Abstract
Deterioration of reproductive traits with age is observed in an increasing number of species. Although such deterioration is often attributed to reproductive senescence, a within-individual decline in reproductive success with age, few studies on wild animals have focused on direct fitness measures while accounting for selective disappearance and terminal effects, and to our knowledge none have determined how senescence effects arise from underlying reproductive traits. We show for female great tits that such an approach helps understanding of the onset, impact and architecture of senescence. Cross-sectional analysis of 49 years of breeding data shows annual recruit production to decline from 3.5 years of age, this decline affecting 9 per cent of females each year. Longitudinal analyses, however, show that selective disappearance of poor-quality breeders partly masks senescence, which in fact starts at 2.8 years and affects 21 per cent of females each year. There is no evidence for abrupt terminal effects. Analyses of underlying traits show no deterioration in clutch size, but significant declines in brood size and fledgling number. Furthermore, these traits contribute −9, 12 and 39 per cent to the senescent decline in recruit production, respectively. Besides providing detailed knowledge of the patterns and architecture of senescence in a natural population, these results illustrate the importance of modelling individual variation, and facilitate study of the underlying mechanisms of senescence.
Keywords: age-specific reproduction, senescence, selective disappearance, terminal effects, life history, Parus major
1. Introduction
Senescence is a within-individual age-specific decline in residual reproductive value owing to deteriorating survival probability and/or reproductive performance, in turn caused by a progressive loss of physiological and cellular function late in life (Williams 1957; Hamilton 1966). The evolution of this seemingly maladaptive process has been explained by the idea that unavoidable extrinsic mortality reduces the strength of selection with age (Fisher 1930; Medawar 1952; Hamilton 1966). Evidence for senescence, and support for evolutionary models of senescence, has come from a number of laboratory studies (Rose 1991; Kirkwood 2002), yet findings in a small number of model species studied under artificial conditions may not easily be generalized to natural settings (Partridge & Gems 2007; Kawasaki et al. 2008).
Similar findings for wild populations are relatively scarce, mainly because obtaining sufficient data on known-age individuals reaching old age is difficult and requires long-term monitoring of large populations. In addition, for analyses of age-specific reproductive performance, analytical limitations have forced many studies to rely on cross-sectional analyses. Population-level patterns do not, however, necessarily provide insight into within-individual processes such as senescence (e.g. Vaupel & Yashin 1985; Forslund & Pärt 1995; Cam et al. 2002; Van de Pol & Verhulst 2006), and may reflect changes in the phenotypic composition of a population instead. Indeed, selective disappearance of poor quality individuals has been found to partly mask age-specific declines in reproductive performance measures in red deer Cervus elaphus (Nussey et al. 2006), roe deer Capreolus capreolus (Nussey et al. 2008) and mute swans Cygnus olor (McCleery et al. 2008), although the extent to which estimates of the onset and strength of deterioration were affected was only reported for roe deer. Moreover, reports of the proportion of individuals suffering from performance declines are currently lacking, although this is of paramount importance when evaluating the strength of selection acting against senescence.
While evidence for within-individual phenotypic deterioration is accumulating for various reproductive traits across a range of animal taxa (reviewed by Nussey et al. 2008), few studies are based on an annual measure of reproductive success beyond offspring independence (Newton & Rothery 1997; Brommer et al. 2007; Wilson et al. 2007; Descamps et al. 2008). Instead, most studies focus on traits that are proxies for reproductive success (such as clutch or litter size or offspring weight, e.g. Reid et al. 2003; Bowen et al. 2006; Balbontin et al. 2007; McCleery et al. 2008; Reed et al. 2008), or physiological measures (e.g. Cichon et al. 2003; Angelier et al. 2007; Palacios et al. 2007; Broggi et al. in press). As performance declines may vary between different components of the reproductive cycle, our knowledge of the fitness cost of reproductive senescence in natural populations is still, therefore, very limited. Moreover, scattered information on the age-specificity of different reproductive traits in different populations does not allow for comparison of the onset and rate of deterioration between these traits. It also does not reveal the extent to which component traits contribute to a decline in annual reproductive success, i.e. the architecture of reproductive senescence. Analysing whether older individuals suffer from reduced performance at all reproductive stages, or experience increasing deterioration at each successive stage of a breeding cycle, would therefore suggest hypotheses as to the origin of senescence effects and provide novel insights into which proximate mechanisms underlying reproductive senescence are most likely to be important.
Finally, most studies of senescence assume performance declines with age to be gradual, but theoretical and empirical studies have also shown the potential for terminal effects to occur, either negative through terminal illness (Coulson & Fairweather 2001; Rattiste 2004), or positive through terminal investment (Clutton-Brock 1984; Bonneaud et al. 2004). Such terminal effects can accompany gradual senescence (e.g. Rattiste 2004), but especially in short-lived species, where there are relatively few data points to determine the slope of age-specific deterioration, they could obscure patterns of age-related reproduction if not accounted for.
In this paper, we present analyses of age-specific reproduction using a large and long-term dataset spanning 49 years of breeding in a small passerine bird species, the great tit (Parus major). In these analyses, reproductive success is measured as the number of recruits (offspring that were recaptured as breeding birds), a measure that is relatively close to the contribution a reproductive attempt makes to parental fitness. We first partition population-level patterns of age-specific reproductive success to those due to senescence and those due to selective disappearance, while testing for terminal effects. We then study the rates of deterioration in recruit production and three underlying reproductive traits, and determine the proportional contribution of the component traits to the decline in recruit production. Taken together, these analyses provide us with novel insights into the patterns and architecture of senescence in a natural bird population.
2. Material and methods
(a). Study population and data collection
The great tit is a small passerine bird abundant in European woodlands and gardens. As a hole-nester, it readily accepts nest-boxes for breeding, which allows monitoring of the whole population if an excess of nest-boxes is provided (Perrins 1979). The data we present here come from a long-term study population in the ca 380 ha mixed deciduous woodland of Wytham Woods, Oxfordshire, UK. From 1960 to 2008, 1020 nest-boxes have been available, of which on average 217 were used for breeding by great tits each year.
Every breeding season, nest-boxes are checked weekly to obtain records of laying date, clutch size (i.e. number of eggs laid), hatching date, brood size (i.e. number of chicks hatched, calculated as the difference between the clutch size and the number of eggs left in the nest) and number of fledglings. Chicks are ringed with individually numbered metal rings at 15 days of age and monitored until fledging at approximately 20 days. Parents are trapped at the nest while feeding their chicks, and identified by their ring number, or ringed if they are newly immigrated birds. Parental age is based on birth year for locally born birds, or plumage characteristics (Svensson 1994) at first catching for immigrants. Although immigration rates are high (47%, McCleery et al. 2004), most immigrants enter the population as yearlings and exact age was therefore known for 92 per cent of birds. Immigrants first caught with adult plumage are assigned a minimal age of 2 years. Birds that are not found breeding for two consecutive years are assumed to have died and their age at last reproduction (ALR) is taken as an approximation for their disappearance from the population. Note that if old birds are more likely to fail early in the breeding cycle (but see Dhondt 1985), or refrain from breeding completely (e.g. Dhondt 1985) ALR does not necessarily reflect longevity.
(b). Data selection
As extra-pair paternity occurs in our population (14% of offspring, Blakey 1994) and is known to vary with paternal age in some great tit populations (Lubjuhn et al. 2007), data on age-specific reproductive performance are less accurate for males than females. In addition, our female life-history trajectories are more complete, since, over the whole study, 90 per cent of females, but only 75 per cent of males of breeding pairs were caught at the nest. We therefore focused our analyses on females.
Wytham great tits very rarely produce a second brood (McCleery & Perrins 1989), so we restricted our dataset to first broods, which were taken to be those initiated within 30 days of the first clutch of the year (Van Noordwijk et al. 1995). Known replacement clutches within this selection, or breeding attempts which were subject to experimental manipulation, were included for assessment of ALR, but excluded from analyses of reproductive performance. In cases of experimental manipulation, we assumed no carry-over effects to the next breeding season (in agreement with results for our population: Doligez et al. 2002) to preserve sample size.
As a fitness measure, we used the number of young from a breeding attempt that recruited into the breeding population. Such local survival only reflects fitness if emigration of chicks is independent of state (as shown for Wytham by Verhulst et al. 1997), and in our case independent of maternal age. We did not have data to relate emigration to maternal age directly, but we could test for a maternal age-effect on natal dispersal distance, which has, for example, been found in common lizards Lacerta vivipara (Ronce et al. 1998). We did not find any evidence for such a relationship (see electronic supplementary material, (a) and table S1), which increased our confidence that local recruitment is an unbiased measure of fitness with respect to maternal age. We restricted our dataset to breeding attempts up to and including 2006 to allow for detection of recruits in subsequent years.
Finally, breeding attempts for which data on parameters included in the analyses (clutch size, brood size, and number of fledglings) were unknown, or breeding attempts by birds that were still alive in 2008, and hence could not be assigned an ALR, were excluded from our dataset. In total, this data selection resulted in 7341 breeding attempts by 4935 females. Breeding age ranged from 1 to 9, averaging 1.81 years.
(c). Statistical analyses
(i). Age-specific reproductive performance
We analysed the maternal age effect on recruitment using additive cross-classified random effect models with Poisson distributed errors and a Markov chain Monte Carlo estimation algorithm with 100 000 iterations (Browne et al. 2007). For the cross-sectional analysis, these models included two random effects accounting for spatio-temporal environmental heterogeneity: year and sector of the wood (Wytham is subdivided into nine sectors which differ in vegetation type and management, see Minot & Perrins 1986). In addition, they included several fixed effects that were taken into account as known sources of variation for reproductive performance at different levels. These were (i) year quality estimated as the total average fledgling number, (ii) nest-box type (wood versus woodcrete, two-level class variable affecting predation risk, McCleery et al. 1996), (iii) local breeding density, and (iv) female status (locally born versus immigrant, two-level class variable). Linear and quadratic effects of maternal age were tested by including both age and its squared term as covariates. Full models were simplified by backward stepwise removal of non-significant terms. Significance (p<0.05, two-tailed) was assessed using the Wald statistic.
For the age effect to reflect an unbiased estimate of within-individual change we extended the population-level model with female identity as a random effect, and ALR as a fixed effect (and these were kept in the model independent of their significance). Female identity controls for the use of repeated, non-independent observations on the same individual, while ALR accounts for potential differential disappearance from the breeding population by individuals of different quality (Van de Pol & Verhulst 2006). A quadratic term for ALR tests for nonlinear effects of reproductive lifespan on performance, such as expected when there is both selective disappearance and a trade off between reproduction and survival (e.g. Reid et al. 2003). Interactions between age, age2 and ALR test whether age effects differ between birds of different reproductive lifespan (table 1 provides an overview of key parameters and their interpretation in the various models). In this model, initially all age classes were included, but as quadratic effects cause one end of the curve to be constrained by the other, we repeated the analysis excluding 21 breeding attempts at ages 7, 8 and 9 to ensure our results were not driven by 18 females of exceptionally long reproductive lifespan.
Table 1.
Parameters used, and their interpretation, in models testing for a relation between age and reproductive performance.
| model | fixed effect | interpretation |
|---|---|---|
| population-level | age, age2 | cross-sectional linear/quadratic change in phenotype with age |
| individual-level | age, age2 | average within-individual linear/quadratic change in phenotype with age |
| ALR, ALR2 | covariation between reproductive lifespan and phenotypic quality | |
| age×ALR | age effects differ for individuals of different reproductive lifespan | |
| terminal effect | age, age2 | average within-individual linear/quadratic change in phenotype with age |
| ALR, ALR2 | covariation between reproductive lifespan and phenotypic quality | |
| age×ALR | age effects differ for individuals of different reproductive lifespan | |
| LR | terminal effect (e.g. through terminal illness or investment) | |
| age×LR | terminal effects are age-dependent |
To investigate terminal effects, we added a two-level class variable to the age model, which defined breeding attempts as being last or not (LR, table 1). If LR were to replace the age effects, this would suggest terminal effects to cause apparent senescence. Alternatively, if LR were to remain in the model besides any age effects, this would suggest that gradual senescence occurs in conjunction with an either more or less successful final breeding attempt. Age×LR interactions would indicate this to be the case for birds of certain lifespan only (as in common gulls Larus canus, Rattiste 2004). If LR were to drop from the model, any age effects found are likely to be gradual and unobscured by terminal effects.
(ii). Senescence
As senescence, by definition, refers to a decline in fitness with age, a significant quadratic effect of the age parameter in models testing for age-specificity of reproductive performance does not necessarily prove its occurrence (Reid et al. 2003; Keller et al. 2008). Instead, such an effect might be driven by improvement early in life, if performance were to level off (but not decline) at later ages. We therefore used the parameter estimates and their standard errors from the minimal adequate model from §1 to calculate at which age (± s.d.) reproductive performance showed its statistical peak. We then performed a post-peak analysis on 1504 old-age breeding records from 1029 females, using the minimal adequate model resulting from the analysis described above, but excluding the quadratic effect of age. If the single age term were to be significantly negative in this model, this would demonstrate that performance declines with age and hence that senescence occurs.
(iii). Partitioning senescence
We repeated the individual-level modelling of recruitment for three underlying reproductive traits: clutch size, brood size and number of fledglings, using similar models, but with normal error distributions. We then investigated the proportional contribution of each of these phases to the age effect found on the recruitment level by re-running the senescence model of §2, while sequentially adding the offspring number in these phases to quantify the change in the parameter estimate for the age effect. The rationale behind this approach is that the addition of a reproductive phase to the model of senescence in recruit production will cause the age-coefficient to change, with the size of this change revealing the importance of that reproductive phase for causing or masking senescence in recruit production.
All models were fitted using MLwiN v. 2.02 (Browne et al. 2001; Rasbash et al. 2005).
3. Results
(a). Age-specific reproductive performance
A cross-sectional analysis of the relationship between age and the production of recruits showed that female great tits exhibit age-specific variation in their reproductive performance. There was a significantly negative quadratic effect of maternal age (figure 1, circles; table 2), and the inflection point of this relationship occurred at the age of 3.45±0.02 years. The minimal adequate model also contained significantly positive fixed effects of year quality and female status (locally born birds produce more recruits than immigrants, in agreement with earlier results, e.g. Wilkin et al. 2007), and a negative fixed effect of local breeding density (table 2).
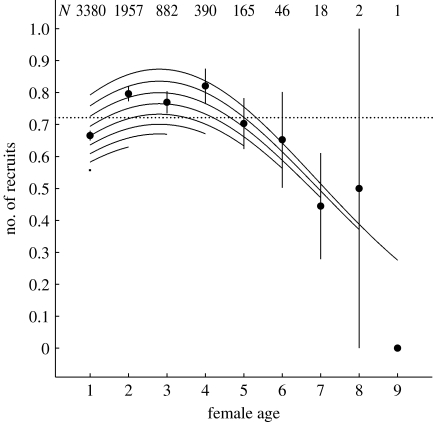
Figure 1.
Age-specific reproductive performance in female great tits. The circles show the population-level average number of recruits produced for each maternal age with standard errors and sample size; the dotted line shows the overall mean. The curves are calculated from the minimal adequate model describing within-individual processes (table 2). Lines represent birds of different reproductive lifespan and the shape of the curves reflects within-individual improvement and senescence.
Table 2.
Results from models testing the effects of age, selective disappearance and selected fixed effects on recruitment at the population-level (left) and at the individual-level (right). (Estimates for random effects are marked by (r). (Shown are parameter estimates with standard errors and significance (*p<0.05, **p<0.01, ***p<0.001). n.a. means a term was not fitted to the model; n.s. implies a term was removed from the model because p>0.05.).)
| population-level model | individual-level model | |||||
|---|---|---|---|---|---|---|
| parameter | est | s.e. | χ12 | est | s.e. | χ12 |
| age | 0.22 | 0.05 | 24.01*** | 0.17 | 0.05 | 11.14*** |
| age2 | −0.03 | 0.01 | 15.97*** | −0.03 | 0.01 | 12.17*** |
| ALR | n.a. | — | — | 0.04 | 0.02 | 9.26** |
| ALR2 | n.a. | — | — | n.s. | — | — |
| ALR×age | n.a. | — | — | n.s. | — | — |
| ALR×age2 | n.a. | — | — | n.s. | — | — |
| status | 0.11 | 0.03 | 14.41*** | 0.11 | 0.03 | 12.80*** |
| density | −0.15 | 0.06 | 7.15** | −0.16 | 0.06 | 6.41* |
| year quality | 0.29 | 0.06 | 22.02*** | 0.31 | 0.06 | 27.59*** |
| female (r) | n.a. | — | — | 0.10 | 0.05 | — |
| year (r) | 0.15 | 0.04 | — | 0.15 | 0.04 | — |
| area (r) | 0.02 | 0.01 | — | 0.02 | 0.01 | — |
To investigate which mechanisms (within-individual change and/or selective disappearance) underlie the population-level pattern of age-specific recruitment, we added female identity, ALR and the interactions between age and ALR to the model. Age2, now reflecting the within-individual process, remained significantly negative (figure 1, curves; table 2). ALR, however, also significantly affected recruitment, reflecting a positive correlation between female reproductive lifespan and annual recruit production, or, in other words, selective disappearance of poor-quality breeders. The parameter estimates and standard errors of the age effects in the individual-level model indicated peak recruit production to be at an average age of 2.80±0.02, almost a year earlier than in the cross-sectional analysis. Interactions between ALR and age or age2 did not contribute significantly to the model, implying that age effects are independent of reproductive lifespan (table 2). Fixed effects on recruitment were again associated with year quality, female status and breeding density.
Removing the 21 breeding records for females aged 7, 8 and 9 did not alter the qualitative conclusions of the model (see electronic supplementary material, (b) and table S2). We thus had no reason to expect our results to be driven by a few females of exceptionally long reproductive lifespan, and included all breeding records of all females in all further analyses. Including the binary factor ‘last reproduction’ (LR) and its interactions with age and ALR in the full age-model showed no sign of terminal effects influencing recruit production: all interactions, as well as the main effect (LR: −0.07±0.05, χ12=2.14, p=0.14), were dropped from the model, leading the minimal adequate model to be identical to the within-individual age-model in table 2.
(b). Senescence
To test whether the significant quadratic effect of age on recruitment can justifiably be attributed, in part, to senescence, we analysed breeding records of females aged 3 years and older. In this reduced dataset, there were still significant fixed effects of year quality, breeding density and ALR, whereas the effect of female status disappeared (status: 0.10±0.07, χ12=2.06, p=0.15). More importantly, there still was a negative linear effect of female age (age: −0.11±0.05, χ12=5.00, p=0.03), revealing senescence to occur in female great tits. The interaction between age and ALR was dropped from the model (age×ALR: −0.02±0.02, χ12=0.46, p=0.50), indicating that females of different reproductive lifespan senesce at similar rates.
(c). Partitioning senescence
Repeating the individual-level age-model for clutch size, brood size and number of fledglings showed that the age and age2 effects were significant and qualitatively similar for all traits (table 3). In contrast to recruitment, there was no ALR effect for the underlying reproductive traits (figure 2), and no effect of female status. Besides similar fixed effects of year quality and local breeding density, there was an additional positive effect of nest-box type, showing that reproductive output increased after nest-boxes were changed from wood to woodcrete to relieve predation pressure (also see McCleery et al. 1996).
Table 3.
Results from models testing the effects of age, selective disappearance and several fixed effects on clutch size (left), brood size (middle) and number of fledglings (right). (Estimates for random effects are marked by (r). (Shown are parameter estimates with standard errors and their significance (*p<0.05, **p<0.01, ***p<0.001). n.s. means a term was removed from the model because p>0.05.).)
| clutch size | brood size | no. of fledglings | |||||||
|---|---|---|---|---|---|---|---|---|---|
| parameter | est | s.e. | χ12 | est | s.e. | χ12 | est | s.e. | χ12 |
| age | 0.52 | 0.05 | 108.27*** | 0.62 | 0.07 | 92.59*** | 0.63 | 0.10 | 39.16*** |
| age2 | −0.06 | 0.01 | 58.51*** | −0.10 | 0.01 | 86.67*** | −0.11 | 0.02 | 40.89*** |
| ALR | −0.02 | 0.02 | 0.80 | −0.02 | 0.02 | 0.50 | 0.04 | 0.03 | 1.53 |
| ALR2 | n.s. | — | — | n.s. | — | — | n.s. | — | — |
| ALR×age | n.s. | — | — | n.s. | — | — | n.s. | — | — |
| ALR×age2 | n.s. | — | — | n.s. | — | — | n.s. | — | — |
| nest box type | 0.35 | 0.15 | 5.69* | 0.52 | 0.15 | 11.26*** | 0.34 | 0.09 | 13.36*** |
| density | −0.25 | 0.05 | 30.66*** | −0.31 | 0.05 | 36.22*** | −0.30 | 0.07 | 18.06*** |
| year quality | 0.57 | 0.07 | 67.55*** | 0.62 | 0.07 | 74.38*** | 0.98 | 0.04 | 766.25*** |
| female (r) | 1.19 | 0.05 | — | 1.04 | 0.06 | — | 1.23 | 0.15 | — |
| year (r) | 0.13 | 0.03 | — | 0.16 | 0.04 | — | 0.00 | 0.00 | — |
| area (r) | 1.21 | 0.03 | — | 2.16 | 0.06 | — | 5.91 | 0.16 | — |
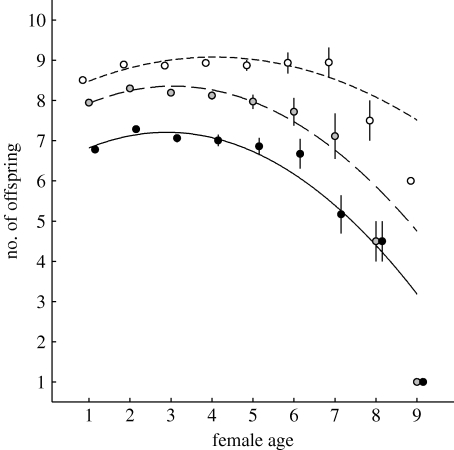
Figure 2.
Age-specificity of three reproductive stages underlying recruitment: clutch size (short dash line, white circles); brood size (long dash line, grey circles); and number of fledglings (solid line, black circles). Circles show the population-level average number of eggs, hatchlings and fledglings produced for each maternal age with standard errors. The curves are calculated from the minimal adequate models describing within-individual processes (table 3). Note that, in contrast to the effect for recruitment in figure 1, there is no effect of reproductive lifespan on any of these characters, but that the age of peak performance is earlier for successive stages in the breeding cycle.
Peak performance occurred at 4.06, 3.05 and 2.90 years of age for clutch size, brood size and number of fledglings respectively, and post-peak declines were found for brood size (age: −0.49±0.11, χ12=18.78, p<0.001) and number of fledglings (age: −0.36±0.09, χ12=14.95, p<0.001), while being non-significant for clutch size (age: −0.05±0.15, χ12=0.09, p=0.77).
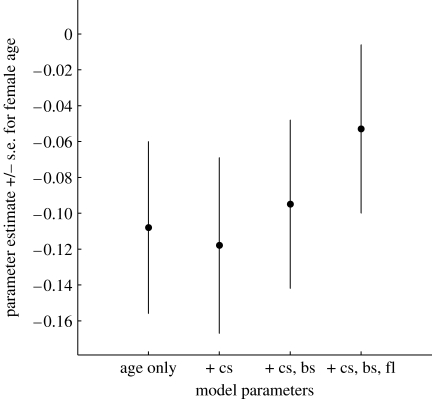
To investigate the proportional contribution of clutch size, brood size and number of fledglings to the age effect on recruit production, we first added clutch size to the minimal adequate model described in §3b. This increased the negative effect of age on recruit production from −0.108±0.048 (χ12=5.00, p=0.03) to −0.118±0.049 (χ12=5.88, p=0.02), an increase of (100×((0.108−0.118)/0.108))=9% (figure 3). The late peak in clutch size, in combination with the non-significant decline after the peak, therefore slightly masks female senescence in recruit production. Adding brood size to the preceding model resulted in an age parameter of −0.095±0.047 (χ12=4.05, p=0.04), a reduction of (100×((0.095−0.108)/0.108))=12%. Finally, adding the number of fledglings reduced the effect of age by another (100×((0.053−0.108)/0.108)) −12%=39% to −0.053±0.047 (χ12=1.25, p=0.26).
Figure 3.
Parameter estimates (±s.e.) for the effect of maternal age on recruitment in senescence models including different breeding stages as covariates. cs, clutch size; bs, brood size; fl, number of fledglings. (See text for statistics.)
4. Discussion
Previous investigation of our study population had already suggested age-specificity, including a late-life decline, for female recruit production at the population level (Perrins & Moss 1974), and our replication with a sixfold larger dataset confirmed this: cross-sectionally, the number of recruits produced declines from 4 years, which encompasses on average 9 per cent of breeding females each year. The decline does not, however, provide unbiased estimates of the rate and age at onset of within-individual reproductive senescence, as it is confounded by selective disappearance. We find that females with low reproductive success cease reproduction at a younger age than females with higher reproductive success, and their disappearance from the breeding population inflates initial improvement, but partly masks senescence. Within individuals, deterioration of recruit production starts on average at the age of three, and affects 21 per cent of breeding females each year. That the process of senescence hence occurs for more than twice as many individuals as would be inferred from a cross-sectional analysis, suggests that senescence may be more common in wild populations than is often assumed.
Our finding of a positive relationship between reproductive lifespan and average annual recruit production in itself is not new (e.g. Van Noordwijk & Van Balen 1988), but at a within-individual level it has not yet been established for a reproductive fitness measure directly affecting population dynamics. We can therefore only compare our result to ALR effects tested for underlying reproductive traits, for which the results appear equivocal. Selective disappearance was found to mask performance declines at the population level for offspring birth weight, but not calving date in red deer (Nussey et al. 2006). Similarly, age-specific laying date and clutch size improved with ALR in mute swans (McCleery et al. 2008), but not in barn swallows Hirundo rustica (Balbontin et al. 2007). Our own results for reproductive traits underlying recruitment show that, for any given age, female great tits of longer reproductive lifespan do not lay more eggs, hatch more chicks or raise more fledglings than females of shorter lifespan. That despite their seemingly similar breeding effort up to fledging, they do differ in the resulting success in terms of recruited offspring, could either reflect a negative relationship between maternal reproductive lifespan and a chick's probability of emigrating from our study population, or a positive relationship between survival of a mother and her offspring. We did not have data to relate emigration to maternal ALR directly, but we did add ALR to our model of natal dispersal distance (see electronic supplementary material, (a) and table S1). We found a significant, negative relationship (ALR: −0.32±0.12√m yr−1, p=0.01), which indicates that offspring of mothers with long reproductive lifespan disperse less far within the study site. Why this is so, and whether this translates to a negative relationship between maternal ALR and a chick's probability of emigrating from the population, or whether maternal breeding dispersal and chick natal dispersal are also related remain intriguing questions for future investigation. In addition, our finding of a positive association between recruit production and reproductive lifespan, comparable to recent findings in kittiwakes Rissa tridactyl a (Cam et al. 2002) and four species of ungulate (Weladji et al. 2008; Hamel et al. 2009), raises the question of how quality differences between females are maintained. The absence of heritability of reproductive lifespan or recruit production (e.g. McCleery et al. (2004) for our population) provides an answer, but raises the new question of how quality differences between females arise.
Unlike some bird species (e.g. black-legged kittiwakes and common gulls: Coulson & Fairweather 2001; Rattiste 2004), Wytham great tits show no sign of net terminal effects, either negative through terminal illness, or positive through terminal investment. Although in principle it is possible that compared to kittiwakes and gulls, great tits are too short-lived for our models to adequately separate gradual senescent effects from abrupt terminal effects, our dataset did comprise 341 females which bred at least twice after the estimated onset of senescence. With the effect size of the term LR being almost half that of the effect of age, we can therefore be confident that late-life age-specific recruit production is best characterized by a gradual, linear decline.
The finding that a substantial proportion of female great tits undergo at least one breeding season of deteriorated reproductive success because of senescence raises the question of its proximate mechanisms. We tested for within-individual age-specificity of clutch size, brood size and number of fledglings, and quantified the proportional contribution of these traits to the fitness-level senescence effect. We found significant quadratic relations that compare to those found for performance traits of other species (reviewed by Nussey et al. 2008), although initial increases with age were followed by performance declines for brood size and number of fledglings only. The fact that clutch size peaks late and does not deteriorate with age is consistent with it not contributing to the overall senescence effect. Instead, its change with age even masked a small part of the senescent decline in recruit production. Late-life declines in brood size and fledgling number did, however, explain 12 and 39 per cent of the effect of age on recruitment respectively, leaving 49 per cent unexplained.
Half of the decline in female reproductive success with age therefore originates from egg and nestling mortality in the pre-fledging period, during which the partner could potentially alleviate female senescence effects (see Rauser et al. (2005) for an example in Drosophila melanogaster). The brood size effect suggests that fertilization success (Pizzari et al. 2008), incubation behaviour (Catry et al. 2006) or egg composition and quality might play a role. In great tits the latter two are female-specific traits (although the male may affect them via territory quality or nuptial feeding), but fertilization success mainly depends on male sperm quality, which has been shown to decline with age in wild birds (e.g. Møller et al. 2009). Chick provisioning is also shared with the male, but little is known about age-specific provisioning behaviour in male or female birds. Detailed future study of male reproductive senescence, and interactions between male and female age-effects, will have to add to our understanding of the proximate mechanisms underlying senescence, as well as that of mate choice and sexual selection.
The remaining 49 per cent of female reproductive senescence could find its origin in the post-fledging period, where the reproductive quality difference between females of different lifespan arises too. At present, comparatively little is known about what happens during this period, as birds are much harder to individually follow, but the substantial rate of chick mortality during post-fledging care (Naef-Daenzer et al. 2001), as well as its duration (Verhulst & Hut 1996) suggest that there is ample scope for further parental age effects. Fledgling condition may play a role too, although in our study fledgling mass was independent of maternal age (S. Bouwhuis, B. C. Sheldon, S. Verhulst & A. Charmantier 2009, unpublished analysis). Either way, its importance for senescence and individual quality does call for further investigation of this reproductive phase.
In conclusion, our results illustrate the importance of modelling individual variation in the study of senescence, show an important part of females in our population to undergo late-life declines in reproductive success, and point to the stages at which this effect is most strongly expressed. This contradicts early ideas that senescence is negligible in wild birds (Holmes et al. 2003), but confirms its ubiquity in a range of vertebrate species (Jones et al. 2008).
Acknowledgments
This paper is dedicated to the memory of Robin McCleery, who besides being a valued colleague and friend, collected part of the data, managed the database and provided us with statistical advice that greatly improved the manuscript. We are very grateful to the many generations of Wytham fieldworkers without whom this study would not have been possible, and also thank William Browne, Olivier Gimenez, Kate Lessells, Martijn Van de Pol, Oscar Vedder and Marcel Visser for very helpful discussion and statistical advice. Hannu Pietiäinen and four anonymous referees provided comments that greatly improved the manuscript. S.V. was supported by an NWO Vici-grant, and the collection of data has been funded by grants and studentships from the UK Natural Environment and Biotechnology and Biological Sciences Research Councils (NERC and BBSRC, respectively).
References
- Angelier F., Weimerskirch H., Dano S., Chastel O.2007Age, experience and reproductive performance in a long-lived bird: a hormonal perspective. Behav. Ecol. Sociobiol 61, 611–621 (doi:10.1007/s00265-006-0290-1.) [Google Scholar]
- Balbontin J., Hermosell I.G., Marzal A., Reviriego M., de Lope F., Moller A.P.2007Age-related change in breeding performance in early life is associated with an increase in competence in the migratory barn swallow Hirundo rustica. J. Anim. Ecol 76, 915–925 (doi:10.1111/j.1365-2656.2007.01269.x.) [DOI] [PubMed] [Google Scholar]
- Blakey J.K.1994Genetic evidence for extra-pair fertilizations in a monogamous passerine, the great tit Parus major. Ibis 136, 457–462 (doi:10.1111/j.1474-919X.1994.tb01122.x.) [Google Scholar]
- Bonneaud C., Mazuc J., Chastel O., Westerdahl H., Sorci G.2004Terminal investment induced by immune challenge and fitness traits associated with major histocompatibility complex in the house sparrow. Evolution 58, 2823–2830 [DOI] [PubMed] [Google Scholar]
- Bowen W.D., Iverson S.J., Mcmillan J.I., Boness D.J.2006Reproductive performance in grey seals: age-related improvement and senescence in a capital breeder. J. Anim. Ecol 75, 1340–1351 (doi:10.1111/j.1365-2656.2006.01157.x.) [DOI] [PubMed] [Google Scholar]
- Broggi J., Hohtola E., Koivula K., Orell M., Nilsson J.-Å.In press Idle slow as you grow old: longitudinal age-related metabolic decline in a wild passerine. Evol. Ecol. (doi:10.1007/s10682-009-9299-z) [Google Scholar]
- Brommer J.E., Wilson A.J., Gustafsson L.2007Exploring the genetics of aging in a wild passerine bird. Am. Nat 170, 643–650 (doi:10.1086/521241.) [DOI] [PubMed] [Google Scholar]
- Browne W.J., Goldstein H., Rasbash J.2001Multiple memberships multiple classification (MMMC) models. Stat. Model 1, 103–124 (doi:10.1191/147108201128113.) [Google Scholar]
- Browne W.J., McCleery R.H., Sheldon B.C., Pettifor R.A.2007Using cross-classified multivariate mixed response models with application to life history traits in great tits (Parus major). Stat. Model 7, 217–238 [Google Scholar]
- Cam E., Link W.A., Cooch E.G., Monnat J.Y., Danchin E.2002Individual covariation in life-history traits: seeing the trees despite the forest. Am. Nat 159, 96–105 (doi:10.1086/324126.) [DOI] [PubMed] [Google Scholar]
- Catry P., Phillips R.A., Phalan B., Croxall J.P.2006Senescence effects in an extremely long-lived bird: the grey-headed albatross Thalassarche chrysostoma. Proc. R. Soc. B 273, 1625–1630 (doi:10.1098/rspb.2006.3482.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichon M., Sendecka J., Gustafsson L.2003Age-related decline in humoral immune function in collared flycatchers. J. Evol. Biol 16, 1205–1210 (doi:10.1046/j.1420-9101.2003.00611.x.) [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.1984Reproductive effort and terminal investment in iteroparous animals. Am. Nat 123, 212–229 (doi:10.1086/284198.) [Google Scholar]
- Coulson J.C., Fairweather J.A.2001Reduced reproductive performance prior to death in the black-legged kittiwake: senescence or terminal illness?. J. Avian Biol 32, 146–152 (doi:10.1034/j.1600-048X.2001.320207.x.) [Google Scholar]
- Descamps S., Boutin S., Berteaux D., Gaillard J.M.2008Age-specific variation in survival, reproductive success and offspring quality in red squirrels: evidence of senescence. Oikos 117, 1406–1416 (doi:10.1111/j.0030-1299.2008.16545.x.) [Google Scholar]
- Dhondt A.A.1985Do old great tits forego breeding? Auk 102, 870–872 [Google Scholar]
- Doligez B., Clobert J., Pettifor R.A., Rowcliffe M., Gustafsson L., Perrins C.M., McCleery R.H.2002Costs of reproduction: assessing responses to brood size manipulation on life-history and behavioural traits using multi-state capture–recapture models. J. Appl. Stat 29, 407–423 (doi:10.1080/02664760120108845.) [Google Scholar]
- Fisher R.A.1930The genetical theory of natural selection. Oxford, UK: Clarendon [Google Scholar]
- Forslund P., Pärt T.1995Age and reproduction in birds:hypotheses and tests. Trends Ecol. Evol 10, 374–378 (doi:10.1016/S0169-5347(00)89141-7.) [DOI] [PubMed] [Google Scholar]
- Hamel S., Cote S.D., Gaillard J.M., Festa-Bianchet M.2009Individual variation in reproductive costs of reproduction: high-quality females always do better. J. Anim. Ecol 78, 143–151 (doi:10.1111/j.1365-2656.2008.01459.x.) [DOI] [PubMed] [Google Scholar]
- Hamilton W.D.1966The moulding of senescence by natural selection. J. Theor. Biol 12, 12–45 (doi:10.1016/0022-5193(66)90184-6.) [DOI] [PubMed] [Google Scholar]
- Holmes D.J., Thomson S.L., Wu J., Ottinger M.A.2003Reproductive aging in female birds. Exp. Gerontol 38, 751–756 (doi:10.1016/S0531-5565(03)00103-7.) [DOI] [PubMed] [Google Scholar]
- Jones O.R., et al. 2008Senescence rates are determined by ranking on the fast–slow life-history continuum. Ecol. Lett 11, 664–673 (doi:10.1111/j.1461-0248.2008.01187.x.) [DOI] [PubMed] [Google Scholar]
- Kawasaki N., Brassil C.E., Brooks R.C., Bonduriansky R.2008Environmental effects on the expression of life span and aging: an extreme contrast between wild and captive cohorts of Telostylinus angusticollis (Diptera: Neriidae). Am. Nat 172, 346–357 (doi:10.1086/589519.) [DOI] [PubMed] [Google Scholar]
- Keller L.F., Reid J.M., Arcese P.2008Testing evolutionary models of senescence in a natural population: age and inbreeding effects on fitness components in song sparrows. Proc. R. Soc. B 275, 597–604 (doi:10.1098/rspb.2007.0961.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood T.B.L.2002Evolution of ageing. Mech. Ageing Dev 123, 737–745 (doi:10.1016/S0047-6374(01)00419-5.) [DOI] [PubMed] [Google Scholar]
- Lubjuhn T., Gerken T., Brun J., Schmoll T.2007Yearling male great tits, Parus major, suffer more strongly from cuckoldry than older males. Zoology 110, 387–397 (doi:10.1016/j.zool.2007.07.005.) [DOI] [PubMed] [Google Scholar]
- McCleery R.H., Perrins C.M.1989Great tit. In Lifetime reproduction in birds (ed Newton I.) pp. 35–74 London, UK: Academic Press [Google Scholar]
- McCleery R.H., Clobert J., Julliard R., Perrins C.M.1996Nest predation and delayed cost of reproduction in the great tit. J. Anim. Ecol 65, 96–104 (doi:10.2307/5703.) [Google Scholar]
- McCleery R.H., Pettifor R.A., Armbruster P., Meyer K., Sheldon B.C., Perrins C.M.2004Components of variance underlying fitness in a natural population of the great tit Parus major. Am. Nat 164, E62–E72 (doi:10.1086/422660.) [DOI] [PubMed] [Google Scholar]
- McCleery R.H., Perrins C.M., Sheldon B.C., Charmantier A.2008Age-specific reproduction in a long-lived species: the combined effects of senescence and individual quality. Proc. R. Soc. B 275, 963–970 (doi:10.1098/rspb.2007.1418.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medawar P.B.1952An unsolved problem in biology. London, UK: H. K. Lewis & Co. Ltd [Google Scholar]
- Minot E.O., Perrins C.M.1986Interspecific interference competition: nest sites for blue and great tits. J. Anim. Ecol 55, 331–350 (doi:10.2307/4712.) [Google Scholar]
- Møller A.P., Mousseau T.A., Rudolfsen G., Balbontin J., Marzal A., Hermosell I., De Lope F.2009Senescent sperm performance in old male birds. J. Evol. Biol 22, 334–344 (doi:10.1111/j.1420-9101.2008.01650.x.) [DOI] [PubMed] [Google Scholar]
- Naef-Daenzer B., Widmer F., Nuber M.2001Differential post-fledging survival of great and coal tits in relation to their condition and fledging date. J. Anim. Ecol 70, 730–738 (doi:10.1046/j.0021-8790.2001.00533.x.) [Google Scholar]
- Newton I., Rothery P.1997Senescence and reproductive value in sparrowhawks. Ecology 78, 1000–1008 [Google Scholar]
- Nussey D.H., Kruuk L.E.B., Donald A., Fowlie M., Clutton-Brock T.H.2006The rate of senescence in maternal performance increases with early-life fecundity in red deer. Ecol. Lett 9, 1342–1350 (doi:10.1111/j.1461-0248.2006.00989.x.) [DOI] [PubMed] [Google Scholar]
- Nussey D.H., Coulson T., Festa-Bianchet M., Gaillard J.M.2008Measuring senescence in wild animal populations: towards a longitudinal approach. Funct. Ecol 22, 393–406 (doi:10.1111/j.1365-2435.2008.01408.x.) [Google Scholar]
- Palacios M.G., Cunnick J.E., Winkler D.W., Vleck C.M.2007Immunosenescence in some but not all immune components in a free-living vertebrate, the tree swallow. Proc. R. Soc. B 274, 951–957 (doi:10.1098/rspb.2006.0192.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L., Gems D.2007Benchmarks for ageing studies. Nature 450, 165–167 (doi:10.1038/450165a.) [DOI] [PubMed] [Google Scholar]
- Perrins C.M.1979British tits. London, UK: Collins [Google Scholar]
- Perrins C.M., Moss D.1974Survival of young great tits in relation to age of female parent. Ibis 116, 220–224 (doi:10.1111/j.1474-919X.1974.tb00242.x.) [Google Scholar]
- Pizzari T., Dean R., Pacey A., Moore H., Bonsall M.B.2008The evolutionary ecology of pre- and post-meiotic sperm senescence. Trends Ecol. Evol 23, 131–140 (doi:10.1016/j.tree.2007.12.003.) [DOI] [PubMed] [Google Scholar]
- Rasbash J., Steele F., Browne W., Prosser B.2005A user's guide to MLwiN, v. 2.0.London, UK: Centre for Multilevel Modelling, University of Bristol [Google Scholar]
- Rattiste K.2004Reproductive success in presenescent common gulls (Larus canus): the importance of the last year of life. Proc. R. Soc. B 271, 2059–2064 (doi:10.1098/rspb.2004.2832.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauser C.L., Hong J.S., Cung M.B., Pham K.M., Mueller L.D., Rose M.R.2005Testing whether male age or high nutrition causes the cessation of reproductive aging in female Drosophila melanogaster populations. Rejuvenation Res 8, 86–95 (doi:10.1089/rej.2005.8.86.) [DOI] [PubMed] [Google Scholar]
- Reed T.E., Kruuk L.E.B., Wanless S., Frederiksen M., Cunningham E.J.A., Harris M.P.2008Reproductive senescence in a long-lived seabird: rates of decline in late-life performance are associated with varying costs of early reproduction. Am. Nat 171, E89–E101 (doi:10.1086/524957.) [DOI] [PubMed] [Google Scholar]
- Reid J.M., Bignal E.M., Bignal S., McCracken D.I., Monaghan P.2003Age-specific reproductive performance in red-billed choughs Pyrrhocorax pyrrhocorax: patterns and processes in a natural population. J. Anim. Ecol 72, 765–776 (doi:10.1046/j.1365-2656.2003.00750.x.) [Google Scholar]
- Ronce O., Clobert J., Massot M.1998Natal dispersal and senescence. Proc. Natl Acad. Sci. USA 95, 600–605 (doi:10.1073/pnas.95.2.600.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M.R.1991Evolutionary biology of aging. Oxford, UK: Oxford University Press [Google Scholar]
- Svensson L.1994Identification guide to European passerines. Stockholm, Sweden: Svensson [Google Scholar]
- Van de Pol M., Verhulst S.2006Age-dependent traits: a new statistical model to separate within and between-individual effects. Am. Nat 167, 766–773 (doi:10.1086/503331.) [DOI] [PubMed] [Google Scholar]
- Van Noordwijk A.J., Van Balen J.H.1988The great tit. In Reproductive success (ed Clutton-Brock T.H.) pp. 119–135 Chicago, IL: University of Chicago Press [Google Scholar]
- Van Noordwijk A.J., McCleery R.H., Perrins C.M.1995Selection for the timing of great tit breeding in relation to caterpillar growth and temperature. J. Anim. Ecol 64, 451–458 (doi:10.2307/5648.) [Google Scholar]
- Vaupel J.W., Yashin A.I.1985Heterogeneity ruses: some surprising effects of selection on population-dynamics. Am. Stat 39, 176–185 (doi:10.2307/2683925.) [PubMed] [Google Scholar]
- Verhulst S., Hut R.A.1996Post-fledging care, multiple breeding and the costs of reproduction in the great tit. Anim. Behav 51, 957–966 (doi:10.1006/anbe.1996.0099.) [Google Scholar]
- Verhulst S., Perrins C.M., Riddington R.1997Natal dispersal of great tits in a patchy environment. Ecology 78, 864–872 [Google Scholar]
- Weladji R.B., Loison A., Gaillard J.M., Holand O., Mysterud A., Yoccoz N.G., Nieminen M., Stenseth N.C.2008Heterogeneity in individual quality overrides costs of reproduction in female reindeer. Oecologia 156, 237–247 (doi:10.1007/s00442-008-0961-x.) [DOI] [PubMed] [Google Scholar]
- Wilkin T.A., Garant D., Gosler A.G., Sheldon B.C.2007Edge effects in the great tit: analyses of long-term data with GIS techniques. Conserv. Biol 21, 1207–1217 (doi:10.1111/j.1523-1739.2007.00767.x.) [DOI] [PubMed] [Google Scholar]
- Williams G.C.1957Pleiotropy, natural selection and evolution of senescence. Evolution 11, 398–411 (doi:10.2307/2406060.) [Google Scholar]
- Wilson A.J., Nussey D.H., Pemberton J.M., Pilkington J.G., Morris A., Pelletier F., Clutton-Brock T.H., Kruuk L.E.B.2007Evidence for a genetic basis of aging in two wild vertebrate populations. Curr. Biol 17, 2136–2142 (doi:10.1016/j.cub.2007.11.043.) [DOI] [PubMed] [Google Scholar]