Abstract
The population-level dynamics of maternally transmitted endosymbionts, including reproductive parasites, depends primarily on the fitness effects and transmission fidelity of these infections. Although experimental laboratory studies have shown that within-host endosymbiont density can affect both of these factors, the existence of such effects in natural populations has not yet been documented. Using quantitative PCR, we survey the density of male-killing Wolbachia in natural populations of Drosophila innubila females from the Chiricahua Mountains of Arizona. We find that there is substantial (20 000-fold) variation in Wolbachia density among wild flies and that within-host Wolbachia density is positively correlated with both the efficacy of male killing and maternal transmission fidelity. Mean Wolbachia density increases three- to five-fold from early to late in the season. This pattern suggests that Wolbachia density declines with fly age, a conclusion corroborated by a laboratory study of Wolbachia density as a function of age. Finally, we suggest three alternative hypotheses to account for the approximately lognormal distribution of Wolbachia density among wild flies.
Keywords: male killing, transmission fidelity, infection prevalence, lognormal distribution, qPCR
1. Introduction
The majority of insect species on Earth harbour one or more species of maternally transmitted endosymbionts (Hilgenboecker et al. 2008). While primary endosymbionts are absolutely required by the host and thus infect all members of the host populations (Baumann 2005), the effects of secondary symbionts—those the host can live without—range from parasitic to apparently commensal to mutualistic (Stouthamer et al. 1999; Haine 2008). The population-level impact of infections by such secondary symbionts depends not only on the fitness effect on individual host insects, but also on the prevalence of infection within the host population.
A variety of models show that the two key variables governing the dynamics of endosymbiont infections are maternal transmission fidelity and the number of female offspring produced by infected versus uninfected females (e.g. Hurst 1991; Turelli 1994). Laboratory studies of Wolbachia and other symbionts have shown that both of these variables are often functions of the within-host density of the endosymbionts (reviewed in Jaenike 2008). Thus, key questions for understanding the dynamics of secondary symbiont infection in natural populations of insects include: (i) do endosymbiont densities vary substantially in nature, (ii) if so, do the observed densities encompass the range over which density-dependent effects on transmission and fitness occur, and (iii) what environmental factors influence endosymbiont density. Answers to these questions will shed light on how environmental conditions can influence insect population dynamics by way of their effects on endosymbiont density. Given the potential importance of within-host endosymbiont density on the dynamics and effects of these infections, it is notable that no studies of endosymbiont density among insects from natural populations have yet been published, although one study has examined Wolbachia density in the offspring of wild-caught female mosquitoes (Ahantarig et al. 2008).
Here we address these questions using Drosophila innubila, a mushroom-feeding member of the quinaria species group that lives in the forests and woodlands of the ‘sky islands’ of southwest North America and that is infected with a male-killing strain of Wolbachia (Jaenike et al. 2003; Dyer et al. 2005). We chose this species for several reasons. First, we already have background information on infection prevalence, transmission fidelity and intensity of male killing in natural populations (Dyer & Jaenike 2004). The mean infection prevalence (35%) is sufficiently high that the approximately 100 per cent male killing represents a substantial selective burden on the population and one that has been expressed for tens of thousands of years (Jaenike & Dyer 2008). Second, experimental manipulation of Wolbachia density in D. innubila has shown that both the transmission fidelity and the intensity of male killing, a proxy for infected female fitness in standard models (Hurst 1991), vary positively with within-host Wolbachia density. Thus, variation in mean Wolbachia density within D. innubila could have substantial population-level effects, if densities in the wild correspond to those at which male killing and transmission fidelity change with Wolbachia density.
Drosophila innubila become active at the onset of the monsoon season, typically in mid to late July in southeast Arizona. The flies feed and breed on the mushrooms brought forth by the summer rains, and they remain active until it becomes too cold in early October, at which point the adult flies go into a long dormant state until the next summer. Because monsoon seasons vary dramatically in intensity and duration from one year to the next, the number of D. innubila generations probably varies from 1 to 3 per year.
We collected D. innubila from the Chiricahua Mountains in southeast Arizona both early and late in the monsoon seasons of 2006 and 2007. We quantified within-host Wolbachia density in infected females, maternal transmission fidelity and offspring sex ratio, the latter serving as a measure of the intensity of male killing. We find that Wolbachia density varies greatly among wild-caught flies and encompasses the range over which transmission fidelity and intensity of male killing vary as a function of density. Consistent with this, we find that both maternal transmission fidelity and offspring sex ratio are correlated with Wolbachia density in these wild females. Finally, the within-host density of Wolbachia is substantially lower in early season flies than in flies collected late in the season, consistent with age-related changes in Wolbachia density we find experimentally in the laboratory. We cast these results in terms of a general selection model on endosymbiont density in natural populations.
2. Material and methods
(a). Fly collections
Drosophila innubila were collected in early August 2006, late September 2006, late July 2007 and late September 2007. Thus, for each of the 2 years, we have one early and one late season collection. For each collection except September 2007 (when flies were primarily obtained at the Southwest Research Station), flies were collected from four sites, all located within 5 km of the Southwest Research Station near Portal, Arizona, on the eastern side of the Chiricahua Mountains. We lured flies using commercial mushrooms (Agaricus bisporus) as bait, and then used a sweep net to catch them and transfer them to vials with sugar agar. Flies were kept at 17°C at the Southwest Research Station until they were shipped (1–4 days after capture) to Rochester, NY, via overnight mail. Min/max thermometers placed in the shipping containers indicated that the flies experienced temperatures in the range of 12–25°C during shipping. Thus, they did not experience unusual or stressful temperatures subsequent to their capture.
(b). Fly processing
Upon arrival at the laboratory, female D. innubila were placed individually in vials with food (instant Drosophila medium (Carolina Biological Supply, Burlington, NC), approximately 1 g of A. bisporus mushroom and a cotton roll) for 5 days to allow them to oviposit. During this time, the flies were kept at 22°C on a 12 L : 12 D light cycle. After the 5-day oviposition period, females were dissected in Drosophila Ringer's solution and ovaries were collected. DNA was isolated from the ovaries using the Puregene DNA purification kit (Qiagen Inc., Valencia, CA) with half the suggested reagents for a single Drosophila melanogaster. Isolated DNA was stored at 4°C until screening.
(c). Wolbachia screening
All females were screened (except those that died before dissection) for Wolbachia, using PCR with the wsp gene (primers 81f and 691r), as described in Zhou et al. (1998). As a control, we also screened for mitochondrial DNA using COI (primers 1490 and 2198; Folmer et al. 1994) and the same PCR conditions as used for wsp. For any ambiguous results, we screened at least three female offspring if available. To assess male infection frequency, 100 wild-caught males were screened from the 2007 collection.
(d). Real-time/quantitative PCR to assess Wolbachia density
To determine the density of Wolbachia in ovaries, we performed real-time/quantitative PCR (qPCR) on all females determined to be infected with Wolbachia. Reactions were performed in duplicate using a 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA), as described in Dyer et al. (2005), with wsp as a target and the single-copy nuclear gene tpi as an endogenous control. Although triplicate reactions are recommended for more precise estimates on individual samples, running duplicate reactions enabled us to test 50 per cent more individuals and thus provides greater statistical power for the same total number of reactions. Our criterion for inclusion of a sample in subsequent statistical analyses was that difference between replicate ΔCT values (as defined below) was less than 0.6, which corresponds approximately to a 50 per cent difference in Wolbachia density between replicate estimates. By this criterion, we excluded 34 out of 793 (4.3%) of our samples. We found no correlation between estimated Wolbachia density and the difference between replicate ΔCT values (r2=0.01); thus, our exclusion criterion did not bias our sample with respect to Wolbachia density. We also quantified Drosophila mitochondrial density, using newly designed primers (COIa-F, 5′-CAGATGACTTGCAACTTTACATGGAGC; COIa-R, 5′-CAGTGGATGAATCCAGCTATAAT) and probe (COIa-P, 5′-FAM-AGCTAAAACAACTCCTGTTAACCCTCCAAC-BHQ1) for the COI gene.
Relative Wolbachia density [W] was estimated as 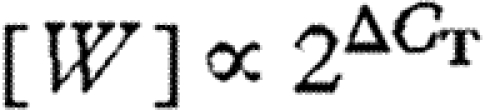 , where ΔCT is the number of PCR cycles required to reach threshold fluorescence for the endogenous control gene (tpi or COI) minus the number of cycles to reach threshold for Wolbachia wsp gene. Because high starting Wolbachia densities require fewer cycles for wsp to reach threshold, high values of ΔCT indicate greater within-host densities of Wolbachia. Since the number of gene copies potentially doubles every PCR cycle, within-host Wolbachia density scales as
, where ΔCT is the number of PCR cycles required to reach threshold fluorescence for the endogenous control gene (tpi or COI) minus the number of cycles to reach threshold for Wolbachia wsp gene. Because high starting Wolbachia densities require fewer cycles for wsp to reach threshold, high values of ΔCT indicate greater within-host densities of Wolbachia. Since the number of gene copies potentially doubles every PCR cycle, within-host Wolbachia density scales as  , if PCR amplification is 100 per cent efficient. The efficiency of qPCR is often less than 100 per cent, and differences in amplification efficiency between wsp and a control gene could bias estimates of Wolbachia density (Giuletti et al. 2001). We therefore tested PCR amplification efficiency of each gene by performing serial dilutions of a sample, carrying out qPCR and examining the slope of the relationship between threshold cycle number and initial concentration (log2 transformed).
, if PCR amplification is 100 per cent efficient. The efficiency of qPCR is often less than 100 per cent, and differences in amplification efficiency between wsp and a control gene could bias estimates of Wolbachia density (Giuletti et al. 2001). We therefore tested PCR amplification efficiency of each gene by performing serial dilutions of a sample, carrying out qPCR and examining the slope of the relationship between threshold cycle number and initial concentration (log2 transformed).
Because endosymbionts have the potential for exponential growth within their hosts, ΔCT is more likely to be normally distributed than  , so we use ΔCT for statistical analysis. To determine which factors influence density, we performed an ANOVA, modelled as ΔCT=year, season (year) and site (year).
, so we use ΔCT for statistical analysis. To determine which factors influence density, we performed an ANOVA, modelled as ΔCT=year, season (year) and site (year).
(e). Offspring sex ratio and transmission efficiency
The total numbers of male and female offspring were determined for all Wolbachia-infected wild-caught females. In 2007, we also counted and sexed all offspring from all uninfected females. All offspring were stored in 100 per cent ethanol at −20°C. The offspring of a subset of the wild-caught females were screened for Wolbachia infection, as described above, in order to determine the relationship between maternal Wolbachia density and transmission fidelity.
Logistic regressions were used to model either offspring sex or infection status as functions of their mother's Wolbachia density (ΔCT). These regressions were not done to assess statistical significance, but rather to infer the relative fitness of infected lineages as a function of Wolbachia density. In models of male-killing dynamics (e.g. Hurst 1991), the fitness of an infected female increases with the level of male killing experienced by her brothers, i.e. with the proportion females in a sibship. We therefore define the male-killing benefit experienced by the offspring of infected females as sMK=proportion of female offspring in a family—0.5, which can range from 0 to 0.5. For simplicity, we limit our analysis to the simplest case, in which the fitness benefit to females increases linearly with the penetrance of male-killing and with perfect fitness compensation. It is likely that fitness compensation in the wild is less than perfect and that there may be a nonlinear relationship between penetrance of male-killing and the fitness benefit to females. In our model, we also ignore the possibility of benefits conferred by Wolbachia that are unrelated to male killing. The infection status of an infected female's daughters provides an estimate of the transmission fidelity of the infection (β). Thus, the fitness of an infected lineage relative to an infected lineage with perfect male killing and transmission is proportional to 2(β×sMK). Using the measured mean and variance of Wolbachia density (ΔCT), we also fitted a normal distribution to the frequency distribution of Wolbachia densities among wild-caught females of D. innubila, pooled across all collections. Together, the fitness function and the frequency distribution of Wolbachia densities enable a crude estimate of the relative fitnesses of infected lineages in the wild.
(f). Effect of age and nutrition on Wolbachia density
We mass reared infected D. innubila by crossing females from an infected isofemale strain (MSR) to males of an uninfected strain (ST1/ST4), both of which are descended from flies collected in the Chiricahua Mountains. To assess the effect of fly age on Wolbachia density, newly emergent flies were placed on instant plus mushroom food (as described above) for 7 days. Subsequently, they were transferred weekly to fresh mushroom–agar medium and held at 8°C. After being aged for periods ranging from 7 to 141 days, the flies (n=87 total) were frozen for subsequent quantification of Wolbachia density using qPCR, as described above.
To assess the effect of nutrient richness on Wolbachia density, newly emergent flies were either placed on sugar agar (nutrient-poor treatment) or instant plus mushroom food (nutrient-rich treatment). After one week, all flies were transferred to fresh instant plus mushroom food. Although this allowed starved flies to feed, this method was necessary to obtain ovaries large enough for dissection and qPCR analysis of Wolbachia density. Flies were dissected at two to three weeks of age and subjected to qPCR to estimate Wolbachia density.
3. Results
(a). Wolbachia infection frequency
The overall infection frequency was 0.344 (n=1523) and 0.318 (n=846) in 2006 and 2007, respectively. These values are in the middle of the range of infection prevalences found previously in this area (Dyer & Jaenike 2004), indicating that the flies used in our sample were collected from populations experiencing typical Wolbachia frequencies. We found significant variation in infection prevalence among sites neither within years (F=0.60, d.f.=6, p=0.73), between early and late collections within years (F=0.06, d.f.=2, p=0.94) nor between years (F=0.13, d.f.=1, p=0.72). The infection frequency of male D. innubila was 0.04 and 0.02 in July and September 2007, respectively. Because this is similar to previous estimates of male infection rate (Dyer & Jaenike 2004), this suggests that the D. innubila populations in 2006 and 2007 harboured ‘typical’ Wolbachia densities, as the production of infected, viable male offspring depends on Wolbachia density (Dyer et al. 2005).
(b). Variation in Wolbachia density among wild-caught flies
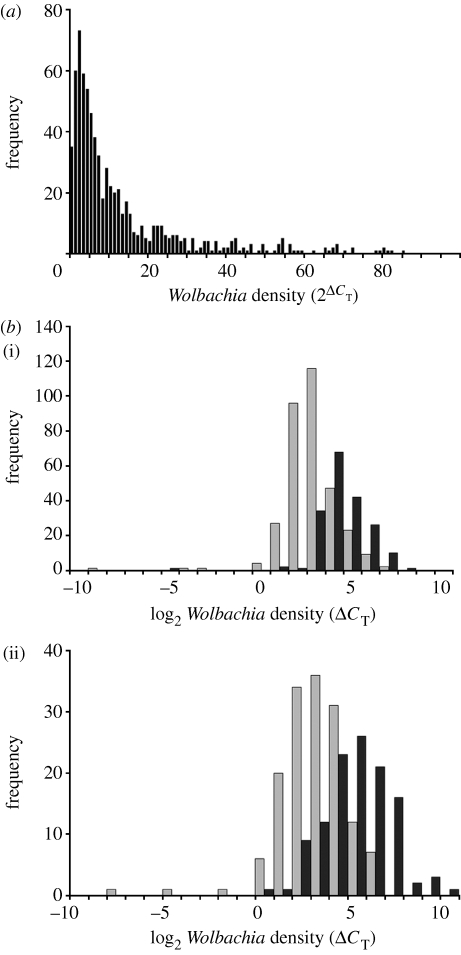
The efficiency of qPCR amplification was not significantly different from 1.0 for any gene, so we assume that for each gene the copy number doubles with every cycle. This enables us to estimate relative Wolbachia density as  . Using a nuclear gene (tpi) as the standard, we find that the relative Wolbachia density varied from 0.035 to 734, i.e. approximately a 20 000-fold difference between the most lightly and most heavily infected individuals in our sample of 759 wild-caught females. If we include only the middle 95 per cent of data, relative Wolbachia density still varied 70-fold, from 1.35 to 96.8 (figure 1a). Using the mtDNA gene COI as the standard, relative Wolbachia density varied by a factor of approximately 100 000, from 6.63×10−5 to 7.06 (n=701). The greater density variation using mtDNA as a baseline may be due to the variable numbers of genomes per mitochondrion and mitochondria per cell. Because the Wolbachia density estimates are correlated between the sets using nuclear and mitochondrial standards (r2=0.27; p<0.0001), we present only the results using the more conservative nuclear DNA (tpi) as a baseline.
. Using a nuclear gene (tpi) as the standard, we find that the relative Wolbachia density varied from 0.035 to 734, i.e. approximately a 20 000-fold difference between the most lightly and most heavily infected individuals in our sample of 759 wild-caught females. If we include only the middle 95 per cent of data, relative Wolbachia density still varied 70-fold, from 1.35 to 96.8 (figure 1a). Using the mtDNA gene COI as the standard, relative Wolbachia density varied by a factor of approximately 100 000, from 6.63×10−5 to 7.06 (n=701). The greater density variation using mtDNA as a baseline may be due to the variable numbers of genomes per mitochondrion and mitochondria per cell. Because the Wolbachia density estimates are correlated between the sets using nuclear and mitochondrial standards (r2=0.27; p<0.0001), we present only the results using the more conservative nuclear DNA (tpi) as a baseline.
Figure 1.
Distribution of Wolbachia densities in ovaries from wild-caught D. innubila in 2006 and 2007. (a) Relative Wolbachia densities within infected females, estimated as 2ΔCT. This includes only females within the middle 95% of infection densities for all collections combined. (b) Log2-transformed Wolbachia density, ΔCT, during four collection periods. Grey bars represent flies from early in the monsoon season and black bars represent flies from late in the monsoon season. Data for (i) 2006 and (ii) 2007 plotted separately.
Mean Wolbachia density did not vary significantly between years (F=0.07; d.f.=1, p=0.77) nor among sites within years (F=0.94, d.f.=6, p=0.47). However, there were highly significant differences between early and late season samples within years (F=88.0, d.f.=2, p<0.0001), with mean Wolbachia density being approximately three times greater among flies collected late in the 2006 season than among those collected early, while in 2007 the Wolbachia density was approximately five times greater in the late versus early season flies (figure 1b). Since there was no difference in density among sites, sites were pooled for subsequent analyses. At any one time, ΔCT (i.e. Wolbachia density on a log scale) exhibited an approximately normal distribution. In other words, Wolbachia density itself appeared to be lognormally distributed.
(c). Offspring sex-ratio and transmission efficiency
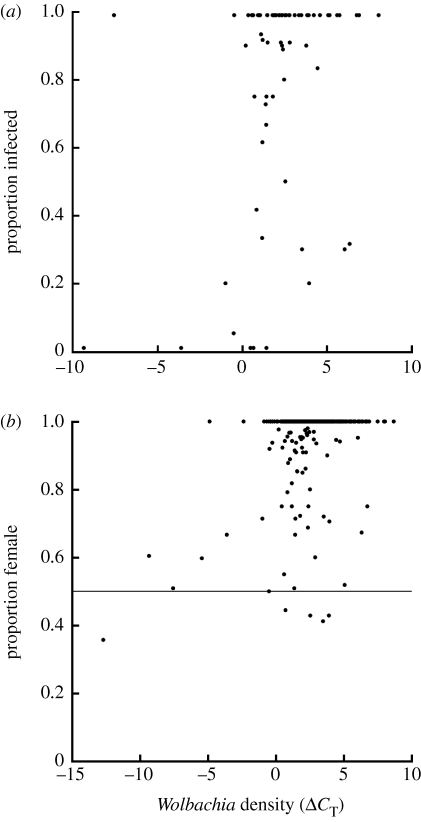
The proportion of female offspring produced by uninfected flies in 2007 was 0.556±0.009 (n=447 wild-caught female parents). Among infected flies in 2006 and 2007, the mean proportion of female offspring was 0.971±0.005 (n=507 female parents). Although there is a great deal of scatter, there are significant positive correlations between Wolbachia density in wild-caught females (ΔCT) and the proportion of female offspring (Spearman ρ=0.20; n=489 families, p<0.0001), and between Wolbachia density and the proportion of female offspring inheriting the Wolbachia infection (Spearman ρ=0.21; n=109 families, p=0.040; figure 2). Because both proportion of female offspring and transmission fidelity are correlated with Wolbachia density, we also find a direct correlation between proportion of female offspring and the transmission fidelity among families produced by wild-caught females (Spearman ρ=0.52; n=109 families; p<0.0001).
Figure 2.
(a) Transmission fidelity and (b) male-killing penetrance as functions of Wolbachia density in wild-caught females. The horizontal line in (b) indicates a 1 : 1 sex ratio, as would be expected if males and females had equal chance of survival.
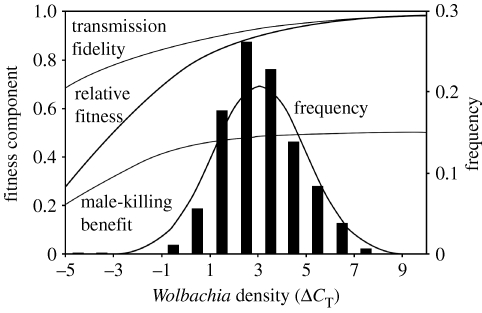
Figure 3 shows the results of logistic regressions of the proportion of female offspring produced by wild-caught females and the proportion of female offspring inheriting the Wolbachia infection as functions of maternal Wolbachia density. The proportion of female offspring is a measure of the fitness benefit to infected cytoplasmic lineages of male killing (sMK), and the proportion of infected offspring is a measure of transmission fidelity (β). From β and sMK, we estimate the fitness of an infected lineage relative to one that has perfect transmission and male killing as 2(β×sMK), and this is also plotted in the figure. Finally, figure 3 shows a normal distribution fitted to the overall Wolbachia density distribution among wild-caught females, pooled across all collections. The fit of ΔCT is close to a normal distribution, but does differ significantly. For the middle 95 per cent of data, ΔCT is much closer to a normal distribution (Wilkes–Shapiro W=0.98) than is 2ΔCt, for which W=0.72; a perfect fit would yield W=1. For the full dataset, W=0.97 and 0.29 for ΔCT and 2ΔCt, respectively. Note that the peak of the Wolbachia density distribution falls just above the densities where β, sMK and relative fitness start to drop off substantially.
Figure 3.
Fitted logistic regressions of transmission fidelity (β) and male-killing benefit (sMK, proportion female offspring in a family—0.5) as functions of maternal Wolbachia density. Also shown is 2(β×sMK) as an indicator of the fitness of an infected cytoplasmic lineage relative to that of an infected lineage with perfect male killing and transmission. A normal distribution has been fitted to the observed frequency distribution (vertical bars) of Wolbachia densities measured in wild-caught females of D. innubila.
(d). Effect of age and nutrition on Wolbachia density
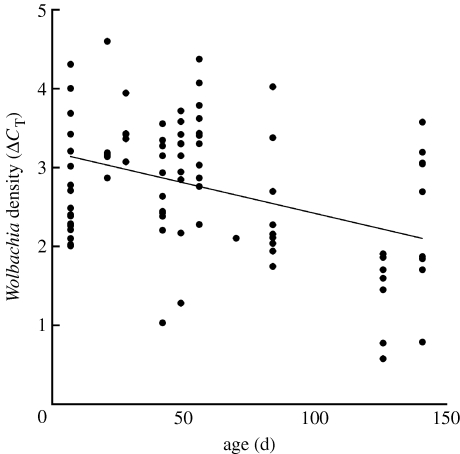
In the age experiment, a small fraction of flies (4%) was not infected with Wolbachia when screened. However, there was no correlation between fly age when assayed and probability of being uninfected (r2=0.0029, p=0.75), suggesting that these flies simply failed to inherit the infection. There was a significant negative correlation between fly age and Wolbachia density (slope=−0.008 ΔCT per day, r2=0.16, n=87, p<0.0001; figure 4). Wolbachia density decreased approximately 50 per cent during the course of experiment. In the nutrient experiment, there was no significant difference in Wolbachia density between flies that had fed on nutrient-poor and nutrient-rich food (t=0.01, p=0.99; mean±s.e. ΔCT=2.25±0.34 and 2.15±0.77 for low- and high-nutrient conditions, respectively).
Figure 4.
Wolbachia density as a function of fly age in laboratory-reared flies.
4. Discussion
We have attempted to quantify within-host density of Wolbachia in natural populations of D. innubila and begun to look into the causes and consequences of that variation among flies. Our measure of within-host density used qPCR to estimate the ratio of Wolbachia to host gene copies. Using a single-copy host nuclear gene (tpi) as a reference, we found that Wolbachia density varied approximately 20 000-fold among female D. innubila from the wild. Using mtDNA COI as a host reference gene suggested an even greater (100 000-fold) range in Wolbachia density. Note that this range excludes flies for which there was more than 50 per cent variation between replicate estimates of Wolbachia density. Thus, experimental qPCR error probably accounts for only a small fraction of the observed variation among flies.
Within collections, most values of ΔCT spanned a range of 4–6 units, which corresponds to 16- to 64-fold variation in Wolbachia density. The ΔCT units can be thought of as equivalent to rounds of Wolbachia replication within flies (increase ΔCT by 1) or half-lives of non-replicating Wolbachia (decrease ΔCT by 1). Thus, even if all flies collected at a given time had started out with the same Wolbachia density, then the variation in ΔCT indicates that the Wolbachia vary among flies by up to six rounds of replication and/or half-lives. Because emergent adult flies probably vary in the Wolbachia densities (Dyer et al. 2005), the observed variation in ΔCT could result from even fewer rounds of replication or half-lives.
A few flies carried extremely low Wolbachia densities. Such flies were often characterized by low fidelity of Wolbachia transmission and low efficiency of male killing. The Wolbachia in such lineages may be living dead, the last vestiges of previously robust infections.
While even our lower estimate of 20 000-fold variation may seem incredible, it is actually less than the 180 000-fold variation in Wolbachia density found among Aedes albopictus mosquitoes (Ahantarig et al. 2008). The mosquitoes studied by Ahantarig et al. were actually the laboratory-reared offspring of wild-caught females, meaning that insect age, nutritional status and rearing conditions were unimportant sources of Wolbachia density variation. Therefore, the density variation evident in the mosquitoes is probably due in part to variation in density among the wild-caught females. Thus, although only two studies of individuals in the wild (or their F1) have been conducted, both have revealed tremendous variation in within-host Wolbachia density. Ours is actually the first estimate of standing variation in Wolbachia density for any natural population of insect. Although Wolbachia density may be influenced by numerous factors, such as insect age or temperature, it is the actual frequency distribution of densities among insects in the wild that is relevant to understanding the dynamics of these infections.
Previous laboratory studies have uncovered a variety of genetic (both host and Wolbachia) and environmental factors that can influence the within-host density of Wolbachia (e.g. Breeuwer & Werren 1993; Hurst et al. 2000; Goto et al. 2006; Mouton et al. 2006; reviewed in Jaenike 2008). In our collections of D. innubila from natural populations, we found consistent and significant differences in mean Wolbachia density between adult flies collected early in the season, when the monsoon rains are just beginning in southeast Arizona, and those collected late in the season, when cooler temperatures and the cessation of the monsoon bring the Drosophila season to an end. The mean density of Wolbachia (2ΔCT) was three times greater in late-season flies than early season flies in 2006, and five times greater in 2007. The natural history of D. innubila in this region suggests that most or all of the flies collected early in the monsoon season are likely to have been very old, having remained dormant since the previous September or October. By contrast, flies collected in late September are most probably the descendants of the early season flies and are thus likely to have been much younger.
We experimentally tested the effect of fly age on Wolbachia density under controlled laboratory conditions. This experiment showed that Wolbachia density declines gradually as a function of fly age, being approximately half as great in flies over 100 days old than in those less than 30 days old. Thus, the low Wolbachia density in early season flies from natural populations is probably a result of their being very old.
Given that the mean Wolbachia density is three to five times lower in the flies collected early in the season and that transmission fidelity and male killing decline with maternal Wolbachia density, one may ask why there was no significant seasonal variation in infection prevalence. One possibility is that the late season flies were two or more generations removed from the early season flies, so that infection prevalence had time to recover from a temporary decline. Alternatively, note that the three- to fivefold difference in mean Wolbachia density occurs within a part of the density distribution where there is little effect of density on either male killing or transmission fidelity (figure 3). Thus, any changes in prevalence resulting from variation in density in this range would be difficult to detect with our samples of several hundred flies.
The two key variables governing the dynamics of endosymbiont infections are the maternal transmission fidelity (β) and the cytoplasmic selection coefficient (s) associated with the infection. Elsewhere, we have shown theoretically how variation in β and s can influence the equilibrium prevalence of an endosymbiont infection (Jaenike 2008). For male-killing infections, the selection coefficient is likely to be a consequence of the male killing itself, whether this comes about through reduced larval competition (Hurst 1991) or reduced sib-mating and inbreeding depression (Werren 1987). In the following, we use the term sMK to refer to the fitness benefit derived specifically from male killing. (The more general term s would incorporate other costs or benefits of the infection, which we have not studied.) We found that both transmission fidelity (β) and offspring sex ratio (and thus sMK) are positively correlated with Wolbachia density among D. innubila collected from natural populations. Thus, the within-host Wolbachia density in natural populations of D. innubila encompasses a range over which β and sMK do change with density. This was not a preordained result: it is entirely possible that even the lowest densities in the wild would be sufficient for perfect transmission and male killing (e.g. Duron et al. 2006; Mouton et al. 2006). Interestingly, the range of Wolbachia densities in wild D. innubila includes the range over which β and sMK change as a function of experimentally manipulated Wolbachia density in the laboratory (Dyer et al. 2005).
The observation that some Wolbachia densities in wild D. innubila are insufficient to bring about perfect transmission and male killing raises the question of how the frequency distribution of densities is determined. We suggest three possibilities: stabilizing selection on density, density drift and a balance between natural selection and density drift. Elsewhere, we have found that, even within a single strain of infected D. innubila, there is a positive correlation in Wolbachia density between mothers and their female offspring (Dyer et al. 2005), indicating that Wolbachia density can be heritable as an epigenetic trait.
With respect to stabilizing selection, natural selection for maximum transmission fidelity and penetrance of a particular phenotype (e.g. male killing or cytoplasmic incompatibility) should favour lineages with the greatest endosymbiont densities. By contrast, if the endosymbiont has any density-dependent adverse effects on the female host, selection will favour less heavily infected host lineages. Depending on the shapes of these functions, cytoplasmic lineage fitness may be the greatest at an intermediate density. Furthermore, because these Wolbachia are male killers, the direction of selection on Wolbachia density is likely to be in opposite directions between host nuclear genes and those of the endosymbiont. In this context, Wolbachia density is considered to be an organismal trait subject to selection.
For density drift, the Wolbachia within a host are considered as a population. Imagine a population of Drosophila flies, each of which experiences or creates conditions that influence rt, the per capita rate of population increase (or decrease) in the resident Wolbachia at time t within a host fly. Thus, the Wolbachia density at time T can be expressed as 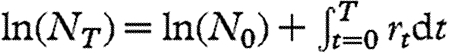 (MacArthur 1960; May 1975). If the values of rt vary randomly through time and among flies, then the integral
(MacArthur 1960; May 1975). If the values of rt vary randomly through time and among flies, then the integral 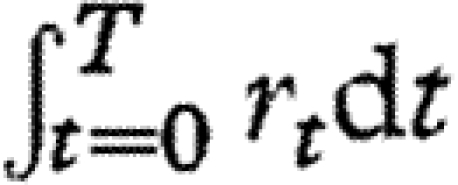 , which is a sum of random variables, will be normally distributed among flies. Thus, if Wolbachia densities in wild-caught flies are determined primarily by cumulative growth rates,
, which is a sum of random variables, will be normally distributed among flies. Thus, if Wolbachia densities in wild-caught flies are determined primarily by cumulative growth rates, 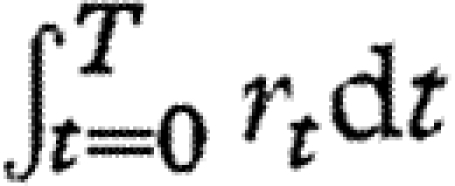 , rather than by initial densities or carrying capacities, then Wolbachia densities should be approximately lognormally distributed among flies.
, rather than by initial densities or carrying capacities, then Wolbachia densities should be approximately lognormally distributed among flies.
Although the accumulated effects of numerous multiplicatively acting factors could account for the lognormal shape of the Wolbachia density distribution, such a process says nothing about where the peak of this distribution should be situated. The observed peak occurs at a relative Wolbachia density of ΔC T values of 3–5 (figure 1). Below this range, transmission fidelity and male-killing penetrance decline notably, both in laboratory-reared flies (Dyer et al. 2005) and among the wild-caught D. innubila examined in the present study (figure 4). Therefore, density drift, as discussed above, may act in conjunction with selection against cytoplasmic lineages in which Wolbachia densities have dropped to levels at which transmission and male killing are incomplete. Such selection could set a lower limit to the position of the overall Wolbachia density distribution.
Regardless of the mechanism responsible for the density distribution, our data show that there is a great deal of variation in Wolbachia density among female D. innubila in natural populations and that this variation is correlated with both transmission fidelity and male killing. We have shown that fly age explains some, but by no means all, of the variation in Wolbachia density. The identification of other factors influencing within-host endosymbiont densities will provide a fuller understanding of the dynamics of infection prevalence in the wild.
Acknowledgements
We would like to thank Dawn Wilson and the staff of the Southwest Research Station for providing an exceptional base of operations for our fieldwork. We also thank members of the Jaenike lab, including Alex Papastrat, Deby Philbrick, Viral Patel, Emily Myers, Meghan Jacobs and Mark Loria for their assistance and comments, and two anonymous referees for very helpful suggestions. This research was supported by an NSF grant DEB-0542094 to J.J. and a Robert and Mary Sproull Fellowship from the University of Rochester to R.L.U.
References
- Ahantarig A., Trinachartvanit W., Kittayapong P.2008Relative Wolbachia density of field-collected Aedes albopictus mosquitoes in Thailand. J. Vector Ecol 33, 173–177 (doi:10.3376/1081-1710(2008)33[173:RWDOFA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Baumann P.2005Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu. Rev. Microbiol 59, 155–189 (doi:10.1146/annurev.micro.59.030804.121041) [DOI] [PubMed] [Google Scholar]
- Breeuwer J.A.J., Werren J.H.1993Cytoplasmic incompatibility and bacterial density in Nasonia vitripennis. Genetics 135, 565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duron O., Labbe P., Berticat C., Rousset F., Guillot S., Raymond M., Weill M.2006High Wolbachia density correlates with cost of infection for insecticide resistant Culex pipiens mosquitoes. Evolution 60, 303–314 [PubMed] [Google Scholar]
- Dyer K.A., Jaenike J.2004Evolutionary stable infection by a male-killing endosymbiont in Drosophila innubila: molecular evidence from the host and parasite genome. Genetics 168, 1443–1455 (doi:10.1534/genetics.104.027854) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer K.A., Minhas M.S., Jaenike J.2005Expression and modulation of embryonic male-killing in Drosophila innubila: opportunities for multilevel selection. Evolution 59, 838–848 [PubMed] [Google Scholar]
- Folmer O., Black M., Hoeh W., Lutz R., Vrijenhoek R.1994DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol 3, 294–299 [PubMed] [Google Scholar]
- Giuletti A., Overbergh L., Valckx D., Decallonne B., Bouillon R., Mathieu C.2001An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods 25, 386–401 (doi:10.1006/meth.2001.1261) [DOI] [PubMed] [Google Scholar]
- Goto S., Anbutsu H., Fukatsu T.2006Asymmetrical interactions between Wolbachia and Spiroplasma endosymbionts coexisting in the same insect host. Appl. Environ. Microbiol 72, 4805–4810 (doi:10.1128/AEM.00416-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haine E.R.2008Symbiont-mediated protection. Proc. R. Soc. B 275, 353–361 (doi:10.1098/rspb.2007.1211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgenboecker K., Hammerstein P., Schlattmann P., Telschow A., Werren J.H.2008How many species are infected with Wolbachia—a statistical analysis of current data. FEMS Microbiol. Lett 281, 215–220 (doi:10.1111/j.1574-6968.2008.01110.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst L.D.1991The incidences and evolution of cytoplasmic male-killers. Proc. R. Soc. Lond. B 244, 91–99 (doi:10.1098/rspb.1991.0056) [Google Scholar]
- Hurst G.D.D., Johnson A.P., Von der Schulenburg J.H.G., Fuyama Y.2000Male-killing Wolbachia in Drosophila: a temperature-sensitive trait with a threshold bacterial density. Genetics 156, 699–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenike J.2008Coupled population dynamics of endosymbionts within and between hosts. Oikos (Early View) 118, 353–362 (doi:101111/j1600-0706.2008.17110.x) [Google Scholar]
- Jaenike J., Dyer K.A.2008No resistance to male-killing after thousands of years of infection. J. Evol. Biol 21, 1570–1577 (doi:10.1111/j.1420-9101.2008.01607.x) [DOI] [PubMed] [Google Scholar]
- Jaenike J., Dyer K.A., Reed L.K.2003Within-population structure of competition and the dynamics of male-killing Wolbachia. Evol. Ecol. Res 5, 1023–1036 [Google Scholar]
- MacArthur R.H.1960On the relative abundance of species. Am. Nat 94, 25–36 (doi:10.1086/282106) [Google Scholar]
- May R.M.1975Patterns of species abundance and diversity. In Ecology and evolution of communities (eds. Cody M.L., Diamond J.M.), pp. 81–120 Cambridge, MA: Belknap Press [Google Scholar]
- Mouton L., Henri H., Bouletreau M., Vavre F.2006Effect of temperature on Wolbachia density and impact on cytoplasmic incompatibility. Parasitology 132, 49–56 (doi:10.1017/S0031182005008723) [DOI] [PubMed] [Google Scholar]
- Stouthamer R., Breeuwer J.A.J., Hurst G.D.D.1999Wolbachia pipientis: microbial manipulators of arthropod reproduction. Annu. Rev. Microbiol 53, 71–102 (doi:10.1146/annurev.micro.53.1.71) [DOI] [PubMed] [Google Scholar]
- Turelli M.1994Evolution of incompatibility-inducing microbes and their hosts. Evolution 48, 1500–1513 (doi:10.2307/2410244) [DOI] [PubMed] [Google Scholar]
- Werren J.H.1987The coevolution of autosomal and cytoplasmic sex ratio factors. J. Theor. Biol 124, 317–334 (doi:10.1016/S0022-5193(87)80119-4) [Google Scholar]
- Zhou W.G., Rousset F., O'Neill S.1998Phylogeny and PCR-based classification of Wolbachia strains using wsp sequences. Proc. R. Soc. Lond. B 265, 509–515 (doi:10.1098/rspb.1998.0324) [DOI] [PMC free article] [PubMed] [Google Scholar]