Abstract
As T cells alone are both necessary and sufficient for the rejection of virtually all allogeneic tissues, much of transplantation immunology has focused on cells of the adaptive immune system. During the past decade, advances in our understanding of innate responses to pathogen-associated molecules have spurred a “rediscovery” of innate immunity. Fueled by this, an increasing body of literature has emerged in which the role of the innate immune system in allograft rejection and tolerance has been examined more closely. This review will give an overview of recent studies and emerging concepts of how the cellular components of the innate immune system participate in the immune response to solid organ transplantation. These important studies highlight the complex interplay between diverse cells of the immune response and provide the basis for optimal strategies of tolerance induction.
For many years, the field of transplantation immunology has focused on cells of the adaptive immune system. Fostered by work showing that T cells are both necessary and sufficient for rejection of virtually all allogeneic tissues, most investigators have emphasized T cell-mediated mechanisms of allograft rejection and tolerance induction. However, advances in our understanding of innate responses to molecules derived from microorganisms has spurred the “rediscovery” of innate immunity and highlighted its critical role in shaping the adaptive response (1). Consistent with these observations, an increasing body of literature has emerged examining the role of the innate immune system in allograft rejection and tolerance. This review will discuss recent studies and emerging concepts of how the cellular components of the innate immune system participate in the immune response to solid organ transplantation.
Pathways of allorecognition and the essentials of acute allograft rejection
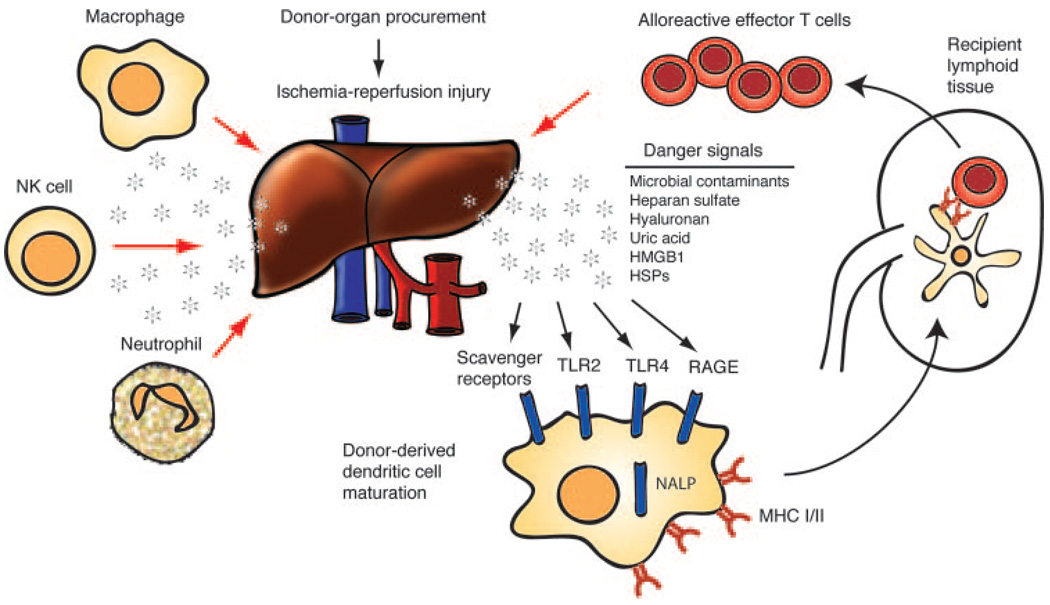
Allorecognition refers to the ability of T cells to recognize genetically different MHC molecules and occurs by two distinct but nonmutually exclusive pathways. In the direct pathway, alloreactive T cells recognize intact donor MHC molecules on APCs that are “passengers” in the transplanted tissue (2). In the indirect pathway, host APCs process Ag derived from donor MHC molecules and present them to alloreactive T cells in a self-restricted manner (3). After naive T cells receive activating signals in lymphoid tissue, they transition to effector cells and engage the graft directly (Fig. 1). Acute graft rejection is considered a T cell-mediated process based on several studies showing mice lacking T cells accept fully mismatched allografts, with rejection occurring only upon T cell reconstitution. Graft damage is caused by mechanisms that include direct T cell cytotoxicity and classic delayed-type hypersensitivity (4).
FIGURE 1.
Innate immune responses in allotransplantation. Soon after solid organ transplantation, Ag-independent insults to the allograft caused by organ procurement and ischemia/reperfusion injury promote immunogenicity via danger signals that lead to the activation of donor-derived APCs. The direct pathway of allorecognition is depicted in which “passenger” DCs undergo functional maturation in response to danger-associated molecules and then traffic to the T cell areas in recipient lymphoid tissue. Thus, naive alloreactive T cells become stimulated, transition to effectors, and engage the graft directly. Other cells of the innate immune system, such as neutrophils, macrophages, and NK cells, rapidly infiltrate the allograft in response to inflammatory signals and promote further injury either by their own proinflammatory mechanisms or by supporting the activity of alloreactive T cells.
However, it is clear from recent data that the paradigm described above is incomplete and does not fully represent the interplay of events associated with allotransplantation. Although T cells have a critical role in acute rejection, it is now recognized that up-regulation of proinflammatory mediators in the allograft occurs before the T cell response; this early inflammation is due to the innate response to tissue injury independent of the adaptive immune system (5–7). For example, using RNase protection assay of cardiac allografts 1 day after transplantation, He et al. (6) found the patterns of cellular infiltration, chemokine receptor, and proinflammatory cytokine expression were similar in RAG-deficient transplant recipients compared with recipients with intact adaptive immunity. Thus, innate, Ag-independent proinflammatory events occur soon after transplantation and may be further shaped and amplified by the graft-specific adaptive response (7).
PRRs as sensors of invading microorganisms and tissue injury
All multicellular organisms have evolved molecular mechanisms to sense danger and rapidly mount protective responses. Such first-line immune mechanisms are collectively referred to as innate immunity. In contrast to cells of the adaptive immune system, innate immune cells perform their sentinel function by using nonrearranged receptors that are referred to as pattern recognition receptors (PRRs)3 (8). PRRs sense conserved pathogen-derived molecules that identify infectious nonself from self, although recent data suggest these receptors may also sense host-derived molecules released from damaged or stressed tissue (9). Because transplantation of some tissues (e.g., skin) is inherently nonsterile, and ischemic and surgical trauma releases endogenous molecules capable of activating PRRs (see below), these systems are relevant to transplantation biology.
TLRs are a well-studied family of PRRs, which play a major role in activating innate responses and directing adaptive immunity (10). These receptors are expressed in a diverse cast of hemopoietic cells, including DCs, B cells, mast cells, and T cells, but are also found in endothelial cells and organ parenchyma cells (11, 12). Furthermore, the expression of TLRs is modulated dynamically by inflammatory mediators and other local or systemic danger signals. As transmembrane proteins, the ligand-binding regions of TLRs survey the extracellular environment or the contents of membrane-enclosed intracellular vesicles. Once stimulated, a cascade of signaling events occur, which in most cases results in the downstream activation of the transcription factors NF-κB and AP-1 (13). Thus, TLR signals lead to the rapid transcription of genes associated with inflammation, resulting in the production of proinflammatory cytokines and chemokines, antimicrobial peptides, adhesion molecules, and, in the case of APCs, enhanced Ag presentation and up-regulation of costimulatory molecules (1, 13).
In addition to TLRs, two other families of PRRs that recognize microbial products have received increasing attention: the NOD-like receptors (NLRs) and the RIG-like helicases (RLHs). In contrast to TLRs, NLRs and RLHs are soluble proteins that survey the intracellular compartment. RLHs are defined as cytoplasmic sensors of viral double-stranded RNA, which upon activation trigger NF-κB and IFN regulatory factor-3/7 transcription factors, resulting in type I IFN responses (14). NLRs represent two large subclasses that consist of the NODs and members of the NALP family (15). NOD1 and NOD2 detect components of bacterial peptidoglycan, which analogous to TLRs, leads to the downstream activation of MAPKs and NF-κB. Regarding NALP proteins, recent data suggest a direct role as intracellular sensors of cellular stress. Of particular interest is NALP3, which in association with two adaptor proteins, ASC and CARDINAL, complexes with caspase-1 to form a structure referred to as the NALP3-inflammasome (16). In-flammasomes are molecular platforms that control the activation of the proinflammatory cytokines IL-1β and IL-18. Endogenous danger signals, such as rapid potassium efflux from the cytosol induced by high ATP concentrations or the presence of monosodium urate crystals, induce caspase-1 activity by a mechanism dependent on the NALP3-inflammasome (17, 18).
Another PRR known as receptor of advanced glycation end products (RAGE) has been recently in the spotlight. RAGE is a cell surface transmembrane receptor expressed on a variety of hemopoietic and parenchymal cells. It was first described as a receptor for the products of nonenzymatic glycation and protein oxidation, which are associated with certain inflammatory disease states such as diabetes and chronic renal failure (19). Further study found RAGE to bind to several other protein molecules with similar tertiary structures, including amyloid fibril components, proinflammatory mediators of the S100/calgranulin family, and high-mobility group box 1 (HMGB1) protein (20, 21). Upon stimulation with its ligands, RAGE signals via a largely unknown pathway, ultimately leading to NF-κB activation (20). In addition to its role in inflammation, recent studies (22, 23) suggest RAGE also serves as a receptor for β2 integrins and directly participates in leukocyte recruitment.
Beyond those listed above, other PRRs have been characterized, some of which include scavenger receptors, mannose binding lectin, complement receptors, dectin-1, and DC-specific ICAM-grabbing nonintegrin, all with as yet unknown roles in allotransplantation.
Injury and endogenous danger in transplantation
The “self-nonself discrimination model” was advanced by Jane-way (24) and others with the discovery that pathogen-associated molecules activate innate immunity through PRRs and direct the adaptive response to infection. In a broader sense, the “danger model” as proposed by Matzinger (25) describes immune surveillance that detects and responds to cellular damage caused by microbial infection or other endogenous alarm signals. In the setting of allotransplantation, Land et al. (26) proposed a similar “injury hypothesis” to describe the clinical finding that intraoperative treatment of cadaver-derived renal allografts with a free-radical scavenger reduced the incidence of acute rejection and improved long-term outcome. In these damage or injury models, Ag-independent insults to the allograft caused by procurement and ischemia/reperfusion injury enhance immunogenicity through the activation of passenger APCs. Thus, allorecognition and rejection are inherently promoted by the unavoidable injurious consequences of organ transplantation (Fig. 1).
Central to the danger model is the ability of tissue to communicate to the immune system the occurrence of cellular stress or injury. Work from the Kupiec-Weglinski group (27, 28) examining a model of ischemia/reperfusion liver injury demonstrated that TLR4 signaling, but not TLR2 signaling, was required for optimal inflammatory responses to this insult. Using a similar model, Tsung et al. (29) show that functional TLR4 on hemopoietic-derived phagocytes, but not organ parenchymal cells, was required for inflammation associated with liver ischemia/reperfusion. Several studies have focused on the specific products of necrotic cells or extracellular matrix disruption as a source of danger signals, particularly through TLR2- and TLR4-dependent responses (9, 30). Such putative endogenous danger-associated molecules include hyaluronan (31, 32), heparan sulfate (33), fibronectin extra domain A (34), and biglycan (35). Additionally, heat-shock proteins 60, 70, and gp96, as well as HMGB1, have been implicated in signaling danger through TLRs (36–39). As discussed by Tsan and Gao (40), these data need to be considered in light of the fact that APCs are extremely sensitive to pathogen-derived agonists of TLR2 and 4, which may contaminate reagents obtained by recombinant DNA technology. For example, a recombinant preparation of heat-shock protein 70 with as little as 0.2 ng/ml LPS contamination was found to induce proinflammatory cytokine release from mouse macrophages while a rigorously purified preparation did not (41). Nevertheless, more recent studies (31, 32, 35) have been designed to specifically address contamination as confounder, substantially strengthening these observations. Furthermore, Tsung et al. (42) demonstrate that HMGB1 levels are increased in liver ischemia/reperfusion as early as 1 h after reperfusion, and neutralization of HMGB1 with Ab decreases markers of liver inflammation.
A corollary of these models is that, in the absence of danger signals, Ag presentation occurs without costimulation favoring peripheral tolerance—a hypothesis that remains difficult to prove in vivo. Testing this model, in studies by the Larsen group (43), T cell-deficient mice received major or multiple minor mismatched skin or cardiac allografts, which were allowed to heal for a period of 50 days. After this period, adoptive transfer of T cells or T cell reconstitution from transplanted bone marrow grafts resulted in acute rejection. The Matzinger group (44) extended these findings performing similar experiments with sex-disparate, H–Y mismatched skin allografts, confirming rejection upon T cell reconstitution. Their experiments revealed that, although the long-standing healed grafts appeared normal histologically, by quantitative PCR subtle differences in multiple transcripts were found, mostly attributable to alterations in GAPDH expression. These data show that well-healed mismatched allografts are rejected by adoptive transfer of T cells and suggest persistence of relevant danger signals in the allografts, although the signals themselves were difficult to specifically identify using the techniques employed in these studies.
More recently, Chalasani et al. (45) used a “healed-in” model in which allograft recipients had an endogenous T cell compartment, eliminating the effect homeostatic proliferation has on tolerance induction (46). Cardiac allografts were allowed to heal for 70 days in splenectomized, alymphoplastic hosts, which have T cells but are devoid of secondary lymphoid organs, thus preventing naive T cell priming. In this model, allografts were not acutely rejected upon adoptive transfer of activated alloreactive T cells, but after 100 days, instead displayed histological evidence of chronic rejection. In further support that injury to the graft plays a major role in shaping the outcome of rejection, cardiac allografts that had been parked for 50 days in splenectomized, alymphoplastic hosts were retransplanted into a second set of identical recipients. Transfer of activated alloreactive T cells 2 days after retransplantation resulted in acute rejection, albeit with more delayed kinetics compared with newly transplanted hearts (45).
Examining PRR signaling in allotransplantation
Several recent studies have examined the role of TLR agonists and TLR signals in allorecognition and rejection. In the first of these reports, Goldstein et al. (47) performed single minor Ag (H–Y Ag) disparate skin transplants using mice with targeted mutations in Tlr2, Tlr4, or Myd88. MyD88 is an adaptor molecule essential for signals via IL-1/IL-18Rs and TLRs, except TLR3, which uses instead the adaptor molecule Toll/IL-1R domain-containing adaptor-inducing IFN-β (TRIF). Goldstein et al. (47) found that allografts from Myd88−/− male mice were not rejected by Myd88−/− female recipients. In contrast, allografts from Tlr2−/−, Tlr4−/−, and caspase-1-deficient male mice were rejected by corresponding gene-knockout female recipients. Because caspase-1 is necessary to convert the precursors of IL-1 and IL-18 into active form, these data indicate that rejection of HY-incompatible skin grafts is MyD88 dependent but not mediated by TLR2, TLR4, or IL-1/18 signals. MyD88 deficiency was associated with impaired accumulation of DCs in draining lymph nodes and a reduced proportion of graft-reactive CD8+ T cells in the spleen. Nonetheless, the presence of MyD88 restricted to either donor tissue or the recipient was sufficient to restore rejection (47).
However, in follow-up studies, the Goldstein group (48) found that rejection of fully mismatched skin and heart allografts occurs independent of MyD88 signaling, implicating other pathways for acute rejection across MHC barriers. To extend these studies, McKay et al. (49) transplanted skin from mice deficient in both Myd88 and Trif to wild-type recipients, either across full MHC disparity or across multiple minor Ag differences. They found grafts from the transgenic mice survived on average 5 days longer than wild-type controls across a full mismatch, with survival slightly extended across a multiple minor Ag difference. Despite this modest effect on graft survival, as pointed out by Goldstein (50), the full impact of dual deletion of Myd88 and Trif is difficult to extrapolate from these studies because TLR signaling was intact in the recipients and indirect allorecognition is sufficient for graft rejection.
The studies above suggest that, except for very weakly immunogenic situations (i.e., isolated H–Y incompatibility), TLR signaling is not required for graft rejection. Additional studies have focused on the role of TLRs and inflammation in tolerance induction. Two groups have reported that long-term graft survival in a highly immunogenic, fully allogeneic skin transplant model can be achieve with costimulatory blockade if both host and recipient are MyD88 deficient (51, 52). In one of these reports, Walker et al. (51) provide evidence to support a model in which absence of MyD88 impairs DC production of IL-6, in turn rendering alloreactive T cells more susceptible to suppression by CD4+CD25+ regulatory T cells (Tregs). These results are interesting given that TLR stimulation of DCs induces IL-6 production, which has been shown to block the suppressive effects of Tregs (53).
Conversely, other studies show that despite costimulatory receptor blockade, challenge with TLR agonists such as LPS and CpG DNA at the time of allotransplantation prevents tolerance induction (52, 54). Using donor-specific transfusion and CD154 Ab with a one-time TLR agonist challenge, Thornley et al. (54) found that blockade of skin graft tolerance induction by TLR stimulation was associated with prevention of alloreactive CD8+ T cell apoptosis. Moreover, Chen et al. (52) found that blockade of heart graft tolerance induction by administering CpG DNA along with CD154 Ab occurred in conjunction with a reduced ratio of Tregs to effector T cells at the graft site.
Turning to another mechanism of innate recognition, Moser et al. (55) report that blockade of RAGE signals delays rejection of fully allogeneic cardiac allografts. Recipient mice treated with the highest dose of soluble RAGE 1 day before transplantation and then daily had a 19.5-day increase in graft survival time (p < 0.001). Interestingly, soluble RAGE suppressed T cell-priming responses in a dose-dependent manner during a one-way in vitro MLR. These results suggest that RAGE signals directly modulate the alloimmune response and provide more data in support of the danger model with regard to allotransplantation.
Lastly, findings based on murine models are complemented by emerging clinical data on the role of PRRs in human solid organ transplantation. Palmer et al. (56, 57) found that lung transplant recipients heterozygous for either of two TLR4 functional polymorphisms (Asp299Gly or Thr399Ile) associated with LPS hyporesponsiveness had a reduced incidence of acute allograft rejection, which was significant and sustained. These effects were limited to the recipient TLR genotype, independent of TLR4 polymorphisms in the donor allograft. Similarly, kidney transplant recipients with either of these TLR4 polymorphisms were found to have a reduced rate of acute allograft rejection but presented more often with bacterial and opportunistic infections during a mean follow-up period of 95 ± 29 mo (58). However, another study found these TLR4 polymorphisms in donor kidney associated with a reduced incidence of acute rejection, but the incidence of rejection analyzed by recipient genotype was not significantly different (59). Furthermore, recent data indicate lung transplant recipients with a polymorphism in the promoter of the LPS receptor CD14 associated with increased transcriptional activity and elevated solubleCD14in blood have an increased incidence of acute allograft rejection and bronchiolitis obliterans syndrome (60). In conjunction with animal models, these clinical studies support a major role for the innate immunity in acute allograft rejection and suggest potential therapeutic targets to improve outcome.
Cells of the innate immune system as participants in allograft rejection and tolerance
The activation of APCs by danger signals is central to priming of alloreactive T cell responses. Seminal experiments depleting and restoring graft “passenger leukocytes” implicated DCs as a key player in alloantigen presentation (61). Upon functional maturation, activated DCs secrete proinflammatory cytokines, up-regulate surface MHC class II, increase expression of T cell costimulatory molecules, and use specific chemokine receptors that facilitate their trafficking to secondary lymphoid organs. Thus, “mature” DCs convey Ag from peripheral tissues and become potent stimulators of T cells. However, it is now recognized that, in the absence of danger signals, DCs exist in an “immature” state expressing little or no costimulatory molecules, and cognate engagement of Ag-specific T cells results in anergy or apoptosis (62, 63). Given this, the potential use of immature or “tolerogenic” DCs as therapy to promote peripheral tolerance upon organ transplantation has been an area of active research (64). In one of the earliest studies, using a mouse model of cardiac transplantation, Fu et al. (62) demonstrated that injection of donor-derived immature DCs 7 days before transplant of a fully mismatched allograft prolonged its median survival by 12.5 days. Recent studies have used immature donor DCs with the addition of costimulation blockade, revealing a synergistic effect in promoting long-term allograft survival (65). Interestingly, recipient-derived immature DCs also prolong allograft survival by a mechanism that is not donor specific and depends in part on the production of NO (66).
Unlike DCs, macrophages probably do not play a direct role in the induction of allorecognition because they inefficiently prime naive T cells. Nonetheless, within 24 h following transplantation, macrophages of both donor and recipient origin infiltrate the allograft and proliferate in situ (67). In the absence of rejection, such as in isografts, the macrophage infiltrate gradually decreases, but in acute rejection, substantial accumulation occurs comprising 40–60% of the cellular infiltrate (68). Activated by danger signals, these cells mount defensive responses, which include phagocytosis of necrotic debris, proinflammatory cytokine secretion, production of reactive nitrogen and oxygen species, and Ag presentation to effector T cells (69). These responses can mediate graft damage as liposomal clodronate administered 1 day after renal transplantation specifically depletes or disables the majority of allograft macrophages and reduces allograft tissue damage (70).
Although acute rejection is associated generally with TH1 responses, TH2-biased inflammation is also capable of mediating acute allograft rejection (71). In the absence of CD8+ T cell alloreactivity, such as in the response of B6 mice to MHC class II-disparate Bm12 skin grafts, numerous eosinophils are found in the inflammatory infiltrate. Other studies similarly indicate that, in the absence of CD8+ T cell activation or IFN-γR expression, the response of alloreactive CD4+ T cells is TH2 biased and promotes eosinophilic inflammation (72, 73). As in allergic-type inflammatory diseases, eosinophils can cause tissue damage through the release of highly cationic granule proteins and the production of several cytokines that further promote inflammation and TH2 polarization (such as IL-1, IL-3, IL-4, IL-5, IL-8, and TNF-α).
Similar to eosinophils, polymorphonuclear neutrophils (PMNs) can mediate tissue damage by an array of cytotoxic and proinflammatory mechanisms. After Ag-independent injury, such as by surgical trauma or ischemia/reperfusion, it has long been known that PMNs infiltrate the organ within hours, and their depletion abrogates tissue damage (74). Despite recent advances in our understanding of the role of innate immunity in transplantation, the influence of PMNs in these processes has received limited attention. Using a mouse model of fully mismatched heart transplantation, the Fairchild group (75) showed that treatment with Abs to the murine chemokine KC/CXCL1, a known chemoattractant for PMNs, attenuated neutrophil infiltration of the allograft and prolonged its survival. More recently, these investigators showed that short-term costimulatory blockade combined with either peritransplant depletion of PMNs or treatment with Abs for KC/CXCL1 plus MIP-2/CXCL2 prolongs survival of fully mismatched cardiac allografts for > 100 days (76). Although induced neutropenia is not clinically practical for organ transplantation, these studies suggest short-term, targeted interruption of neutrophil trafficking combined with other immune modulating agents may provide a more feasible approach.
Data from several groups has reshaped the role of NK cells in both allograft rejection and tolerance (77). NK cells are innate immune lymphocytes that contribute to surveillance against transformed cells, certain viruses, and other intracellular pathogens. Without prior Ag priming, NK cells perform rapid effector functions that include cytokine release (such as IFN-γ and TNF) and contact mediated cytotoxicity through perforin, granzymes, and Fas ligand. Their activation is regulated by a balance of positive and negative signals transmitted via stimulatory and inhibitory surface receptors, which engage the target cell directly. Relevant to transplantation biology, as posed by the “missing self” response, NK cells are cytotoxic to target cells mismatched for MHC class I molecules. Despite this, prior studies show that NK cells are not sufficient to reject solid organs directly because cardiac and skin allografts transplanted into mice that have intact NK cell function but absent adaptive immunity survive indefinitely (such as in Rag1−/− or Scid mice) (43, 77). However, recent studies show NK cells to act as facilitators of solid organ rejection by amplifying early graft inflammation and supporting the activity of alloreactive T cells (78–80). Maier et al. (79) demonstrated that in Cd28−/− recipients, which have impaired ability to receive T cell costimulatory signals, specific depletion of NK cells prolongs survival of fully mismatched cardiac allografts. Using the same model, McNerney et al. (80) show that NK cells promote the expansion and effector function of CD28-deficient alloreactive T cells by a mechanism independent of the NK cell-activating receptors Ly49D and NKG2D.
In addition to their role promoting solid organ rejection, NK cells also facilitate tolerance induction (81–83). In a series of well-controlled experiments, Beilke et al. (81) show that tolerance induction to fully mismatched islet allografts by either costimulatory blockade with CD154 Ab or Ab blockade of CD11a required the presence of both MHC class I expression and NK cells. Furthermore, using a model of fully mismatched skin transplantation, Yu et al. (83) demonstrate a novel role for NK cells in regulating T cell alloreactivity. In these elegant studies, recipient NK cells were critical for tolerance induction strategies by reducing the survival and dissemination of graft-derived donor APCs in transplant recipients. These studies suggest that NK cells, like DCs, may have dual roles in solid organ transplantation and that therapies that interfere with NK cell function may actually hinder the induction of tolerance.
Last but not least, mast cells are functionally diverse innate immune cells and possess immunoregulatory potential that influences both innate and adaptive immunity (84). Interestingly, analysis of gene expression in tolerant allografts revealed an increase in transcripts that could be associated with mast cells (85). Recognizing this, the Noelle group (86) found that the presence of mast cells was essential for Treg-dependent allograft tolerance. The induction of tolerance with CD154 Ab and donor-specific transfusion was not possible when allogeneic skin was transplanted onto mast-cell deficient mice. Moreover, the investigators provided evidence linking the production of IL-9 by Tregs to mast cell accumulation and allograft tolerance (86). These studies are testaments to the exciting body of recent work that has expanded our knowledge of the complex interplay between diverse cells of the immune response.
It should also be noted that soluble components of the innate immune system, such as complement, also participate in allograft responses. A comprehensive review of the role of complement in allograft rejection has been provided recently (87).
Implications for the development of tolerance strategies
While in evolutionary terms, the innate immune system is far older than the adaptive immune system, it is far newer in its recognition as an important factor in transplantation immunobiology. It is clear that innate immune mechanisms are responsible for the initial inflammatory events following engraftment. While these alone are not sufficient to lead to graft rejection itself, they are important for optimal adaptive immune responses to the graft and may play a major role in resistance to tolerance induction. The well-known association of infection with graft rejection may also be, at least in part, mediated by reactivation of innate immunity and “re-creation” of inflammation. The development of methods to blunt innate immune responses, which has potential implications for a wide variety of diseases, is likely to have a significant impact on transplantation as well.
Footnotes
This work was supported by the National Institutes of Health Grant R01-AI-062789 (to L.A.T.). D.F.L. is supported by National Institutes of Health Grant T32-HL-007586.
Abbreviations used in this paper: PRR, pattern recognition receptor; DC, dendritic cell; HMGB1, high-mobility group box 1; NLR, NOD-like receptor; PMN, polymorphonuclear neutrophil; RAGE, receptor of advanced glycation end product; RLH, RIG-like helicase; Treg, regulatory T cell; TRIF, Toll/IL-1R domain-containing adaptor-inducing IFN-β.
References
- 1.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 2.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens: a novel pathway for initiation of rejection. J. Exp. Med. 1990;171:307–314. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Game DS, Lechler RI. Pathways of allorecognition: implications for transplantation tolerance. Transpl. Immunol. 2002;10:101–108. doi: 10.1016/s0966-3274(02)00055-2. [DOI] [PubMed] [Google Scholar]
- 4.Le Moine A, Goldman M, Abramowicz D. Multiple pathways to allograft rejection. Transplantation. 2002;73:1373–1381. doi: 10.1097/00007890-200205150-00001. [DOI] [PubMed] [Google Scholar]
- 5.Christopher K, Mueller TF, Ma C, Liang Y, Perkins DL. Analysis of the innate and adaptive phases of allograft rejection by cluster analysis of transcriptional profiles. J. Immunol. 2002;169:522–530. doi: 10.4049/jimmunol.169.1.522. [DOI] [PubMed] [Google Scholar]
- 6.He H, Stone JR, Perkins DL. Analysis of robust innate immune response after transplantation in the absence of adaptive immunity. Transplantation. 2002;73:853–861. doi: 10.1097/00007890-200203270-00005. [DOI] [PubMed] [Google Scholar]
- 7.He H, Stone JR, Perkins DL. Analysis of differential immune responses induced by innate and adaptive immunity following transplantation. Immunology. 2003;109:185–196. doi: 10.1046/j.1365-2567.2003.01641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 9.Mollen KP, Anand RJ, Tsung A, Prince JM, Levy RM, Billiar TR. Emerging paradigm: Toll-like receptor 4-sentinel for the detection of tissue damage. Shock. 2006;26:430–437. doi: 10.1097/01.shk.0000228797.41044.08. [DOI] [PubMed] [Google Scholar]
- 10.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 11.Kabelitz D. Expression and function of Toll-like receptors in T lymphocytes. Curr. Opin. Immunol. 2007;19:39–45. doi: 10.1016/j.coi.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Andonegui G, Bonder CS, Green F, Mullaly SC, Zbytnuik L, Raharjo E, Kubes P. Endothelium-derived Toll-like receptor-4 is the key molecule in LPS-induced neutrophil sequestration into lungs. J. Clin. Invest. 2003;111:1011–1020. doi: 10.1172/JCI16510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 15.Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 2005;26:447–454. doi: 10.1016/j.it.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 17.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 18.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt AM, Vianna M, Gerlach M, Brett J, Ryan J, Kao J, Esposito C, Hegarty H, Hurley W, Clauss M, et al. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J. Biol. Chem. 1992;267:14987–14997. [PubMed] [Google Scholar]
- 20.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 21.Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–360. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 22.Orlova VV, Choi EY, Xie C, Chavakis E, Bierhaus A, Ihanus E, Ballantyne CM, Gahmberg CG, Bianchi ME, Nawroth PP, Chavakis T. A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. EMBO J. 2007;26:1129–1139. doi: 10.1038/sj.emboj.7601552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chavakis T, Bierhaus A, Al-Fakhri N, Schneider D, Witte S, Linn T, Nagashima M, Morser J, Arnold B, Preissner KT, Nawroth PP. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J. Exp. Med. 2003;198:1507–1515. doi: 10.1084/jem.20030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janeway CA., Jr The immune system evolved to discriminate infectious non-self from noninfectious self. Immunol. Today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 25.Matzinger P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 26.Land W, Schneeberger H, Schleibner S, Illner WD, Abendroth D, Rutili G, Arfors KE, Messmer K. The beneficial effect of human recombinant superoxide dismutase on acute and chronic rejection events in recipients of cadaveric renal transplants. Transplantation. 1994;57:211–217. doi: 10.1097/00007890-199401001-00010. [DOI] [PubMed] [Google Scholar]
- 27.Zhai Y, Shen XD, O’Connell R, Gao F, Lassman C, Busuttil RW, Cheng G, Kupiec-Weglinski JW. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J. Immunol. 2004;173:7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 28.Shen XD, Ke B, Zhai Y, Gao F, Busuttil RW, Cheng G, Kupiec-Weglinski JW. Toll-like receptor and heme oxygenase-1 signaling in hepatic ischemia/reperfusion injury. Am. J. Transplant. 2005;5:1793–1800. doi: 10.1111/j.1600-6143.2005.00932.x. [DOI] [PubMed] [Google Scholar]
- 29.Tsung A, Hoffman RA, Izuishi K, Critchlow ND, Nakao A, Chan MH, Lotze MT, Geller DA, Billiar TR. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J. Immunol. 2005;175:7661–7668. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- 30.Boros P, Bromberg JS. New cellular and molecular immune pathways in ischemia/reperfusion injury. Am. J. Transplant. 2006;6:652–658. doi: 10.1111/j.1600-6143.2005.01228.x. [DOI] [PubMed] [Google Scholar]
- 31.Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J. Immunol. 2006;177:1272–1281. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- 32.Tesar BM, Jiang D, Liang J, Palmer SM, Noble PW, Goldstein DR. The role of hyaluronan degradation products as innate alloimmune agonists. Am. J. Transplant. 2006;6:2622–2635. doi: 10.1111/j.1600-6143.2006.01537.x. [DOI] [PubMed] [Google Scholar]
- 33.Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J. Immunol. 2002;168:5233–5239. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- 34.Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF., III The extra domain A of fibronectin activates Toll-like receptor 4. J. Biol. Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 35.Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, Gotte M, et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J. Clin. Invest. 2005;115:2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the Toll-like receptor-4 complex. J. Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 37.Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J. Biol. Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- 38.Vabulas RM, Braedel S, Hilf N, Singh-Jasuja H, Herter S, Ahmad-Nejad P, Kirschning CJ, Da Costa C, Rammensee HG, Wagner H, Schild H. The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. J. Biol. Chem. 2002;277:20847–20853. doi: 10.1074/jbc.M200425200. [DOI] [PubMed] [Google Scholar]
- 39.Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, Tracey KJ, Yang H. HMGB1 signals through Toll-like receptor (TLR)4 and TLR2. Shock. 2006;26:174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 40.Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J. Leukocyte Biol. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- 41.Gao B, Tsan MF. Endotoxin contamination in recombinant human heat shock protein 70 (Hsp70) preparation is responsible for the induction of tumor necrosis factor α release by murine macrophages. J. Biol. Chem. 2003;278:174–179. doi: 10.1074/jbc.M208742200. [DOI] [PubMed] [Google Scholar]
- 42.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J. Exp. Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bingaman AW, Ha J, Waitze SY, Durham MM, Cho HR, Tucker-Burden C, Hendrix R, Cowan SR, Pearson TC, Larsen CP. Vigorous allograft rejection in the absence of danger. J. Immunol. 2000;164:3065–3071. doi: 10.4049/jimmunol.164.6.3065. [DOI] [PubMed] [Google Scholar]
- 44.Anderson CC, Carroll JM, Gallucci S, Ridge JP, Cheever AW, Matzinger P. Testing time-, ignorance-, and danger-based models of tolerance. J. Immunol. 2001;166:3663–3671. doi: 10.4049/jimmunol.166.6.3663. [DOI] [PubMed] [Google Scholar]
- 45.Chalasani G, Li Q, Konieczny BT, Smith-Diggs L, Wrobel B, Dai Z, Perkins DL, Baddoura FK, Lakkis FG. The allograft defines the type of rejection (acute versus chronic) in the face of an established effector immune response. J. Immunol. 2004;172:7813–7820. doi: 10.4049/jimmunol.172.12.7813. [DOI] [PubMed] [Google Scholar]
- 46.Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Huang X, Markmann JF, Kassaee A, Rosengard BR, Hancock WW, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nat. Med. 2004;10:87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldstein DR, Tesar BM, Akira S, Lakkis FG. Critical role of the Toll-like receptor signal adaptor protein MyD88 in acute allograft rejection. J. Clin. Invest. 2003;111:1571–1578. doi: 10.1172/JCI17573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tesar BM, Zhang J, Li Q, Goldstein DR. TH1 immune responses to fully MHC mismatched allografts are diminished in the absence of MyD88, a Toll-like receptor signal adaptor protein. Am. J. Transplant. 2004;4:1429–1439. doi: 10.1111/j.1600-6143.2004.00544.x. [DOI] [PubMed] [Google Scholar]
- 49.McKay D, Shigeoka A, Rubinstein M, Surh C, Sprent J. Simultaneous deletion of MyD88 and Trif delays major histocompatibility and minor antigen mismatch allograft rejection. Eur. J. Immunol. 2006;36:1994–2002. doi: 10.1002/eji.200636249. [DOI] [PubMed] [Google Scholar]
- 50.Goldstein D. Role of MyD88 and Trif in acute allograft rejection: glass half full or empty? Eur. J. Immunol. 2006;36:2820. doi: 10.1002/eji.200636546. [DOI] [PubMed] [Google Scholar]
- 51.Walker WE, Nasr IW, Camirand G, Tesar BM, Booth CJ, Goldstein DR. Absence of innate MyD88 signaling promotes inducible allograft acceptance. J. Immunol. 2006;177:5307–5316. doi: 10.4049/jimmunol.177.8.5307. [DOI] [PubMed] [Google Scholar]
- 52.Chen L, Wang T, Zhou P, Ma L, Yin D, Shen J, Molinero L, Nozaki T, Phillips T, Uematsu S, et al. TLR engagement prevents transplantation tolerance. Am. J. Transplant. 2006;6:2282–2291. doi: 10.1111/j.1600-6143.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- 53.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 54.Thornley TB, Brehm MA, Markees TG, Shultz LD, Mordes JP, Welsh RM, Rossini AA, Greiner DL. TLR agonists abrogate costimulation blockade-induced prolongation of skin allografts. J. Immunol. 2006;176:1561–1570. doi: 10.4049/jimmunol.176.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moser B, Szabolcs MJ, Ankersmit HJ, Lu Y, Qu W, Weinberg A, Herold KC, Schmidt AM. Blockade of RAGE suppresses alloimmune reactions in vitro and delays allograft rejection in murine heart transplantation. Am. J. Transplant. 2007;7:293–302. doi: 10.1111/j.1600-6143.2006.01617.x. [DOI] [PubMed] [Google Scholar]
- 56.Palmer SM, Burch LH, Davis RD, Herczyk WF, Howell DN, Reinsmoen NL, Schwartz DA. The role of innate immunity in acute allograft rejection after lung transplantation. Am. J. Respir. Crit. Care Med. 2003;168:628–632. doi: 10.1164/rccm.200303-447OC. [DOI] [PubMed] [Google Scholar]
- 57.Palmer SM, Burch LH, Trindade AJ, Davis RD, Herczyk WF, Reinsmoen NL, Schwartz DA. Innate immunity influences long-term outcomes after human lung transplant. Am. J. Respir. Crit. Care Med. 2005;171:780–785. doi: 10.1164/rccm.200408-1129OC. [DOI] [PubMed] [Google Scholar]
- 58.Ducloux D, Deschamps M, Yannaraki M, Ferrand C, Bamoulid J, Saas P, Kazory A, Chalopin JM, Tiberghien P. Relevance of Toll-like receptor-4 polymorphisms in renal transplantation. Kidney Int. 2005;67:2454–2461. doi: 10.1111/j.1523-1755.2005.00354.x. [DOI] [PubMed] [Google Scholar]
- 59.Palmer SM, Burch LH, Mir S, Smith SR, Kuo PC, Herczyk WF, Reinsmoen NL, Schwartz DA. Donor polymorphisms in Toll-like receptor-4 influence the development of rejection after renal transplantation. Clin. Transplant. 2006;20:30–36. doi: 10.1111/j.1399-0012.2005.00436.x. [DOI] [PubMed] [Google Scholar]
- 60.Palmer SM, Klimecki W, Yu L, Reinsmoen NL, Snyder LD, Ganous TM, Burch L, Schwartz DA. Genetic regulation of rejection and survival following human lung transplantation by the innate immune receptor CD14. Am. J. Transplant. 2007;7:693–699. doi: 10.1111/j.1600-6143.2007.01669.x. [DOI] [PubMed] [Google Scholar]
- 61.Lechler RI, Batchelor JR. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J. Exp. Med. 1982;155:31–41. doi: 10.1084/jem.155.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fu F, Li Y, Qian S, Lu L, Chambers F, Starzl TE, Fung JJ, Thomson AW. Costimulatory molecule-deficient dendritic cell progenitors (MHC class II+, CD80dim, CD86−) prolong cardiac allograft survival in nonimmunosuppressed recipients. Transplantation. 1996;62:659–665. doi: 10.1097/00007890-199609150-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu L, McCaslin D, Starzl TE, Thomson AW. Bone marrow-derived dendritic cell progenitors (NLDC 145+, MHC class II+, B7-1dim, B7-2−) induce alloantigen-specific hyporesponsiveness in murine T lymphocytes. Transplantation. 1995;60:1539–1545. doi: 10.1097/00007890-199560120-00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McCurry KR, Colvin BL, Zahorchak AF, Thomson AW. Regulatory dendritic cell therapy in organ transplantation. Transpl. Int. 2006;19:525–538. doi: 10.1111/j.1432-2277.2006.00306.x. [DOI] [PubMed] [Google Scholar]
- 65.Wang Q, Zhang M, Ding G, Liu Y, Sun Y, Wang J, Zhang W, Fu Z, Cao X. Anti-ICAM-1 antibody and CTLA-4Ig synergistically enhance immature dendritic cells to induce donor-specific immune tolerance in vivo. Immunol. Lett. 2003;90:33–42. doi: 10.1016/s0165-2478(03)00160-3. [DOI] [PubMed] [Google Scholar]
- 66.Peche H, Trinite B, Martinet B, Cuturi MC. Prolongation of heart allograft survival by immature dendritic cells generated from recipient type bone marrow progenitors. Am. J. Transplant. 2005;5:255–267. doi: 10.1111/j.1600-6143.2004.00683.x. [DOI] [PubMed] [Google Scholar]
- 67.Grau V, Herbst B, Steiniger B. Dynamics of monocytes/macrophages and T lymphocytes in acutely rejecting rat renal allografts. Cell Tissue Res. 1998;291:117–126. doi: 10.1007/s004410050985. [DOI] [PubMed] [Google Scholar]
- 68.Hancock WW, Thomson NM, Atkins RC. Composition of interstitial cellular infiltrate identified by monoclonal antibodies in renal biopsies of rejecting human renal allografts. Transplantation. 1983;35:458–463. doi: 10.1097/00007890-198305000-00013. [DOI] [PubMed] [Google Scholar]
- 69.Wyburn KR, Jose MD, Wu H, Atkins RC, Chadban SJ. The role of macrophages in allograft rejection. Transplantation. 2005;80:1641–1647. doi: 10.1097/01.tp.0000173903.26886.20. [DOI] [PubMed] [Google Scholar]
- 70.Jose MD, Ikezumi Y, van Rooijen N, Atkins RC, Chadban SJ. Macrophages act as effectors of tissue damage in acute renal allograft rejection. Transplantation. 2003;76:1015–1022. doi: 10.1097/01.TP.0000083507.67995.13. [DOI] [PubMed] [Google Scholar]
- 71.Goldman M, Le Moine A, Braun M, Flamand V, Abramowicz D. A role for eosinophils in transplant rejection. Trends Immunol. 2001;22:247–251. doi: 10.1016/s1471-4906(01)01893-2. [DOI] [PubMed] [Google Scholar]
- 72.Foucras G, Coudert JD, Coureau C, Guery JC. Dendritic cells prime in vivo alloreactive CD4 T lymphocytes toward type 2 cytokine- and TGF-β-producing cells in the absence of CD8 T cell activation. J. Immunol. 2000;165:4994–5003. doi: 10.4049/jimmunol.165.9.4994. [DOI] [PubMed] [Google Scholar]
- 73.Braun MY, Desalle F, Le Moine A, Pretolani M, Matthys P, Kiss R, Goldman M. IL-5 and eosinophils mediate the rejection of fully histoincompatible vascularized cardiac allografts: regulatory role of alloreactive CD8+ T lymphocytes and IFN-γ. Eur. J. Immunol. 2000;30:1290–1296. doi: 10.1002/(SICI)1521-4141(200005)30:5<1290::AID-IMMU1290>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 74.Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990;4:3355–3359. [PubMed] [Google Scholar]
- 75.Morita K, Miura M, Paolone DR, Engeman TM, Kapoor A, Remick DG, Fairchild RL. Early chemokine cascades in murine cardiac grafts regulate T cell recruitment and progression of acute allograft rejection. J. Immunol. 2001;167:2979–2984. doi: 10.4049/jimmunol.167.5.2979. [DOI] [PubMed] [Google Scholar]
- 76.El-Sawy T, Belperio JA, Strieter RM, Remick DG, Fairchild RL. Inhibition of polymorphonuclear leukocyte-mediated graft damage synergizes with short-term costimulatory blockade to prevent cardiac allograft rejection. Circulation. 2005;112:320–331. doi: 10.1161/CIRCULATIONAHA.104.516708. [DOI] [PubMed] [Google Scholar]
- 77.Kitchens WH, Uehara S, Chase CM, Colvin RB, Russell PS, Madsen JC. The changing role of natural killer cells in solid organ rejection and tolerance. Transplantation. 2006;81:811–817. doi: 10.1097/01.tp.0000202844.33794.0e. [DOI] [PubMed] [Google Scholar]
- 78.Obara H, Nagasaki K, Hsieh CL, Ogura Y, Esquivel CO, Martinez OM, Krams SM. IFN-γ, produced by NK cells that infiltrate liver allografts early after transplantation, links the innate and adaptive immune responses. Am. J. Transplant. 2005;5:2094–2103. doi: 10.1111/j.1600-6143.2005.00995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maier S, Tertilt C, Chambron N, Gerauer K, Huser N, Heidecke CD, Pfeffer K. Inhibition of natural killer cells results in acceptance of cardiac allografts in CD28−/−mice. Nat. Med. 2001;7:557–562. doi: 10.1038/87880. [DOI] [PubMed] [Google Scholar]
- 80.McNerney ME, Lee KM, Zhou P, Molinero L, Mashayekhi M, Guzior D, Sattar H, Kuppireddi S, Wang CR, Kumar V, Alegre ML. Role of natural killer cell subsets in cardiac allograft rejection. Am. J. Transplant. 2006;6:505–513. doi: 10.1111/j.1600-6143.2005.01226.x. [DOI] [PubMed] [Google Scholar]
- 81.Beilke JN, Kuhl NR, Van Kaer L, Gill RG. NK cells promote islet allograft tolerance via a perforin-dependent mechanism. Nat. Med. 2005;11:1059–1065. doi: 10.1038/nm1296. [DOI] [PubMed] [Google Scholar]
- 82.Goldstein DR, Thomas JM, Kirklin JK, George JF. An essential role for natural killer cells in augmentation of allograft survival mediated by donor spleen cells. Transplantation. 2001;72:954–956. doi: 10.1097/00007890-200109150-00035. [DOI] [PubMed] [Google Scholar]
- 83.Yu G, Xu X, Vu MD, Kilpatrick ED, Li XC. NK cells promote transplant tolerance by killing donor antigen-presenting cells. J. Exp. Med. 2006;203:1851–1858. doi: 10.1084/jem.20060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 85.Zelenika D, Adams E, Humm S, Graca L, Thompson S, Cobbold SP, Waldmann H. Regulatory T cells overexpress a subset of Th2 gene transcripts. J. Immunol. 2002;168:1069–1079. doi: 10.4049/jimmunol.168.3.1069. [DOI] [PubMed] [Google Scholar]
- 86.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, et al. Mast cells are essential intermediaries in regulatory T cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 87.Sacks SH, Zhou W. Allograft rejection: effect of local synthesis of complement. Springer Semin. Immunopathol. 2005;27:332–344. doi: 10.1007/s00281-005-0005-0. [DOI] [PubMed] [Google Scholar]