Abstract
Approximately 10% of gastric cancer cases show familial clustering but only 1-3% of gastric carcinomas arise as a result of inherited gastric cancer predisposition syndromes. Direct proof that Hereditary Gastric Cancer a genetic disease with a germline gene defect has come from the demonstration of co-segregation of germline E-cadherin (CDH1) mutations with early onset diffuse gastric cancer in families with an autosomal dominant pattern of inheritance (HDGC). E-cadherin is a transmembrane calcium-dependent cell-adhesion molecule involved in cell-junction formation and the maintenance of epithelial integrity. In this review, we describe frequency and type of CDH1 mutations in sporadic and familial gastric cancer. Further we demonstrate the functional significance of some CDH1 germline missense mutations found in HDGC. We also discuss the CDH1 polymorphisms that have been associated to gastric cancer. We report other types of malignancies associated to HDGC, besides diffuse gastric cancer. Moreover, we review the data available on putative alternative candidate genes screened in familial gastric cancer. Finally, we briefly discuss the role of low-penetrance genes and Helicobacter pylori in gastric cancer. This knowledge is a fundamental step towards accurate genetic counselling, in which a highly specialised pre-symptomatic therapeutic intervention should be offered.
Keywords: gastric cancer, familial gastric cancer, E-cadherin, CDH1, HDGC, hereditary diffuse gastric cancer, inheritance, germline mutation, missense mutation, Helicobacter pylori, low penetrance genes, IL1, TNFα, early onset, genetic counselling, functional analysis
Introduction
Gastric cancer is one of the most common gastrointestinal malignancies world-wide, although in recent decades a decline has been observed in its incidence and associated mortality [1,2]. Gastric cancer is highly heterogeneous morphologically, but there are two main histotypes of gastric carcinoma: the glandular (intestinal) type and the isolated-cell type (diffuse). A proportion of cases displays a mixed phenotype harbouring in a single tumour of two histological components (glandular and diffuse) [3,4].
Although the incidence of gastric cancer in older patients is decreasing, the incidence of gastric cancer in younger patients and cases with familial clustering remains quite stable. This suggests that a genetic predisposition may play an important role in the pathogenesis of some forms of gastric cancer [5]. About 10% of cases of gastric cancer show familial clustering [2,6] but only 1-3% of gastric carcinomas arise as a result of an inherited gastric cancer predisposition syndrome [7].
In formulating a definition of familial gastric cancer syndromes, a distinction must be made between the histopathological subtypes (diffuse or diffuse with glandular component/mixed versus intestinal) which segregate within families [8]. Gastric cancer was proved to be an inherited disease, primarily in families with aggregations of diffuse gastric cancer.
The syndrome of hereditary diffuse gastric cancer (HDGC) was defined by the International Gastric Cancer Linkage Consortium (IGCLC) [8], as any family that fits the following criteria: (1) two or more documented cases of diffuse gastric cancer in first/second degree relatives, with at least one diagnosed before the age of 50, or (2) three or more cases of documented diffuse gastric cancer in first/second degree relatives, independently of age. The identification of the germline gene defect underlying HDGC came from segregation studies in early onset diffuse gastric cancer families [9-12]. Germline mutations of the CDH1 gene (EMBL/GenBank Data Libraries# CDH1 - Z13009) resulting in E-cadherin inactivation have been identified in HDGC (OMIM# Gastric cancer - 137215). Families with aggregation of gastric cancer and index cases with diffuse gastric cancer but not fulfilling the IGCLC criteria for HDGC are termed familial diffuse gastric cancer (FDGC).
The criteria to define hereditary intestinal gastric cancer (HIGC) families were adjusted by the IGCLC depending on the incidence of gastric cancer in the population. Thus, countries with a high incidence like Japan and Portugal should use the diagnostic criteria analogous to the Amsterdam criteria for HNPCC [13]: (1) at least three relatives should have intestinal gastric cancer and one of them should be a first degree relative of the other two; (2) at least two successive generations should be affected; (3) in one of the relatives, gastric cancer should be diagnosed before the age of 50. In countries with a low incidence (USA, UK) HIGC was defined as (1) at least two first/second degree relatives affected by intestinal gastric cancer, one diagnosed before the age of 50; or (2) three or more relatives with intestinal gastric cancer at any age. No germline genetic defect has been found to date in this type of predisposing disease.
Families with aggregations of gastric cancer and an index case with intestinal gastric cancer are termed familial intestinal gastric cancer (FIGC).
Families with aggregation of gastric cancer, but without histology available on the tumours are termed familial gastric cancer (FGC).
Patients who developed gastric cancer at an early age (< 50 years old) without a familial history of gastric cancer were considered early onset gastric cancer patients.
The CDH1 gene
E-cadherin is a 120 kD glycoprotein localised at adherens junctions of epithelial cells, where it mediates homophilic calcium-dependent cell-adhesion [14,15]. The CDH1 gene maps to 16q22.1, comprises 16 exons spanning approximately 100 kb of genomic DNA which are transcribed into a 4.5 Kb mRNA [16]. The E-cadherin modular structure consists of five extracellular domains each ~110 aa in length, with conserved calcium-binding motifs, a transmembrane region and a cytoplasmic domain, which interacts with filaments of actin through catenins [17]. Disruption of the E-cadherin complex is expected to induce loss of cell-adhesion with a concomitant increased cell invasion [18,19].
E-cadherin and sporadic cancer
Loss of E-cadherin function is one of the crucial steps for tumour progression in several types of human cancer. Despite what has been observed in other types of epithelial cancers, in which E-cadherin expression is down regulated without harbouring gene mutations, namely thyroid, skin, lung, ovary and colon, in sporadic diffuse gastric carcinoma E-cadherin down regulation is often associated with gene mutation [20-22]. CDH1 mutations have been described not only in diffuse gastric cancers but also in a specific histological type of breast cancer namely infiltrative lobular breast cancers, another epithelial cancer in which neoplastic cells are dispersed in the stromal tissue [23]. Most of the somatic CDH1 mutations found in sporadic diffuse gastric carcinomas are missense and in-frame deletions [23]. In contrast to stomach, the mutations found in infiltrating lobular breast cancers were out-of-frame mutations, causing premature stop codons. In both models somatic mutations cluster in exons 7 to 9, in the extracellular domain of the protein.
Inactivation of CDH1 in diffuse gastric cancer cell lines and primary lobular breast carcinomas is achieved by 2 genetic hits. Infiltrative lobular breast carcinomas show LOH at the CDH1 locus as a second hit. But in the majority of diffuse gastric cancer cases with CDH1 mutations it was demonstrated that hypermethylation of the CDH1 promoter region accounts for the inactivation of the second allele [24].
Mutation of CDH1 in HDGC and Early-onset Gastric Cancer
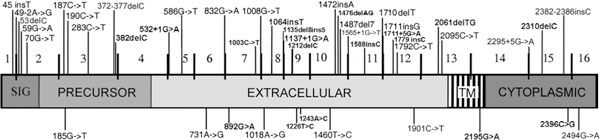
Germline truncating and missense mutations of the CDH1 gene resulting in E-cadherin inactivation and/or segregating with the disease have been identified in hereditary diffuse gastric carcinoma. To date, forty eight families harbouring CDH1 germline mutations have been described, 41 HDGC (40%) and 7 FDGC (8%) (see Table 1 for details) [21,25-31]. In these families, 45 different CDH1 germline mutations were found and dispersed along the gene (see Table 2 and Fig. 1 for details). The majority (76.0%) of these CDH1 germline mutations are frameshift, splice-site and nonsense changes resulting in truncated non active proteins. Guilford et al described for the first time germline CDH1 mutations in a large percentage of New Zealand Maori HDGC families [9]. Shortly thereafter, CDH1 germline mutations were described in a broad range of ethnic backgrounds. CDH1 mutations were also found in a significant percentage of HDGC families of European and American origin [21]. In families of Asian ethnicity no truncating mutations have been identified to date [21]. In 24% of the families CDH1 germline missense mutations have also been reported [26,27,29-33]. These missense mutations are also distributed along the gene, eight germline missense mutations cluster in the extracellular region of the protein, one in the transmembrane domain and two are localised in the intracellular domain of the protein (see Table 2 and Fig. 1 for details).
Table 1.
Summary of the germline CDH1 mutation screening studies in families with gastric cancer
| Study | Total of families | HDGC families | FDGC families | FIGC families | FGC families | CDH1 truncating mutations | CDH1 missense mutations | % CDH1 mutations in HDGC | % CDH1 mutations in FDGC |
|---|---|---|---|---|---|---|---|---|---|
| Guilford et al, 1998 [9] | 3 | 3 | 0 | 0 | 0 | 3 | 0 | 100% | 0% |
| Gayther et al, 1998 [10] | 18 | 10 | 0 | 8 | 0 | 3 | 0 | 30.0% | 0% |
| Richards et al, 1998 [11] | 8 | 8 | 0 | 0 | 0 | 2 | 0 | 25.0% | 0% |
| Guilford et al, 1999 [12] | 6 | 4 | 2** | 0 | 0 | 6** | 0 | 100% | 100% |
| Shinmura et al, 1999 [13] | 13 | 3 | 0 | 10 | 0 | 0 | 1 | 33.3% | 0% |
| Yoon et al, 1999 [85] | 5 | 5 | 0 | 0 | 0 | 0 | 2 | 40% | 0% |
| Iida et al, 1999 [86] | 14 | 0 | 6 | 6 | 2 | 0 | 0 | 0% | 0% |
| Keller et al, 1999 [44] | 7 | 2 | 5 | 0 | 0 | 1 | 0 | 50.0% | 0% |
| Avizienyte et al, 2001 [87] | 11 | 5 | 4 | 1 | 1 | 0 | 0 | 0% | 0% |
| Dussaulx-Garin et al, 2001 [88] | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 100% | 0% |
| Humar et al, 2002 [89] | 10 | 7 | 3* | 0 | 0 | 5* | 0 | 57.1% | 33.3% |
| Oliveira et al, 2002 [32] | 39 | 11 | 24 | 4 | 0 | 3 | 1 | 36.4% | 0% |
| Yabuta et al, 2002 [26] | 17 | 2 | 3 | 0 | 12 | 0 | 1 | 50.0% | 0% |
| Wang et al, 2003 [27] | 78 | 0 | 2** | 0 | 76 | 0 | 2** | 0% | 100% |
| Oliveira et al, 2004 [28] | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 100% | 0% |
| Jonsson et al, 2002 [25] | 3 | 3 | 0 | 0 | 0 | 1 | 0 | 33.3% | 0% |
| Oliveira et al, in press [30] | 32 | 9 | 10 | 3 | 10 | 0 | 1 | 11.1% | 0% |
| Keller et al, 2004 [29] | 28*** | 2 | 21 | 5 | 0 | 0 | 1* | 0% | 4.8% |
| Brooks-Wilson et al, in press [31] | 34 | 26 | 7* | 1 | 0 | 10 | 3* | 46.2% | 14.3% |
| TOTAL | 328 | 102 | 87 | 38 | 101 | 36 | 12 | 40.2% | 8.0% |
*One FDGC families with a germline mutation
**Two FDGC families with missense germline mutations
*** the numbers of families, not included in Ref. (Keller et al 1999) are listed
Table 2.
Details from all the CDH1 germline mutations described to date in familial gastric cancer
| CDH1 Mutation | Gene location | Mutation type | Predicted premature stop codon | Reference |
|---|---|---|---|---|
| 45insT | Exon 1 | Frameshift | Codon 32 | Oliveira et al, 2002 [32] |
| 49-2A>G | Intron 1 | Splice-site | Unknown | Richards et al, 1999 [11] |
| 53delC | Exon 2 | Frameshift | Codon 32 | Humar et al, 2002 [89] |
| 59G>A | Exon 2 | Nonsense (W20X) | Codon 20 | Richards et al, 1999 [11] |
| 70G>T | Exon 2 | Nonsense (E24X) | Codon 24 | Guilford et al, 1999 [12] |
| 185G>T | Exon 3 | Missense (G62V) | Ns | Shinmura et al, 1999 [13] |
| 187C>T | Exon 3 | Nonsense (R63X) | Codon 63 | Gayther et al, 1998 [10] |
| 190C>T | Exon 3 | Nonsense (Q64X) | Codon 64 | Guilford et al, 1999 [12] |
| 283C>T | Exon 3 | Nonsense (Q95X) | Codon 95 | Dussaulx-Garin et al, 2001 [88] |
| 372-377delC | Exon 3 | Frameshift | Codon 249 | Keller et al, 1999 [44] |
| 382delC | Exon 3 | Frameshift | Codon 215 | Brooks-Wilson et al, in press [31] |
| 531+1G>A | Intron 5 | Splice-site | Unknown | Brooks-Wilson et al, in press [31] |
| 586G>T | Exon 5 | Nonsense (G196X) | Codon 196 | Guilford et al, 1999 [12] |
| 731A>G | Exon 6 | Missense (D244G) | Ns | Yoon et al, 1999 [85] |
| 832G>A | Exon 6 | Frameshift | Codon 281 Codon 336+18bp int 7 |
Oliveira et al, 2002 [32] |
| 892G>A | Exon 7 | Missense (A298T) | Ns | Brooks-Wilson et al, in press [31] |
| 1003C>T | Exon 7 | Nonsense (R335X) | Codon 335 | Jonsson et al, 2002 [25] |
| 1008G>T | Exon 7 | Splice-site | Codon 349 | Guilford et al, 1998 [9] |
| 1018A>G | Exon 8 | Missense (T340A) | Ns | Oliveira et al, 2002 [32] |
| 1064insT | Exon 8 | Frameshift | Codon 393 | Brooks-Wilson et al, in press [31] |
| 1135del8ins5 | Exon 8 | Splice-site | Codon 386 | Oliveira et al, 2004 [28]; Brooks-Wilson et al, in press [31] |
| 1137+1G>A | Intron 8 | Donor splice-site | Unknown | Guilford et al, 1999 [12] |
| 1212delC | Exon 9 | Frameshift | Codon 417 | Brooks-Wilson et al, in press [31] |
| 1226T>C | Exon 9 | Missense (W409R) | Ns | Brooks-Wilson et al, in press [31] |
| 1243A>C | Exon 9 | Missense (I415L) | Ns | Wang et al, 2003 (two families) [27] |
| 1460T>C | Exon 10 | Missense (V487A) | Ns | Yoon et al, 1999 [85] |
| 1472insA | Exon 10 | Frameshift | Codon 536 | Oliveira et al, 2002 [32] |
| 1476delAG | Exon 10 | Frameshift | Codon 547 | Brooks-Wilson et al, in press [31] |
| 1487del7 | Exon 10 | Frameshift | Codon 556 | Guilford et al, 1999 [12] |
| 1565+1G>T | Intron 10 | Splice-site | Unknown | Humar et al, 2002 [89] |
| 1588insC | Exon 11 | Frameshift | Codon 536 | Guilford et al, 1999 [12] |
| 1710delT | Exon 11 | Frameshift | Unknown | Humar et al, 2002 [89] |
| 1711insG | Exon 11 | Frameshift | Codon 587 | Gayther et al, 1998 [10] |
| 1711+5G>A | Intron 11 | Splice-site | Unknown | Brooks-Wilson et al, in press [31] |
| 1779insC | Exon 12 | Frameshift | Codon 604 | Brooks-Wilson et al, in press [31] |
| 1792C>T | Exon 12 | Nonsense (R598X) | Codon 598 | Gayther et al, 1998, Humar et al, 2002 [10,89] |
| 1901C>T | Exon 12 | Missense (A634V) | Codon 653 | Oliveira et al, in press [30] |
| 2061delTG | Exon 13 | Frameshift | Codon 783 | Brooks-Wilson et al, in press [31] |
| 2095C>T | Exon 13 | Nonsense (Q699X) | Codon 699 | Guilford et al, 1998 [9] |
| 2195G>A | Exon 14 | Missense (R732Q) | Ns | Brooks-Wilson et al, in press [31] |
| 2295+5G>A | Intron 14 | Splice-site | Unknown | Humar et al, 2002 [89] |
| 2310delC | Exon 15 | Frameshift | Codon 783 | Brooks-Wilson et al, in press [31] |
| 2382-2386insC | Exon 15 | Frameshift | Codon 349 | Guilford et al, 1998 [9] |
| 2396C>G | Exon 15 | Missense (P799R) | Ns | Keller et al, 2004 [29] |
| 2494G>A | Exon 16 | Missense (V832M) | Ns | Yabuta et al, 2002 [26] |
Figure 1.
Scheme of the CDH1 gene with germline mutations described to date in HDGC. Truncating mutations are shown above and missense mutations below the gene. Sig: signal peptide; Precursor: protein precursor domain; TM: transmembrane domain; Cyto. Domain: protein cytoplasmic domain
A total of 104 early-onset apparently sporadic gastric cancer patients were studied for CDH1 germline mutations. Eighty seven of them had diffuse type or mixed gastric cancer with a diffuse component. Only two of the 104 patients had germline CDH1 mutations (Table 3). These two mutations were identified in patients with diffuse gastric cancer [29,33].
Table 3.
Details from CDH1 germline mutations described to date in early onset gastric cancer patients
Initially it was reported that the mechanism of inactivation of the CDH1 wild-type allele in tumour cells from HDGC by families was either by promoter methylation or by somatic mutation [34]. The biallelic inactivation leads to diminished or absent E-cadherin immunoreactivity in the neoplastic cells [34]. Recently it was found in a Caucasian family with a CDH1 germline splice-site mutation in all members affected by gastric cancer, a CDH1 intragenic deletion of CDH1, affecting at least exon 8, as the second hit in one of the tumours [28]. This observation highlights the need of developing experimental protocols to identify, in the setting of HDGC families, the presence of germline or somatic intragenic deletions in CDH1, which are easily missed by mutation detection methods based on PCR of genomic DNA.
Functional significance of CDH1 germline missense mutations
The functional significance associated to CDH1 germline missense sequence variants is not straightforward. Moreover, due to the lethal nature of the disease there are rarely enough available affected individuals in any family to perform segregation analysis in families carrying germline missense sequence variants. The lack of such knowledge represents a major limitation for the clinical management of these patients and families.
To address this problem, a functional in vitro screen for gastric cancer missense mutations was created [33]. Cell-lines stably expressing the germline E-cadherin sequence variants were established and their effect on the protein ability to mediate cell-cell adhesion and suppress invasion was addressed. To date, we have analysed nine germline missense sequence variants and showed that some of these variants cause impaired or reduced cell-cell adhesion, increased cell motility and invasion, resulting in a scattered cell morphology and invasive phenotype similar to that observed in diffuse gastric carcinoma [29,31,33,35-37] (see Table 4 for details).
Table 4.
Functional characterization of missense mutations found in gastric cancer probands
| CDH1 Construct | Aggregation | Invasion | Pathogenic significance |
|---|---|---|---|
| Wild type | Yes | No | Not applicable |
| A298T | No | Yes | Yes |
| T340A | No | Yes | Yes |
| W409R | No | Yes | Yes |
| A592T | Yes | No | No |
| A617T | Yes | No | No |
| A634V | No | Yes | Yes |
| R732Q | No | Yes | Yes |
| P799R | No | Yes | Yes |
| V832M | No | Yes | Yes |
In addition, it was shown that the effect of different E-cadherin germline missense mutations in cell morphology and motility was distinct, demonstrating the existence of a genotype-phenotype correlation between different E-cadherin mutations and cell behaviour, likely dependent on the specific E-cadherin domain affected by each mutation [38].
The aforementioned studies indicate that functional assays should be used as an adjunct in deciding on the potential pathogenic role of germline sequence variants, with significant potential to help clinical counselling of the CDH1 mutation carriers.
CDH1 polymorphisms
There is an increasing number of manuscripts reporting CDH1 sequence variants in gastric cancer families and also in controls (see Table 5 for details). Two good examples of these sequence variants are single nucleotide polymorphisms located at the promoter region of CDH1, the -347G->GA and the -160C/A. Both sequence variants were described to affect the transcriptional activity of CDH1.
Table 5.
Polymorphisms identified in CDH1 in gastric cancer probands and normal controls reported to date
| Sequence variant | Gene location | Codon | Effect | % patients | % controls | Reference |
|---|---|---|---|---|---|---|
| -71C>G | Promoter | Unknown | 1/13 (7.7%) | 2/51 (3.9%) | Avizienyte et al, 2000 [87] | |
| -160C>A | Promoter | See text | 17/32 (53.1%) | 63/114 (55.3%) | Oliveira et al, 2002 [32] | |
| 2/5 (40%) | 38/94 (40.4%) | Humar et al, 2002 [89] | ||||
| 7/28 (25%) | 32/142 (22.5%) | Shin et al, 2004 [39] | ||||
| 31/87 (35.6%) | 18/50 (36%) | Wang et al, 2003 [27] | ||||
| -347G>GA | Promoter | See text | 12/28 (42.9%) | 39/142 (27.5%) | Shin et al, 2004 [39] | |
| 48+6T>C | Intron 1 | Unknown | 5/13 (38%) | 18/51 (35%) | Avizienyte et al, 2000 [87] | |
| 11/28 (39.3%) | 27/100 (27%) | Oliveira et al, 2002 [32] | ||||
| 1/10 (10%) | 75/350 (21.4%) | Humar et al, 2002 [89] | ||||
| 5/17 (29.4%) | nd | Yabuta et al, 2002 [26] | ||||
| 531+10G>C | Intron 4 | Unknown | 2/34 (5.9%) | nd | Oliveira et al, 2002 [32] | |
| ns | nd | Guilford et al, 1999 [12] | ||||
| ns | nd | Gayther et al, 1998 [10] | ||||
| 532-18C>T | Intron 4 | Unknown | 2/66 (3.0%) | 0/100 (0%) | Suriano and Oliveira et al, 2003 [33] | |
| 2/34 (5.9%) | 1/50 (2.0%) | Keller et al, 2004 [29] | ||||
| 918C>T | Exon 7 | 306 | Silent | 1/34 (2.9%) | nd | Oliveira et al, 2002 [32] |
| 1029C>G | Exon 8 | 343 | Silent | 1/34 (2.9%) | nd | Oliveira et al, 2002 [32] |
| 1774G>A | Exon 12 | 592 | A592T | 1/34 (2.9%) | 1/50 (2.0%) | Keller et al, 2004 [29] |
| 1849G>A | Exon 12 | 617 | A617T | 2/66 (3%) | 2/193 (1%) | Suriano and Oliveira et al, 2004 [33] |
| 1896C>T | Exon 12 | 632 | Silent | 1/34 (2.9%) | 5/100 (5%) | Oliveira et al, 2002 [32] |
| ns | nd | Gayther et al, 1998 [10] | ||||
| 1937-13T>C | Intron 12 | Unknown | 2/27 (7.4%) | 25/100 (25%) | Oliveira et al, 2002 [32] | |
| ns | nd | Guilford et al, 1998, 1999 [9,12] | ||||
| 1937-27T>G | Intron 12 | Unknown | ns | nd | Guilford et al, 1999 [12] | |
| 2076C>T | Exon 13 | 692 | Silent | 8/13 (61.5%) | nd | Avizienyte et al, 2000 [87] |
| 15/27 (55.6%) | 29/100 (59.0%) | Oliveira et al, 2002 [32] | ||||
| 1/5 (20%) | nd | Richards et al, 1999 [11] | ||||
| 7/16 (43.8%) | nd | Iida et al, 1999 [86] | ||||
| ns | nd | Guilford et al, 1998, 1999 [9,12] | ||||
| ns | nd | Gayther et al, 1998 [10] | ||||
| ns | ns | Yabuta et al, 2002 [26] | ||||
| 82/87 (94.3%) | 48/50 (96%) | Wang et al, 2003 [27] | ||||
| 2253C>T | Exon 14 | 751 | Silent | ns | ns | Yabuta et al, 2002 [26] |
| 2292C>T | Exon 14 | 764 | Silent | 1/34 (2.9%) | nd | Oliveira et al, 2002 [32] |
| 2634C>T | Exon 16 | 878 | Silent | 1/34 (2.9%) | nd | Oliveira et al, 2002 [32] |
nd, Not done; ns, Not specified.
The -347G->GA single nucleotide polymorphism was shown to down regulate the transcriptional activity of the E-cadherin gene by measuring the promoter activity of the -347G->GA polymorphism. The GA allele decreased the transcriptional efficiency by 10-fold (p < 0.001) and had a weak transcription factor binding compared to the G allele [39]. In a case-control study performed in a Korean population of 170 individuals (28 probands from gastric cancer families and 142 normal controls) the -347G/GA heterozygous or GA homozygous was associated with FGC patients (p < 0.05) compared with the G homozygous genotype [39].
The A-allele of the -160C/A polymorphism was shown to decrease the transcriptional efficiency by 68% compared with the C-allele, down regulating E-cadherin expression [40]. Wu and colleagues [41] suggested that individuals who have inherited two copies of the A-allele that reduce transcription of CDH1 may have a decreased risk of developing gastric cancer in a Taiwanese population. However, no consistent data has been reported about the association between the -160C/A CDH1 sequence variant and gastric cancer. In a case-control study performed in an Italian population this variant was associated with an increased susceptibility to diffuse gastric cancer. The frequency of the -160A allele was significantly higher (P < 0.005) in 53 diffuse gastric cancer cases compared to 70 matched controls. The odds ratio associated with the A-allele was 2.27 for CA-heterozygotes (95% CI 1.16-4.44) and 7.84 for AA-homozygotes (95% CI 2.89-21.24) [42]. However, these results were not confirmed in a large series of gastric cancer patients and control populations from Portugal, Canada and Germany who have found no significant evidence for an association between stomach cancer and the -160C/A polymorphism in the promoter of CDH1. In this report a total of 899 individuals (433 patients and 466 controls) were analysed. The genotype frequencies did not differ significantly between cases and controls, and the genotype-specific risks were not significantly different from unity, with an odds ratio for heterozygotes compared with the common homozygote of 1.3 (95% CI 0.98-1.8) and 1.2 (0.68-2.0) for rare homozygotes compared with common homozygotes [43].
In summary, it is mandatory to clarify the functional relevance of the A allele in vivo and to disclose the association of the A/A genotype with GC in larger epidemiologic studies.
Other cancers in HDGC families
In the CDH1 positive families, family members show other types of malignancy besides diffuse gastric cancer. Breast, colon (namely signet ring cell cancer of the colon), prostate and ovarian carcinomas have been shown to occur in families carrying CDH1 germline mutations suggesting that non-gastric malignancies can be associated with HDGC [21,31].
Importantly, breast carcinoma, in particular of the lobular type, has been associated to a positive history of gastric carcinoma [44]. There was reported a gastric cancer patient carrying a germline mutation of CDH1 who had a mother affected with bilateral breast carcinoma at the age of 49 [29]. An overrepresentation of this tumour type in families with E-cadherin germline mutations has been demonstrated [45]. In a recent study, 17 cases of breast cancer were found in families carrying CDH1 germline mutations, three of which were histologically confirmed as lobular breast carcinomas. This data highlights the need for screening of CDH1 germline mutations in families with both types of malignancy, diffuse gastric carcinoma and lobular breast carcinoma occurring in the same family.
Familial Gastric Cancer and genes involved in other inherited syndromes
Gastric cancer might also be seen as part of the tumour spectrum in other inherited cancer predisposition syndromes. In particular, gastric cancer has been identified as part of the HNPCC syndrome [46]. As a consequence, the tumours of patients with germline MMR deficiency exhibit a particular phenotype called MSI-H, characterised by a global instability phenomenon affecting microsatellite repetitive sequences [47,48]. The MSI-H phenotype has been extensively used to pre-screen tumours in cases in which patients should be analysed for hMLH1 and hMSH2 [47]. Recently, two tumours from familial gastric cancer probands were detected with MSI-H (one with HDGC and the other with familial gastric cancer). In these two probands germline mutations in hMLH1 and hMSH2 were excluded, though other mismatch repair genes may be involved [30].
Gastric cancer has also been recognised as a component of other hereditary cancer syndromes, such as the Li-Fraumeni syndrome [49]. Most of the cases harbouring germline mutations of the p53 gene have been found in approximately 70% of the families with Li-Fraumeni syndrome. Recently, two gastric cancer families with p53 germline mutations were identified. One mutation was previously described in a Li-Fraumeni kindred and the other was localised in a highly conserved region of p53 [29,30] (Table 6). In these gastric cancer families with p53 germline mutations, gastric, liver, pancreatic, colon cancers and leukaemia occurred in different members of the families [29,30]. The presence of p53 germline mutations in families with a predominance of gastric cancer strengthens the need for p53 mutation screening in families with aggregations of gastric cancer and no CDH1 mutations.
Table 6.
Candidate genes analysed in gastric cancer families
| Candidate gene | No of families analysed | Germline mutations | Observations | Reference |
|---|---|---|---|---|
| TP53 | 66 | 471C>G (FGC) 847C>T (FDGC) |
Family reclassified as Li-Fraumeni Highly conserved residue (Arg 283) |
Oliveira et al, in press [30] Keller et al, 2004 [29] |
| SMAD4 | 32 | 0 | Probably not relevant for familial gastric cancer | Oliveira et al, in press [30] |
| Caspase10 | 32 | 0 | Probably not relevant for familial gastric cancer | Oliveira et al, in press [30] |
| RUNX3 | 34 | 0 | Probably not relevant for familial gastric cancer | Keller et al, 2004 [29] |
| HPP1 | 34 | 0 | Probably not relevant for familial gastric cancer | Keller et al, 2004 [29] |
In addition to HNPCC and Li-Fraumeni syndrome, stomach cancer can also occur in breast and ovarian cancer families. Recently, twenty nine families harbouring gastric and breast malignancies were screened for germline mutations in BRCA2 and in six of the 29 (20.7%), three frameshift mutations and three missense mutations were identified [50]. Moreover, a BRCA2 mutation was found in eight of 34 women with ovarian cancer and a family history of stomach cancer [51]. In gastric cancer families with an excess of breast and ovarian tumours, lacking CDH1, p53 or MSI-H tumour phenotype, BRCA2 is likely to be a candidate gene.
Other candidate genes in Familial Gastric Cancer
In kindreds negative for CDH1 or p53 germline mutations, other genes are probably involved. We will address RUNX3, HPP1, Caspase-10 and SMAD4, which have been shown to be involved in gastric cancer development (mutated in sporadic gastric carcinoma or associated with gastric cancer phenotype in knockout models).
Putative tumour suppressor genes, which are commonly inactivated in sporadic gastric cancers, could also represent good candidate susceptibility genes to familial gastric cancer. RUNX3, which belongs to the family of runt domain transcription factors, as well as HPP1, encoding a cell surface receptor, which is suggested to play multiple roles in cell growth, maturation and adhesion, have recently been shown to be inactivated by promoter hypermethylation at a high frequency in gastric cancer [52,53]. Moreover, in the Runx3/Pebp2alphaC null mouse gastric mucosa exhibits hyperplasias due to stimulated proliferation and suppressed apoptosis in epithelial cells. Gastric cancer families that were screened for RUNX3 and HPP1, do not show germline mutations in both genes, suggesting that RUNX3 and HPP1 are not important alternative gastric cancer predisposition genes [29]. In 3% of sporadic gastric carcinomas alterations of caspase-10 were described [54]. In vitro expression studies have shown that cells carrying caspase-10 mutations harbour impaired caspase-10-mediated apoptosis, suggesting that somatic alterations of the caspase-10 gene might contribute to the pathogenesis of gastric cancers through the loss of their apoptotic function [54]. Germline mutations in caspase-10 were recently screened in families with gastric cancer and early-onset gastric carcinoma patients, but only a high frequency of sequence variants was found. All variants showed similar frequencies in cases (gastric cancer probands) and in control populations demonstrating its polymorphic nature [30].
In knockout studies SMAD4 heterozygous mice revealed the presence of foci of signet ring carcinoma cells in the stomach [55]. Germline mutations in the SMAD4 gene were described in a minority of hereditary juvenile polyposis (JPS) [56,57]. The tumour suppressor gene, SMAD4, is a transcription activator that binds specific DNA sequences and whose nuclear localisation is induced after exposure to TGFβ. This gene was searched for germline mutations in gastric cancer families but only sequence variants were found. These sequence variants were either silent or intronic alterations that were present with the same frequency in normal controls pointing to its polymorphic nature [30].
In summary, RUNX3, HPP1, Caspase-10 and SMAD4 can be ruled out as major gastric cancer predisposition genes in families with an excess of gastric carcinoma (see Table 6 for details).
Genetic counselling in HDGC
The IGCLC recommends pre- and post-test genetic counselling for families that either meet or exceed the minimum requirements for HDGC [8].
Testing of asymptomatic at-risk adults for HDGC is available only after an affected family member has been tested and a mutation found. Testing of an asymptomatic at-risk individual is considered predictive testing, not diagnostic testing. Lynch et al [58] describe the genetic counselling process they followed with a large kindred with HDGC. Relevant issues should be discussed with family members seeking predictive testing for HDGC. Discussion should include: (1) the genetics of cancer development and HDGC; (2) the individual's knowledge of HDGC; (3) the individual's reasons for requesting the test; (4) the individual's understanding of the risk for having inherited the mutation based on a family history of HDGC; (5) availability of molecular genetic testing; (6) cancer risk if the individual has inherited the mutation; (7) recommendations for cancer screening and prophylactic surgery; and (8) the possible social impact of positive and negative test results.
Genetic testing in children has always been a controversial issue. Since there have been reports of patients diagnosed with HDGC under the age of 18, it has been suggested that genetic testing in children may be beneficial [8]. Overall, a request from parents for testing of asymptomatic at-risk children requires sensitivity and understanding and thorough rigorous counselling for both the parents and child.
Requests for prenatal testing for conditions such as HDGC that do not affect intellect and have some available treatment are uncommon. Differences in perspective may exist among medical professionals and in families regarding the use of prenatal testing, particularly if the testing is being considered for the purpose of pregnancy termination rather than early diagnosis. Although most centres would consider decisions about prenatal testing to be the choice of the parents, careful discussion of these issues is appropriate.
Helicobacter pylori infection and Familial Gastric Cancer
Among the possible causes of familial aggregation of gastric cancer, exposure to similar environmental factors such as H. pylori infection may contribute to a higher number of affected individuals within the same family.
H. pylori is one of the most common chronic infections in a man, and once acquired early in childhood and if left untreated, persists for the host's lifetime [59]. Risk factors for H. pylori acquisition include a low socioeconomic status, household crowding, country of origin and ethnicity, and transmission occurs from person to person [60]. Intrafamilial clustering of the infection also reinforces the importance of person-to-person transmission [61].
A large number of studies provided evidence for the aetiological role of H. pylori in gastric carcinoma, and the infection significantly increases the risk of developing both subtypes of gastric carcinoma [62-65]. Despite the well established role of H. pylori as a risk factor for gastric cancer, the mechanism of carcinogenesis is still not very clear. The first consequence of H. pylori infection is the induction of chronic superficial gastritis. The initiation and promotion of gastric neoplasia may occur via disruption of the epithelial cell proliferation/apoptosis balance and direct damage to host-cell DNA through the synthesis of reactive oxygen species [66,67].
Nevertheless, only a small fraction of infected individuals develop gastric cancer. This probably depends on a combination of factors, including variation in bacterial pathogenicity. H. pylori is genomically diverse and strain differences in virulence factors, including the cag pathogenicity island and the vacuolating cytotoxin, have an important role in the development of gastric carcinoma [68-70].
Within the context of familial gastric cancer, it has been shown that first degree relatives of gastric cancer patients have an increased prevalence of H. pylori infection [71-73]. Furthermore, H. pylori-infected first degree relatives of gastric cancer patients have an increased prevalence of histological and physiological preneoplastic changes, such as atrophic gastritis, intestinal metaplasia and high levels of hypochlorhydria [71,74].
Although it has not been proven that the eradication of H. pylori will result in protection against gastric cancer in first degree relatives of gastric cancer patients, this group is at a significantly higher risk than the general population [72,74,75]. Therefore, international consensus guidelines strongly recommended H. pylori eradication in first degree relatives of gastric cancer patients [75].
In summary, although familial aggregation of gastric cancer may be mediated by familial clustering of H. pylori infection [61], the infection alone cannot explain all of the family aggregation of gastric cancer. Additional genetic and environmental risk factors are likely to contribute to this finding.
Low penetrance genes and genetic susceptibility to gastric cancer
The low frequency of germline mutations in high penetrance genes in familial gastric cancer may be related to an increased susceptibility of these patients to gastric cancer due to low penetrance predisposing genes in association with environmental factors. Patients infected with H. pylori are at an increased risk of developing gastric carcinoma [62]. The risk of developing this type of tumour relates to the physiological and histological changes that H. pylori infection induces in the stomach [76,77]. Although there is evidence showing that H. pylori infection plays a crucial role in the pathogenesis of gastric carcinoma, a striking difference exists between the number of infected individuals and the number that go on to develop malignancy. Hence, progression towards disease is likely to depend on the combined effects of bacterial pathogenicity, host susceptibility and environmental factors.
The IL1B-511*T and IL1RN*2 alleles - which are putatively associated with increased levels of IL1β production [78,79] - and the TNFA-308*A allele - which is thought to increase the production of TNFα [80] - have been found to confer an increased risk of development of gastric carcinoma [69,81,82]. The combined effect of pro-inflammatory host genetic polymorphisms in the IL1B, IL1RN and TNFA genes in the risk of gastric carcinoma development has also been investigated. For gastric carcinoma the odds of developing disease increased with the number of high-risk genotypes. Individuals carrying the three high-risk genotypes are at an increased risk of gastric carcinoma with an OR of 9.7 (95% CI 2.6-36.0) [83]. Very similar findings were also reported by El-Omar and colleagues [84].
Results on record support the hypothesis that the extent of gastric mucosal injury may be related to H. pylori strain differences, inflammatory responses governed by host genetics, and interactions between host and bacterial determinants. The combination of these factors, favouring a set of responses with higher magnitude, can eventually result in hypochlorhydria, corpus atrophy, and an increased risk of gastric carcinoma. In this context, the IL1B, IL1RN and TNFA genes would play a role in gastric carcinogenesis as low penetrance genes.
In conclusion
Sporadic diffuse gastric cancer cases harbour somatic mutations within the CDH1 gene of the truncating and missense type, clustered in exons seven to nine. Similarly, approximately 40% of the families that fulfil the criteria for HDGC show germline mutations of the same gene, ~80% of which are of the truncating type and evenly distributed along the gene. In ~20% of cases, germline CDH1 missense mutations were found and their functional significance was determined by functional assays using an in vitro cell model system. p53 was found to be mutated in families with an excess of gastric cancer and negative for CDH1 germline mutations indicating the need of p53 screen in these types of families. These p53 germline mutation carriers should have a distinct clinical follow-up. It is of great importance to perform an early and comprehensive screening for CDH1 mutations in families at an increased risk of developing diffuse gastric cancer, to allow adequate genetic counselling in these families. Efforts must be made to disclose the genetic basis underlying HDGC in families that lack CDH1 mutations. Familial aggregation of gastric cancer, in the absence of mutations in high penetrance genes, may be in part explained by familial clustering of H. pylori infection in combination with an increased host susceptibility.
Acknowledgements
Raquel Seruca would like to thank all the young members of IPATIMUP that were involved in the work reported herein.
References
- Howson CP, Hiyama T, Wynder EL. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol Rev. 1986;8:1–27. doi: 10.1093/oxfordjournals.epirev.a036288. [DOI] [PubMed] [Google Scholar]
- La Vecchia C, Lucchini F, Negri E, Boyle P, Maisonneuve P, Levi F. Trends of cancer mortality in Europe, 1955-1989: I, Digestive sites. Eur J Cancer. 1992;28(1):132–235. doi: 10.1016/0959-8049(92)90402-N. [DOI] [PubMed] [Google Scholar]
- Carneiro F. Classification of gastric carcinomas. Curr Diag Pathol. 1997;4:51–9. doi: 10.1016/S0968-6053(97)80008-7. [DOI] [Google Scholar]
- Laurén P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma: An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- Goldgar DE, Easton DF, Cannon-Albright LA, Skolnick MH. Systematic population-based assessment of cancer risk in first-degree relatives of cancer probands. J Natl Cancer Inst. 1994;86(21):1600–8. doi: 10.1093/jnci/86.21.1600. [DOI] [PubMed] [Google Scholar]
- Zanghieri G, Di Gregorio C, Sacchetti C, Fante R, Sassatelli R, Cannizzo G, Carriero A, Ponz de Leon M. Familial occurrence of gastric cancer in the 2-year experience of a population-based registry. Cancer. 1990;66(9):2047–51. doi: 10.1002/1097-0142(19901101)66:9<2047::AID-CNCR2820660934>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Palli D, Galli M, Caporaso NE, Cipriani F, Decarli A, Saieva C, Fraumeni JF Jr, Buiatti E. Family history and risk of stomach cancer in Italy. Cancer Epidemiol Biomarkers Prev. 1994;3(1):15–8. [PubMed] [Google Scholar]
- Caldas C, Carneiro F, Lynch HT, Yokota J, Wiesner GL, Powell SM, Lewis FR, Huntsman DG, Pharoah PD, Jankowski JA, MacLeod P, Vogelsang H, Keller G, Park KG, Richards FM, Maher ER, Gayther SA, Oliveira C, Grehan N, Wight D, Seruca R, Roviello F, Ponder BA, Jackson CE. Familial gastric cancer: overview and guidelines for management. J Med Genet. 1999;36(12):873–80. [PMC free article] [PubMed] [Google Scholar]
- Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, Taite H, Scoular R, Miller A, Reeve AE. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392(6674):402–5. doi: 10.1038/32918. [DOI] [PubMed] [Google Scholar]
- Gayther SA, Gorringe KL, Ramus SJ, Huntsman D, Roviello F, Grehan N, Machado JC, Pinto E, Seruca R, Halling K, MacLeod P, Powell SM, Jackson CE, Ponder BA, Caldas C. Identification of germ-line E-cadherin mutations in gastric cancer families of European origin. Cancer Res. 1998;58(18):4086–9. [PubMed] [Google Scholar]
- Richards FM, McKee SA, Rajpar MH, Cole TR, Evans DG, Jankowski JA, McKeown C, Sanders DS, Maher ER. Germline E-cadherin gene (CDH1) mutations predispose to familial gastric cancer and colorectal cancer. Hum Mol Genet. 1999;8(4):607–10. doi: 10.1093/hmg/8.4.607. [DOI] [PubMed] [Google Scholar]
- Guilford PJ, Hopkins JB, Grady WM, Markowitz SD, Willis J, Lynch H, Rajput A, Wiesner GL, Lindor NM, Burgart LJ, Toro TT, Lee D, Limacher JM, Shaw DW, Findlay MP, Reeve AE. E-cadherin germline mutations define an inherited cancer syndrome dominated by diffuse gastric cancer. Hum Mutat. 1999;14(3):249–55. doi: 10.1002/(SICI)1098-1004(1999)14:3<249::AID-HUMU8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Shinmura K, Kohno T, Takahashi M, Sasaki A, Ochiai A, Guilford P, Hunter A, Reeve AE, Sugimura H, Yamaguchi N, Yokota J. Familial gastric cancer: clinicopathological characteristics, RER phenotype and germline p53 and E-cadherin mutations. Carcinogenesis. 1999;20(6):1127–31. doi: 10.1093/carcin/20.6.1127. [DOI] [PubMed] [Google Scholar]
- Shore EM, Nelson WJ. Biosynthesis of the cell adhesion molecule uvomorulin (E-cadherin) in Madin-Darby canine kidney epithelial cells. J Biol Chem. 1991;266(29):19672–80. [PubMed] [Google Scholar]
- Shapiro L, Fannon AM, Kwong PD, Thompson A, Lehmann MS, Grubel G, Legrand JF, Als-Nielsen J, Colman DR, Hendrickson WA. Structural basis of cell-cell adhesion by cadherins. Nature. 1995;374(6520):327–37. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- Berx G, Cleton-Jansen AM, Nollet F, de Leeuw WJ, Vijver M van de, Cornelisse C, van Roy F. E-cadherin is a tumour/invasion suppressor gene mutated in human lobular breast cancers. EMBO J. 1995;14(24):6107–15. doi: 10.1002/j.1460-2075.1995.tb00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckband D, Sivasankar S. Mechanism of homophilic cadherin adhesion. Curr Opin Cell Biol. 2000;12(5):587–92. doi: 10.1016/S0955-0674(00)00136-8. [DOI] [PubMed] [Google Scholar]
- Christofori G, Semb H. The role of the cell-adhesion molecule E-cadherin as a tumour-suppressor gene. Trends Biochem Sci. 1999;24(2):73–6. doi: 10.1016/S0968-0004(98)01343-7. [DOI] [PubMed] [Google Scholar]
- Van Aken E, De Wever O, Correia da Rocha AS, Mareel M. Defective E-cadherin/catenin complexes in human cancer. Virchows Arch. 2001;439(6):725–51. doi: 10.1007/s004280100516. [DOI] [PubMed] [Google Scholar]
- Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, Hofler H. E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res. 1994;54(14):3845–52. [PubMed] [Google Scholar]
- Oliveira C, Seruca R, Caldas C. Genetic screening for hereditary diffuse gastric cancer. Expert Rev Mol Diagn. 2003;3(2):201–15. doi: 10.1586/14737159.3.2.201. [DOI] [PubMed] [Google Scholar]
- Soares P, Berx G, van Roy F, Sobrinho-Simoes M. E-cadherin gene alterations are rare events in thyroid tumors. Int J Cancer. 1997;70(1):32–8. doi: 10.1002/(SICI)1097-0215(19970106)70:1<32::AID-IJC5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Berx G, Becker KF, Hofler H, van Roy F. Mutations of the human E-cadherin (CDH1) gene. Hum Mutat. 1998;12(4):226–37. doi: 10.1002/(SICI)1098-1004(1998)12:4<226::AID-HUMU2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Machado JC, Oliveira C, Carvalho R, Soares P, Berx G, Caldas C, Seruca R, Carneiro F, Sobrinho-Simoes M. E-cadherin gene (CDH1) promoter methylation as the second hit in sporadic diffuse gastric carcinoma. Oncogene. 2001;20(12):1525–8. doi: 10.1038/sj.onc.1204234. [DOI] [PubMed] [Google Scholar]
- Jonsson BA, Bergh A, Stattin P, Emmanuelsson M, Gronberg H. Germline mutations in E-cadherin do not explain association of hereditary prostate cancer, gastric cancer and breast cancer. Int J Cancer. 2002;98(6):838–43. doi: 10.1002/ijc.10258. [DOI] [PubMed] [Google Scholar]
- Yabuta T, Shinmura K, Tani M, Yamaguchi S, Yoshimura K, Katai H, Nakajima T, Mochiki E, Tsujinaka T, Takami M, Hirose K, Yamaguchi A, Takenoshita S, Yokota J. E-cadherin gene variants in gastric cancer families whose probands are diagnosed with diffuse gastric cancer. Int J Cancer. 2002;101(5):434–41. doi: 10.1002/ijc.10633. [DOI] [PubMed] [Google Scholar]
- Wang Y, Song JP, Ikeda M, Shinmura K, Yokota J, Sugimura H. Ile-Leu substitution (I415L) in germline E-cadherin gene (CDH1) in Japanese familial gastric cancer. Jpn J Clin Oncol. 2003;33(1):17–20. doi: 10.1093/jjco/hyg002. [DOI] [PubMed] [Google Scholar]
- Oliveira C, de Bruin J, Nabais S, Ligtenberg M, Moutinho C, Nagengast FM, Seruca R, van Krieken H, Carneiro F. Intragenic deletion of CDH1 as the inactivating mechanism of the wild-type allele in a HDGC tumour. Oncogene. 2004;23(12):2236–40. doi: 10.1038/sj.onc.1207335. [DOI] [PubMed] [Google Scholar]
- Keller G, Vogelsang H, Becker I, Plaschke S, Ott K, Suriano G, Mateus AR, Seruca R, Biedermann K, Hundsman D, Döring C, Holinski-Feder E, Neutzling A, Siewert JR, Höfler H. Germline mutations of the E-cadherin (CDH1) and TP53 genes rather than of RUNX3 and HPP1 contribute to genetic predisposition in german gastric cancer patients. J Med Genet. 2004;41(6):E89. doi: 10.1136/jmg.2003.015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira C, Ferreira P, Nabais S, Campos L, Ferreira A, Cirnes L, Alves CC, Veiga I, Fragoso M, Regateiro F, Dias LM, Moreira H, Suriano G, Machado JC, Lopes C, Castedo S, Carneiro F, Seruca R. E-Cadherin (CDH1) and TP53 rather than SMAD4 and Caspase-10 germline mutations contribute to genetic predisposition in Portuguese gastric cancer patients. Eur J Cancer. in press . [DOI] [PubMed]
- Brooks-Wilson AR, Kaurah P, Suriano G, Leach S, Senz J, Grehan N, Buttefield YSN, Jeyes J, Schinas J, Bacani J, Kelsey M, Ferreira P, MacGillivray B, MacLeod P, Micek M, Ford J, Foulkes W, Australie K, Greenberg C, LaPointe M, Gilpin CR, Nikkel S, Gilchrist D, Hughes R, Jackson C, Monaghan KG, Oliveira MJ, Seruca R, Gallinger S, Caldas C, Huntsman DG. Germline E-Cadherin Mutations in Hereditary Diffuse Gastric Cancer: Assessment of 42 New Families and Review of Genetic Screening Criteria. J Med Genet. in press . [DOI] [PMC free article] [PubMed]
- Oliveira C, Bordin MC, Grehan N, Huntsman D, Suriano G, Machado JC, Aaltonen L, Jackson CE, Seruca R, Caldas C. Screening of E-Cadherin in gastric cancer families reveals germ-line mutations only in hereditary diffuse gastric cancer kindred. Hum Mutat. 2002;19(5):510–517. doi: 10.1002/humu.10068. [DOI] [PubMed] [Google Scholar]
- Suriano G, Oliveira C, Ferreira P, Machado JC, Bordin MC, De Wever O, Bruyneel EA, Moguilevsky N, Grehan N, Porter TR, Richards FM, Hruban RH, Roviello F, Huntsman D, Mareel M, Carneiro F, Caldas C, Seruca R. Identification of CDH1 germline missense mutations associated with functional inactivation of the E-cadherin protein in young gastric cancer probands. Hum Mol Genet. 2003;12(5):575–582. doi: 10.1093/hmg/ddg048. [DOI] [PubMed] [Google Scholar]
- Grady WM, Willis J, Guilford PJ, Dunbier AK, Toro TT, Lynch H, Wiesner G, Ferguson K, Eng C, Park JG, Kim SJ, Markowitz S. Methylation of the CDH1 promoter as the second genetic hit in hereditary diffuse gastric cancer. Nat Genet. 2000;26(1):16–17. doi: 10.1038/79120. [DOI] [PubMed] [Google Scholar]
- Handschuh G, Luber B, Hutzler P, Hofler H, Becker KF. Single amino acid substitutions in conserved extracellular domains of E-cadherin differ in their functional consequences. J Mol Biol. 2001;314(3):445–454. doi: 10.1006/jmbi.2001.5143. [DOI] [PubMed] [Google Scholar]
- Vecsey-Semjen B, Becker KF, Sinski A, Blennow E, Vietor I, Zatloukal K, Beug H, Wagner E, Huber LA. Novel colon cancer cell lines leading to better understanding of the diversity of respective primary cancers. Oncogene. 2002;21(30):4646–4662. doi: 10.1038/sj.onc.1205577. [DOI] [PubMed] [Google Scholar]
- Suriano G, Mulholland D, de Wever O, Ferreira P, Mateus AR, Bruyneel E, Nelson CC, Mareel MM, Yokota J, Huntsman D, Seruca R. The intracellular E-cadherin germline mutation V832 M lacks the ability to mediate cell-cell adhesion and to suppress invasion. Oncogene. 2003;22(36):5716–9. doi: 10.1038/sj.onc.1206672. [DOI] [PubMed] [Google Scholar]
- Suriano G, Oliveira MJ, Huntsman D, Mateus AR, Ferreira P, Casares F, Oliveira C, Carneiro F, Machado JC, Mareel M, Seruca R. E-cadherin germline missense mutations and cell phenotype: evidence for the independence of cell invasion on the motile capabilities of the cells. Hum Mol Genet. 2003;12(22):3007–3016. doi: 10.1093/hmg/ddg316. [DOI] [PubMed] [Google Scholar]
- Shin Y, Kim IJ, Kang HC, Park JH, Park HR, Park HW, Park MA, Lee JS, Yoon KA, Ku JL, Park JG. The E-cadherin -347G->GA promoter polymorphism and its effect on transcriptional regulation. Carcinogenesis. 2004;25(6):895–899. doi: 10.1093/carcin/bgh073. [DOI] [PubMed] [Google Scholar]
- Li LC, Chui RM, Sasaki M, Nakajima K, Perinchery G, Au HC, Nojima D, Carroll P, Dahiya R. A single nucleotide polymorphism in the E-cadherin gene promoter alters transcriptional activities. Cancer Res. 2000;60(4):873–876. [PubMed] [Google Scholar]
- Wu MS, Huang SP, Chang YT, Lin MT, Shun CT, Chang MC, Wang HP, Chen CJ, Lin JT. Association of the -160 C -> a promoter polymorphism of E-cadherin gene with gastric carcinoma risk. Cancer. 2002;94(5):1443–8. doi: 10.1002/cncr.10371. [DOI] [PubMed] [Google Scholar]
- Humar B, Graziano F, Cascinu S, Catalano V, Ruzzo AM, Magnani M, Toro T, Burchill T, Futschik ME, Merriman T, Guilford P. Association of CDH1 haplotypes with susceptibility to sporadic diffuse gastric cancer. Oncogene. 2002;21(53):8192–8195. doi: 10.1038/sj.onc.1205921. [DOI] [PubMed] [Google Scholar]
- Pharoah PD, Oliveira C, Machado JC, Keller G, Vogelsang H, Laux H, Becker KF, Hahn H, Paproski SM, Brown LA, Caldas C, Huntsman D. CDH1 c-160a promotor polymorphism is not associated with risk of stomach cancer. Int J Cancer. 2002;101(2):196–197. doi: 10.1002/ijc.10590. [DOI] [PubMed] [Google Scholar]
- Keller G, Vogelsang H, Becker I, Hutter J, Ott K, Candidus S, Grundei T, Becker KF, Mueller J, Siewert JR, Hofler H. Diffuse type gastric and lobular breast carcinoma in a familial gastric cancer patient with an E-cadherin germline mutation. Am J Pathol. 1999;155(2):337–342. doi: 10.1016/S0002-9440(10)65129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharoah PD, Guilford P, Caldas C. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology. 2001;121(6):1348–1353. doi: 10.1053/gast.2001.29611. [DOI] [PubMed] [Google Scholar]
- Aarnio M, Salovaara R, Aaltonen LA, Mecklin JP, Jarvinen HJ. Features of gastric cancer in hereditary non-polyposis colorectal cancer syndrome. Int J Cancer. 1997;74(5):551–555. doi: 10.1002/(SICI)1097-0215(19971021)74:5<551::AID-IJC13>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Peltomaki P. Role of DNA mismatch repair defects in the pathogenesis of human cancer. J Clin Oncol. 2003;21(6):1174–1179. doi: 10.1200/JCO.2003.04.060. [DOI] [PubMed] [Google Scholar]
- Suraweera N, Duval A, Reperant M, Vaury C, Furlan D, Leroy K, Seruca R, Iacopetta B, Hamelin R. Evaluation of tumor microsatellite instability using five quasimonomorphic mononucleotide repeats and pentaplex PCR. Gastroenterology. 2002;123(6):1804–1811. doi: 10.1053/gast.2002.37070. [DOI] [PubMed] [Google Scholar]
- Varley JM, McGown G, Thorncroft M, Santibanez-Koref MF, Kelsey AM, Tricker KJ, Evans DG, Birch JM. Germ-line mutations of TP53 in Li-Fraumeni families: an extended study of 39 families. Cancer Res. 1997;57(15):3245–3252. [PubMed] [Google Scholar]
- Jakubowska A, Nej K, Huzarski T, Scott RJ, Lubinski J. BRCA2 gene mutations in families with aggregations of breast and stomach cancers. Br J Cancer. 2002;87(8):888–891. doi: 10.1038/sj.bjc.6600562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowska A, Scott R, Menkiszak J, Gronwald J, Byrski T, Huzarski T, Gorski B, Cybulski C, Debniak T, Kowalska E, Starzynska T, Lawniczak M, Narod S, Lubinski J. A high frequency of BRCA2 gene mutations in Polish families with ovarian and stomach cancer. Eur J Hum Genet. 2003;11(12):955–958. doi: 10.1038/sj.ejhg.5201064. [DOI] [PubMed] [Google Scholar]
- Li QL, Ito K, Sakakura C, Fukamachi H, Inoue K, Chi XZ, Lee KY, Nomura S, Lee CW, Han SB, Kim HM, Kim WJ, Yamamoto H, Yamashita N, Yano T, Ikeda T, Itohara S, Inazawa J, Abe T, Hagiwara A, Yamagishi H, Ooe A, Kaneda A, Sugimura T, Ushijima T, Bae SC, Ito Y. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002;109(1):113–124. doi: 10.1016/S0092-8674(02)00690-6. [DOI] [PubMed] [Google Scholar]
- Shibata DM, Sato F, Mori Y, Perry K, Yin J, Wang s, Xu Y, Olaru A, Selaru F, Spring K, Youing J, Abraham JM, Meltzer SJ. Hypermethylation of HPP1 is associated with hMLH1 hypermethylation in gastric adenocarcinomas. Cancer Res. 2002;62(20):5637–5640. [PubMed] [Google Scholar]
- Park WS, Lee JH, Shin MS, Park JY, Kim HS, Lee JH, Kim YS, Lee SN, Xiao W, Park CH, Lee SH, Yoo NJ, Lee JY. Inactivating mutations of the caspase-10 gene in gastric cancer. Oncogene. 2002;21(18):2919–2925. doi: 10.1038/sj.onc.1205394. [DOI] [PubMed] [Google Scholar]
- Takaku K, Miyoshi H, Matsunaga A, Oshima M, Sasaki N, Taketo MM. Gastric and duodenal polyps in Smad4 (Dpc4) knockout mice. Cancer Res. 1999;59(24):6113–6117. [PubMed] [Google Scholar]
- Houlston R, Houlston R, Bevan S, Williams A, Young J, Dunlop M, Rozen P, Eng C, Markie D, Woodford-Richens K, Rodriguez-Bigas MA, Leggett B, Neale K, Phillips R, Sheridan E, Hodgson S, Iwama T, Eccles D, Bodmer W, Tomlinson I. Mutations in DPC4 (SMAD4) cause juvenile polyposis syndrome, but only account for a minority of cases. Hum Mol Genet. 1998;7(12):1907–1912. doi: 10.1093/hmg/7.12.1907. [DOI] [PubMed] [Google Scholar]
- Howe JR, Roth S, Ringold JC, Summers RW, Jarvinen HJ, Sistonen P, Tomlinson IP, Houlston RS, Bevan S, Mitros FA, Stone EM, Aaltonen LA. Mutations in the SMAD4/DPC4 gene in juvenile polyposis. Science. 1998;280(5366):1086–1088. doi: 10.1126/science.280.5366.1086. [DOI] [PubMed] [Google Scholar]
- Lynch HT, Grady W, Lynch JF, Tsuchiya KD, Wiesner G, Markowitz SD. E-cadherin mutation-based genetic counseling and hereditary diffuse gastric carcinoma. Cancer Genet Cytogenet. 2000;122(1):1–6. doi: 10.1016/S0165-4608(00)00273-9. [DOI] [PubMed] [Google Scholar]
- Blaser MJ. The changing relationships of Helicobacter pylori and humans: implications for health and disease. J Infect Dis. 1999;179(6):1523–1530. doi: 10.1086/314785. [DOI] [PubMed] [Google Scholar]
- Everhart JE. Recent developments in the epidemiology of Helicobacter pylori. Gastroenterol Clin N Am. 2000;29(3):559–578. doi: 10.1016/S0889-8553(05)70130-8. [DOI] [PubMed] [Google Scholar]
- Drumm B, Perez-Perez GI, Blaser MJ, Sherman PM. Intrafamilial clustering of Helicobacter pylori infection. N Engl J Med. 1990;322(6):359–363. doi: 10.1056/NEJM199002083220603. [DOI] [PubMed] [Google Scholar]
- Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Huang J-Q, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114(6):1169–1179. doi: 10.1016/S0016-5085(98)70422-6. [DOI] [PubMed] [Google Scholar]
- Ekstrom AM, Serafini M, Nyren O, Hansson LE, Ye W, Wolk A. Dietary antioxidant intake and the risk of cardia cancer and noncardia cancer of the intestinal and diffuse types: a population-based case-control study in Sweden. Int J Cancer. 2000;87(1):133–140. doi: 10.1002/1097-0215(20000701)87:1<133::AID-IJC20>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345(11):784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- Cover TL, Krishna US, Israel DA, Peek RM Jr. Induction of gastric epithelial cell apoptosis by Helicobacter pylori vacuolating cytotoxin. Cancer Res. 2003;63(5):951–957. [PubMed] [Google Scholar]
- Smoot DT, Elliott TB, Verspaget HW, Jones D, Allen CR, Vernon KG, Bremner T, Kidd LC, Kim KS, Groupman JD, Ashktorab H. Influence of Helicobacter pylori on reactive oxygen-induced gastric epithelial cell injury. Carcinogenesis. 2000;21(11):2091–2095. doi: 10.1093/carcin/21.11.2091. [DOI] [PubMed] [Google Scholar]
- Figueiredo C, van Doorn LJ, Nogueira C, Soares JM, Pinho C, Figueira P, Quint WGV, Carneiro F. Helicobacter pylori genotypes are associated with clinical outcome in Portuguese patients and show a high prevalence of infections with multiple strains. Scand J Gastroenterol. 2001;36(2):128–135. doi: 10.1080/003655201750065861. [DOI] [PubMed] [Google Scholar]
- Figueiredo C, Machado JC, Pharoah P, Seruca R, Sousa S, Carvalho R, Capelinha AF, Quint W, Caldas C, van Doorn LJ, Carneiro F, Sobrinho-Simoes M. Helicobacter pylori and interleukin-1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst. 2002;94(22):1680–1687. doi: 10.1093/jnci/94.22.1680. [DOI] [PubMed] [Google Scholar]
- Peek RM, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2(1):28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- Carneiro F, Taveira-Gomes A, Cabral-Correia A, Vasconcelos-Teixeira A, Barreira R, Cardoso-Oliveira M, Sobrinho-Simoes M. Characteristics of the gastric mucosa of direct relatives of patients with sporadic gastric carcinoma. Eur J Cancer Prev. 1993;2(3):239–46. doi: 10.1097/00008469-199305000-00008. [DOI] [PubMed] [Google Scholar]
- Brenner H, Bode G, Boeing H. Helicobacter pylori infection among offspring of patients with stomach cancer. Gastroenterology. 2000;118(1):31–35. doi: 10.1016/S0016-5085(00)70411-2. [DOI] [PubMed] [Google Scholar]
- Brenner H, Arndt V, Sturmer T, Stegmaier C, Ziegler H, Dhom G. Individual and joint contribution of family history and Helicobacter pylori infection to the risk of gastric carcinoma. Cancer. 2000;88(2):274–279. doi: 10.1002/(SICI)1097-0142(20000115)88:2<274::AID-CNCR5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- El-Omar EM, Oien K, Murray LS, El-Nujumi A, Wirz A, Gillen D, Williams C, Fullarton G, McColl KEL. Increased prevalence of precancerous changes in relatives of gastric cancer patients: critical role of Helicobacter pylori. Gastroenterology. 2000;118(1):22–30. doi: 10.1016/S0016-5085(00)70410-0. [DOI] [PubMed] [Google Scholar]
- Malfertheiner P, Megraud F, O'Morain C, Hungin AP, Jones R, Axon A, Graham DY, Tytgat G. the European Helicobacter Pylori Study Group (EHPSG) Current concepts in the management of Helicobacter pylori infection-the Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther. 2002;16(2):167–180. doi: 10.1046/j.1365-2036.2002.01169.x. [DOI] [PubMed] [Google Scholar]
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process - First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52(24):6735–6740. [PubMed] [Google Scholar]
- Kuipers EJ, Uyterlinde AM, Pena AS, Roosendaal R, Pals G, Nelis GF, Festen HP, Meuwissen SG. Long-term sequelae of Helicobacter pylori gastritis. Lancet. 1995;345(8964):1525–1528. doi: 10.1016/S0140-6736(95)91084-0. [DOI] [PubMed] [Google Scholar]
- Santtila S, Savinainen K, Hurme M. Presence of the IL-1RA allele 2 (IL1RN*2) is associated with enhanced IL-1beta production in vitro. Scand J Immunol. 1998;47(3):195–198. doi: 10.1046/j.1365-3083.1998.00300.x. [DOI] [PubMed] [Google Scholar]
- Pociot F, Molvig J, Wogensen L, Worsaae H, Nerup J. A TaqI polymorphism in the human interleukin-1 beta (IL-1 beta) gene correlates with IL-1 beta secretion in vitro. Eur J Clin Invest. 1992;22(6):396–402. doi: 10.1111/j.1365-2362.1992.tb01480.x. [DOI] [PubMed] [Google Scholar]
- Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Natl Acad Sci USA. 1997;94(7):3195–3199. doi: 10.1073/pnas.94.7.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, Lanyon G, Martin M, Fraumeni JFJ, Rabkin CS. Interleukin-1 polymorphisms associated with increased risk of gastric cancer (published erratum appears in Nature 2001; 412: 99) Nature. 2000;404(6842):398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- Machado JC, Pharoah P, Sousa S, Carvalho R, Oliveira C, Figueiredo C, Amorim A, Seruca R, Caldas C, Carneiro F, Sobrinho-Simoes M. Interleukin 1B and interleukin 1RN polymorphisms are associated with increased risk of gastric carcinoma. Gastroenterology. 2001;121(4):823–829. doi: 10.1053/gast.2001.28000. [DOI] [PubMed] [Google Scholar]
- Machado JC, Figueiredo C, Canedo P, Pharoah P, Carvalho R, Nabais S, Alves CC, Campos ML, van Doorn LJ, Caldas C, Seruca R, Carneiro F, Sobrinho-Simoes M. A pro-inflammatory genetic profile increases the risk of chronic atrophic gastritis and gastric carcinoma. Gastroenterology. 2003;125(2):364–371. doi: 10.1016/S0016-5085(03)00899-0. [DOI] [PubMed] [Google Scholar]
- El Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA, Schoenberg JB, Stanford JL, Mayne ST, Goedert J, Blot WJ, Fraumeni JF Jr, Chow WH. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124(5):1193–1201. doi: 10.1016/S0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- Yoon KA, Ku JL, Yang HK, Kim WH, Park SY, Park JG. Germline mutations of E-cadherin gene in Korean familial gastric cancer patients. J Hum Genet. 1999;44(3):177–180. doi: 10.1007/s100380050137. [DOI] [PubMed] [Google Scholar]
- Iida S, Akiyama Y, Ichikawa W, Yamashita T, Nomizu T, Nihei Z, Sugihara K, Yuasa Y. Infrequent germ-line mutation of the E-cadherin gene in Japanese familial gastric cancer kindreds. Clin Cancer Res. 1999;5(6):1445–1447. [PubMed] [Google Scholar]
- Avizienyte E, Launonen V, Salovaara R, Kiviluoto T, Aaltonen L. E-cadherin is not frequently mutated in hereditary gastric cancer. J Med Genet. 2001;38(1):49–52. doi: 10.1136/jmg.38.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussaulx-Garin L, Blayau M, Pagenault M, Le Berre-Heresbach N, Raoul JL, Campion JP, David V, Bretagne JF. A new mutation of E-cadherin gene in familial gastric linitis plastica cancer with extra-digestive dissemination. Eur J Gastroenterol Hepatol. 2001;13(6):711–715. doi: 10.1097/00042737-200106000-00016. [DOI] [PubMed] [Google Scholar]
- Humar B, Toro T, Graziano F, Muller H, Dobbie Z, Kwang-Yang H, Eng C, Hampel H, Gilbert D, Winship I, Parry S, Ward R, Findlay M, Christian A, Tucker M, Tucker K, Merriman T, Guilford P. Novel germline CDH1 mutations in hereditary diffuse gastric cancer families. Hum Mutat. 2002;19(5):518–525. doi: 10.1002/humu.10067. [DOI] [PubMed] [Google Scholar]