Abstract
Immune activation via TLRs is known to prevent transplantation tolerance in multiple animal models. To investigate the mechanisms underlying this barrier to tolerance induction, we used complementary murine models of skin and cardiac transplantation in which prolonged allograft acceptance is either spontaneous or pharmacologically induced with anti-CD154 mAb and rapamycin. In each model, we found that prolonged allograft survival requires the presence of natural CD4+Foxp3+ T regulatory cells (Tregs), and that the TLR9 ligand CpG prevents graft acceptance both by interfering with natural Treg function and by promoting the differentiation of Th1 effector T cells in vivo. We further demonstrate that although Th17 cells differentiate from naive alloreactive T cells, these cells do not arise from natural Tregs in either CpG-treated or untreated graft recipients. Finally, we show that CpG impairs natural Treg suppressor capability and prevents Treg-dependent allograft acceptance in an IL-6-independent fashion. Our data therefore suggest that TLR signals do not prevent prolonged graft acceptance by directing natural Tregs into the Th17 lineage or by using other IL-6-dependent mechanisms. Instead, graft destruction results from the ability of CpG to drive Th1 differentiation and interfere with immunoregulation established by alloreactive natural CD4+Foxp3+ Tregs.
The induction of donor-specific transplantation tolerance has been an elusive goal in transplantation immunology since its original description by Billingham et al. (1). Although donor-specific tolerance of solid organs from genetically disparate individuals has been achieved in numerous rodent models (2–5), translation of this work to primates has only rarely succeeded (6–11). These generally disappointing results indicate that our understanding of the basic mechanisms that promote or prevent acquired immunologic tolerance remains limited.
Recently, the activation of innate immunity via TLRs was shown by several groups to be a barrier to the induction of transplantation tolerance (12). Transplanted animals treated with co-stimulatory blockade cannot be induced to accept their grafts when they are concomitantly treated with known TLR ligands (13, 14). Conversely, mice that cannot normally respond to TLR signals (e.g., mice deficient in the adaptor protein MyD88) accept allografts more readily than their wild-type littermates when stringent transplantation models are used (15). Taken together, these data may explain in part why tolerance is notoriously difficult to achieve in animals receiving allografts routinely exposed to pathogens and commensal organisms (i.e., skin, intestine, lung, etc.). These data also suggest that TLR ligation may be a factor contributing to the failure of tolerance strategies in primates; in contrast to relatively pathogen-free rodent facilities where TLR signals may be significantly commuted, TLR signals may be more readily available in primates living in less sterile environments. Hence, pathogen exposure in primates may impede tolerance in a multitude of ways, not only through the development of heterologous immunity and an increased frequency of memory T cells (16, 17), but also through immune activation as a consequence of TLR ligation.
In this study, we used a well-established model of CD4+-mediated allograft rejection to investigate the mechanisms by which TLR ligation prevents allograft acceptance. We found that CD4+Foxp3+ natural T regulatory cells (Tregs)3 are required for graft acceptance and that CpG impairs their suppressive capabilities. We also observed that CpG promotes Th1 differentiation from alloreactive T cells both in the presence and absence of tolerogenic conditions. Although Th1 and Th17 cells both differentiate in response to the allograft, Th17 cells do not arise from CD4+Foxp3+ natural Tregs, and Th1 cells ultimately dominate the effector response. Finally, we determined that the ability of CpG to promote graft rejection is not mediated through the proinflammatory cytokine IL-6. Altogether, these data suggest that TLR ligation with CpG interferes with tolerance induction both by compromising the function of graft-protective Tregs and by promoting the survival and differentiation of graft-destructive alloreactive T cells.
Materials and Methods
Mice
Anti-bm12 (ABM) CD4+ TCR transgenic mice have been previously described (18, 19) and possess CD4+ T cells whose TCR recognizes the MHC class II variant I-Abm12 found on B6(C)-H2-Ab1bm12/KhEgJ mice (i.e., bm12). ABM mice are maintained as a breeding colony in our animal facility and have been bred to B6.PL-Thy1a/CyJ congenic mice purchased from The Jackson Laboratory to generate ABM-Thy1.1 mice. ABM mice have also been bred with Foxp3-GFP reporter mice (H-2b; C57BL/6 background) (20) to generate ABM-Foxp3-GFP mice whose Foxp3+ Tregs are identified by expression of the GFP reporter. C57BL/6J (i.e., B6) and B6.129S7-Rag1tm1MoM/J (i.e., Rag−/−) were also purchased from The Jackson Laboratory. IL-6-deficient bm12 mice (bm12.IL-6−/−) were generated in our laboratory by crossing bm12 mice and B6.129S2-Il6tm1Kopf/J (i.e., B6.IL-6−/−) mice purchased from The Jackson Laboratory. All colonies were maintained in accordance with the protocols of the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Abs, FACS analysis, and cell sorting
The following mAbs were purchased from BD Pharmingen and used in flow cytometric analysis: anti-CD4 (PerCP Cy5.5 conjugated; clone RM4–5), anti-CD25 (PE conjugated; clone PC61), anti-CD90.2 (allophycocyanin conjugated; clone 53-2.1), anti-CD45R/B220 (PECy7 conjugated; clone RA3-6B2), anti-NK1.1 (PECy7 conjugated; clone PK36), anti-Gr-1 (PECy7 conjugated; clone RB6-8C5), anti-CD11c (PECy7 conjugated; clone HL3), anti-CD11b (PECy7 conjugated; clone M1/70), anti-CD8 (PECy7 conjugated; clone 53-6.7), anti-IFN-γ (Alexa-Fluor 700 conjugated; clone XMG1.2), anti-IL-17 (PE conjugated; clone TC11-18H10), and anti-IL-4 (allophycocyanin conjugated; clone 11B11). Anti-Foxp3 (Pacific Blue conjugated; clone FJK-16S) and anti-IFN-γ (allophycocyanin conjugated; clone XMG1.2) were purchased from eBioscience. Flow cytometric analysis was performed on FACSCalibur and LSRII cytometers (BD Biosciences), and high-speed cell sorting was performed on FACSAria and FACSVantage SE cytometers (BD Biosciences). Flow cytometric data were analyzed using FlowJo software (v. 8.4.2; Tree Star).
Skin transplantation
Skin transplantation was performed using a modification of the technique originally described by Billingham and Medawar (21). In brief, recipient mice were anesthetized with ketamine and xylazine, and a 2 × 2-cm area of dermis was removed from the lateral trunk. A full-thickness donor skin graft was sutured to the exposed s.c. tissue bed using 4.0 chromic absorbable suture, and animals were bandaged after application of antibiotic ointment to the graft.
Cardiac transplantation
Vascularized cardiac allografts were transplanted into the peritoneal cavity of mice using microvascular techniques described by Corry et al. (22). Allograft viability was determined by daily palpation of the graft through the abdominal wall, and rejection was defined as complete arrest of contractility. Cessation of contractility was confirmed by direct visualization.
Treatment of skin allograft recipients
Mice receiving anti-CD154 and rapamycin therapy were coinjected i.p. on days 0, 2, and 4 after transplantation with 250 µg of anti-CD154 mAb (Bio-express) and rapamycin (1 mg/kg; LC Laboratories) every other day from day 0 to 12 posttransplantation. Groups of mice were also injected i.p. with 100 µg of CpG 1668 phosphorothioated oligodeoxynucleotide (Integrated DNA Technologies; 5′-TCCATGACGTTCCTGATGCT-3′) every other day from day 0 to 8 posttransplantation or a control, nonstimulatory oligodeoxynucleotide (AP-1; 5′-GCTTGATGACTCAGCCGGAA-3′; Integrated DNA Technologies).
In vitro MLR
Bm12 splenocytes were T depleted using magnetic beads, according to the manufacturer’s protocol (Miltenyi Biotec), and 1 × 105 stimulators were cocultured with 1 × 105 sorted CFSE-labeled responder CD4+ T cells (23) for 4 days in complete medium (RPMI 1640 (Invitrogen Life Technologies) + 10% FCS (HyClone) + 1% penicillin-streptomycin solution (Invitrogen Life Technologies) + .05% 2-ME (Sigma-Aldrich)) in a 96-well round-bottom plate at 37°C (5% CO2). Individual wells were cocultured in the presence of rapamycin (20 ng/ml), anti-CD154 mAb (50 µg/ml), and/or CpG (3 µM). Cells were recovered after 4 days for FACS analysis.
In vitro suppression assay
A total of 2 × 105 FACS purified CD4+CD25− cells was stimulated in the presence or absence of CD4+CD25+ Tregs with latex beads (1 bead/1 T cell) coated with anti-CD3 (2C11; 1 µg/ml) and anti-CD28 (2.5 µg/ml) for 72 h. Culture medium was supplemented with CpG DNA (3.3 µM) or left untreated. For the final 16 h of culture before recovery for analysis, cells were pulsed with 0.5 µCi of tritiated thymidine.
Intracellular cytokine analysis
A total of 2 × 105 cells was suspended in 250 µl of complete medium for 6 h in the presence of 10 ng/ml PMA (Sigma-Aldrich), 1 µM ionomycin (Sigma-Aldrich), and monensin (BD Pharmingen; 4 µl of monensin/6 ml of medium) in a 96-well plate. After 6 h of stimulation in vitro, cells were washed and surface stained. Cells were subsequently fixed and permeabilized using either eBioscience or BD Biosciences fixation/permeabilization buffers, according to the manufacturer’s instructions, and Abs against the intracellular proteins of interest were added for 1 h before FACS analysis.
ELISPOT assay
Single-cell suspensions of splenocytes from transplanted animals were prepared 2 wk after heart engraftment and plated in complete medium on 96-well ELISPOT plates precoated with 100 µl of 4 µg/ml rat anti-mouse IFN-γ, anti-mouse IL-4, or anti-mouse IL-5 capture Abs (BD Pharmingen). Cells were tested in duplicate against medium alone (negative control) or cells stimulated with Con A (positive control). Plates were incubated 24 h for IFN-γ and 48 h for IL-4 and IL-5 at 37°C. After washing with PBS, followed by washing with PBS containing 0.05% Tween 20 (PBST), 2 µg/ml biotinylated rat anti-mouse IFN-γ detection mAb or 4 µg/ml biotinylated anti-mouse IL-4 mAb or IL-5 mAb (BD Pharmingen) was added overnight. The plates were then washed four times in PBST, followed by 2 h of incubation with HRP-conjugated streptavidin (DakoCytomation) diluted at 1/2000 in PBS/1% BSA. After washing three times with PBST, followed by PBS, the plates were developed using 3-amino-9-ethylcarbazole (Sigma-Aldrich). The resulting spots were counted on a computer-assisted enzyme-linked immunospot image analyzer (Cellular Technology), and frequencies were expressed as the number of cytokine-producing spots per million splenocytes.
Immunopathology
Cryostat sections of snap-frozen allografts were stained by immunoperoxidase using an En Vision kit (DakoCytomation) and affinity-purified rat anti-mouse CD4 (clone L3T4) and Foxp3 mAbs (clone FJK-16s; eBioscience) or rabbit anti-GFP Ab (Santa Cruz Biotechnology). Control sections were stained with isotype-matched mAbs or rabbit IgG.
Statistical analysis
All analyses were performed using SPSS statistical software (v.15.0). Continuous variables were compared using Student’s t test, and categorical variables were compared using Fisher’s exact test. One-way ANOVA was used to compare means between more than two groups where appropriate. Statistical analysis between Kaplan-Meier survival curves was performed using the log-rank test. Values of p < .05 indicated statistical significance.
Results
CpG prevents skin and cardiac allograft acceptance
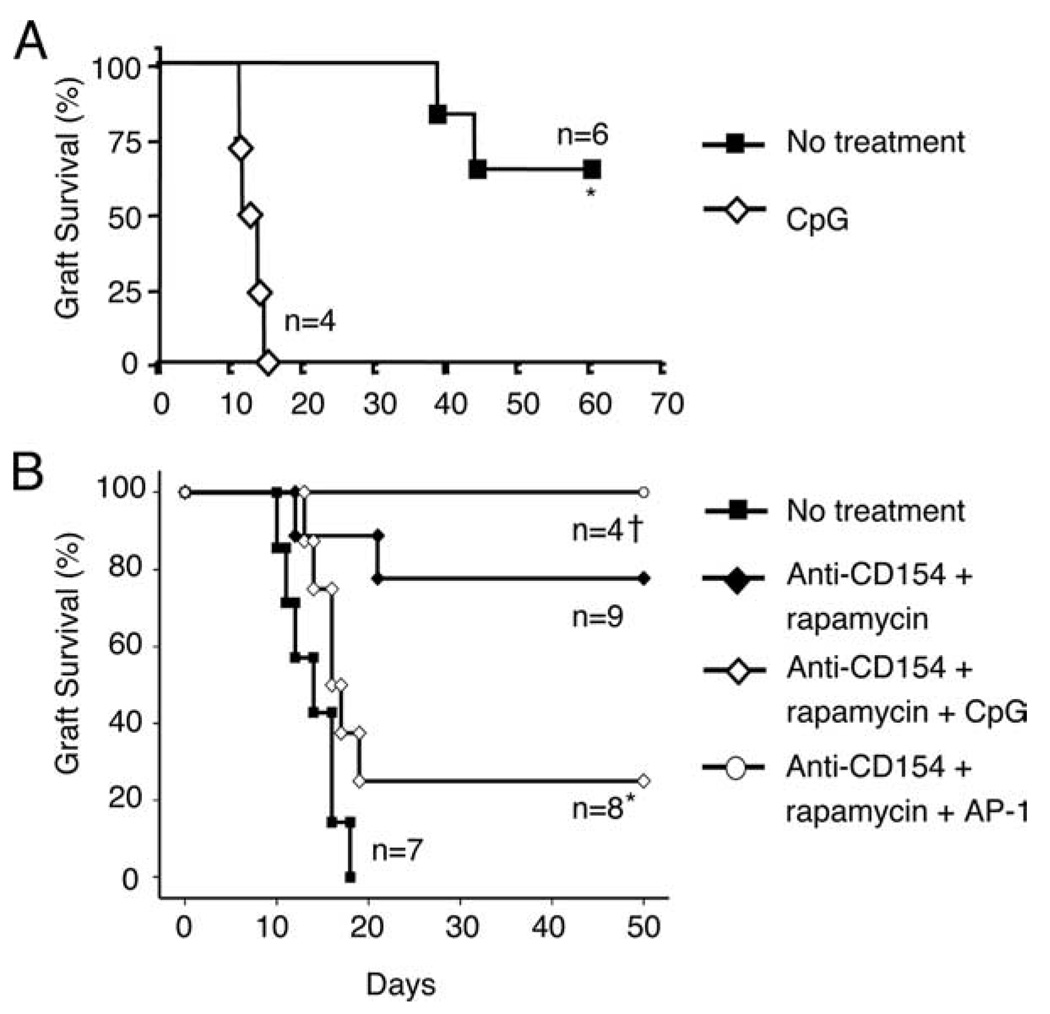
To study the mechanisms by which TLR ligation impairs long-term allograft survival, we used two previously reported models of transplantation (19, 24). In the bm12→B6 model, B6 mice spontaneously accept cardiac allografts from bm12 donors despite the MHC class II mismatch between donor (I-Abm12) and recipient (I-Ab) (Fig. 1A). In the complementary bm12→B6 skin transplantation model, however, B6 mice rapidly reject bm12 skin allografts with kinetics similar to other major MHC mismatch models (median survival time (MST) = 14 days; Fig. 1B), although a brief course of anti-CD154 mAb (250 µg, i.p.: days 0, 2, and 4) in combination with rapamycin (1 mg/kg i.p.: days 0–12 every other day) delayed graft rejection beyond 50 days in the skin transplant recipients (Fig. 1B). Hence, long-term graft survival can be achieved either spontaneously (e.g., cardiac model) or pharmacologically (e.g., skin model) in both bm12→B6 transplant systems.
FIGURE 1.
CpG prevents allograft acceptance. A, Bm12 (I-Abm12) cardiac allografts were transplanted into B6 (I-Ab) recipients, and recipient mice were injected perioperatively with CpG (50 µg i.p.; days 0, 2, and 4; ◇) or PBS (control; ■). *, p = 0.001. B, Bm12 (I-Abm12) skin allografts were transplanted onto B6 (I-Ab) recipients. Transplanted animals received either no treatment (n = 7; ■); treatment with anti-CD154 mAb (250 µg i.p.; days 0, 2, and 4) and rapamycin (1 mg/kg i.p. every other day for 12 days) (n = 9; ♦); treatment with anti-CD154, rapamycin, and CpG (100 µg i.p. every other day for 8 days) (n = 8; ◇); or treatment with anti-CD154, rapamycin, and AP-1 (100 µg i.p. every other day for 8 days) (n = 4; ○). *, p = 0.03 (♦ vs ◇). †, p = NS (♦ vs ○).
We subsequently asked whether the TLR9 ligand CpG would prevent allograft acceptance in either or both of these models. Indeed, the i.p. administration of CpG prevented both spontaneous and drug-induced graft acceptance (Fig. 1). Of note, graft rejection was specific to CpG, because skin-grafted animals that received anti-CD154 and rapamycin did not reject their grafts when treated with the nonstimulatory oligodeoxynucleotide AP-1 (Fig. 1B). Although AP-1 appears to enhance graft survival over anti-CD154 plus rapamycin treatment alone (100% graft survival (anti-CD154 plus rapamycin plus AP-1) vs 80% (anti-CD154 plus rapamycin); Fig. 1B), this result was not statistically significant, and the improved graft survival among AP-1-treated animals is most likely due to the lower number of animals in this group (n = 4 in anti-CD154 plus rapamycin plus AP-1 vs n = 9 anti-CD154 plus rapamycin; Fig. 1B). Consistent with the findings of both the Greiner and Chong groups (13, 14), our data confirm that CpG administration prevents the establishment of acquired transplantation tolerance and further demonstrate that CpG prevents spontaneous allograft acceptance, a finding that may have important clinical implications for well-matched allografts.
Prolonged allograft acceptance requires CD4+Foxp3+ natural Tregs
To determine how CpG prevents tolerance, we first needed to understand the mechanisms underlying graft acceptance in this system. Given that allograft survival in many tolerance models requires the presence of Tregs (25–28), our hypothesis was that CpG abrogates tolerance by altering graft-protective Treg number or function in transplant recipients. Although we have previously shown that the spontaneous acceptance of a bm12 cardiac allograft by a B6 recipient requires CD4+CD25+ natural Tregs (24), the use of rapamycin in the bm12→B6 skin transplant system implicated adaptive Tregs in the tolerance process, given the ability of rapamycin to induce alloreactive Foxp3+ T cells from Foxp3− cells (29).
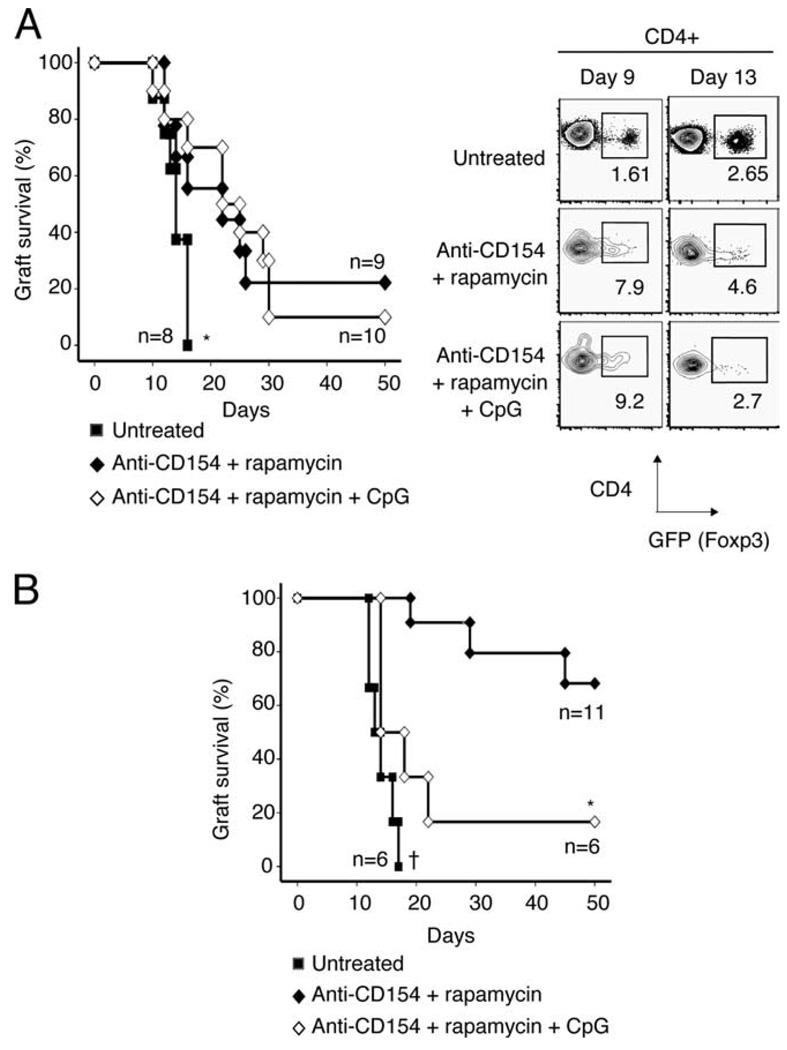
We therefore focused our initial efforts on defining the role of adaptive vs natural Tregs in graft acceptance in recipients treated with rapamycin and costimulatory blockade. To accomplish this task, we monitored bm12 graft survival in Rag−/− recipients whose graft-protective Treg complement was derived from either adaptive or natural CD4+Foxp3+ Tregs (Fig. 2, A and B, respectively). In Rag−/− recipients that receive CD4+GFP−Foxp3− naive T cells and a bm12 skin graft (Fig. 2A), CD4+Foxp3+ Tregs that promote graft survival must differentiate from GFP−Foxp3− precursors (i.e., adaptive Tregs). In contrast, in bm12-grafted Rag−/− recipients that receive effector cells and CD4+GFP+Foxp3+ T cells (Fig. 2B), graft survival is conferred by Tregs that already express Foxp3 (i.e., natural Tregs).
FIGURE 2.
Prolonged allograft acceptance requires CD4+Foxp3+ natural Tregs. A, Left panel, Bm12 skin allografts were transplanted onto Rag−/− recipient mice, and recipients were injected i.v. with 1 × 106 CD4+GFP− effector T cells (sorted from ABM-Foxp3-GFP mice) (n = 27). Recipients received no treatment (■), or were treated with anti-CD154 and rapamycin (♦) or anti-CD154, rapamycin, and CpG (◇). Data are pooled from three different experiments. *, p < 0.04 for ■ vs ♦ or ◇ . p = NS for ♦ vs ◇ . MST = 14.5, 22, and 23.5 days (■, ♦, ◇), respectively. Right panel, Rag−/− recipients received a bm12 skin graft and were injected with 1 × 106 CD4+GFP− effector T cells (sorted from ABM-Foxp3-GFP mice) and treated as above. On days 9 and 13, animals were sacrificed and the draining lymph nodes were analyzed for GFP+ adaptive Tregs. Plots are representative of two experiments with two animals per group. B, Rag−/− recipients received bm12 skin allografts and were injected i.v. with 9 × 105 CD4+CD25− effector T cells (sorted from ABM mice) and 1 × 105 CD4+GFP+ Tregs (sorted from ABM-Foxp3-GFP mice) (n = 23). Mice were divided into the three treatment groups, as described above, and monitored for graft rejection. Data are pooled from three different experiments. *, p = 0.004 (♦ vs ◇). †, p = 0.04 (■ vs ◇). MST = 13.5, 47.5, and 16 days (■, ♦, ◇), respectively.
Using this bm12→Rag−/− system, we observed that in the absence of natural Tregs, bm12 skin graft survival was only slightly prolonged in anti-CD154:rapamycin-treated recipient animals (Fig. 2A, left), despite the emergence of adaptive Tregs in the draining lymph nodes of these animals (Fig. 2A, right). However, the reconstitution of Rag−/− recipients with a physiologic ratio of natural CD4+Foxp3+ Tregs to effector T cells (i.e., 10% CD4+Foxp3+ T cells and 90% CD4+Foxp3− T cells) once again permitted long-term graft survival in animals treated with anti-CD154 and rapamycin (Fig. 2, B vs A). Importantly, allograft acceptance in these animals was not due solely to the presence of Tregs, because animals who received Tregs, but did not receive anti-CD154:rapamycin therapy still rejected the skin graft with normal kinetics (Fig. 2B). These data suggest that even under conditions that favor adaptive Treg development (e.g., treatment with rapamycin), prolonged graft acceptance requires the presence of natural CD4+Foxp3+ Tregs. Moreover, these data further demonstrate that CpG prevents allograft acceptance in transplantation models in which allograft survival depends on operational CD4+Foxp3+ Tregs.
CpG does not decrease natural Treg number in vivo, but alters CD4+Foxp3+ frequency
Both in vivo and in vitro assays of Treg suppressive capability have demonstrated that Treg suppression is dose dependent (30). Given the importance of Treg number vs effector T cell number in establishing immunoregulation and promoting graft acceptance, we asked whether CpG subverted long-term graft survival in our transplant system by decreasing the overall number of Tregs in vivo.
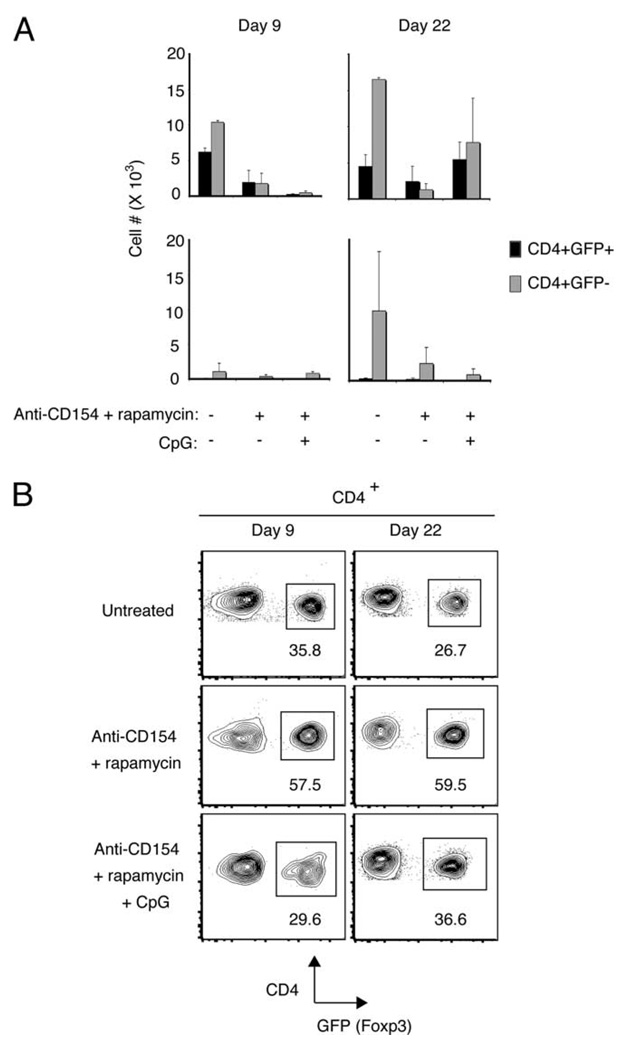
We cotransferred CD4+GFP+Foxp3+ ABM Tregs with CD4+GFP−CD25− ABM T effector cells (1 × 105 and 9 × 105 cells, respectively) into Rag−/− recipients grafted with bm12 skin. We subsequently enumerated both Tregs (CD4+GFP+) and effector T cells (CD4+GFP−) in the graft’s draining lymph nodes early after transplantation, when alloreactive T cells are initially encountering alloantigen in the draining lymph node (day 9), and late after transplantation, when anti-CD154:rapamycin-treated animals have accepted their grafts and anti-CD154:rapamycin:CpG-treated animals are rejecting their grafts (day 22).
Surprisingly, we found that whereas alloreactive Tregs were most abundant in the draining lymph nodes of animals that ultimately rejected their grafts (i.e., untreated animals (days 9 and 22) and anti-CD154:rapamycin:CpG-treated animals after cessation of therapy (day 22)) (Fig. 3A, top row), Tregs were least frequent in the draining lymph nodes of animals that accepted their grafts (anti-CD154:rapamycin group, days 9 and 22) (Fig. 3A, top row). Despite the decrease in Treg number in anti-CD154:rapamycin-treated animals, however, the CD4+GFP− effector population was more profoundly diminished by these agents, and anti-CD154: rapamycin therapy therefore resulted in a dramatic increase in the proportion of Foxp3+ cells in the alloreactive CD4+ population (Fig. 3B). Notably, we observed that CpG prevents this rapamycin-induced increase in frequency of Foxp3+ cells in the CD4+ population, because Foxp3+ T cell frequency in CpG-treated animals remained similar to untreated animals at both time points (Fig. 3B). Importantly, control experiments in which ungrafted Rag−/− recipients received cell transfer and anti-CD154:rapamycin:CpG treatment demonstrated that CpG rescued only alloantigen-driven Treg and effector T cell expansion, because both regulatory and effector T cell populations remained diminished in treated Rag−/− control animals that did not receive a bm12 skin graft at both early and late time points (Fig. 3A, bottom row).
FIGURE 3.
CpG does not decrease absolute Treg number in vivo, but alters CD4+Foxp3+ frequency. A total of 1 × 105 CD4+GFP+ T cells sorted from ABM-Foxp3-GFP mice was cotransferred with 9 × 105 CD4+ CD25− ABM T cells i.v. into Rag−/− mice. Rag−/− mice received either a bm12 skin graft (A (top row) and B) or no graft (A, bottom row). Animals received either of the following: 1) no additional treatment; 2) treatment with anti-CD154 and rapamycin (anti-CD154 mAb (250 µg i.p. on days 0, 2, and 4); rapamycin (1 mg/kg i.p. every other day for 12 days)); or 3) treatment with anti-CD154, rapamycin, and CpG (100 µg i.p. every other day for 8 days). Data are representative of two to three mice per group. A, Top row, CD4+GFP+ vs CD4+GFP− cell number in the draining lymph nodes of skin-grafted animals (e.g., axillary and inguinal lymph nodes ipsilateral to the graft). Bottom row, CD4+GFP+ vs CD4+GFP− cell number in the equivalent lymph nodes in ungrafted animals. B, CD4+GFP+ frequency in the draining lymph nodes in skin-grafted animals.
To ensure that Treg or T effector migration from the lymph node to the graft did not confound our results, we examined graft histology for Foxp3+ and Foxp3− T cells in a parallel experiment. Consistent with the work of previous investigators, we found that Foxp3+ T cells were detectable in small numbers in both rejected and accepted grafts (data not shown). Notably, Foxp3+ T cells were present in the grafts of animals in each of the three treatment groups, but the overall number of these infiltrating cells was highly variable and no discernible trend was noted between the groups (data not shown). In rejected grafts from untreated or CpG-treated animals, the overwhelmingly predominant cellular infiltrate consisted of CD4+Foxp3− T cells.
In sum, we observed that both Treg and effector T cell number are greatest in untreated animals that reject their grafts early after transplantation. Although anti-CD154 and rapamycin decreased absolute Treg number, these drugs significantly increased the proportion of Foxp3+ cells among the alloreactive responder population. After cessation of anti-CD154 and rapamycin therapy, both Treg and effector T cell number rebounded in CpG-treated animals, and this increase in cell number required alloantigen. We conclude from these results that CpG does not promote graft rejection by decreasing absolute Treg number in vivo.
CpG abrogates Treg suppression and allograft acceptance independent of IL-6
Although CpG did not diminish CD4+Foxp3+ Treg number in our transplanted animals, we have previously observed that CpG impairs the suppressive function of Tregs in vitro (31). Given the fact that the proinflammatory cytokine IL-6 has been implicated in the abrogation of Treg suppressor function (32) and the fact that IL-6 is readily detectable in the sera of CpG-treated animals (data not shown), we asked whether IL-6 was responsible for the ability of CpG to break Treg suppression and prevent allograft acceptance.
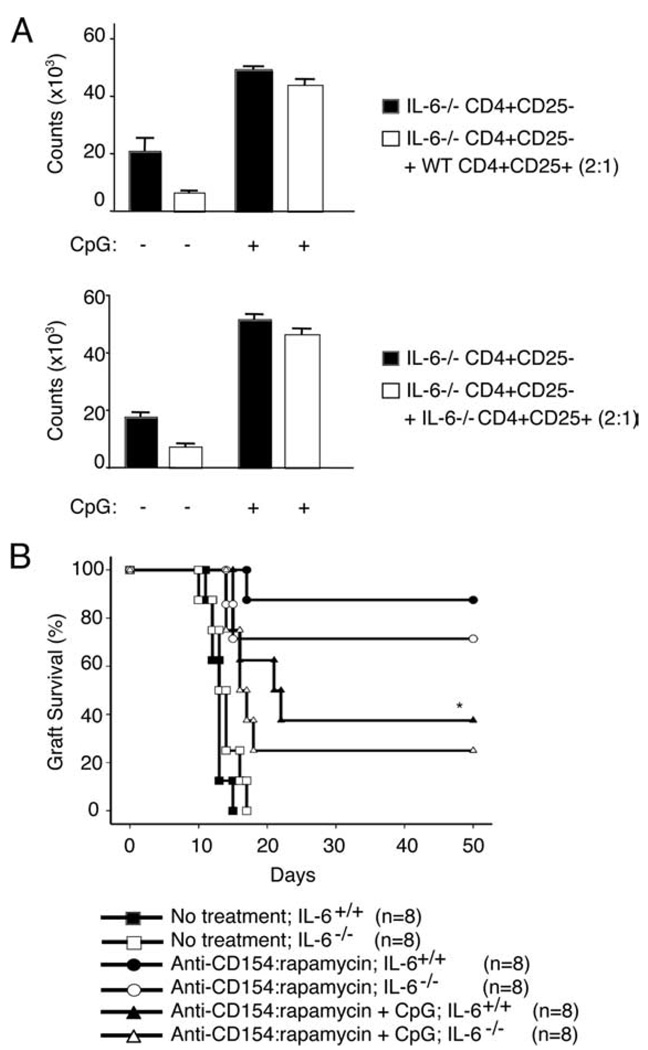
We assayed Treg function in vitro by coculturing Tregs and effector T cells in the presence or absence of CpG. Consistent with our previously published results (31), we found that even in the absence of APCs, CpG effectively abrogated Treg suppression (Fig. 4A). Notably, when CD4+CD25+ Tregs and CD4+CD25− T effectors were isolated from IL-6-deficient animals, CpG again impaired Treg suppression under all conditions tested (Fig. 4A). These data strongly suggest that CpG does not abrogate Treg suppression via signals mediated by IL-6.
FIGURE 4.
CpG abrogates Treg suppression and allograft acceptance independent of IL-6. A, 2 × 105 CD4+CD25− T cells were cocultured in complete medium for 72 h in the presence or absence of CD4+CD25+ Tregs (2:1 ratio) and anti-CD3 plus anti-CD28-coated latex beads for 72 h. T cells were FACS sorted from either C57BL/6J or B6.IL-6−/− mice. Culture medium was supplemented with CpG DNA (3.3 µM) or left untreated. Tritiated thymidine was added to the cultures for the last 16 h of incubation. B, C57BL/6J (n = 24) and B6.IL-6−/− (n = 24) mice received bm12 (■, ●, ▲) or bm12.IL-6−/− skin grafts (□, ○, Δ), respectively, and received no treatment (■, □); treatment with anti-CD154 and rapamycin (●, ○); or treatment with anti-CD154, rapamycin, and CpG (▲, Δ), as previously described. Data are pooled from two experiments. *, p < 0.001 (circles vs triangles). p = NS between IL-6+/+ and IL-6−/− recipients within any treatment group (■ vs □; ●vs ○; ▲ vs Δ).
To determine whether IL-6 was comparably dispensable in vivo, we monitored bm12 skin graft survival in untreated animals, anti-CD154:rapamycin-treated animals, and anti-CD154:rapamycin:CpG-treated animals in the absence of IL-6. As shown in Fig. 4B, IL-6−/− recipients that received bm12.IL-6−/− grafts rejected their grafts with kinetics identical with wild-type controls, and CpG continued to prevent allograft acceptance in both wild-type and IL-6-deficient animals. These in vitro and in vivo experiments therefore suggest that whereas CpG impairs Treg suppressive ability, these effects are not mediated solely by IL-6.
CpG does not promote Th17 differentiation from alloreactive Tregs in vivo
Although we did not find a nonredundant role for IL-6 in the abrogation of natural Treg suppressor function, other investigators have noted in vitro that coculture of natural Tregs with IL-6 results in Th17 production and Foxp3 down-regulation (33). We therefore asked whether CpG was preventing allograft acceptance by diverting graft-protective natural Tregs into this alternate fate in vivo. To do this, we used congenic ABM animals in our colony with disparate CD90 (Thy) alleles to track regulatory (CD4+ GFP+Thy1.2+) vs effector (CD4+CD25−Thy1.1+) T cells in vivo. We cotransferred 1 × 105 CD4+GFP+Thy1.2 ABM Tregs with 9 × 105 CD4+25−Thy1.1 effector ABM T cells into Rag−/− mice that had received a bm12 skin graft. Animals were once again left untreated; treated with anti-CD154:rapamycin; or treated with anti-CD154, rapamycin, and CpG. Fourteen days after transplantation, we isolated lymphocytes from the draining lymph nodes of the grafted animals and assayed for intracellular IL-17, IFN-γ, and IL-4 production after restimulation in vitro.
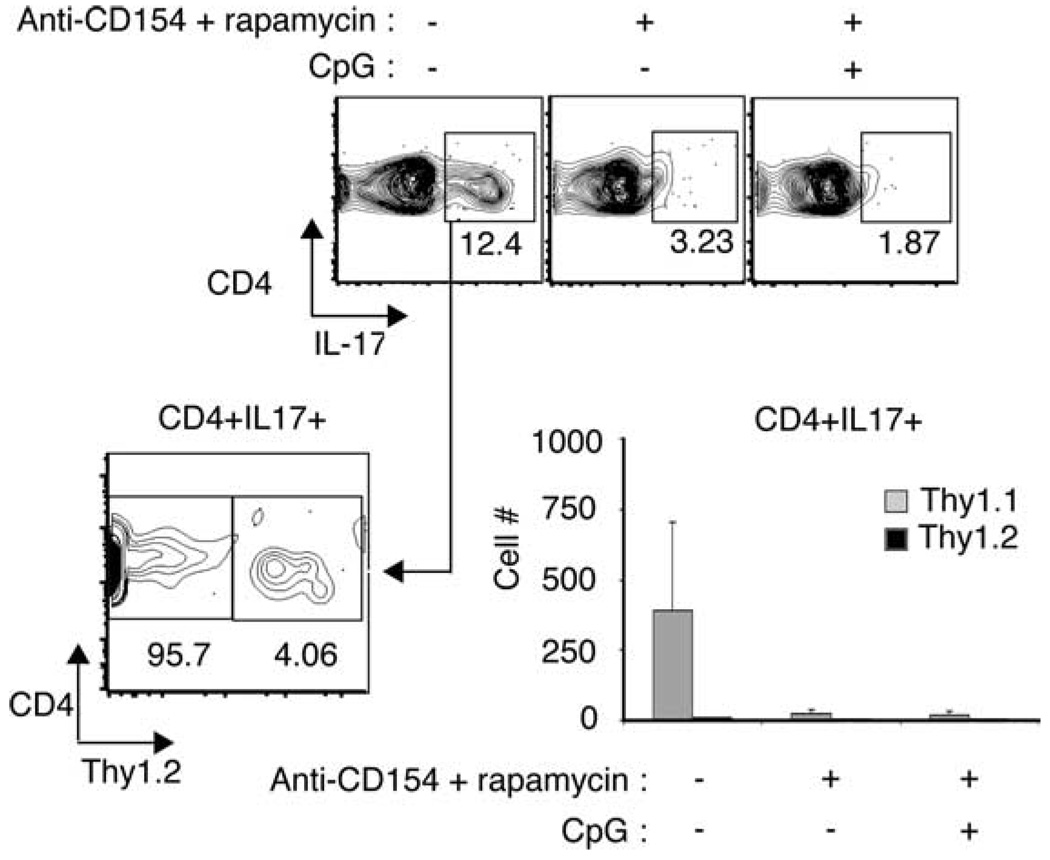
We found that very few CD4+ T cells produced IL-4 after restimulation and that a large number of alloreactive CD4+ T cells in the draining lymph node produced IFN-γ (~40–50% of CD4+ T cells; data not shown). In addition to Th1 differentiation, we observed that ~5–10% of graft-reactive CD4+ T cells in the draining lymph node differentiated into Th17 cells (Fig. 5). When we subsequently evaluated the allelic expression of CD90 protein on the Th17 cells, we found that virtually all of the Th17-expressing cells had differentiated from naive T cells transferred into the recipient mice (Thy1.1) and not from the GFP+Foxp3+ regulatory population (Thy1.2) (Fig. 5). Th17 cells were not readily detectable in the lymph nodes of anti-CD154:rapamycin-treated animals whether or not they were treated with CpG. Our results therefore demonstrate that Th17 cells do differentiate during the alloresponse, but these cells are unlikely to promote graft rejection in anti-CD154:rapamycin:CpG-treated animals. Furthermore, these cells arise from naive allospecific precursors and not natural CD4+Foxp3+ Tregs in vivo.
FIGURE 5.
CpG does not promote Th17 differentiation from alloreactive Tregs in vivo. A total of 1 × 105 CD4+GFP+Thy1.2+ T cells sorted from ABM-Foxp3-GFP mice was cotransferred with 9 × 105 CD4+CD25−Thy1.1+ ABM T cells i.v. into Rag−/− mice transplanted with bm12 skin allografts. Animals received either no additional treatment (untreated); treatment with anti-CD154 and rapamycin; or anti-CD154, rapamycin, and CpG, as previously described. Fourteen days after transplantation, animals were sacrificed, and lymphocytes from the graft’s draining lymph nodes were restimulated in vitro in the presence of PMA and ionomycin and stained for IL-17 protein. Top row, CD4+IL-17+ cells in the draining lymph nodes of untreated animals. Bottom left, Thy1.2 expression on gated CD4+IL-17+ cells in untreated animals. Bottom right, Number of CD4+IL-17+ T cells from draining lymph nodes of untreated animals derived from Thy1.1 or Thy1.2 T cell donors.
CpG promotes Th1 effector differentiation from naive alloreactive T cells
TLR ligation up-regulates costimulatory molecules on APCs (34) and also provides costimulation and prosurvival signals to CD4+ T cells independent of APC effects (35, 36). We therefore hypothesized that CpG was preventing graft acceptance not only by impairing natural Treg suppressor function, but also by promoting helper cell differentiation from naive alloreactive CD4+ T cells. To test this, we studied Th cell differentiation from alloreactive naive CD4+ T cells in the presence and absence of CpG both in vitro and in vivo.
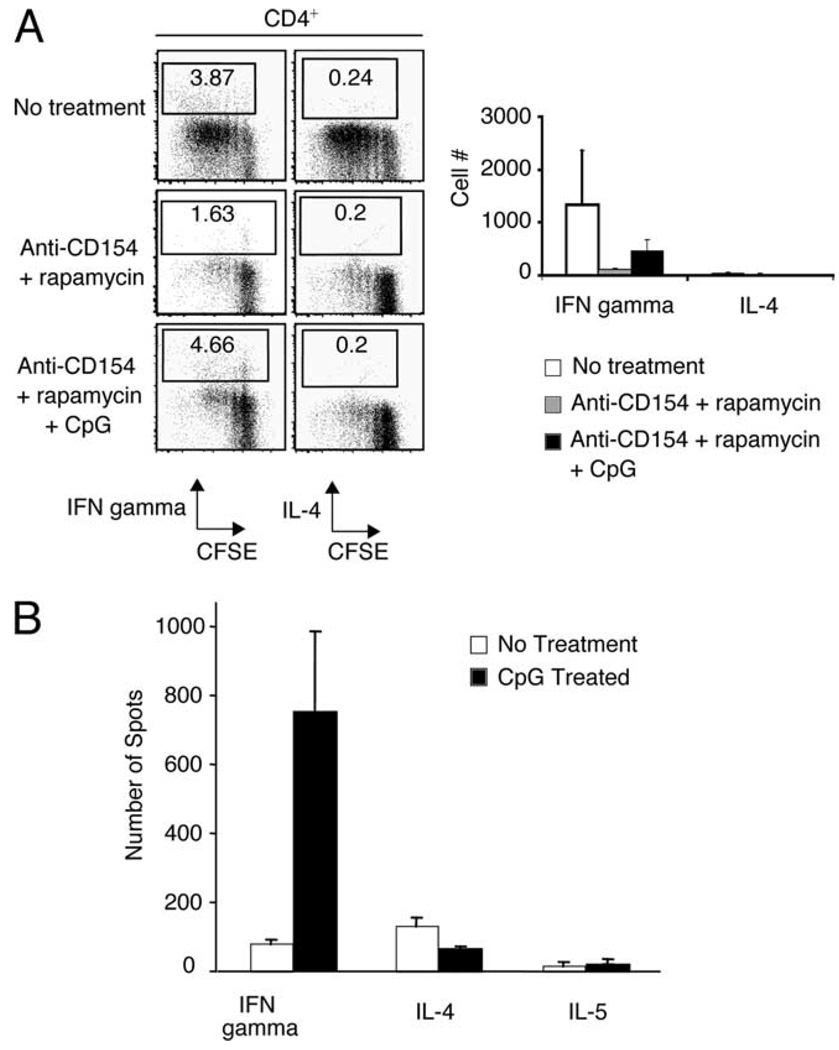
In vitro, we asked whether CpG promoted Th differentiation from naive T cells in the presence of costimulatory blockade and rapamycin. Using intracellular cytokine stains, we assayed for the production of IFN-γ and IL-4 in CFSE-labeled CD4+CD25− T cells purified from ABM mice that were cocultured with T-depleted bm12 splenocytes in the presence or absence of rapamycin (20 ng/ml), anti-CD154 mAb (50 µg/ml), and CpG (3 µM) for 4 days. As expected, Th1 cells, but not IL-4-producing Th2 cells differentiated in response to bm12 stimulators (Fig. 6A). Although CpG did not promote significant proliferation of alloreactive T cells in the presence of rapamycin and anti-CD154 mAb treatment, we found that CpG rescued Th1 differentiation (Fig. 6A). To confirm these results in vivo, we performed an ELISPOT assay on splenocytes isolated from CpG-treated and PBS-treated recipients of bm12 cardiac allografts. Consistent with our in vitro results, we found that Th1 cells were much more abundant in animals treated with CpG than with PBS (Fig. 6B). Taken together, these results suggest that TLR ligation promotes Th1 effector differentiation from naive alloreactive T cells under conditions of both spontaneous and pharmacologically induced graft acceptance.
FIGURE 6.
CpG promotes Th1 differentiation from naive alloreactive T cells in vitro and in vivo. A, 1 × 105 T-depleted bm12 splenocytes were cocultured for 96 h with 1 × 105 CFSE-labeled CD4+CD25− T cells sorted from ABM mice. Anti-CD154 mAb (50 µg/ml), rapamycin (20 ng/ml), and CpG (3 µM) were added to individual culture wells. After 96 h of stimulation, the cells were washed and restimulated in vitro for intracellular cytokine analysis. Left panel, Frequency of CD4+IFN-γ and CD4+IL-4+ T cells in representative culture wells after 96 h. Right panel, Number of CD4+IFN-γ and CD4+IL-4+ T cells in culture wells after 96 h. Mean ± SD is shown of triplicate wells. Data are representative of two different experiments. B, B6 mice transplanted with bm12 cardiac allografts were treated with CpG (50 µg i.p.; days 0, 2, and 4) or PBS. Mice were subsequently sacrificed 2 wk after transplantation, and cytokine production from splenocytes from individual animals was assayed using an ELISPOT assay.
Discussion
The recent discovery that TLR signals prevent transplantation tolerance represents a major breakthrough in the ongoing effort to establish reliable allograft tolerance in humans. Throughout the process of transplantation, organ recipients are inundated by endogenous and exogenous TLR ligands alike, because surgical trauma and ischemic injury to the graft cause the release of endogenous TLR ligands (hyaluronan, heat shock proteins, heparan sulfate, etc.) (37–39), and ubiquitous pathogens provide a continuous source of exogenous TLR stimulation. Given the unavoidable nature of TLR stimulation in humans, it therefore seems unlikely that allograft tolerance will be achieved unless this barrier can be surmounted therapeutically. However, rational therapy cannot be designed without a significantly improved understanding of the mechanisms by which TLR ligation prevents allograft acceptance.
In this study, we demonstrate that the TLR9 ligand CpG prevents allograft acceptance both by promoting the differentiation of Th1 effector T cells from naive precursor T cells and by interfering with Treg suppression. Although it remains unclear whether these effects are mediated by cytokines produced by TLR-responsive cells or whether they result from TLR9 ligation directly on T cells, our data suggest that IL-6 produced in response to TLR ligation does not by itself account for the ability of CpG to abrogate transplantation tolerance. We specifically evaluated the role of IL-6 in preventing transplantation tolerance in our Treg-dependent tolerance models because of its reported effects on both adaptive and natural Tregs. Notably, IL-6 promotes the differentiation of Th17 cells from naive T cells in lieu of adaptive Foxp3+ Tregs (40). Moreover, it may release effector T cells from Treg suppression (32), and it may promote Th17 differentiation from natural CD4+Foxp3+ Tregs (33). In light of these data highlighting the ability of this cytokine to impair Treg differentiation and/or function, we were thus surprised that Treg-dependent allograft acceptance could be prevented in its absence. Although IL-6 may therefore not be necessary to either disable Tregs or enable effector T cells to escape Treg suppression, it is important to recognize the limitations imposed by the cytokine-deficient system used in our study. Given that cytokine redundancy is extremely prevalent (41) and that a variety of other cytokines signal through the gp130 component of the IL-6R (e.g., IL-11, leukemia inhibitory factor, etc.), other pleiotropic cytokines produced in response to TLR ligation may compensate for the absence of IL-6 either by engaging the gp130 receptor or by using shared signaling pathways downstream of the receptor (42). Hence, our data do not necessarily exclude a role for IL-6 in the prevention of transplantation tolerance, and instead predict that therapies that attempt to block IL-6 in isolation will be ineffective in overcoming the barrier to tolerance imposed by TLR ligation.
In addition to its potential influence on Tregs, we tested the hypothesis that CpG abrogated transplant tolerance by influencing effector T cell differentiation in our transplanted animals. Both in vitro and in vivo, we found that CpG promoted Th1 differentiation from naive alloreactive T cells. We also specifically investigated the possibility that Th17 effector T cells produced in response to the allograft prevented transplantation tolerance in CpG-treated animals, because Th17 cells are known to be pathogenic in a variety of animal models (20, 43), although their role in transplantation tolerance or rejection is currently unclear (44). Although a small proportion of bm12-specific Th17 cells differentiated in response to the graft in untreated animals, the emergence of these cells was blunted by anti-CD154:rapamycin treatment and, unlike Th1 cells, was not rescued by treatment with CpG.
Finally, to further our understanding of how TLR ligation subverts tolerance induction, our study explored the underlying mechanisms responsible for tolerance itself. Although a number of studies over the last 15 years have described the importance of Tregs in the tolerance process (3, 5, 25–30), we present evidence that thymic-derived natural CD4+Foxp3+ T cells may be indispensable mediators of immunoregulation under certain transplantation conditions. This may be particularly relevant in lymphopenic transplant recipients, because natural Tregs may be important in controlling the homeostatic proliferation of effector cells that may ultimately contribute to graft rejection (45). Although it is known that natural Tregs can control homeostatic proliferation (46), it is currently unknown whether homeostatic proliferation can be controlled by rapamycin-induced adaptive Tregs. Moreover, lymphopenia might in theory be more effective in promoting the expansion of CD4+Foxp3+ natural Tregs compared with adaptive Tregs generated from naive CD4+Foxp3− T cells, given that natural Tregs may possess high-affinity receptors that recognize endogenous self-peptide:MHC complexes (47, 48).
Although we have previously shown that rapamycin-induced CD4+Foxp3+ adaptive Tregs generated in vivo from polyclonal CD4+GFP− effector T cells can prevent the rejection of DBA skin grafts transplanted onto Rag−/− recipients (29), our current study suggests that adaptive Tregs are not always sufficient to establish tolerance. Although it is not immediately clear why adaptive Tregs promote tolerance in one skin transplant model and not another, we speculate that the low frequency of adaptive Treg conversion observed in our study (4–10%) may not generate a sufficient number of Tregs to consistently protect the graft. Alternatively, the higher dose of rapamycin used in our earlier study (3 mg/kg) may have prolonged adaptive Treg survival in vivo, because the suppressive capabilities of rapamycin-induced adaptive Tregs depend on the continued presence of this drug (49). Clearly, additional experiments will be needed to define the role of these various Treg populations in transplantation tolerance and delineate the multitude of factors that affect their specificity, survival, and function.
In conclusion, our data indicate that TLR ligation concomitantly prevents the establishment of immunoregulation and promotes effector differentiation in transplanted animals. Importantly, TLR ligation abrogates graft acceptance both in the presence and absence of pharmacologic treatments designed to maintain alloreactive T cells in a quiescent state. Our data thus support the view that immunoregulatory mechanisms critical to transplantation tolerance dominate only during a state of global immune quiescence that encompasses both the innate and adaptive immune systems. We must therefore endeavor to incorporate more integrated models of adaptive and innate immunity into the design of future tolerance protocols if we are to achieve durable donor-specific tolerance in humans.
Footnotes
This work was supported by National Institutes of Health Grant R01 AI41521.
Abbreviations used in this paper: Treg, T regulatory cell; ABM, anti-bm12; MST, median survival time.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 2.Parker DC, Greiner DL, Phillips NE, Appel MC, Steele AW, Durie FH, Noelle RJ, Mordes JP, Rossini AA. Survival of mouse pancreatic islet allografts in recipients treated with allogeneic small lymphocytes and antibody to CD40 ligand. Proc. Natl. Acad. Sci. USA. 1995;92:9560–9564. doi: 10.1073/pnas.92.21.9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, Davies J, Waldmann H. Infectious transplantation tolerance. Science. 1993;259:974–977. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 4.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, Cho HR, Aruffo A, Hollenbaugh D, Linsley PS, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 5.Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J. Immunol. 2002;168:1080–1086. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 6.Kawai T, Cosimi AB, Colvin RB, Powelson J, Eason J, Kozlowski T, Sykes M, Monroy R, Tanaka M, Sachs DH. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59:256–262. [PubMed] [Google Scholar]
- 7.Barber WH, Mankin JA, Laskow DA, Deierhoi MH, Julian BA, Diethelm AG. Long-term results of a controlled prospective study with transfusion of donor-specific bone marrow in 57 cadaveric renal allograft recipients. Transplantation. 1991;51:70–75. doi: 10.1097/00007890-199101000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Kean LS, Gangappa S, Pearson TC, Larsen CP. Transplant tolerance in non-human primates: progress, challenges, and unmet needs. Am. J. Transpl. 2006;6:884–893. doi: 10.1111/j.1600-6143.2006.01260.x. [DOI] [PubMed] [Google Scholar]
- 9.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubinc N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N. Engl. J. Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Millan MT, Shizuru JA, Hoppe RT, Lowsky R, Engleman EG, Strober S. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N. Engl. J. Med. 2008;358:362–368. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 11.Alexander SI, Smith N, Hu M, Verran D, Shun A, Dorney S, Smith A, Webster B, Shawn PJ, Lammi A, Stormon MO. Chimerism and tolerance in a recipient of a deceased-donor liver transplant. N. Engl. J. Med. 2008;358:369–374. doi: 10.1056/NEJMoa0707255. [DOI] [PubMed] [Google Scholar]
- 12.LaRosa DF, Rahman AH, Turka LA. The innate immune system in allograft rejection and tolerance. J. Immunol. 2007;178:7503–7509. doi: 10.4049/jimmunol.178.12.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Wang T, Zhou P, Ma L, Yin D, Shen J, Molinero L, Nozaki T, Phillips T, Uematsu S, et al. TLR engagement prevents transplantation tolerance. Am. J. Transp. 2006;6:2282–2291. doi: 10.1111/j.1600-6143.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- 14.Thornley TB, Brehm MA, Markees TG, Shultz LD, Mordes JP, Welsh RM, Rossini AA, Greiner DL. TLR agonists abrogate costimulation blockade-induced prolongation of skin allografts. J. Immunol. 2006;176:1561–1570. doi: 10.4049/jimmunol.176.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker WE, Nasr IW, Camirand G, Tesar BM, Booth CJ, Goldstein DR. Absence of innate MyD88 signaling promotes inducible allograft acceptance. J. Immunol. 2006;177:5307–5316. doi: 10.4049/jimmunol.177.8.5307. [DOI] [PubMed] [Google Scholar]
- 16.Taylor DK, Neujahr D, Turka LA. Heterologous immunity and homeostatic proliferation as barriers to tolerance. Curr. Opin. Immunol. 2004;16:558–564. doi: 10.1016/j.coi.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Yang J, Brook MO, Carvalho-Gaspar M, Zhang J, Ramon HE, Sayegh MH, Wood KJ, Turka LA, Jones ND. Allograft rejection mediated by memory T cells is resistant to regulation. Proc. Natl. Acad. Sci. USA. 2007;104:19954–19959. doi: 10.1073/pnas.0704397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bäckström BT, Muller U, Hausmann B, Palmer E. Positive selection through a motif in the αβ T cell receptor. Science. 1998;281:835–838. doi: 10.1126/science.281.5378.835. [DOI] [PubMed] [Google Scholar]
- 19.Sandner S, Salama AD, Houser SL, Palmer E, Turka LA, Sayegh MH. New TCR transgenic model for tracking allospecific CD4+ T-cell activation and tolerance in vivo. Am. J. Transpl. 2003;3:1242–1250. doi: 10.1046/j.1600-6143.2003.00220.x. [DOI] [PubMed] [Google Scholar]
- 20.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic Th17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 21.Billingham R, Medawar P. The technique of free skin grafting in mammals. J. Exp. Biol. 1951;28:385–402. [Google Scholar]
- 22.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of heart in mice. Transplantation. 1973;16:343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Wells AD, Gudmundsdottir H, Turka LA. Following the fate of individual T cells throughout activation and clonal expansion. J. Clin. Invest. 1997;12:3173–3183. doi: 10.1172/JCI119873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schenk S, Kish DD, He C, El-Sawy T, Chiffoleau E, Chen C, Wu Z, Sandner S, Gorbachev AV, Fukamachi K, et al. Alloreactive T cell response and acute rejection of single class II MHC-disparate heart allografts are under strict regulation by CD4+CD25+ T cells. J. Immunol. 2005;174:3741–3748. doi: 10.4049/jimmunol.174.6.3741. [DOI] [PubMed] [Google Scholar]
- 25.Quezada SA, Bennett K, Blazar BR, Rudensky AY, Sakaguchi S, Noelle RJ. Analysis of the underlying cellular mechanisms of anti-CD154-induced graft tolerance: the interplay of clonal anergy and immune regulation. J. Immunol. 2005;175:771–779. doi: 10.4049/jimmunol.175.2.771. [DOI] [PubMed] [Google Scholar]
- 26.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat. Rev. Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 27.Graca L, Cobbold SP, Waldmann H. Identification of regulatory T cells in tolerated allografts. J. Exp. Med. 2002;195:1641–1646. doi: 10.1084/jem.20012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng XX, Sanchez-Fueyo A, Sho M, Domenig C, Sayegh MH, Strom TB. Favorably tipping the balance between cytopathic and regulatory T cells to create transplantation tolerance. Immunity. 2003;19:503–514. doi: 10.1016/s1074-7613(03)00259-0. [DOI] [PubMed] [Google Scholar]
- 29.Gao W, Lu Y, El Essawy B, Oukka M, Kuchroo VK, Strom TB. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am. J. Transpl. 2007;7:1–11. doi: 10.1111/j.1600-6143.2007.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Maurik A, Herber M, Wood KJ, Jones ND. Cutting edge: CD4+CD25+ alloantigen-specific immunoregulatory cells that can prevent CD8+ T cell-mediated graft rejection: implications for anti-CD154 immunotherapy. J. Immunol. 2002;169:5401–5404. doi: 10.4049/jimmunol.169.10.5401. [DOI] [PubMed] [Google Scholar]
- 31.Larosa DF, Gelman AE, Rahman AH, Zhang J, Turka LA, Walsh PT. CpG DNA inhibits CD4+CD25+ Treg suppression through direct MyD88-dependent costimulation of effector CD4+ T cells. Immunol. Lett. 2007;108:183–188. doi: 10.1016/j.imlet.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 33.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25−Foxp3− T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-β. J. Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 34.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 35.Gelman AE, Zhang J, Choi Y, Turka LA. Toll-like receptor ligands directly promote activated CD4+ T cell survival. J. Immunol. 2004;172:6065–6073. doi: 10.4049/jimmunol.172.10.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gelman AE, LaRosa DF, Zhang J, Walsh PT, Choi Y, Sunyer JO, Turka LA. The adaptor molecule MyD88 activates PI-3 kinase signaling in CD4+ T cells and enables CpG oligodeoxynucleotide-mediated costimulation. Immunity. 2006;25:783–793. doi: 10.1016/j.immuni.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tesar BM, Jiang D, Liang J, Palmer SM, Noble PW, Goldstein DR. The role of hyaluronan degradation products as innate alloimmune agonists. Am. J. Transpl. 2006;6:2622–2635. doi: 10.1111/j.1600-6143.2006.01537.x. [DOI] [PubMed] [Google Scholar]
- 38.Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J. Immunol. 2002;168:5233–5239. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- 39.Kaczorowski DJ, Nakao A, Mollen KP, Vallabhaneni R, Sugimoto R, Kohmoto J, Tobita K, Zuckerbraun BS, McCurry KR, Murase N, Billiar TR. Toll-like receptor 4 mediates the early inflammatory response after cold ischemia/reperfusion. Transplantation. 2007;84:1279–1287. doi: 10.1097/01.tp.0000287597.87571.17. [DOI] [PubMed] [Google Scholar]
- 40.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGF-β in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Kelso A. The enigma of cytokine redundancy. Immunol. Cell Biol. 1994;72:97–101. doi: 10.1038/icb.1994.14. [DOI] [PubMed] [Google Scholar]
- 42.Ozaki K, Leonard WJ. Cytokine and cytokine receptor pleiotropy and redundancy. J. Biol. Chem. 2002;277:29355–29358. doi: 10.1074/jbc.R200003200. [DOI] [PubMed] [Google Scholar]
- 43.Steinman L. A brief history of Th17, the first major revision of the Th1/Th2 hypothesis of T cell-mediated tissue damage. Nat. Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, Wood KJ. Interleukin 23 and Th17 cells in transplantation immunity: does 23 + 17 equal rejection? Transplantation. 2007;84:1071–1074. doi: 10.1097/01.tp.0000287126.12083.48. [DOI] [PubMed] [Google Scholar]
- 45.Moxham VF, Karegli J, Phillips RE, Brown KL, Tapmeier TT, Hangartner R, Sacks SH, Wong W. Homeostatic proliferation of lymphocytes results in augmented memory-like function and accelerated allograft rejection. J. Immunol. 2008;180:3910–3918. doi: 10.4049/jimmunol.180.6.3910. [DOI] [PubMed] [Google Scholar]
- 46.Shen S, Ding Y, Tadokoro CE, Olivares-Villagómez D, Camps-Ramirez M, Curotto de Lafaille MA, Lafaille JJ. Control of homeostatic proliferation by regulatory T cells. J. Clin. Invest. 2005;115:3517–3526. doi: 10.1172/JCI25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Picca CC, Larkin J, Boesteanu A, Lerman MA, Rankin AL, Caton AJ. Role of TCR specificity in CD4+CD25+ regulatory T-cell selection. Immunol. Rev. 2006;212:74–85. doi: 10.1111/j.0105-2896.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 48.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 49.Valmori D, Tosello V, Souleimanian NE, Godefroy E, Scotto L, Wang Y, Ayyoub M. Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J. Immunol. 2006;177:944–949. doi: 10.4049/jimmunol.177.2.944. [DOI] [PubMed] [Google Scholar]