23 and IL-12 were predicted to have similar functions. However, in contrast to IL-12, which enhances Th1 responses, IL-23 is important to maintain Th17 cells. Early experiments performed by Aggarval et al. showed that addition of IL-23 in T cell cultures generated a population of cells which produced IL-17 [13]. Later, additional in vitro experiments including the analysis of p19 deficient mice, which are resistant to the development of experimental autoimmune encephalomyelitis (EAE), showed a defect in the newly described Th17 subset [3,4]. Based on these observations, Cua and colleagues suggested that IL-23 was an important cytokine for the differentiation of Th17 cells. Clearly, IL-23 promotes proliferation of IL-17-producing cells in the pool of activated memory cells [4,13] and is important to maintain the Th17 phenotype [5]. This is in contrast to IL-2 which is an important growth factor for CD4+ Th1, Th2 and CD8+ T cells, but strongly inhibits the differentiation of Th17 cells [5,10].
Several pieces of evidence support the fact that IL-23 promotes inflammation and autoimmune responses through its action on the adaptive, but also the innate immune system. IL-23 transgenic mice suffer from systemic inflammation involving multiple organs, including the skin. Proinflammatory cytokine concentrations are increased in the serum of these animals [24,25]. IL-23 is highly expressed in the skin of psoriasis patients [26]. Furthermore, intradermal injection of IL-23 in mice leads to the development of psoriasis-like lesions, which is dependant on TNF-α but not IL-17 or IL-12 production [27]. Although not tested, it is also possible that this pathology results from increased IL-22 production since this cytokine was recently shown to be preferentially produced by Th17 cells [28,29]. The role of both IL-12 and IL-23 in the regulation of the innate immune system has been investigated, among others, by Powrie and colleagues [30]. They show that administration of an agonistic anti-CD40 antibody leads to a systemic and local inflammation characterized by wasting disease, splenomegaly, increases in serum proinflammatory cytokines and colitis. The analysis of p40, p35 and p19 in mice on RAG deficient background shows that the systemic inflammatory response and the elevated concentrations of proinflammatory cytokines in the serum are driven by IL-12 while the local intestinal inflammation and production of IL-17 in the intestine is controlled by IL-23. These experiments carried out in RAG deficient mice highlight the effect of IL-23 on non-T cell populations and show that IL-23 can also enhance IL-17 production from cells of the innate immune system. Although the specific cellular source of IL-17 in this system and the mechanisms by which IL-23 mediates intestinal inflammation is not fully elucidated, this report stresses the importance of the IL-17/IL-23 network in immune pathology via T cell-independent mechanisms.
4. The cytokines involved in the differentiation of Th17 cells
Despite a clear role of IL-23 in Th17-mediated immunopathology, IL-23 is not required for initial Th17 differentiation from naïve T cells [5,6]. Three independent studies, including our own, independently observed that a combination of the pro-inflammatory cytokine IL-6 and TGF-β could induce the differentiation of Th17 cells from naïve T cells in vitro [5–7]. The importance of TGF-β in this process has been shown in vivo as well. We have demonstrated that mice, which express TGF-β under the CD2 promoter produced TGF-β upon ex vivo stimulation, but developed Th17 cells in vivo under inflammatory conditions and elevated concentrations of IL-6 [6]. The increased number of Th17 cells in these animals resulted in the exacerbation of EAE. Consequently, CD4-DNTGFβRII mice, which express a dominant negative mutant of the TGF-β receptor are deficient in Th17 cells and are resistant to the development of EAE [31]. The analysis of IL-6 deficient animals also points to an important role of IL-6 in the differentiation of Th17 cells since these animals are resistant to the development of EAE and do not develop a competent Th17 response [6]. Therefore, under inflammatory conditions and constant surveillance by regulatory mechanisms, IL-6 and TGF-β are key cytokines for the differentiation of Th17 cells in vivo.
TGF-β is a regulatory cytokine with pleiotropic functions in T cell development, homeostasis and tolerance [32]. TGF-β is produced by multiple lineages of leukocytes and stromal cells. However, the essential cellular sources of TGF-β in vivo are unknown. In a recent study, Flavell and coworkers generated mice in which the TGF-β1 gene can be selectively inactivated in T cells. These mice developed a lethal inflammatory disorder characterized by enhanced Th1 and Th2 responses indicating that T-reg cell-derived TGF-β is required to control these responses. Interestingly, ablation of TGF-β production from T cells in these mice resulted in a defect in the generation of Th17 cells and the development of EAE [33]. Although, the present study does not elucidate which or which combination of T cell subsets (T-reg, Th3 cells [34], non T-reg) represent the major source of TGF-β, it clearly indicates that T cell-derived TGF-β plays a critical role in the differentiation of Th17 cells in vivo. Identifying the T cell-subset that is the essential source of TGF-β will certainly be critical to understand how Th17 cells are generated and regulated in vivo.
5. IL-27, a negative regulator of Th17 cells
Th1 and Th2 cells as well as the cytokines that they produce antagonize each other. Similarly, the development of Th17 cells is also negatively regulated by IL-4 and IFN-γ. IL-4 and IFN-γ can inhibit IL-23-driven expansion of Th17 populations [35,36]. Whether IL-17A and IL-17F can modulate the development and expansion of Th1 and Th2 populations has not been fully evaluated. However, there might be a reciprocal inhibition of other subsets by Th17 cells since IL-17 and IL-23 were shown to inhibit Th1 development [37].
In addition to the role of the “usual suspects”, IL-4 and IFN-γ, in the inhibition of Th17 development, recent data indicate that, the IL-12 family member IL-27 is a negative regulator of Th17 cell development. IL-27 is a heterodimeric cytokine composed of Epstein-Barr virus–induced gene 3 (EBI3) and p28 chains. Similar to IL-12 and IL-23, IL-27 is produced by dendritic cells and macrophages. IL-27 signals through a receptor complex composed of the IL-27 receptor chain (IL-27R; also called WSX-1 or TCCR) and the gp130 chain shared with the IL-6 receptor [38,39]. The absence of IL-27-mediated signaling enhances the generation of Th17 cells, increases the number of IL-17–expressing T cells in tissue infiltrates, and exacerbates neuroinflammation [40,41]. Furthermore, IL-27-mediated inhibition of Th17 cells is independent of IFN-γR- and IL-6R-signaling, and T-bet, but requires intact STAT1 signaling [40,41]. The factors ‘downstream’ of STAT1 responsible for IL-27–mediated suppression of Th17 differentiation remain to be identified. Further understanding of the mechanisms involved in the inhibition of Th17 cells by IL-27 and other cytokines will provide valuable information on ways to regulate these cells.
6. Differentiation and regulation of Th17 cells by transcription factors
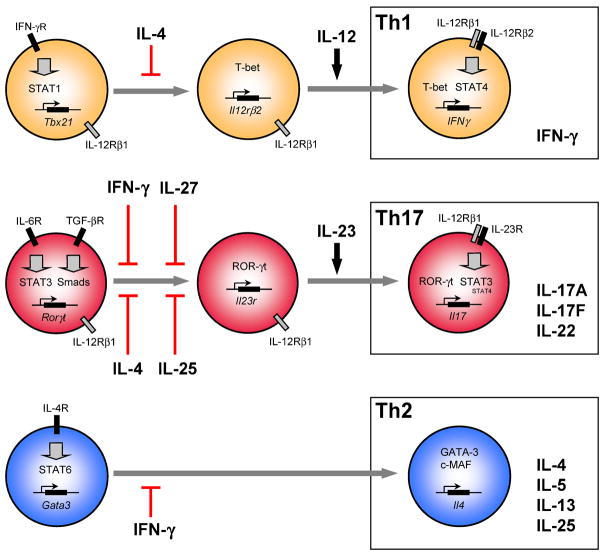
It is now clear that distinct signaling pathways govern the differentiation of Th1, Th2 and Th17 cells (Figure 1). IFN-γ and IL-12 signals are important for Th1 cell differentiation. The Th1 cytokine IFN-γ signals through STAT1 which, in turn activates the T-box transcription factor T-bet, a key inducer of IFN-γ and Th1 cell differentiation [42–44]. The transcription of T-bet then leads to the expression of the IL-12Rβ2 subunit, which associates with the IL-212Rβ1 chain to form the IL-12R complex. IL-12-mediated activation of STAT4 through the IL-12R is essential for the stabilization of IFN-γ production and the development of terminally differentiated Th1 cells [42,43] (Figure 1). In differentiating Th2 cells, IL-4 induces STAT6 activation, which promotes the expression of GATA3. GATA3 and c-maf then promote IL-4 production and the development of Th2 cells [42,43].
Figure 1. Differentiation and effector cytokines of T helper cell subsets.
Th1, Th17, and Th2 cells constitute different T helper cell subsets with different cytokine signatures. The induction of the various T helper cell subsets requires different cytokine stimuli to induce distinct transcriptional programs. Other cytokines are necessary to stabilize the transcriptional phenotype (For details see text). Cross-inhibition of Th17 cell development by Th1 and Th2 associated cytokines has been shown in vitro and in vivo.
The involvement of different JAK/STAT complexes in the differentiation of Th17 cells has been investigated. CD4+ T cells isolated from mice lacking the Th1- or Th2-specific transcription factors STAT1, STAT4, T-bet, or STAT6 retain the ability to differentiate into Th17 cells in vitro when activated in the presence of IL-23 [36,45]. Moreover, Stat1−/−, Stat4−/−, T-bet−/−, and Stat6−/− mice develop Th17 cells in vivo following immunization [35,46], indicating that these transcription factors are not critical for the differentiation of Th17 cells. These observations are not entirely surprising since engagement of IL-6 with its receptor leads to the recruitment of STAT3 and to a lesser extend STAT1 and engagement of TGF-β with its receptor leads to the phosphorylation of Smad molecules (Figure 1). IL-23 signaling in T cells seems to require both STAT3 and to a lesser extend STAT4. While STAT4 is dispensable for the initial generation of Th17 cells [36,47], STAT4 appears important for IL-23–driven expansion of Th17 cells [48]. This may further explain why STAT4 deficient mice are resistant to the development of EAE [49]. In agreement with its importance in IL-6 signaling, STAT3 was recently shown to be critical for IL-6 (plus TGF-β)-driven differentiation and IL-23-mediated expansion of Th17 cells [9,10]. On the other hand, little is currently known about the importance for Th17 differentiation of the factors downstream of the TGF-β receptor.
In addition to proximal factors which associate with cytokine receptors, the differentiation of Th subsets is governed by key or master transcription factors such as T-bet for Th1 cells and GATA3 for Th2 cells. Th17 cells do not express the transcription factors T-bet, Hlx, or GATA3 [5,36] indicating that they represent a distinct subset of Th cells. However, it remains to be determined if Th1- and Th2-specific transcription factors play an inhibitory role in the expansion and/or maintenance of Th17 cells. Experiments using T cells from c-maf transgenic mice, suggested that c-maf, a Th2-specific transcription factor, might inhibit the generation of both IL-17– and IFN-γ–producing CD4+ T cells [35]. In addition, T-bet–deficient lymph node cells produce IL-17 upon stimulation with anti-CD3 antibody, but exogenous IL-23 does not further boost IL-17 production. These observations suggest that T-bet may regulate IL-23 responsiveness during early Th17 cell development [35]. Similarly, T-bet–deficient mice have increased numbers of Th17 cells, raising the possibility that T-bet might antagonize Th17 differentiation [50]. However, to date it is not clear whether the effects observed in animals deficient in Th1 and Th2 specific transcription factors result from the lack of inhibitory cytokines (i.e. IFN-γ and IL-4) or the direct impact of transcription factors on Th17 cells.
Although the precise mechanism by which IL-6 and TGF-β cooperate to induce Th17 cells has not been determined, a combination of these two cytokines leads to subsequent upregulation of the transcription factor retinoid acid-related orphan nuclear hormone receptor-γt (ROR-γt). ROR-γt is a transcription factor expressed by fetal lymphocyte tissue inducer cells (LTi), which participate in the formation of lymph nodes and payers patches, intestinal LTi-like cells and immature thymocytes [51]. Littman and colleagues recently described that ROR-γt was also expressed in developing Th17 cells and more specifically in IL-17–producing T cells present in the intestinal lamina propria [8]. This latter population is absent in ROR-γt-deficient animals. Transduction of naïve T cells with a ROR-γt-encoding retrovirus induces IL-17 production. Together, these data demonstrate the importance of ROR-γt in the differentiation of Th17 cells and place ROR-γt in the rank of master regulator of Th differentiation [8]. The mechanisms by which ROR-γt mediates Th17 differentiation remain to be determined. ROR-γt could directly transactivate Il-17A and IL-17F genes. Alternatively, ROR-γt may act as a chromatin-remodeling factor that opens the Il17 locus and allows other factors to bind directly to Il17 promoters. ROR-γt might also control Th17 development by regulating IL-23 responsiveness and by controlling the expression of IL-23R. In addition, since TGF-β induces the transcription factor Foxp3 and that Foxp3 is a potent inhibitor of cytokine expression [52,53], ROR-γt might promote TGF-β plus IL-6-induced Th17 differentiation via the inhibition of Foxp3 expression or activity. Identification of the factors that interact with ROR-γt as well as the elucidation of their mode of interaction, will enhance our understanding of Th17 differentiation and regulation in vivo.
7. Role of IL-17 and Th17 cells in host protection
Since it was initially cloned almost 15 years ago, IL-17 has been found to induce several chemokines including MCP-1 and a variety of CXC chemokines, but also IL-1β, TNF, IL-6, and colony-stimulating factors from a series of different cells types [14]. This pattern of target molecules was suggestive of an important role of IL-17 in immune cell recruitment and inflammatory effector functions and thus prompted the investigation of IL-17 in tissue inflammation in response to different pathogens. The analysis of IL-17 receptor and IL23p19 deficient mice proved that the IL-17/IL-17RA system was essential for host defense against infection with Klebsiella that causes atypical forms of pneumonia and Citrobacter that is an intestinal pathogen [7,54,55]. In addition, the production of IL-17 appears to be required for abscess formation and to generate an appropriate host response during infection with gram-negative bacteria like Bacteroides species [56]. Moreover, the production of IL-17 is induced by infection with Borrelia burgdorferi which causes Lyme disease [57]. There is a strong link between the infection with this pathogen, the induction of IL-17 and the development of immune-mediated arthritis in an experimental model of Lyme disease [58]. Th17 cells also play an important role in host protection against systemic fungal infections. Here, polysaccharide constituents of fungal cell walls like β-glucans trigger cells of the innate immune system to prime Th17 cells. For example, curdlan, a ligand for dectin-1 on dendritic cells, has been demonstrated to induce a MyD88-independent signaling pathway in dendritic cells leading to preferential production of IL-23 and enhanced IL-17 production by co-cultured T cells [59]. IL-17 receptor deficient mice are very susceptible to systemic infection with Candida albicans and eventually succumb to the pathogen since they cannot clear it [60]. This suggests that IL-17 is an essential effector molecule in host defense against Candida infection. In the absence of IL-17 signaling, the influx and activation of neutrophils in the infected organs is severely delayed which might explain why IL-17 receptor deficient mice fail to control the infection [60]. Conversely, transgenic over-expression of IL-17 in bronchial epithelial cells induces the expression of CCL7, CCL11, CCL20, CCL22, and CX3CL1 and results in the accumulation of mononuclear cells within the lung parenchyma [35]. Apart from orchestrating the infiltration of mononuclear cells and evoking a strong neutrophil response by induction of IL-8 and G-CSF, IL-17 may also control the migration of various other cell types. In mycobacterial infection, an early pathogen-specific Th17 response triggers the induction of chemokines like CXCL9, CXCL10, and CXCL11 that attract IFN-γ-producing CD4+ Th1 cells into the lung parenchyma which eventually control the infection [61]. Thus, in a broader perspective, Th17 cells may be responsible for shaping the type of inflammatory cells in tissue infiltrates.
8. Role of IL-17 and Th17 cells in autoimmunity
The first report on IL-17 producing CD4+ T cells came from a study in which the addition of B. burgdorferi lysate to TcR transgenic T cells induced the production of IL-17 [57]. However since then, the role of IL-17 and Th17 cells in autoimmune tissue inflammation has been recognized as well. IL-17 is directly involved in cartilage and bone destruction as observed in an experimental model for human rheumatoid arthritis [62]. Consistent with these observations, IL-17–deficient mice develop reduced collagen-induced arthritis [63] and treatment with an IL-17R antagonist is sufficient to significantly attenuate adjuvant-induced arthritis in rats [64]. Similarly, IL-17–deficient animals develop experimental autoimmune encephalomyelitis (EAE) with delayed onset and diminished severity [65]. In addition, administration of an IL-17-blocking antibody in mice immunized with a myelin antigen prevents chemokine expression in the brain and the subsequent development of EAE [4,66].
Since the expression of IL-17 appeared to be increased in human autoimmune diseases like Multiple Sclerosis [67,68], rheumatoid arthritis [69], and psoriasis [70] as well as in animal models of autoimmunity, much attention has been focused on defining the role of Th17 cells in the pathogenic process of tissue inflammation [71]. In the last 3 years the importance of Th17 cells in the pathogenesis of organ-specific autoimmune inflammation has been demonstrated in different animal models. This was paradigm changing, since previously, Th1 cells were considered to be almost exclusively responsible for driving autoimmune tissue damage [72]. This concept was challenged when it became clear that IFN-γ and IFN-γ-receptor deficient mice as well as mice that lack other molecules involved in the differentiation and stabilization of the Th1 phenotype like IL-12p35, IL-12 receptor-β2, and IL-18 were not protected from EAE, but developed more severe disease [73–77]. Furthermore, it was shown that IL-23 and not IL-12 was crucial for mounting an autopathogenic T cell response in the CNS [3]. Finally, Th17 cells were more potent than Th1 cells in transferring EAE to naïve wild type host animals [4]. This suggested that Th17 cells might be responsible for the induction of tissue specific autoimmunity. In addition, in chronic inflammatory bowel disease, Th17 cells seem to be essential in inducing the break down of the intestinal epithelial barrier [78]. Once induced, Th17 cells inhibit the turn-over of colonic epithelial cells and drive immunopathology [79,80]. Collectively, these data corroborate the importance of Th17 cells for the induction of autoimmune tissue inflammation.
In the current understanding of the pathogenic process in EAE, antigen specific priming of encephalitogenic T cells as well as their commitment to a certain T helper cell lineage happens in secondary lymphoid tissue outside the central nervous system (CNS). Myelin-specific T cells then traffic to the CNS where they are re-activated [81,82]. The efficiency of the activation in situ as well as the acquisition of further effector functions is critical for the maintenance of specific effector populations within the CNS. IL-23 and possibly other cytokines seem to be critical for this process. Indeed, when IL-23 is not available in order to maintain and expand a population of already primed Th17 cells, EAE is markedly attenuated [3]. Certain adjuvants like zymosan, a constituent of fungal cell walls, are potent inducers of Th17 cells, but do not result in a sufficiently robust IL-23 production by dendritic cells. Using this adjuvant, it has been illustrated that a temporary Th17 response in the secondary lymphoid tissue only resulted in limited tissue inflammation [31]. Furthermore, it has been shown that both resident microglial cells and infiltrating macrophages were producers of IL-23 [3]. Given that the lack of p40 in the CNS-APC compartment results in significantly reduced encephalitogenicity of T cells infiltrating the CNS [83] whereas IL-12p35 deficient mice are extremely susceptible to EAE [75,84], it appears that IL-23 expression in the target organ is essential for inducing autoimmune tissue destruction. Collectively, these findings suggest that IL-23p19 is not only required to shape a stable Th17 population in the secondary lymphoid tissue, but also to maintain an encephalitogenic Th17 population in the CNS. Besides IL-23, other cytokines may have a role in stabilizing the phenotype of Th17 cells. For example, in IL-1RI deficient mice, the Th17 response is severely compromised and the animals are resistant to EAE. Thus, IL-1 may contribute to the recruitment and maintenance of Th17 cells [85]. Although, IL-23 can expand Th17 cells independently of IL-1, it has been shown that IL-23 and TNF may also cooperate to induce IL-1 and drive the expansion of Th17 cells [85]. Moreover, in animal models of rheumatoid arthritis, IL-17 induces IL-1 in the inflamed synovial tissue [86]. Hence, IL-17 may be part of a positive feedback loop driven by IL-1.
While IL-23 and possibly IL-1 potentiate Th17 responses, other cytokines like IL-25 (IL-17E) may inhibit Th17 responses. Il25−/− mice are highly susceptible to EAE due to an enhanced Th17 response. Accordingly, treatment with recombinant IL-25 or IL-25 delivered by a viral vector system is sufficient to suppress EAE in wild type mice [87]. Although IL-25 is produced by activated Th2 cells, resident cells of the innate immune system like microglial cells are believed to be the major source of IL-25 in CNS autoimmunity. IL-25 may down-regulate the Th17 response in an indirect manner through the induction of IL-13 which inhibitis the production of IL-23, IL-1 and IL-6 in antigen presenting cells [87]. As previously mentioned, IL-27 is another cytokine with inhibitory effect on the generation of Th17 cells. IL-27 antagonizes the initial lineage commitment of Th17 cells and conversely, IL-27 receptor deficient mice exhibit increased immunopathology in EAE and chronic Toxoplasmosis due to an enhanced Th17 response [40,41]. Together, these studies illustrate that the Th17 response is under tight control by a cytokine network whose complexity we are only beginning to understand.
Although Th17 cells are potent inducers of autoimmunity, it is clear that Th1 cells are also involved in the development of autoimmune responses. However, to date, it is not established what are the specific roles and the kinetics of action of these two T helper cell subsets during the development of an autoimmune response. Several points needs to be considered and could explain why Th17 cells are more pathogenic than Th1 cells (see also Figure 2): 1) Th17 might migrate in the target organ before Th1 cells, 2) Th17 cells might be necessary to recruit effector T cells to the CNS and 3) Th1 cells might need to cooperate with Th17 cells in order to induce tissue inflammation and destruction. In the natural course of MOG35-55-induced EAE, the number of Th17 producing CD4+ T cells in the CNS peaks earlier than that of Th1 cells [88]. Therefore, it is conceivable that Th17 cells are generated and expand faster in response to antigenic challenge and constitute the first wave of effector T cells migrating to the CNS. Consequently, Th17 cells might play a role in the recruitment of further waves of effector T cells, especially Th1 cells (Figure 2). In support of this hypothesis, IL-17 is an inducer of MCP-1 [35] that plays a prominent role in the recruitment of mononuclear cells to the CNS [89]. Furthermore, by inducing IP-10, Th17 cells might drive the migration of Th1 cells that express the IP-10 receptor CXCR3 [90] into the inflamed tissue. The concept of a specific temporal sequence of various T helper cell subsets infiltrating the target tissue of autoimmune inflammation assumes that the lineage commitment of T helper cells is stable in the target organ. However, whereas in vitro-differentiation of naive T cells leads to stable lineage commitment, a considerable population of cells secreting both IL-17 and IFN-γ can consistently be observed in vivo in the target tissue of autoimmune inflammation. This suggests that there may be transitional stages of lineage commitment in vivo. However, the significance of these cells for the disease process is very unclear at present.
Figure 2. Development of tissue inflammation driven by Th17 cells.
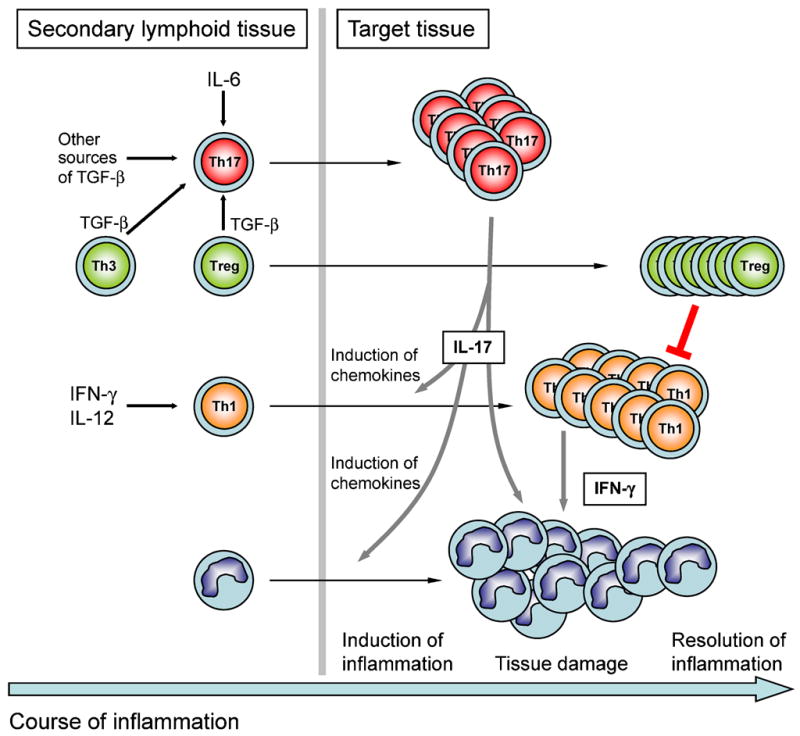
Antigen specific T cells are primed in secondary lymphoid tissue and driven into the Th17 developmental pathway in the presence of IL-6 and TGF-β. Sources of TGF-β are other T cells like Foxp3-expressing regulatory T cells (Treg) or Th3 cells. Similarly, antigen specific T cells are committed to the Th1 subset in the presence of IFN-γ and IL-12. Activated Th17 cells are among the first to infiltrate the target organ. By induction of chemokines, secondary waves of inflammatory cells like mononuclear cells and Th1 cells are attracted into the inflamed tissue. Both IL-17 produced by Th17 cells and IFN-γ produced by Th1 cells may act on myeloid cells to induce their effector functions that eventually lead to tissue damage. Whereas Th17 cells are prone to apoptosis, Th1 cells might finally be suppressed by T-reg cells that accumulate in the target organ and thus induce the resolution of inflammation.
To date, it is also uncertain whether Th1 and Th17 cells are equally susceptible to T-reg-mediated suppression in vivo. The fact that one of the potent immunosuppressive cytokines, TGF-β, which is produced by T-reg, is also required for the differentiation of Th17 cells needs to be taken in consideration. Under inflammatory conditions, notably in the presence of IL-6, T-reg, through their production of TGF-β, might therefore fuel the generation of Th17 cells rather than control it. Moreover, T-reg express the high affinity IL-2 receptor CD25 and may be major consumers of IL-2. Since, IL-2 has been shown to suppress the generation of Th17 cells [10], the presence of T-reg may also facilitate the differentiation of Th17 by restraining the availability of IL-2.
Collectively, Th17 cells are highly potent inflammatory cells that initiate tissue inflammation and induce the infiltration of other inflammatory cells into the target organ. Hence, at least in relevant mouse models of organ specific autoimmunity, Th17 cells are indispensable for the induction of massive immunopathology. Paradoxically and unlike Th1 cells, Th17 may not be subject to T-reg mediated suppression. However, it appears that in order for Th17 populations to be maintained in the target tissue a complex network of cytokines has to be operative which provides the necessary means to control Th17 cells and may be the basis for the fact that this T cell subset is relatively short-lived and prone to rapid attrition.
9. Concluding remarks
It is now established that Th17 cells constitute an independent T helper cell subset with major functions in the induction of tissue inflammation. IL-6 plus TGF-β have been identified as differentiation factors for the commitment of naive T cells to the Th17 lineage [5–7]. The identification of the cellular sources of these cytokines, as well as the stimuli leading to their production will be critical to determine how Th17 cells are generated in vivo. It is clear that TGF-β is readily available in mucosa associated lymphoid tissue. In secondary lymphoid organs, TGF-β may be provided by T cells themselves as suggested by recent findings [33]. By itself, TGF-β is an ‘immunosuppressive’ cytokine creating an environment that is favorable for the generation of induced regulatory T cells. However, following infection, IL-6 is produced by cells of the innate immune system and specifically triggered upon activation of TLRs. Thus, the presence of an inflammatory environment and specifically IL-6, in the context of continuous production of TGF-β appears to dictate the generation of Th17 cells. We propose that there is a close relationship between T-reg and Th17 cells since TGF-β, which is required for the generation of induced T-reg cells, is also necessary for the differentiation of Th17 cells [6], and the transcription factors required for the development of these two T cell subsets might antagonize each other. The close connection between T-reg and Th17 cells could represent an important mechanism by which the immune system rapidly generates Th17 cells specialized in the clearance of specific pathogens. By the production of IL-22 and IL-17, and hence the induction of chemokines and the attraction of highly specialized effector cells like neutrophils and macrophages into parenchymal tissue, Th17 cells appear to be efficient in orchestrating an immune response against tissue infiltrating pathogens. But on the other hand, this also correlates with a high potency to initiate substantial immunopathology and autoimmune diseases. There, the association between Th17 and T-reg cells might initially be detrimental since TGF-β produced by T-reg cells might amplify the pathogenic Th17 population. Eventually, Th17 responses may ware off either because Th17 cells may be short-lived or further regulated by the environment.
Clearly, although IL-23 does not induce the differentiation of Th17, it is an important cytokine for the maintenance and possibly the final commitment of these cells. However, the mechanisms involved in this process have not been fully elucidated. The discovery of new cytokines and new functions for “old” cytokines introduced new players, which participate in the establishment and regulation of Th17 responses adding new layers of complexity to the cytokine network that controls Th17 responses. Specifically, IL-1 and possibly other cytokines, perhaps by an autocrine feedback loop, amplify and stabilize Th17 cells. On the other hand, IL-27 and cytokines produced by the other two subsets of Th cells, such as IFN-γ and IL-25 antagonize Th17 responses and could explain why later phases of chronic inflammation are then dominated by IFN-γ producing Th1 cells.
Taken together, the identification of Th17 cells and the analysis of their functions helped to resolve some inconsistencies of the Th1/Th2 paradigm. However, the introduction of this third arm of adaptive immunity also raises new questions as to how Th17 cells and Th1 cells cooperate in the pathogenesis of organ-specific autoimmunity. Thus, in order to approach chronic autoimmune diseases therapeutically in a phase-specific manner, it is not only important to understand the factors that drive the various T helper subsets, but also their temporal sequence and potential interaction in the induction of immunopathology.
Acknowledgments
This work was supported by grants from the National Multiple Sclerosis Society, the National Institutes of Health, the Juvenile Diabetes Research Foundation Center for Immunological Tolerance at Harvard, and the Deutsche Forschungsgemeinschaft.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 3.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–8. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 4.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 7.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 8.Ivanov, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The Orphan Nuclear Receptor RORgammat Directs the Differentiation Program of Proinflammatory IL-17(+) T Helper Cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 9.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–63. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 10.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O’Shea JJ. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–81. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol. 2002;71:1–8. [PubMed] [Google Scholar]
- 12.Kawaguchi M, Adachi M, Oda N, Kokubu F, Huang SK. IL-17 cytokine family. J Allergy Clin Immunol. 2004;114:1265–73. doi: 10.1016/j.jaci.2004.10.019. quiz 1274. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–4. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 14.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–76. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–74. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 16.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–95. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 17.Shalom-Barak T, Quach J, Lotz M. Interleukin-17-induced gene expression in articular chondrocytes is associated with activation of mitogen-activated protein kinases and NF-kappaB. J Biol Chem. 1998;273:27467–73. doi: 10.1074/jbc.273.42.27467. [DOI] [PubMed] [Google Scholar]
- 18.Schwandner R, Yamaguchi K, Cao Z. Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. J Exp Med. 2000;191:1233–40. doi: 10.1084/jem.191.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang SH, Park H, Dong C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J Biol Chem. 2006;281:35603–7. doi: 10.1074/jbc.C600256200. [DOI] [PubMed] [Google Scholar]
- 20.Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, Kordula T, Zhang QW, Vallance B, Swaidani S, Aronica M, Tuohy VK, Hamilton T, Li X. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8:247–56. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 21.Kastelein RA, Hunter CA, Cua DJ. Discovery and Biology of IL-23 and IL-27: Related but Functionally Distinct Regulators of Inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 22.Sheibanie AF, Tadmori I, Jing H, Vassiliou E, Ganea D. Prostaglandin E2 induces IL-23 production in bone marrow-derived dendritic cells. Faseb J. 2004;18:1318–20. doi: 10.1096/fj.03-1367fje. [DOI] [PubMed] [Google Scholar]
- 23.Schnurr M, Toy T, Shin A, Wagner M, Cebon J, Maraskovsky E. Extracellular nucleotide signaling by P2 receptors inhibits IL-12 and enhances IL-23 expression in human dendritic cells: a novel role for the cAMP pathway. Blood. 2005;105:1582–9. doi: 10.1182/blood-2004-05-1718. [DOI] [PubMed] [Google Scholar]
- 24.Kopp T, Lenz P, Bello-Fernandez C, Kastelein RA, Kupper TS, Stingl G. IL-23 production by cosecretion of endogenous p19 and transgenic p40 in keratin 14/p40 transgenic mice: evidence for enhanced cutaneous immunity. J Immunol. 2003;170:5438–44. doi: 10.4049/jimmunol.170.11.5438. [DOI] [PubMed] [Google Scholar]
- 25.Wiekowski MT, Leach MW, Evans EW, Sullivan L, Chen SC, Vassileva G, Bazan JF, Gorman DM, Kastelein RA, Narula S, Lira SA. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. J Immunol. 2001;166:7563–70. doi: 10.4049/jimmunol.166.12.7563. [DOI] [PubMed] [Google Scholar]
- 26.Lee E, Trepicchio WL, Oestreicher JL, Pittman D, Wang F, Chamian F, Dhodapkar M, Krueger JG. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125–30. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, Abbondanzo S, Lucian L, Geissler R, Brodie S, Kimball AB, Gorman DM, Smith K, de Waal Malefyt R, Kastelein RA, McClanahan TK, Bowman EP. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203:2577–87. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2006 doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 30.Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, Stepankova R, Robinson N, Buonocore S, Tlaskalova-Hogenova H, Cua DJ, Powrie F. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309–18. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 31.Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006;7:1151–6. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- 32.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 33.Li MO, Wan YY, Flavell RA. T Cell-Produced Transforming Growth Factor-beta1 Controls T Cell Tolerance and Regulates Th1- and Th17-Cell Differentiation. Immunity. 2007 doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 35.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 37.Nakae S, Iwakura Y, Suto H, Galli SJ. Phenotypic differences between Th1 and Th17 cells and negative regulation of Th1 cell differentiation by IL-17. J Leukoc Biol. 2007;81:1258–68. doi: 10.1189/jlb.1006610. [DOI] [PubMed] [Google Scholar]
- 38.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–90. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 39.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–31. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 40.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–36. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 41.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O’Shea JJ, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–45. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 42.Ho IC, Glimcher LH. Transcription: tantalizing times for T cells. Cell. 2002;109 (Suppl):S109–20. doi: 10.1016/s0092-8674(02)00705-5. [DOI] [PubMed] [Google Scholar]
- 43.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–44. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 44.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 45.Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein W, Churakovsa T, Low J, Presta L, Hunter CA, Kastelein RA, Cua DJ. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116:1317–26. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rangachari M, Mauermann N, Marty RR, Dirnhofer S, Kurrer MO, Komnenovic V, Penninger JM, Eriksson U. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med. 2006;203:2009–19. doi: 10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O’Shea JJ. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006;103:8137–42. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O’Malley JT, Kapur R, Levy DE, Kansas GS, Kaplan MH. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178:4901–7. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 49.Chitnis T, Najafian N, Benou C, Salama AD, Grusby MJ, Sayegh MH, Khoury SJ. Effect of targeted disruption of STAT4 and STAT6 on the induction of experimental autoimmune encephalomyelitis. J Clin Invest. 2001;108:739–47. doi: 10.1172/JCI12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mathur AN, Chang HC, Zisoulis DG, Kapur R, Belladonna ML, Kansas GS, Kaplan MH. T-bet is a critical determinant in the instability of the IL-17-secreting T-helper phenotype. Blood. 2006;108:1595–601. doi: 10.1182/blood-2006-04-015016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 52.Bettelli E, Dastrange M, Oukka M. Foxp3 interacts with nuclear factor of activated T cells and NF-kappa B to repress cytokine gene expression and effector functions of T helper cells. Proc Natl Acad Sci U S A. 2005;102:5138–43. doi: 10.1073/pnas.0501675102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, Mathis D, Benoist C, Chen L, Rao A. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–87. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 54.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–27. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, Kolls JK. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–9. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chung DR, Kasper DL, Panzo RJ, Chitnis T, Grusby MJ, Sayegh MH, Tzianabos AO. CD4+ T cells mediate abscess formation in intra-abdominal sepsis by an IL-17-dependent mechanism. J Immunol. 2003;170:1958–63. doi: 10.4049/jimmunol.170.4.1958. [DOI] [PubMed] [Google Scholar]
- 57.Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000;165:6107–15. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 58.Burchill MA, Nardelli DT, England DM, DeCoster DJ, Christopherson JA, Callister SM, Schell RF. Inhibition of interleukin-17 prevents the development of arthritis in vaccinated mice challenged with Borrelia burgdorferi. Infect Immun. 2003;71:3437–42. doi: 10.1128/IAI.71.6.3437-3442.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leibundgut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Reis ESC. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007 doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 60.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–31. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 61.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–77. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 62.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–82. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–7. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 64.Bush KA, Farmer KM, Walker JS, Kirkham BW. Reduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with interleukin-17 receptor IgG1 Fc fusion protein. Arthritis Rheum. 2002;46:802–5. doi: 10.1002/art.10173. [DOI] [PubMed] [Google Scholar]
- 65.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–73. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 66.Hofstetter HH, Ibrahim SM, Koczan D, Kruse N, Weishaupt A, Toyka KV, Gold R. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237:123–30. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 67.Matusevicius D, Kivisakk P, He B, Kostulas N, Ozenci V, Fredrikson S, Link H. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1999;5:101–4. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- 68.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–8. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 69.Aarvak T, Chabaud M, Miossec P, Natvig JB. IL-17 is produced by some proinflammatory Th1/Th0 cells but not by Th2 cells. J Immunol. 1999;162:1246–51. [PubMed] [Google Scholar]
- 70.Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA, Bos JD. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645–9. doi: 10.1046/j.1523-1747.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- 71.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–45. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 72.O’Garra A, Steinman L, Gijbels K. CD4+ T-cell subsets in autoimmunity. Curr Opin Immunol. 1997;9:872–83. doi: 10.1016/s0952-7915(97)80192-6. [DOI] [PubMed] [Google Scholar]
- 73.Krakowski M, Owens T. Interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur J Immunol. 1996;26:1641–6. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- 74.Tran EH, Prince EN, Owens T. IFN-gamma shapes immune invasion of the central nervous system via regulation of chemokines. J Immunol. 2000;164:2759–68. doi: 10.4049/jimmunol.164.5.2759. [DOI] [PubMed] [Google Scholar]
- 75.Gran B, Zhang GX, Yu S, Li J, Chen XH, Ventura ES, Kamoun M, Rostami A. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol. 2002;169:7104–10. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- 76.Zhang GX, Gran B, Yu S, Li J, Siglienti I, Chen X, Kamoun M, Rostami A. Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-beta 2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. J Immunol. 2003;170:2153–60. doi: 10.4049/jimmunol.170.4.2153. [DOI] [PubMed] [Google Scholar]
- 77.Gutcher I, Urich E, Wolter K, Prinz M, Becher B. Interleukin 18-independent engagement of interleukin 18 receptor-alpha is required for autoimmune inflammation. Nat Immunol. 2006;7:946–53. doi: 10.1038/ni1377. [DOI] [PubMed] [Google Scholar]
- 78.Schwartz S, Beaulieu JF, Ruemmele FM. Interleukin-17 is a potent immuno-modulator and regulator of normal human intestinal epithelial cell growth. Biochem Biophys Res Commun. 2005;337:505–9. doi: 10.1016/j.bbrc.2005.09.075. [DOI] [PubMed] [Google Scholar]
- 79.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–6. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, McKenzie BS, Cua DJ, Powrie F, Cheever AW, Maloy KJ, Sher A. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203:2485–94. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wekerle H, Kojima K, Lannes-Vieira J, Lassmann H, Linington C. Animal models. Ann Neurol. 1994;36 (Suppl):S47–53. doi: 10.1002/ana.410360714. [DOI] [PubMed] [Google Scholar]
- 82.Flugel A, Berkowicz T, Ritter T, Labeur M, Jenne DE, Li Z, Ellwart JW, Willem M, Lassmann H, Wekerle H. Migratory activity and functional changes of green fluorescent effector cells before and during experimental autoimmune encephalomyelitis. Immunity. 2001;14:547–60. doi: 10.1016/s1074-7613(01)00143-1. [DOI] [PubMed] [Google Scholar]
- 83.Becher B, Durell BG, Noelle RJ. IL-23 produced by CNS-resident cells controls T cell encephalitogenicity during the effector phase of experimental autoimmune encephalomyelitis. J Clin Invest. 2003;112:1186–91. doi: 10.1172/JCI19079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Becher B, Durell BG, Noelle RJ. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J Clin Invest. 2002;110:493–7. doi: 10.1172/JCI15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–91. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koenders MI, Kolls JK, Oppers-Walgreen B, van den Bersselaar L, Joosten LA, Schurr JR, Schwarzenberger P, van den Berg WB, Lubberts E. Interleukin-17 receptor deficiency results in impaired synovial expression of interleukin-1 and matrix metalloproteinases 3, 9, and 13 and prevents cartilage destruction during chronic reactivated streptococcal cell wall-induced arthritis. Arthritis Rheum. 2005;52:3239–47. doi: 10.1002/art.21342. [DOI] [PubMed] [Google Scholar]
- 87.Kleinschek MA, Owyang AM, Joyce-Shaikh B, Langrish CL, Chen Y, Gorman DM, Blumenschein WM, McClanahan T, Brombacher F, Hurst SD, Kastelein RA, Cua DJ. IL-25 regulates Th17 function in autoimmune inflammation. J Exp Med. 2007;204:161–70. doi: 10.1084/jem.20061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–31. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fife BT, Huffnagle GB, Kuziel WA, Karpus WJ. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:899–905. doi: 10.1084/jem.192.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu L, Huang D, Matsui M, He TT, Hu T, Demartino J, Lu B, Gerard C, Ransohoff RM. Severe disease, unaltered leukocyte migration, and reduced IFN-gamma production in CXCR3−/− mice with experimental autoimmune encephalomyelitis. J Immunol. 2006;176:4399–409. doi: 10.4049/jimmunol.176.7.4399. [DOI] [PubMed] [Google Scholar]