Abstract
Diet-induced atherosclerotic lesions in the descending thoracic segment of rabbit aorta were analysed ex vivo by micro-attenuated total reflection (ATR)–Fourier transform infrared (FTIR) spectroscopic imaging. The distribution and chemical character of lipid deposits within the arterial wall near intercostal branch ostia were assessed in histological sections from immature and mature rabbits fed cholesterol with or without l-arginine supplements. Previous studies have shown that both these properties change with age in cholesterol-fed rabbits, putatively owing to changes in the synthesis of nitric oxide (NO) from l-arginine. Immature animals developed lesions at the downstream margin of the branch ostium, whereas lipid deposition was observed at the lateral margins in mature animals. Dietary l-arginine supplements had beneficial effects in mature rabbit aorta, with overall disappearance of the plaques; on the other hand, they caused only a slight decrease of the lipid load in lesions at the downstream margin of the ostium in immature rabbits. ATR–FTIR imaging enabled differences in the lipid to protein density ratio of atherosclerotic lesions caused by age and diet to be visualized. Lipid deposits in immature rabbits showed higher relative absorbance values of their characteristic spectral bands compared with those in immature l-arginine-fed rabbits and mature rabbits. The multivariate methods of principal component analysis (PCA) and factor analysis (FA) were employed, and relevant chemical and structural information were obtained. Two distinct protein constituents of the intima–media layer at different locations of the wall were identified using the method of FA. This approach provides a valuable means of investigating the structure and chemistry of complex heterogeneous systems. It has potential for in vivo diagnosis of pathology.
Keywords: ageing, l-arginine, atherosclerosis, rabbit model, Fourier transform infrared imaging, attenuated total reflection
1. Introduction
Atherosclerosis, the disease underlying most heart attacks and strokes, is characterized by local deposits of fat, cells and collagen within the intima and inner media of systemic arteries. The precise composition of the lesions is of considerable interest because it gives information on the pathogenesis of the disease (e.g. concerning the role of lipids circulating in the blood, influx of inflammatory cells, etc.) and because lesion composition is related to clinical outcome, lipid-rich lesions being more prone to catastrophic rupture. The use of non-invasive techniques for the detection of unstable atherosclerotic plaques is currently explored in the literature (Langer & Gawaz 2006; Tan & Lip 2008). Methods for in situ lesion characterization, such as those based on magnetic resonance imaging (Briley-Saebo et al. 2007) and ultrasound (Dumont et al. 2006; Wetterholm et al. 2007), are of great clinical importance but generally give imprecise details of composition and have poor spatial resolution. Ex vivo techniques based on light or electron microscopy give better resolution; compositional information is provided either by staining with dyes, which are somewhat non-specific, or by antibody or antisense probes, which require considerable effort to produce. Here, we use a method based on Fourier transform infrared (FTIR) spectroscopy in combination with a focal plane array (FPA) detector (Chan & Kazarian 2003) to obtain chemical images showing the distribution of specific compounds within atherosclerotic lesions. This approach gives a resolution comparable to that obtained by optical microscopy without the need for staining or specific probes and with added chemical specificity.
FTIR spectroscopic imaging (Kazarian & Chan 2006) involves the simultaneous measurement of many spectra of a sample, each containing the chemical signatures (absorption bands related to vibrational modes of specific chemical bonds) of compounds at a specific location within the specimen. With an FPA detector, the spectra for different positions can be acquired simultaneously, greatly increasing the speed of acquisition of chemical information from the sample. Post-processing of the spectra gives images showing the spatial distribution within the specimen of absorbance of defined bands in the spectrum. The method has been dubbed ‘chemical photography’ (Kazarian & Chan 2003). Quantitative information can be obtained from direct consideration of spectra or, using standard image processing software, from the images themselves. Preliminary work from this laboratory (Colley et al. 2004) demonstrated the feasibility of using FTIR imaging to study atherosclerosis by obtaining chemical images of cross sections of an atherosclerotic rabbit aorta. In that study, we obtained images in transmission and by using attenuated total reflection (ATR) in micro-mode (with a germanium (Ge) microscope objective) and in macro-mode (with a single reflection diamond accessory). These methods gave different image sizes and spatial resolutions, and hence different insights into the atherosclerotic deposits. With the micro-ATR–FTIR imaging, we obtained chemical images with a spatial resolution of 3–4 μm (Colley et al. 2004).
Aortic lipid deposits in people and cholesterol-fed rabbits have an age-related distribution and composition (Spagnoli et al. 1991; Barnes & Weinberg 1998, 1999, 2001; Orlandi et al. 2000). In immature rabbit and human aortas, lesions develop at the downstream margin of intercostal branch ostia and are restricted to fatty streaks, whereas in mature aortas of both species lesions occur at the sides and upstream of the branch ostia and have a more complex, fibrous composition (Weinberg 2002). The age-related changes are thought to depend on differences in the synthesis of nitric oxide (NO) from l-arginine (Orlandi et al. 2000; Staughton & Weinberg 2004), and our data show that dietary l-arginine has a stronger atheroprotective effect in mature than immature rabbits (Weinberg & Cremers 2003). In the present study, micro-ATR–FTIR imaging was used to investigate quantitatively the effects of age and dietary l-arginine on atherosclerosis in cholesterol-fed rabbits. The area imaged by contact of the surface of the Ge crystal with the surface of the sample corresponds to 63×63 μm2. Characteristic bands of proteins and lipids, principally amide I, amide II and ester carbonyl stretching, were analysed, yielding images with a spatial resolution of approximately 3–4 μm. These bands constitute the spectral signatures of the main components of atherosclerotic arteries (Colley et al. 2004; Wang & Mizaikoff 2008). The method successfully revealed differences in lesion distribution and composition with age and diet.
Multivariate analysis was performed on the ATR–FTIR images of atherosclerotic aorta for the different rabbit groups to gain further insight into the structure and chemical composition of the tissue. Chemometric methods are valuable tools in analytical studies, allowing the handling and interpretation of large datasets such that the relevant information contained therein can be extracted. They have been applied in a number of biomedical studies using spectroscopic methods for in vitro analysis, especially to discriminate between healthy and pathological tissues (Bonnier et al. 2006, 2008; Wang et al. 2006, 2007; Wang & Mizaikoff 2008). In this work, principal component analysis (PCA) and factor analysis (FA) were successfully applied to the hyperspectral datasets. FA revealed the presence of two spatially and spectrally distinct protein-containing constituents of the wall, a finding that was not achieved via univariate analysis owing to overlapping spectral features in the mid-infrared spectrum of the tissue.
2. Methods
2.1. Animal feeding trial
Animal procedures complied with the Animals (Scientific Procedures) Act 1986. Male New Zealand white rabbits (HSDIF strain, Harlan) of two different age groups, immature (two months old) and mature (six months old), were housed at 18(±2)°C under a 12 hour light cycle, and fed 75 g per day of a normal diet (9603 TRB Rabbit; Harlan Teklad, Bicester, UK) supplemented for eight weeks with 1 per cent (w/w) cholesterol (Sigma). The cholesterol was added in ether (inhibitor-free spectrophotometer grade, Sigma), which was then evaporated. For half of the rabbits in each age group, the diet was additionally supplemented with 6 per cent (w/w) l-arginine (Sigma); this was added in water, which was then allowed to evaporate.
2.2. Tissue collection and sectioning
Descending aortas were fixed in situ at physiological pressure for 10 min with 10 per cent neutral buffered formalin before being excised. The major part of each aorta was stained for lipid and examined en face. Preliminary data concerning lesion patterns and frequencies have been published (Weinberg & Cremers 2003), and further details will be reported elsewhere. A segment of each thoracic aorta containing the third pair of intercostal branches was retained for the present study. It was post-fixed for at least 24 hours in further formalin solution and then opened along the ventral wall. The segment was immersed in a 2 M sucrose solution overnight to inhibit the subsequent formation of ice crystals, before being embedded in Bright cryo-m-bed (Bright Instruments, Huntingdon, UK) and frozen. Longitudinal sections of 15 μm thickness were cut in a cryostat and mounted on standard microscope slides. Sections were not rinsed in order to avoid leaching sucrose (used as an internal standard—see below) and washing Bright cryo-m-bed compound into the tissue (Wong & Rigas 1990).
For comparative purposes, unfixed and undiseased tissue was also used. The thoracic aorta of a mature (10 months) rabbit fed a normal diet without additional cholesterol or l-arginine was collected and stored for 1 day in Ringer's solution (composition in g l−1: NaCl, 9.0; KCl, 0.2; CaCl2, 0.2; NaHCO3, 0.1) before cryosectioning. An aortic segment, which contained a pair of intercostal branches, was immersed in Bright cryo-m-bed compound and frozen before cryosections were cut.
2.3. Microscopy and spectroscopy
Two aortic sections per animal (eight rabbits) were analysed giving a total of 16 specimens. The geometry of the sections was recorded in light microscope images, obtained using a 10× objective on a standard light microscope and a CCD camera (AX-2 Viper, Axiom Research), or a 15× objective on an FTIR microscope (Varian 600 UMA) and a 64×64 FPA detector.
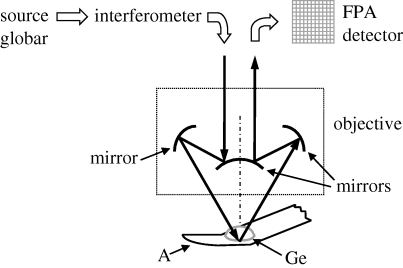
Micro-ATR–FTIR images were collected with a rapid scan system consisting of a Bio-Rad spectrometer (type FTS-60A) coupled to a Varian 600 UMA FTIR microscope with a 15× Cassegrain objective, to which was attached a Varian slide-on ATR accessory equipped with a germanium crystal (refractive index 4, numerical aperture 2.4), and a liquid nitrogen-cooled FPA detector (figure 1). The detector had 4096 pixels, each 40×40 μm in size and arranged in a 64×64 array, corresponding to a 63×63 μm2 area of the specimen.
Figure 1.
Scheme of the 15× Cassegrain objective with germanium (Ge) ATR crystal used in the micro-ATR–FTIR imaging measurements; the IR beam emitted by a globar is sent to the sample (A, aorta section) through the ATR crystal. An evanescent wave resulting from internal reflection of light at the crystal surface propagates into and interacts with the sample, which is in contact with the crystal. The internally reflected light is collected and detected by a FPA detector.
The germanium objective gives a lateral resolution of approximately 3–4 μm in the fingerprint part of the infrared spectrum (Chan & Kazarian 2003; Chan et al. 2005) despite the long wavelengths being used because it has a refractive index of 4 and is placed in direct contact with the sample. A low contact pressure was applied in order not to damage the tissue; this generally ensures a good signal-to-noise ratio of the spectra. Good contact between the objective and the specimen is required across the whole imaged area to obtain a homogeneous optical path length. An evanescent wave from the objective has a depth of penetration of 1.2 μm at a wavenumber of 1000 cm−1.
Accurate focus adjustment of the ATR accessory was performed before each series of measurements. Also, the crystal was centred with respect to the objective by ensuring alignment of optical and micro-ATR–FTIR images of a standard comprising gold strips on a microscope slide. Varian Resolution Pro v. 4.0 software was used for acquisition and manipulation of the data. An IR absorption spectrum was obtained for each detector pixel by co-adding 256 interferograms. Spectra were measured over the range 4000–900 cm−1 at 4 cm−1 resolution with a zero filling factor of 2, achieving a collection time of approximately 10 min per image. For all image measurements, a background obtained in the absence of a sample was measured.
The data can be regarded as a three-dimensional dataset with two spatial dimensions and a third dimension corresponding to wavenumber; values in the dataset corresponded to absorbance at each combination of position and wavenumber. Absorbance is related to the concentration of a particular chemical component through the Beer–Lambert Law. In the present work, the fingerprint region of the infrared spectrum between 1800 and 900 cm−1, containing characteristic absorption bands of esters and amide groups, was analysed for each detector pixel. To calculate the absorbance of each band, a linear baseline was drawn through the troughs either side of the peak, and absorbance values above this line were integrated with respect to wavenumber, as shown in figure 2 a.
Figure 2.
(a) Spectrum extracted from a medial lamella upstream of an aortic branch ostium in an immature rabbit; the integration method used to produce an image of the specimen is displayed for the amide II peptide band. (b) A spectrum of a lamella in the formalin-fixed sucrose-treated aortic tissue of a mature cholesterol-fed rabbit is displayed along with one from an unfixed, untreated aorta of a mature non-cholesterol-fed rabbit. Solid curve, fixed tissue; circles, untreated tissue.
The bands analysed in this way were the main peptide absorptions in the mid-infrared spectral region, i.e. amide I and II bands at approximately 1640 and 1540 cm−1, respectively, assigned to C=O stretching and mainly NH bending modes (Parker 1975), as well as the ester C=O stretching band and CH stretching pattern due to lipids. (Some contribution to the region of the ν(CH) absorption also derives from proteins.) To improve the reliability of comparisons between sections, prominent bands below 1200 cm−1 due to the sucrose cryopreservative were used as an internal reference for normalization (Kačurácová & Mathlouthi 1996). The small contribution of residual formalin in the C=O stretching region (aldehydes typically absorb at slightly lower frequency than esters) can be neglected when the carbonyl stretching band of lipid esters at approximately 1735 cm−1 is integrated.
Figure 2 b shows the effect of sucrose treatment and formalin fixation on the IR features of aortic tissue. (Absorbance of spectral bands was normalized with respect to the amide I band.) Formalin accounts for the differences in spectral position and intensity ratios for the main peptide bands (vibrational wavenumbers of amide I, II and III are shown on the graph) as it promotes the formation of chemical bonds between protein molecules (Ó Faoláin et al. 2005).
Because analysis of each spectral band gives a single value (representing the integrated absorbance at the range of wavenumbers characterizing a certain vibration), two-dimensional images could be reconstructed corresponding to the density distribution of each chemical species within the specimen. Using ISys v. 4.0 data analysis software (Spectral Dimensions Inc., Maryland, USA), 5×5 pixel regions rich in lipid were selected and a characteristic spectral profile for each of these regions was obtained by averaging the 25 spectra (one per pixel). Also, to demonstrate that quantitative information could be directly and rapidly obtained from the images, as in conventional histological techniques, standard image processing software (Adobe Photoshop CS2 v. 9.0 by Adobe) was used to calculate mean intensity values for 3×3 pixel areas of the lipid clusters in images that had been converted from colour to greyscale. Note that in the conversion from colour to greyscale images the same scale of absorbance values was maintained, this scale being linear. Therefore, the mean intensities calculated for the greyscale images correspond to mean integrated absorbance of the spectral band from which the image was derived, and hence to the concentration of a particular functional group.
2.4. Multivariate analysis
Multivariate analysis was also performed on the micro-ATR–FTIR imaging datasets using ISys v. 4.0 software. PCA and FA were employed for the data processing. The fingerprint region of the mid-infrared spectrum (1800–900 cm−1), which shows signatures of proteins and lipids in biomedical samples, was selected for the analysis. A linear baseline was drawn through the extremes of this spectral region to prevent spurious background effects in the calculation.
Since each dataset had three dimensions, values for any pixel in the FPA detector can be considered a vector of three variables, the values of which can be transformed in different ways using chemometric strategies (Geladi et al. 2007). For instance, PCA and FA determine new axes in the multivariate space. These mathematical tools allow a reduction in the number of variables in the measured dataset by determining the principal components (PCs) or factors that better reproduce the variance of the dataset.
PCA calculates PCs that are visualized as score images, each coupled to its loading spectrum. The PCs are ranked in such a way that the first component accounts for the highest percentage of the total variance explained, while the last component refers to the lowest percentage of the variance. FA also gives score images for the different calculated factors, each with an associated loading spectrum. The difference between these two methods lies in the way they account for the error of measurement in the development of the algorithm. For a description of the PCA and FA methods, see Keenan (2007). In both the cases, owing to the sensitivity of the method and computational limitations, generally only three to five components were used to describe the space of variables in the dataset.
3. Results
3.1. Immature rabbits
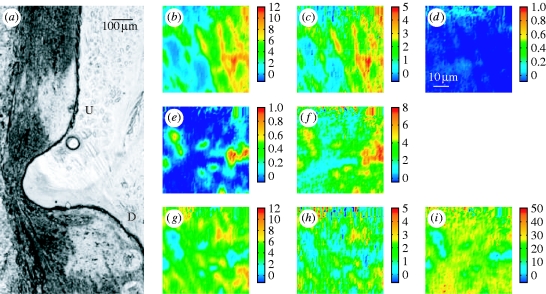
For this class of samples, longitudinal sections of aortic wall through the branch ostium were analysed. Figure 3 a shows a photomicrograph of such a section obtained by transmitted light microscopy with a 10× objective. The orientation of the section is easily distinguished because the arterial wall is thicker downstream than upstream (Weinberg 1988). The apparent blockage of the branch reflects the non-planar curvature of the intercostal artery. The two large impressions in the upstream and downstream parts of the tissue (indicated by U and D, respectively) are caused by contact with the ATR crystal during sample measurement.
Figure 3.
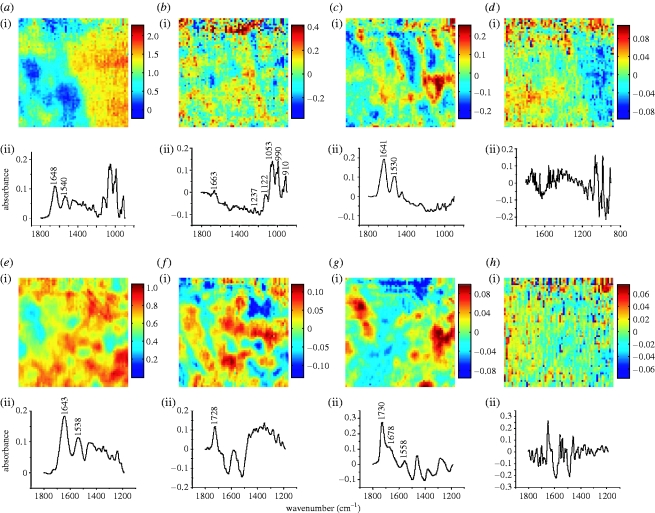
Visible and micro-ATR–FTIR images of a longitudinal section of thoracic aorta of immature rabbit containing a branch ostium. (a) Photomicrograph collected after the experiment with an epifluorescence microscope in transmission mode using 10× objective; U and D indicate the regions upstream and downstream of the ostium, which appears blocked due to three-dimensional curvature. The two large marks at the upper and lower locations correspond to the contact regions between the crystal and the sample. (b,c) Micro-ATR–FTIR images of the tunica media at the upstream margin of the ostium obtained from the spatial distribution of the integrated absorbance of the (b) amide I and (c) amide II bands, in the range of 1715–1585 cm−1 and 1585–1485 cm−1, respectively. (d) No lesion is revealed by integrating between 1753 and 1707 cm−1, in the ν(C=O)ester region. The intensity scale associated with each image is such that blue and red extremes respectively correspond to low and high absorption values as given by spectral integration; this is directly related to a density scale (Beer–Lambert Law). (e–i) Micro-ATR–FTIR images of the intima–media boundary at the downstream margin of the branch ostium; the distributions of the integrated absorbance refer to: (e) ν(C=O)ester in the 1753–1707 cm−1 range, (f) ν(CH) in the 3020–2750 cm−1 range, (g) amide I (1715–1585 cm−1), (h) amide II (1585–1485 cm−1), (i) ν(NH) and ν(OH) in the region of 3650–3020 cm−1.
Micro-ATR–FTIR images from these two locations are presented in figure 3 b–i. Each image represents the integrated absorbance of a specific band of the IR spectrum for each pixel of the FPA detector. At the upstream margin of the branch, the lamellar structure of the tunica media is clearly visible in images derived from the amide I and II peptide bands (figure 3 b,c). Lamellae made of elastin and collagen fibres are the structural motifs that characterize this layer of the arterial wall (Clark & Glagov 1985). No lipid is detected at this position in the image of the distribution of absorbance of the ν(C=O)ester band (1753–1707 cm−1) (figure 3 d). On the other hand, data for the intima–media boundary in the area downstream of the ostium reveal the occurrence of an atherosclerotic lesion. Figure 3 e,f displays, respectively, images of the plaque given by integration of the ν(C=O)ester and the ν(CH) bands; clusters of lipid esters, thought mainly to be cholesteryl esters (Kodali et al. 1991; Manoharan et al. 1993), are heterogeneously distributed in this subendothelial portion of the aortic wall. Images (figure 3 g,h) of the peptide bands obtained from the same measurement are complementary: there is little peptide in areas with a high concentration of lipid esters, and vice versa. The aggregates of lipid ester embedded in a protein matrix may reflect the formation of the macrophage-derived foam cells typical of early lesions. An image was also obtained for the absorbance of the ν(NH) band above 3020 cm−1 (figure 3 i); the lipid-rich, hydrophobic regions occupied by the clusters appear as low-intensity areas in this image of the protein distribution. (Sucrose also contributes to the absorbance in the 3650–3020 cm−1 region due to the stretching mode of its OH groups.) The presence of lipid downstream but not upstream of the ostium is consistent with maps of lesion prevalence obtained by optically scanning in the visible region the luminal surface of the intact aorta en face after staining for lipid (Weinberg & Cremers 2003).
3.2. Immature l-arginine-fed rabbits
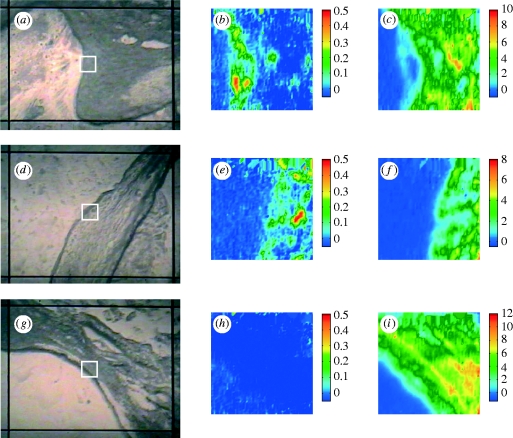
Sections through aortic branches were examined for comparison with the results from immature rabbits not given arginine supplements. The analysis of the upstream region gave similar results: a lamellar structure of the medial layer without any occurrence of lesions. Figure 4 a shows an image of the downstream region of this specimen obtained using the FTIR microscope with its visible reflection mode coupled to the imaging system; the white square indicates the area of the white sample (approx. 63×63 μm2) from which the FTIR imaging dataset was obtained. Lipid esters forming aggregates were observed at the downstream margin of the ostium within the intima–media layer (figure 4 b); they appeared to overlie a protein-rich area that might correspond to the uppermost media (figure 4 c). Lipid was observed downstream but not upstream of branches in such animals in our studies using en face scanning in the visible region (Weinberg & Cremers 2003).
Figure 4.
Photomicrograph and micro-ATR–FTIR images of various rabbit aortic sections. (a–c) Downstream margin of the ostium at the intima–media boundary in a branch-containing section from an immature rabbit fed an l-arginine-supplemented diet: (a) photomicrograph; the FTIR images refer to the distribution of the integrated absorbance of (b) ν(C=O)ester and (c) amide I bands in the range of 1753–1707 cm−1 and 1715–1585 cm−1 respectively. (d–f) Lateral margin of the ostium at the intima–media boundary in a section of mature rabbit aorta, cut at the branch side; integrated bands: (e) ν(C=O)ester (1753–1707 cm−1) and (f) amide I (1715–1585 cm−1). (g–i) Lateral margin of the ostium at the intima–media boundary in a section of mature rabbit fed an l-arginine-supplemented diet; integration of (h) ν(C=O)ester region between 1753 and 1707 cm−1 and (i) amide I band between 1715 and 1585 cm−1.
3.3. Mature and mature l-arginine-fed rabbits
In mature cholesterol-fed rabbits, lipid deposition occurs at the lateral margins of branches and not upstream or downstream of them (Barnes & Weinberg 1998, 1999, 2001). Hence, longitudinal aortic sections were cut at the right and left sides of the branch so that the intima–media layer of the lateral margins of the ostium could be analysed in both these groups of rabbits. Figure 4 d–i shows visible and spectroscopic images related to these measurements; lipid ester deposition (figure 4 e), embedded within a protein-rich matrix (figure 4 f), was seen in the mature rabbit samples but not in the mature l-arginine-fed rabbit samples (figure 4 h). Figure 4 i clearly depicts the internal elastic lamina that defines the boundary between the intima and the media. The location of the lesion and the strong inhibitory effect of l-arginine on lesion formation in mature animals are again consistent with previously reported lesion maps obtained by en face scanning (Weinberg & Cremers 2003).
3.4. Plaque analysis
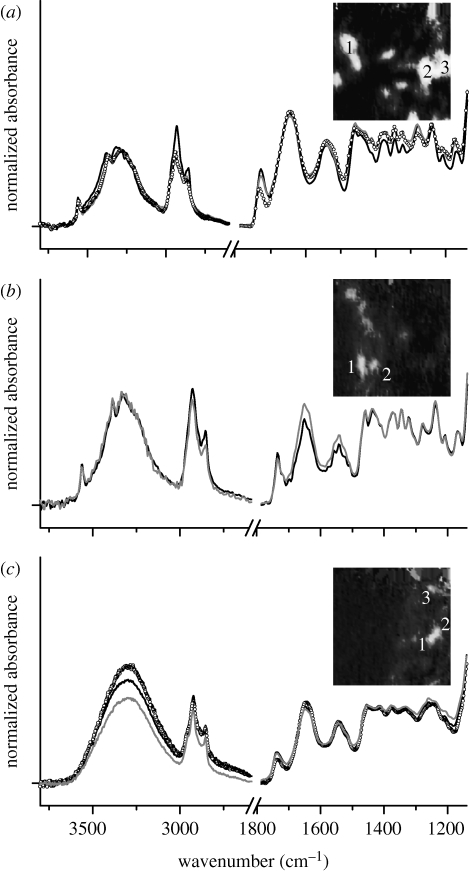
Average spectra, each obtained from 25 individual spectra (5×5 pixels) and representing a lipid cluster in the images above, are shown in figure 5 for the different rabbit groups (with the exception of the mature l-arginine-fed group, in which no lipid clusters could be detected). The main absorption features here are due to ν(CH) with a band maximum approximately 2924 cm−1, ν(C=O) at approximately 1730 cm−1, ν asym(PO2) at 1280 cm−1 and ν(C—O) at 1169 cm−1, from the lipid esters and phospholipids within the plaque (Colley et al. 2004; Wang et al. 2007; Wang & Mizaikoff 2008). For the three classes of samples, the average spectra are very similar to one another with respect to the relative intensity of the ν(C=O)ester peak and peptide bands, which is an approximate measure of the relative concentration in lipid esters in the different lesions. Accordingly to the Beer–Lambert Law, measured absorbance of spectral bands is proportional to the corresponding concentrations of chemical components. However, the actual path length may vary as a result of variation of refractive index within the sample. In addition, the use of the integration protocol, described above, makes measurement of absolute concentrations approximate.
Figure 5.
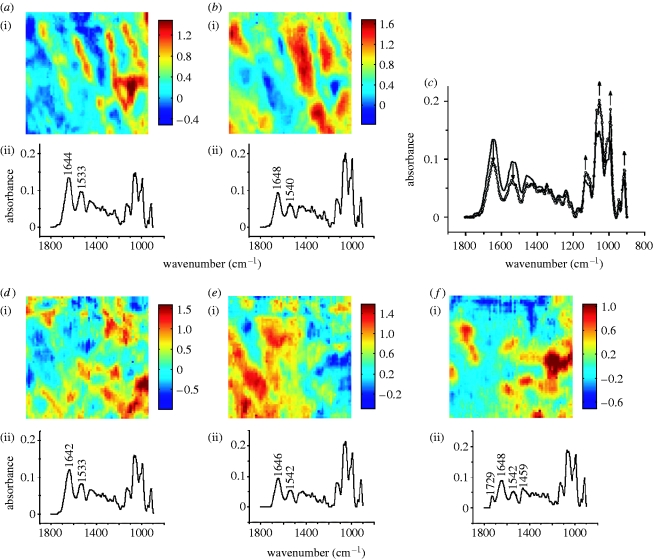
Average spectra obtained from 5×5 pixel regions within the lipid ester clusters in the images of lesions; these profiles are representative of the spectra of clusters shown in the figure insets. (a) Average spectra of three lipid ester clusters in immature rabbit aortic lesion (see inset). (b,c) The same for immature l-arginine-fed rabbit and mature rabbit, respectively. Insets: greyscale images obtained by converting the images in figures 3 e and 4 b,e, using a fixed intensity scale from −0.07 to 0.7 integrated absorbance units. Black curve, cluster 1; grey curve, cluster 2; circles, cluster 3.
In the case of immature and immature l-arginine-fed rabbits, the ratio of absorbance between the carbonyl band and the amide II band is approximately 0.7 (figure 5 a,b), indicating a similar plaque composition and consequently no evidence of a substantial beneficial effect of the dietary l-arginine. For the clusters of lesions in mature rabbit aorta at the lateral margins of the ostium, this ratio is approximately 0.5 (figure 5 c); no lesion was detected in mature l-arginine-fed rabbits, thus confirming the efficacy of l-arginine in preventing the plaques.
To simplify the analysis of the images, we converted them to greyscale images and assessed the average pixel intensity, corresponding to the average integrated absorbance of the ester carbonyl stretching band, in different regions (figure 5, insets). In these images, light regions are those with high absorbance and dark grey regions represent low absorbance. Mean grey values, calculated for 3×3 pixel regions of the greyscale images, are reported in table 1. Small differences in the absorbance values were found between sections within an animal and between animals within a group. On the other hand, the difference between the mature arginine and mature control rabbits is very large, unlike the difference between immature arginine and immature control rabbits.
Table 1.
Intensities (mean±s.d.) of 3×3 pixel regions of the greyscale images corresponding to integrated absorbance of the lipid ester band. (Light grey regions within the images represent ksion clusters, whereas dark grey regions represent areas of low lipid concentration.)
| light grey region | ||||||
|---|---|---|---|---|---|---|
| groups | animals | samples | cluster 1 | cluster 2 | cluster 3 | dark grey region |
| rabbit 1 | section 1 | 235±6 | 240±3 | 240±4 | 8.9±0.3 | |
| section 2 | 238±4 | 231±7 | 231±8 | 9.3±0.8 | ||
| rabbit 2 | section 1 | 240±2 | 241±1 | 241±1 | 8.9±0.3 | |
| immature | section 2 | 244±0 | 244±1 | 244±0 | 23±2 | |
| rabbit 1 | section 1 | 207±7 | 209±8 | 218±10 | 8.9±0.3 | |
| section 2 | 194±4 | 161±11 | 181±19 | 10±1 | ||
| rabbit 2 | section 1 | 205±7 | 218±9 | 222±11 | 11±2 | |
| immature with arginine | section 2 | 222±17 | 187±40 | 197±22 | 8.9±0.3 | |
| rabbit 1 | section 1 | 200±4 | 196±3 | 154±20 | 19±2 | |
| section 2 | 217±6 | 209±9 | 181±18 | 8.9±0.4 | ||
| rabbit 2 | section 1 | 204±9 | 196±6 | 215±8 | 9.2±0.7 | |
| mature | section 2 | 173±17 | 167±21 | 198±3 | 8.9±0.3 | |
| rabbit 1 | section 1 | 10±1 | 9±1 | 9.1±0.8 | 8.9±0.3 | |
| section 2 | 12±3 | 12±4 | 13±3 | 11±3 | ||
| rabbit 2 | section 1 | 9±1 | 9.0±0.8 | 9±1 | 8.9±0.3 | |
| mature with arginine | section 2 | 9.1±0.8 | 9±1 | 9±1 | 8.9±0.3 | |
3.5. Multivariate analysis
PCA and FA were applied to the datasets with the aim of identifying individual contributions to the spectral data. The analyses were applied to the results obtained for immature rabbit aorta at both upstream and downstream margins of the branch ostium, within the intima–media layer. Figure 6 shows the results of PCA applied to the micro-ATR–FTIR image of the upstream region (figure 3). Four components were selected based on the percentage of variance explained and on the spectral features of the loading vectors. The first component (PC1), which accounted for 97.3 per cent of the total data variance, provided the average spectrum of the specimen in this region (figure 6 a) and the corresponding score image simply represents the area of contact between the ATR crystal and the specimen. The second and third components (PC2 and PC3; figure 6 b,c) explained a considerably lower percentage of the variance (1.6 and 0.6%, respectively); despite some baseline effects in the loading spectra, they could be assigned, respectively, to the sucrose used as a cryopreservative (Kačurácová & Mathlouthi 1996) and elastin forming the medial lamellae (see pure elastin spectrum in Dehghani et al. 2008). The PC3 score image closely resembles the image of the elastic lamellae obtained via univariate analysis (figure 3 b,c). The distributions depicted by PC2 and PC3 are mutually complementary, implying that sucrose accumulates preferentially in regions of the tissue between adjacent lamellae (where smooth muscle cells (SMCs) are found). The fourth component (PC4; figure 6 d) represented the noise, which is higher in the zones of poor contact.
Figure 6.
Image scores and loading plots of the PCs obtained from the analysis of a micro-ATR–FTIR image of immature rabbit aorta at the (a–d) upstream and (e–h) downstream margins of the ostium. (a,e) PC1, (b,f) PC2, (c,g) PC3, and (d,h) PC4.
For the FTIR image collected at the downstream margin of the ostium, PCA generated the components shown in figure 6 e–h. In this calculation, the spectral region below 1190 cm−1 was discarded owing to problems with the data processing. As for the upstream region, the first component accounted for most of the explained variance (approx. 99%), and yielded the average spectrum of the sample for this location. The second component (approx. 0.5% of the explained variance) was of more complex interpretation, due to the absence of the low-frequency region of the spectrum. The third component (approx. 0.2%) could be assigned to the lipid esters forming the plaque, also shown by univariate analysis (figure 3 e,f). The fourth component represented the noise.
FA was also applied to the micro-ATR–FTIR images and the results for immature rabbit are presented here. Figure 7 a–c shows the results of FA applied to the intima–media layer at the upstream margin of the ostium. Two factors were selected by visual inspection of the score images and loading spectra. The first factor (F1) clearly showed the lamellar structure of the media already depicted by univariate analysis (figure 3 b,c), while the second factor (F2) yielded a distribution complementary to the previous one, which could be assigned to SMCs. (The peak position of the peptide bands in the F2 loading profile was similar to that in the ATR–FTIR spectrum of actin, the dominant protein in SMCs, reported by Böhl et al. (2007).) Lamellae and SMCs are the major structural motifs that characterize the medial layer of the aortic wall (Clark & Glagov 1985). Figure 7 c displays the loading spectra of the two selected factors plotted in the same graph; it can be seen that the peptide bands are relatively more intense (and sucrose bands relatively less intense) in spectrum F1 compared with spectrum F2. This finding supported our assignment, as lamellae are essentially protein structures (elastin and collagen) while SMCs contain proteins along with other compounds. Therefore, two spatially distinct distributions of protein-containing constituents of the wall, with different but overlapping absorption spectra, were identified through FA. Analogous results (not shown) were obtained by processing the same dataset in the reduced spectral range of amide I and II bands (1750–1480 cm−1; no relevant absorption due to sucrose), indicating that the calculated factors are independent of the sucrose distribution in the tissue.
Figure 7.
Image scores and loading plots of the factors obtained from the analysis of a micro-ATR–FTIR image of immature rabbit aorta at the (a,b) upstream and (d–f) downstream margins of the ostium. (c) Loading spectra of F1 (solid curve) and F2 (circles) factors for the upstream measurement. (a,d) F1, (b,e) F2, and (f) F3.
At the downstream margin of the ostium, three meaningful factors were recognized (figure 7 d–f). Two factors (F1 and F2) depicted spectrally different protein-containing structures of the intima–media layer, with spectral profiles similar to those of the corresponding factors above (figure 7 a,b). Additionally, lipid ester clusters were depicted by factor F3, which provided evidence for the presence of a plaque in this region of the wall, as previously derived by univariate analysis (figure 3 e,f). We assigned factor F1 to the lamellae and factor F2 to the SMCs at the plaque base (media).
4. Discussion
Micro-ATR–FTIR spectroscopic imaging was used to characterize the distribution and composition of aortic lesions around intercostal branch ostia in rabbits fed a cholesterol-enhanced diet. The location (Barnes & Weinberg 1998, 1999, 2001) and composition (Spagnoli et al. 1991; Orlandi et al. 2000) of such lesions are known to change with age. The change in location can be explained by a parallel switch in the pattern of transport of plasma macromolecules into the arterial wall (Sebkhi & Weinberg 1994, 1996); the coupling of this transport process to NO synthesis changes with age (Forster & Weinberg 1997). The change in lesion composition has also been attributed to an alteration of NO synthesis by the aortic endothelium (Orlandi et al. 2000), for which l-arginine is the precursor. Rabbits of two ages, two and six months, and treated or not treated with dietary l-arginine supplements, were studied. A previous en face examination of the same aortas indicated that lesions occurred downstream of branches in the two-month group but at the lateral margins of the ostia in the six-month group and that l-arginine had a much stronger atheroprotective effect in the mature animals (Weinberg & Cremers 2003).
The spectroscopic imaging confirmed both the change in distribution and the greater efficacy of dietary l-arginine with age observed en face. In cryosections of thoracic aorta cut along the main axis of the vessel, lesions were detected at the downstream margin of the branch ostium in immature rabbits, and some reduction of the lesions at this location was observed with dietary l-arginine supplementation. No lesions were found at this location in sections from mature animals; however, sections of aortic wall cut at the lateral margins of the branch showed evidence of lesions and these entirely disappeared as a result of dietary supplementation with l-arginine. This is an important result because lipid deposits may be obscured by overlying fibrous caps when using en face visualization techniques (Richards & Weinberg 2000); hitherto, it has therefore been uncertain whether the apparent change with age in the location of lesions and the atheroprotective effects of l-arginine might in fact reflect changes in the visibility of arterial lesions from the endothelial surface. The result may have clinical implications because the location and composition of atherosclerotic lesions in human aortas also appear to change with age.
It is noteworthy that the lipid and protein largely occupied different regions in the ATR–FTIR images. That corresponds to the known structure of atherosclerotic lesions, in which lipid tends to accumulate within foam cells, so called because the large lipid droplets within them give a foamy appearance under the microscope, or within a lipid-rich core from which most cells and fibrous proteins are excluded. These deposits, which include cholesterol and other fat molecules (triglycerides and phospholipids), occur in the intima and inner medial layers of the wall. In normal areas of the wall, SMCs are embedded in a protein matrix, principally consisting of elastin and collagen; the lamellar structure of this matrix was also apparent in the spectroscopic images.
The same conclusions could have been reached by using standard histological techniques. However, FTIR spectroscopic imaging has a number of significant advantages. First, there is no need to pretreat the specimens with stains, antibodies or other probes prior to the microscopy, although more sophisticated equipment is required. More importantly, the images contain precise, well-defined chemical information because they are derived from integrated absorbance of bands within the spectrum obtained for each point in the specimen. A group of FTIR spectroscopic images representing different bands, typically in the fingerprint region (below 1800 cm−1), provides a compositional map of the specimen. Images of the lipid in lesions are generated by integration of the ester carbonyl stretching band (at approx. 1735 cm−1) or the methyl/methylene C–H stretching pattern (3020–2750 cm−1), whereas images of the protein structure of the arterial wall are derived from the integrated absorbance of the amide I and II bands. (Amide I can be used only for imaging dehydrated samples, as in our case, where the water bending band contribution to its spectral intensity is negligible.) Quantitative or semi-quantitative results can be obtained by applying standard image processing techniques to the spectroscopic images, or by referring back to the spectra from which the images are derived. The resolution is comparable to that obtained by standard histological techniques using cryosections.
The results demonstrate the potential of micro-ATR–FTIR imaging for studying atherosclerosis. Furthermore, the method we used was extended by the application of multivariate analysis, which allowed the decomposition of the highly overlapped features forming the mid-IR spectrum of such complex specimens, and hence the recognition of different biochemical constituents. The results of PCA and FA demonstrated structural motifs of the arterial wall that are difficult to separate via univariate analysis. In particular, PCA provided evidence of individual components of the tissues such as elastin, lipid esters and sucrose (cryopreservative). FA was able to distinguish two protein-containing structures having different spectral signatures. Lipid deposits giving rise to atherosclerotic lesions were also identified by both PCA and FA. Thus, by taking into account spatial correlations among spectral features, multivariate analysis was able to discriminate between different components of the wall, while a univariate approach showed limitations. This demonstrated that the application of chemometric techniques allows compositional information to be obtained from chemically specific datasets, such as those measured by micro-ATR–FTIR imaging.
Since the FTIR spectroscopic imaging does not involve staining of, and hence chemical interference with, the specimen, it is ideally suited to human in vivo applications, where staining is not possible. FTIR spectroscopic imaging with multivariate data analysis could evolve into an important tool for routine diagnosis of vascular disease if suitable fibre-optic devices become available. Analysis of the chemical composition of plaques might, for example, allow identification of those likely to rupture and cause clinical events.
Acknowledgments
This project was funded by EPSRC grant no. EP/E003281. We thank Dr Bettina A. Nier for her contribution to the sample preparation.
References
- Barnes S.E., Weinberg P.D.1998Contrasting patterns of spontaneous aortic disease in young and old rabbits. Arterioscler. Thromb. Vasc. Biol. 18, 300–308. [DOI] [PubMed] [Google Scholar]
- Barnes S.E., Weinberg P.D.1999Two patterns of lipid deposition in the cholesterol-fed rabbit. Arterioscler. Thromb. Vasc. Biol. 19, 2376–2386. [DOI] [PubMed] [Google Scholar]
- Barnes S.E., Weinberg P.D.2001Strain-dependent differences in the pattern of aortic lipid deposition in cholesterol-fed rabbits. Exp. Mol. Pathol. 71, 161–170. 10.1006/exmp.2001.2395. [DOI] [PubMed] [Google Scholar]
- Böhl M., Tietze S., Sokoll A., Madathil S., Pfennig F., Apostolakis J., Fahmy K., Gutzeit H.O.2007Flavonoids affect actin functions in cytoplasm and nucleus. Biophys. J. 93, 2767–2780. 10.1529/biophysj.107.107813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnier F., Rubin S., Ventéo L., Krishna C.M., Pluot M., Baehrel B., Manfait M., Sockalingum G.D.2006In-vitro analysis of normal and aneurismal human ascending aortic tissues using FT-IR micro spectroscopy. Biochim. Biophys. Acta. 1758, 968–973. 10.1016/j.bbamem.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Bonnier F., Bertrand D., Rubin S., Ventéo L., Pluot M., Baehrel B., Manfait M., Sockalingum G.D.2008Detection of pathological aortic tissues by infrared multispectral imaging and chemometrics. Analyst. 133, 784–790. 10.1039/b717164a. [DOI] [PubMed] [Google Scholar]
- Briley-Saebo K.C., Mulder W.J.M., Mani V., Hyafil F., Amirbekian V., Aguinaldo J.G.S., Fisher E.A., Fayad Z.A.2007Magnetic resonance imaging of vulnerable atherosclerotic plaques: current imaging strategies and molecular imaging probes. J. Magn. Reson. Imaging. 26, 460–479. 10.1002/jmri.20989. [DOI] [PubMed] [Google Scholar]
- Chan K.L.A., Kazarian S.G.2003New opportunities in micro- and macro-attenuated total reflection infrared spectroscopic imaging: spatial resolution and sampling versatility. Appl. Spectrosc. 57, 381–389. 10.1366/00037020360625907. [DOI] [PubMed] [Google Scholar]
- Chan K.L.A., Kazarian S.G., Mavraki A., Williams D.R.2005Fourier transform infrared imaging of human hair with a high spatial resolution without the use of a synchrotron. Appl. Spectrosc. 59, 149–155. 10.1366/0003702053085070. [DOI] [PubMed] [Google Scholar]
- Clark J.M., Glagov S.1985Transmural organization of the arterial media. The lamellar unit revisited. Arterioscler. Thromb. Vasc. Biol. 5, 19–34. [DOI] [PubMed] [Google Scholar]
- Colley C.S., Kazarian S.G., Weinberg P.D., Lever M.J.2004Spectroscopic imaging of arteries and atherosclerotic plaques. Biopolymers. 74, 328–335. 10.1002/bip.20069. [DOI] [PubMed] [Google Scholar]
- Dehghani F., Annabi N., Valtchev P., Mithieux S.M., Weiss A.S., Kazarian S.G., Tay F.H.2008Effect of dense gas CO2 on the coacervation of elastin. Biomacromolecules. 9, 1100–1105. 10.1021/bm700891b. [DOI] [PubMed] [Google Scholar]
- Dumont D., Behler R.H., Nichols T.C., Merricks E.P., Gallippi C.M.2006ARFI imaging for noninvasive material characterization of atherosclerosis. Ultrasound Med. Biol. 32, 1703–1711. 10.1016/j.ultrasmedbio.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Forster B.A., Weinberg P.D.1997Changes with age in the influence of endogenous nitric oxide on transport properties of the rabbit aortic wall near branches. Arterioscler. Thromb. Vasc. Biol. 17, 1361–1368. [DOI] [PubMed] [Google Scholar]
- Geladi P.L.M., Grahn H.F., Burger J.E.Multivariate images, hyperspectral imaging: background and equipment. In Techniques and applications of hyperspectral image analysis Grahn H.F., Geladi P.Eds.2007. pp. 89–126. Chichester, UK:Wiley. [Google Scholar]
- Kačurácová M., Mathlouthi M.1996FTIR and laser-Raman spectra of oligosaccharides in water: characterization of the glycosidic bond. Carbohydr. Res. 284, 145–157. 10.1016/0008-6215(95)00412-2. [DOI] [PubMed] [Google Scholar]
- Kazarian S.G., Chan K.L.A.2003“Chemical photography” of drug release. Macromolecules. 36, 9866–9872. 10.1021/ma035210l. [DOI] [Google Scholar]
- Kazarian S.G., Chan K.L.A.2006Applications of ATR–FTIR spectroscopic imaging to biomedical samples. Biochim. Biophys. Acta Biomembr. 1758, 858–867. 10.1016/j.bbamem.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Keenan M.R.Multivariate analysis of spectral images composed of count data. In Techniques and applications of hyperspectral image analysis Grahn H.F., Geladi P.Eds.2007. pp. 89–126. Chichester, UK:Wiley. [Google Scholar]
- Kodali D.R., Small D.M., Powell J., Krishnan K.1991Infrared micro-imaging of atherosclerotic arteries. Appl. Spectrosc. 45, 1310–1317. 10.1366/0003702914335878. [DOI] [Google Scholar]
- Langer H., Gawaz M.2006Molecular imaging of vulnerable atherosclerotic plaques. Fut. Cardiol. 2, 113–122. 10.2217/14796678.2.1.113. [DOI] [PubMed] [Google Scholar]
- Manoharan R., Baraga J.J., Rava R.P., Dasari R.R., Fitzmaurice M., Feld M.S.1993Biochemical analysis and mapping of atherosclerotic human artery using FT-IR microspectroscopy. Atherosclerosis. 103, 181–193. 10.1016/0021-9150(93)90261-R. [DOI] [PubMed] [Google Scholar]
- Ó Faoláin E., Hunter M.B., Byrne J.M., Kelehan P., McNamara M., Byrne H.J., Lyng F.M.2005A study examining the effects of tissue processing on human tissue sections using vibrational spectroscopy. Vib. Spectrosc. 38, 121–127. 10.1016/j.vibspec.2005.02.013. [DOI] [Google Scholar]
- Orlandi A., Marcellini M., Spagnoli L.G.2000Aging influences development and progression of early aortic atherosclerotic lesions in cholesterol-fed rabbits. Arterioscler. Thromb. Vasc. Biol. 20, 1123–1136. [DOI] [PubMed] [Google Scholar]
- Parker F.S.1975Biochemical applications of infrared and Raman spectroscopy. Appl. Spectrosc. 29, 129–147. 10.1366/000370275774455266. [DOI] [Google Scholar]
- Richards J.P., Weinberg P.D.2000Distribution of disease around the aortocoeliac branch of White Carneau pigeons at different ages. Exp. Mol. Pathol. 68, 95–103. 10.1006/exmp.1999.2293. [DOI] [PubMed] [Google Scholar]
- Sebkhi A., Weinberg P.D.1994Age-related variations in transport properties of the rabbit arterial wall near branches. Atherosclerosis. 106, 1–8. 10.1016/0021-9150(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Sebkhi A., Weinberg P.D.1996Effect of age on the pattern of short-term albumin uptake by the rabbit aortic wall near intercostal branch ostia. Arterioscler. Thromb. Vasc. Biol. 16, 317–327. [DOI] [PubMed] [Google Scholar]
- Spagnoli L.G., Orlandi A., Mauriello A., Santeusanio G., Deangelis C., Lucreziotti R., Ramacci M.T.1991Aging and atherosclerosis in the rabbit. 1. Distribution, prevalence and morphology of atherosclerotic lesions. Atherosclerosis. 89, 11–24. 10.1016/0021-9150(91)90003-L. [DOI] [PubMed] [Google Scholar]
- Staughton T.J., Weinberg P.D.Investigation of the role of endogenous nitric oxide synthesis in determining patterns of arterial wall permeability and diet-induced lipid deposition in the rabbit. In Trends in atherosclerosis research Clark L.V.Eds.2004. pp. 123–144. New York, NY:Nova Biomedical Books. [Google Scholar]
- Tan K.T., Lip G.Y.H.2008Imaging of the unstable plaque. Int. J. Cardiol. 127, 157–165. 10.1016/j.ijcard.2007.11.054. [DOI] [PubMed] [Google Scholar]
- Wang L., Mizaikoff B.2008Application of multivariate data-analysis techniques to biomedical diagnostics based on mid-infrared spectroscopy. Anal. Bioanal. Chem. 391, 1641–1654. 10.1007/s00216-008-1989-9. [DOI] [PubMed] [Google Scholar]
- Wang L., Chapman J., Palmer R.A., Alter T.M., Hooper B.A., van Ramm O., Mizaikoff B.2006Classification of atherosclerotic rabbit aorta samples with an infrared attenuated total reflection catheter and multivariate analysis. Appl. Spectrosc. 60, 1121–1126. 10.1366/000370206778664608. [DOI] [PubMed] [Google Scholar]
- Wang L., Chapman J., Palmer R.A., van Ramm O., Mizaikoff B.2007Classification of atherosclerotic rabbit aorta samples by mid-infrared spectroscopy using multivariate data analysis. J. Biomed. Opt. 12, 024006-1–024006-10. 10.1117/1.2714030. [DOI] [PubMed] [Google Scholar]
- Weinberg P.D.1988Application of fluorescence densitometry to the study of net albumin uptake by the rabbit aortic wall up- and downstream of intercostal ostia. Atherosclerosis. 74, 139–148. 10.1016/0021-9150(88)90200-6. [DOI] [PubMed] [Google Scholar]
- Weinberg P.D.2002Disease patterns at arterial branches and their relation to flow. Biorheology. 39, 533–537. [PubMed] [Google Scholar]
- Weinberg P., Cremers S.2003Changes with age in the atheroprotective effect of dietary l-arginine. Atheroscl. Suppl. 4, 303. 10.1016/S1567-5688(03)91297-9. [DOI] [Google Scholar]
- Wetterholm R., Caidahl K., Volkmann R., Brandt-Eliasson U., Fritsche-Danielson R., Gan L.M.2007Imaging of atherosclerosis in WHHL rabbits using high-resolution ultrasound. Ultrasound Med. Biol. 33, 720–726. 10.1016/j.ultrasmedbio.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Wong P.T.T., Rigas B.1990Infrared spectra of microtome sections of human colon tissues. Appl. Spectrosc. 44, 1715–1718. 10.1366/0003702904417481. [DOI] [Google Scholar]