Abstract
Local alpha-band synchronization has been associated with both cortical idling and active inhibition. Recent evidence, however, suggests that long-range alpha synchronization increases functional coupling between cortical regions. We demonstrate increased long-range alpha and beta band phase synchronization during short-term memory retention in children 6–10 years of age. Furthermore, whereas alpha-band synchronization between posterior cortex and other regions is increased during retention, local alpha-band synchronization over posterior cortex is reduced. This constitutes a functional dissociation for alpha synchronization across local and long-range cortical scales. We interpret long-range synchronization as reflecting functional integration within a network of frontal and visual cortical regions. Local desynchronization of alpha rhythms over posterior cortex, conversely, likely arises because of increased engagement of visual cortex during retention.
Keywords: Child development, Functional integration, Connectivity, MEG, Neural synchrony, Oscillatory network dynamics, Alpha
Introduction
Synchronization of local alpha rhythms has been associated with cortical idling and inhibition of processing, whereas engagement of a cortical region typically yields local alpha desynchronization (see Klimesch et al. 2007; Pfurtscheller et al. 1996 for reviews). Tasks such as the retention of visual information in short-term memory (STM) and working memory entail paradoxical predictions about local alpha synchronization in visual cortex as (1) active processing in visual cortex is required for retention, suggesting local alpha desynchronization, and (2) successful retention of a memory trace may require inhibition of potentially disruptive incoming visual information, suggesting local alpha synchronization similar to that which is observed when attention is allocated away from the visual modality (Doesburg et al. 2009; Foxe et al. 1998; Fu et al. 2001) or away from one visual hemifield (Thut et al. 2006; Sauseng et al. 2005a; Worden et al. 2000). Accordingly, increased engagement of STM and working memory has been associated with local alpha-band synchronization (Busch and Herrmann 2003; Jensen et al. 2002; Jokisch and Jensen 2007; Schack and Klimesch 2002), desynchronization (Gevins et al. 1997; Stipacek et al. 2003) or both synchronization and desynchronization (Krause et al. 2000; Sauseng et al. 2005b). It has been shown that children aged 8–12 express load-dependent local alpha desynchronization during visual STM processing (Gomarus et al. 2006). As research on the oscillatory mechanisms of visual STM retention in children is scarce, however, the role of long-range synchronization remains unexplored in this age group.
Recent evidence suggests that long-range alpha-band synchronization, rather than reflecting cortical idling or inhibition, increases functional coupling between brain regions (see Palva and Palva 2007 for review). In this view, synchronously oscillating neural populations are able to consistently exchange burst of action potentials during the optimal phase of the receiving neurons’ fluctuations in dendritic excitability, thereby increasing information exchange (see Fries 2005). Neural synchronization thus functions to transiently increase functional connectivity within a distributed neural ensemble to instantiate a particular task, percept, or memory trace (Llinás and Ribary 1993; Varela et al. 2001; Ward 2003). In the context of STM retention, this has been previously shown to manifest as alpha coherence between midline parietal and left temporal/parietal electrodes (Payne and Kounios 2009). A continuous arithmetic working memory task has also been associated with widespread long-range alpha-band synchronization in adults (Palva et al. 2005). Such results suggest a functional dissociation across cortical scales for alpha rhythms wherein long-range synchronization within a network of cortical regions establishes a large-scale neural ensemble to maintain information in STM, while local desynchronization reflects the active engagement of cortical regions. This leads to the hypothesis that the retention of visual information in STM will induce long-range alpha-band synchronization as a distributed assembly is recruited to represent the memory trace, whereas local alpha rhythms will become desynchronized as cortical regions become engaged in processing. The exact relationship between local and long-range task-dependent alpha synchronization, however, remains unclear. Palva et al. (2005) showed task-dependent increases in both local and long-range alpha synchronization. Conversely, visual recognition and sensory integration are associated with decreased local alpha synchronization and increased long-range alpha synchronization (Freunberger et al. 2008; Hummel and Gerloff 2005). The present study aimed to investigate the role of long-range neural synchronization in STM retention in children and to examine the relationship between local and long-range alpha synchronization. To this end we recorded magnetoencephalographic (MEG) data while 17 children age 6–10 years performed a visual STM task and we analyzed local and long-range synchronization.
Methods
Subjects
Data were recorded from 17 normal healthy children aged 6–10 years (mean age 7.88; 10 females). All children had normal or corrected-to-normal vision and did not have any known neurological abnormalities. Vision was corrected, when necessary, using nonmagnetic Medigoggle system. Informed written consent was obtained from the parents of all children.
Task
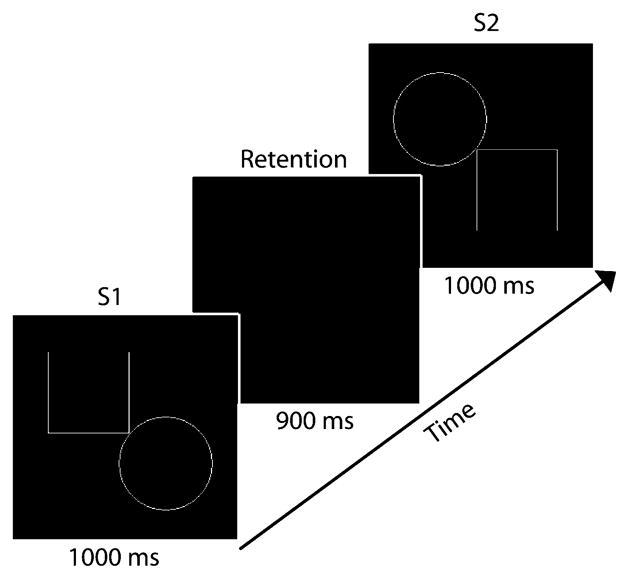
Subjects lay supine and viewed stimuli projected onto a screen above them (40 cm from nasion). On each trial of the visual STM task subjects viewed the first stimulus (S1) for 1,000 ms, followed by a 900 ms delay period, followed by the presentation of the second stimulus (S2) which lasted 1,000 ms (Fig. 1). Stimuli were adapted from Beery™ VMI (The Beery–Buktenica Developmental Test of Visual-Motor Integration 5th Edition, © 2004), with the permission of the publisher. Each stimulus pair consisted of geometric shapes composed of white lines on a black background and were surrounded by a box (10 cm; 14.25° visual angle) which appeared and disappeared simultaneously with S1 and S2. During the 900 ms delay period the screen was black. Following the onset of S2 subjects were instructed to press one of two buttons on a response box to indicate whether S2 was the same as, or different from, S1. S2 was considered to be different from S1 if (1) it was a different size, (2) it had been rotated, or (3) part of the shape had been added or taken away. Subjects were instructed to respond as quickly and accurately as possible.
Fig. 1.
The experimental paradigm and its time course on a single trial
To ensure that subjects remained engaged in the task responses were monitored online during recording. All trials were included in our analysis, regardless of whether or not a correct response was made. Following the offset of S2 a 1,700–2,000 ms intertrial interval occurred during which the screen was black. Each trial consisted of a unique shape pair. Trials were presented in random order across three blocks of 60 trials (50% same; 50% different). A brief rest period was given between each block. Prior to the experiment all subjects received training on the task in order to clarify the criteria for same and different shape pairs, to practice mapping the ‘same’ and ‘different’ responses to the appropriate buttons, and to become familiar with the task.
Data recording and analysis
Magnetoencephalographic data were digitized continuously at 1,200 Hz using a 151 channel MEG (CTF Systems, Burnaby, Canada) throughout task performance and stored for offline analysis. Coils were attached to subjects at the nasion and immediately anterior to the left and right ear. Each of these coils was energized at a specific narrowband frequency to provide a signature of head location throughout recording. To address the issue of measuring neural activity from sensors which might change positions relative to the head throughout the recording session, as well as across subjects, we corrected for head position. This was accomplished by computing dipole solutions to locate the position of each of the coils (indexed by their characteristic energized frequency). Data were then standardized in sensor space by computing an inverse solution, rotating it into alignment with a template location, and subsequently performing a forward solution (see Wilson et al. 2007). This procedure was performed 30 times per second. In this manner the sensor data were adjusted to reflect neural activation generated from a common head position, and the record of the energized coils was then removed from the MEG data using notch filters. Data epochs were then extracted from 2,100 ms before until 1,500 ms after the onset of the delay period. A principal components analysis (PCA) based procedure was used to reduce the influence of ocular and nonocular artifacts of the resulting time series (see Herdman and Cheyne 2009). This approach was chosen, rather than simply rejecting all epochs contaminated by artifacts, as the preservation of trials was paramount to our analysis approach because the small number of trials imposed by recording from a child population.
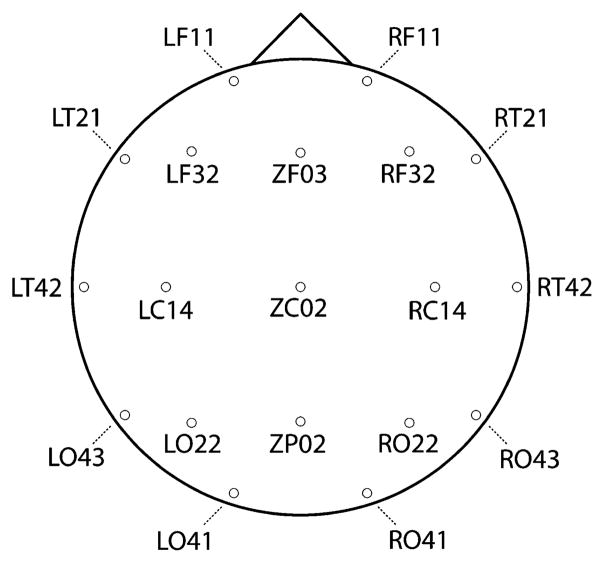
Due to computational limitations imposed by pair-wise comparisons across multiple frequencies and data points, data were downsampled to 300 Hz. In order to image the topography of synchronization while avoiding combinatorial explosion entailed by pair-wise comparisons we selected 19 sensors, distributed roughly evenly about the head, on which to perform the synchronization analysis. This montage included the sensors LF11, RF11, LF32, RF32, LC14, RC14, L022, R022, L041, R041, LT21, RT21, LT42, RT42, L043, R043, ZF03, ZC03 and ZP02. The location of these sensors relative to the head is depicted in Fig. 2.
Fig. 2.
Location of each sensor in the 19 channel montage
Data were band-pass filtered digitally at 1 Hz intervals between 6 and 60 Hz (passband = f ± 0.05f, where f represents the filter frequency). Using algorithms in the MATLAB Signal Processing Toolbox we then calculated the analytic signal
of the filtered waveform for each epoch, f(t), where f̃(t) is the Hilbert transform of f(t) and , to obtain the instantaneous phase, φ(t), and amplitude, A(t), at each sample point. Digital filtering and the Hilbert transform were performed on epoched data obtained from each subject. In order to avoid contamination by edge effects near the beginning and end of these data segments, analyzed epochs extended beyond the periods of interest (the baseline and retention interval), and data points at the beginning and end were discarded. Instantaneous amplitudes from this analysis represent the envelope of the filtered waveform. We interpreted these amplitudes as reflecting variations in local neural synchrony.
We measured long-range phase synchrony by calculating phase locking values (PLVs) for each subject. PLVs were obtained by comparing the instantaneous phases of the signals recorded by various pairs of sensors, for example, sensors j and k, at each point in time, t, across the N epochs available (Lachaux et al. 1999):
PLV is a real value between 0 (random phase difference, no phase locking) and 1 (constant phase difference, maximum phase locking).
To remove the record of ongoing synchrony unrelated to the task, we standardized PLVs and amplitudes relative to a baseline interval lasting from 500 ms prior to the onset of S1 until the onset of S1. This was done by subtracting the mean PLV for the baseline interval at a given frequency from the PLV for every data point at that frequency and dividing the difference by the standard deviation of PLV for the baseline interval at that frequency. The resulting index, PLVz, indicates standardized changes from the average baseline PLV at a given frequency in the direction of increased synchrony (positive values) or decreased synchrony (negative values). The same standardization was applied to the amplitude values.
To characterize the statistical reliability of changes in synchronization and desynchronization in MEG time series between pairs of sensors we obtained the distribution of PLVs using a bootstrapping procedure across subjects. A normal distribution was fit to 4096 bootstrapped PLVs for each time sample (which very closely approximate a normal distribution) in the baseline and active intervals. These distributions were then used to determine statistical confidence intervals for each time–frequency point. To provide protection against false positives we required that significant PLV values be sustained for at least ten consecutive data points (P < 0.05). Additionally, if ten consecutive data points were detected as significant (P < 0.05) in the baseline period the alpha level would be adjusted downward until ten consecutive data points were no longer detected during the baseline period, or until the Bonferroni corrected alpha level was reached (P < 0.0000000001; a statistical threshold exceeding the level at which a false positive would be expected (P < 0.05) across all analyzed time points, frequencies and sensor pairs). In this manner, we ensured that observed effects rose above a level of significance that could not be generated by false positives originating from multiple comparisons.
Results
Task performance
The subjects generally performed well on the task. Subject’s responded correctly on 71.9% of trials (SD across subjects 12), incorrectly on 18.8% of trials (SD 8.6) and did not make a response on 9.3% of trials (SD 10.5). Subjects correct responses occurred with an average reaction time (RT) of 905 ms (SD across subjects 150 ms), and incorrect responses occurred with an RT of 826 ms (SD 182 ms), producing an average RT of 866 ms (SD 151 ms).
Neural synchrony during short-term memory retention
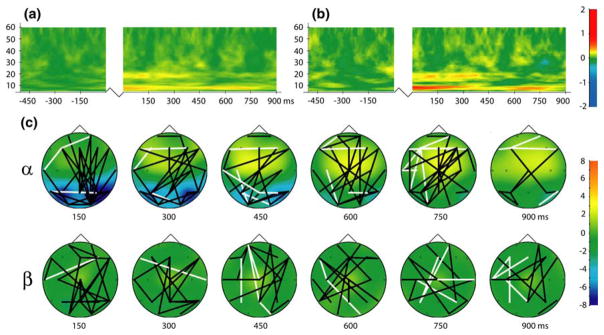
Analysis of global long-range phase locking, assessed by averaging standardized PLV across all 171 analyzed sensor pairs for each time–frequency point during the retention period, revealed sustained increases the alpha and beta frequency bands (Fig. 3a). Due to the prevalence of frontoposterior coupling in previous studies of STM and working memory retention (i.e. Sarnthein et al. 1998; Sauseng et al. 2005b) we plotted the time–frequency distribution of frontoposterior phase locking. This was accomplished by averaging PLV across the 49 sensor pairs which included a frontal sensor (LF11, RF11, LF32, RF32, LT21, RT21, and ZF03) and a posterior sensor (LO22, RO22, LO41, RO41, LO43, RO43, ZP02). This revealed a pattern of effects similar to that found for global long-range phase locking but much more distinct and pronounced (Fig. 3b), suggesting that frontoposterior synchronization was dominant in the generation of the global pattern.
Fig. 3.
a Global long-distance phase synchronization obtained by averaging PLV across all 171 analyzed sensor pairs. b Frontoposterior phase locking obtained by averaging PLV across all connections between frontal (LF11, RF11, LF32, RF32, LT21, RT21, ZFO3) and posterior (LO22, RO22, LO41, RO41, LO43, RO43, ZP02) sensors (49 pairs). Jagged lines in time–frequency plots represent temporal discontinuity (presentation of S1 between baseline and retention interval). c Topography of local and long-range alpha (9 Hz) and beta (18 Hz) band synchronization during short-term memory retention. Black lines indicate statistically significant phase synchronization between sensors (P < 0.05); white lines denote significant desynchronization (P < 0.05). Colors represent changes in amplitude (local synchronization) in units of standard deviation from the baseline; legends at right (color figure online)
The topography of long-range alpha- and beta-band phase synchronization during STM retention, together with corresponding amplitude changes, is presented in Fig. 3c. Long-range alpha-band synchronization was characterized by abundant frontoposterior connections. Bilateral sustained reductions in alpha-band amplitude were observed over posterior cortex and were most pronounced early during the retention interval. Increased frontocentral alpha amplitude was also observed and but peaked later in the retention interval. Long-range beta-band synchronization was also found to include considerable frontoposterior connections, but the topography of beta-band synchronization was more diffuse than was observed for alpha-band synchronization. Beta-band synchronization was not accompanied by distinct topographic amplitude changes (see Fig. 3c).
Posterior cortex: local and long-range alpha-band synchronization
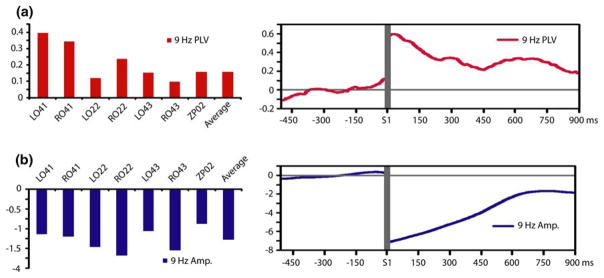
Sustained increases in alpha-band phase locking were observed between sensors over posterior cortex (LO22, RO22, LO41, RO41, LO43, RO43, ZP02) and all other analyzed sensors throughout STM retention (Fig. 4a). Alpha amplitude at posterior sensors, conversely, was found to be decreased during visual STM retention (Fig. 4b). Posterior alpha amplitude decreases were also sustained throughout the retention interval. These results demonstrate a functional dissociation for alpha synchronization across cortical scales, as task-demands increased long-range synchrony between posterior cortex and other regions while local synchronization in posterior cortex was decreased. Both local alpha desynchronization over posterior cortex and long-range synchronization between posterior cortex and other cortical regions were most pronounced early in the retention interval.
Fig. 4.
Functional dissociation for alpha-band synchronization across local and long-range cortical scales during STM retention. a Average alpha-band phase locking between sensors over posterior cortex and all other analyzed sensors during STM retention (left) and the time course of this long-range synchronization throughout STM retention and the baseline period averaged across posterior sensors (right). b Average alpha-band amplitude at posterior sensors during STM retention (left) and the time course of this local desynchronization throughout STM retention and the baseline period averaged across posterior sensors (right). Amplitude changes and phase synchronization are expressed in units of standard deviation from the baseline. Gray horizontal lines indicates temporal discontinuity (the period of S1 presentation between the baseline and retention intervals)
Discussion
Functional dissociation for alpha-band synchronization across scales
Our results constitute a functional dissociation for alpha-band desynchronization across local and long-range cortical scales. We demonstrate local alpha-band desynchronization during visual STM retention, similar to results from previous studies conducted in adults (Gevins et al. 1997; Krause et al. 2000; Sauseng et al. 2005b; Stipacek et al. 2003) and children (Gomarus et al. 2006) examining alpha activity during the delay phase of STM and working memory tasks. The topography of our MEG results is also consistent with previous research showing load-dependent decreases in local alpha activity posterior cortex and load-dependent increases over frontocentral locations during working memory using both scalp electrodes (Krause et al. 2000; Sauseng et al. 2005b) and intracranial electrodes (Meltzer et al. 2008). We interpret the local posterior alpha desynchronization observed in our study as an index of task-dependent activation of visual cortex, consistent with the view that local alpha rhythms reflect cortical idling (Pfurtscheller et al. 1996).
It remains unclear what differences in task demands account for the variability of alpha oscillatory responses during STM and working memory processing, as some previous studies conducted in adults have reported increases in local posterior alpha synchronization (Busch and Herrmann 2003; Jokisch and Jensen 2007; Jensen et al. 2002; Schack and Klimesch 2002). Such inconsistencies may be due to the multidetermined nature of the alpha response. Recognition memory tasks, for example, have been shown to elicit both alpha increases and decreases which are related, at least in that context, to evoked and induced processes, respectively (Klimesch et al. 2000). Local synchronization and desynchronization during working memory processing, moreover, can occur in distinct bandwidths within the alpha frequency range (Krause et al. 2000). Differences between adults and children in local alpha desynchronization during memory retrieval have also been shown to be specific to narrow frequency ranges within the alpha-band (Krause et al. 2001). Such results underline the need for future research into the component processes which give rise to the task-dependent changes in alpha oscillations recorded using EEG and MEG.
We demonstrate, in the presence of local alpha-band desynchronization over posterior cortex, increased alpha-band phase synchronization between sensors over posterior cortex and those over other, widespread, cortical regions during STM retention. This result is consistent with the emerging view that long-distance synchronization in the alpha-band, as is the case for other frequency ranges, is associated with the formation of transient, task relevant, neural assemblies (see Palva and Palva 2007 for review). In the context of STM retention this would imply that a large-scale coalition of neurons synchronously oscillating in the alpha frequency range contributes to the maintenance of the memory trace. Such a role for long-range alpha synchronization would be quite different from that of locally synchronous alpha rhythms, which are thought to reflect cortical idling or inhibitory processes (see Klimesch et al. 2007; Pfurtscheller et al. 1996 for reviews). Further evidence for such a functional dissociation for alpha-band synchronization across cortical scales comes from the recent finding that attending to one side of the visual field increases long-range occipital–parietal alpha-band synchronization in the hemisphere contralateral to the attended hemifield, whereas alpha-band amplitude is increased in ipsilateral occipital cortex (Doesburg et al. 2009). Observations that sensory integration and visual recognition are associated with local desynchronization and long-range synchronization in the alpha-band (Freunberger et al. 2008; Hummel and Gerloff 2005) also lend credence to the notion that synchronization of alpha rhythms may play distinct roles at different cortical scales and may also fit very well with earlier reported co-activation of subsequent specific/nonspecific thalamocortical circuitry of gamma band activations required for the temporal binding of sensory input or “content” into long-range circuitry or “context” (Llinás and Ribary 1993).
The results of the present study are similar to those of Sauseng et al. (2005b), who showed using EEG in adults that alpha rhythms during the manipulation of visual information in working memory, relative to simple retention, were (1) reduced over occipital cortex, (2) increased over frontal cortex, and (3) displayed increased functional coupling between frontal and occipital areas. Palva et al. (2005) similarly showed in adults that long-range alpha synchronization was increased during the performance of a continuous arithmetic working memory task. Task-dependent long-range alpha synchronization in their study, however, also coincided with increased local alpha activity, highlighting the variability of alpha oscillatory responses across task demands. The complexity of the relationship between local and long-range synchronization of alpha rhythms is further underscored by our observation that long-range synchronization occurs between posterior cortex, which is locally desynchronized, and frontal cortex, which is locally synchronized (see also Sauseng et al. 2005b). Local synchronization of alpha rhythms over frontal cortex also peaked after local posterior desynchronization in our data (see Fig. 3c), indicating that these effects reflect signals from distinct sets of neural sources. This observation, together with the distances at which synchronizations are observed, confirm that the patterns of synchronization reported here are not artefactual. The distributed lead fields of the axial gradiometers, however, leave open the possibility that synchronization between nearby sensors may have occurred because of the detection of a single oscillating source at both locations, highlighting the importance of future research investigating connectivity between localized cortical sources (see Doesburg and Ward 2009; Schoffelen and Gross 2009).
We interpret the sustained long-range alpha-band synchronization observed in our study as a mechanism for maintaining memory traces during STM retention. Recent research has also implicated long-range alpha-band synchronization in other memory processes. Large differences in the topography of long-range alpha-band synchronization were observed during the encoding of abstract, relative to concrete, spoken words (Schack et al. 2003). Mima et al. (2001) also demonstrated increased interhemispheric alpha-band coherence over visual cortex during object recognition. Interestingly, this study also reported decreased local alpha-band synchronization over visual cortex, suggesting that the inverse relationship between local and long-range synchronization may apply to alpha rhythms across a variety of memory processes. Using depth electrodes, it has been shown that the fusiform gyrus (a cortical region known to relevant to the visual processing of faces) became synchronized with other cortical regions during the recognition of a face, but not a nonface, stimulus (Klopp et al. 2000). Kujala et al. (2006) showed alpha-band synchronization between a network of cortical areas and the cerebellum during semantic encoding. The presence of alpha-band synchronization across such an array of memory processes suggests that it could be relevant for the instantiation and activation of a Hebbian ensemble representing the memory trace. This outlook is consistent with general conceptions of the role of neural synchronization (Varela et al. 2001) and research on the role of neural oscillations and memory in other frequency ranges such as the gamma band (e.g. Miltner et al. 1999; Supp et al. 2007; see Jensen et al. 2007 for review).
Oscillatory dynamics during short-term memory retention in children
We uniquely demonstrate increased long-range alpha and beta band synchronization during STM retention in children age 6–10 years. These results are comparable to reports of increased long-range alpha and beta band synchronization during working memory processing (Palva et al. 2005) and increased beta-band synchrony between implanted cortical electrodes during STM retention (Tallon-Baudry et al. 2001) in adults. Palva et al. (2005) observed that increased long-range alpha and beta band phase synchronization was accompanied by abundant cross-frequency coupling between alpha and beta oscillations during working memory processing in adults, suggesting that the long-range alpha and beta band phase synchronization observed in the present study may reflect oscillatory mechanisms of STM retention that are interacting across frequency bands. We also reveal decreased alpha-band amplitude over posterior cortex and increased alpha-band amplitude over frontal cortex during STM retention in children. These findings parallel results from previous experiments conducted in adults, which have associated posterior decreases (Gevins et al. 1997; Krause et al. 2000; Sauseng et al. 2005b; Stipacek et al. 2003) and frontal increases (Krause et al. 2000; Sauseng et al. 2005b) in local alpha activity with STM and working memory. Load-dependent decreases in local posterior alpha activity have also been previously demonstrated in children aged 8–12, consistent with our results (Gomarus et al. 2006). In our study, both local alpha amplitude changes and long-range alpha synchronization were already manifest at the onset of the STM retention interval. This most likely occurred because the children had already committed S1 to STM before the termination of stimulus presentation. Moreover, both local alpha desynchronization over posterior cortex and long-range synchronization between posterior cortex and other, widespread, cortical regions was most pronounced during the early phase of the retention interval. This may be due to the superposition of STM-related processes and responses relevant to the offset of a visual stimulus early during retention, whereas synchronization late in the retention interval is relevant only to STM-related processing.
While research on alpha oscillatory responses in children is scarce, several lines of evidence indicate that these mechanisms play an important role in cognitive development. Krause et al. (2001) demonstrated that children (mean age 12 years) exhibited less local alpha-band desynchronization during memory retrieval than adults. Children age 6–11 years have also been shown to differ from adults in the location and amplitude of alpha responses in an auditory oddball paradigm, and strikingly, the responses of the eldest children (age 10–11) were found to most similar to those of adults (Yordanova and Kolev 1996, 1997). The view that alpha mechanisms play a critical role in cognitive performance is supported by findings that individual visual STM spans are correlated with individual differences in the parameters of alpha oscillations (Maltseva and Maslobev 1997) and that resting alpha power is related to performance on both perceptual and memory tasks (Hanslmayr et al. 2005). It also appears likely that alpha oscillatory mechanisms are relevant to healthy cognitive development. Decreased resting alpha oscillatory activity and increased theta rhythmicity, due to thalamic cell hyperpolarization and increased low-threshold calcium spike bursts, in conjunction with a widespread and marked increase of power correlation among high and low frequency oscillations have been demonstrated as a thalamocortical dysrhythmia syndrome and are correlated to some neuropsychiatric positive symptoms (Llinás et al. 1999). Altered patterns of alpha synchrony have also been associated with conditions such as ADHD and autism (Dockstader et al. 2008; Murias et al. 2007). Such findings suggest that increased understanding of how alpha oscillations develop during childhood may help to illuminate neural mechanisms of cognitive maturation and uncover specific alterations in neurocognitive processing underlying developmental conditions.
A limitation of the present study is that no control condition was included in our experimental paradigm. The decision not to include a control condition was made because of the signal-to-noise ratio demands of phase synchronization analysis and the limited duration of challenging cognitive tasks that can be reliably performed by children 6–10 years of age. Without comparison to a control condition it is not possible to definitively relate the observed patterns of synchronization to STM processing only as there is no means to separate out activity relevant to other task demands such as attention. We believe that our results reflect oscillatory mechanisms of STM retention, however, due to the similarity of the observed topography of amplitude changes to previous studies of STM and working memory processing in adults (Gevins et al. 1997; Krause et al. 2000; Sauseng et al. 2005b; Stipacek et al. 2003) and children (Gomarus et al. 2006). In addition, load-dependent long-range synchronization effects comparable to ours have also been observed during working memory processing in adults (Palva et al. 2005).
Acknowledgments
This research was supported by grant R01 HD039783 from the Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH) to R.E.G. We thank Ivan Cepeda and Gisela Gosse for coordinating the study, Katia Jitlina and Julie Unterman for help with data collection, and John Gaspar for assistance with data analysis. We also thank Dr. Keiichi Kitajo, RIKEN Institute, and Dr. Kentaro Yamanaka, University of Tokyo, for help in developing the phase synchrony analysis program. R. E. G. is supported by a Distinguished Scholar award from the Child and Family Research Institute and a Senior Scholar award from the Human Early Learning Partnership (HELP). S. M. D. is partially supported by the CIHR Pain in Child Health (PICH) Strategic Training Initiative in Health Research.
Footnotes
Conflict of interest statement None declared.
References
- Busch NA, Herrmann CS. Object-load and feature-load modulate EEG in a short-term memory task. Neuroreport. 2003;14(13):1721–1724. doi: 10.1097/00001756-200309150-00013. [DOI] [PubMed] [Google Scholar]
- Dockstader C, Gaetz W, Cheyne D, et al. MEG event-related desynchronization and synchronization deficits during basic somatosensory processing in individuals with ADHD. Behav Brain Funct. 2008;4(8):1–13. doi: 10.1186/1744-9081-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doesburg SM, Ward LM. Synchronization between sources: emerging methods for understanding large-scale functional networks in the human Brain. In: Perez-Velazquez J-L, Wennberg R, editors. Coordinated activity in the human brain: measurements and relevance to brain function and behavior. Springer; New York: 2009. pp. 25–42. [Google Scholar]
- Doesburg SM, Green JJ, McDonald JJ, Ward LM. From local inhibition to long-range integration: a functional dissociation of alpha-band synchronization across cortical scales in visuospatial attention. Brain Res. 2009;1303:97–110. doi: 10.1016/j.brainres.2009.09.069. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV, Ahlfors SP. Parietal-occipital 10 Hz activity reflects anticipatory state of visual attention mechanisms. Neuroreport. 1998;9(17):3929–3933. doi: 10.1097/00001756-199812010-00030. [DOI] [PubMed] [Google Scholar]
- Freunberger R, Klimesch W, Greismayr B, et al. Alpha phase coupling reflects object recognition. Neuroimage. 2008;42(2):928–935. doi: 10.1016/j.neuroimage.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Fu KG, Foxe JJ, Murray MM, et al. Attention-dependent suppression of distracter visual input can be cross-modally cued as indexed by parietal-occipital alpha-band oscillations. Cogn Brain Res. 2001;12:145–152. doi: 10.1016/s0926-6410(01)00034-9. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D. High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cereb Cortex. 1997;7:1047–3211. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- Gomarus HK, Althaus M, Wijers AA, Minderaa RB. The effects of memory load and stimulus relevance on the EEG during a visual selective memory search task: an ERP and ERD/ERS study. Clin Neurophysiol. 2006;117:871–884. doi: 10.1016/j.clinph.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Klimesch W, Sauseng P, Gruber W, Doppelmayr M, et al. Visual discrimination performance is related to decreased alpha amplitude but increased phase locking. Neurosci Lett. 2005;375:65–68. doi: 10.1016/j.neulet.2004.10.092. [DOI] [PubMed] [Google Scholar]
- Herdman AT, Cheyne D. A practical guide to MEG and beamforming. In: Handy TC, editor. Brain signal analysis: advances in neuroelectric and neuromagnetic methods. MIT Press; Cambridge: 2009. pp. 99–140. [Google Scholar]
- Hummel F, Gerloff G. Larger interregional synchrony is associated with greater behavioral success in a complex sensory integration task in humans. Cereb Cortex. 2005;15:670–678. doi: 10.1093/cercor/bhh170. [DOI] [PubMed] [Google Scholar]
- Jensen O, Gelfand J, Kounios J, Lisman JE. Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cereb Cortex. 2002;12:877–882. doi: 10.1093/cercor/12.8.877. [DOI] [PubMed] [Google Scholar]
- Jensen O, Kaiser J, Lachaux JP. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007;30(7):317–324. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Jokisch D, Jensen O. Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. J Neurosci. 2007;27:3244–3251. doi: 10.1523/JNEUROSCI.5399-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayer M, Rohm D, Pollhuber D, Stadler W. Simultaneous desynchronization and synchronization of different alpha responses in the human electroencephalograph: a neglected paradox? Neurosci Lett. 2000;284:97–100. doi: 10.1016/s0304-3940(00)00985-x. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition timing hypothesis. Brian Res Rev. 2007;53(1):63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Klopp J, Marinkovic K, Chauvel P, et al. Early widespread cortical distribution of coherent fusiform face selective activity. Hum Brain Mapp. 2000;11:286–293. doi: 10.1002/1097-0193(200012)11:4<286::AID-HBM80>3.0.CO;2-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause CM, Sillanmaki L, Koivisto M, Saarela C, Haggqvist A, et al. The effects of memory load on event-related EEG desynchronization and synchronization. Clin Neurophysiol. 2000;112:2233–2240. doi: 10.1016/s1388-2457(00)00429-6. [DOI] [PubMed] [Google Scholar]
- Krause CM, Salminen PA, Sillanmaki L, Holopainen Event-related desynchronization and synchronization during a memory task in children. Clin Neurophysiol. 2001;111:2071–2078. doi: 10.1016/s1388-2457(01)00684-8. [DOI] [PubMed] [Google Scholar]
- Kujala J, Prammer K, Cornelissen P, et al. Phase coupling in a cerebro-cerebellar network at 8–13 Hz Turing reading. Cereb Cortex. 2006;17:1476–1485. doi: 10.1093/cercor/bhl059. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum Brain Mapp. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R, Ribary U. Coherent 40-Hz oscillation characterizes dream state in humans. Proc Natl Acad Sci USA. 1993;20:2078–2081. doi: 10.1073/pnas.90.5.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci USA. 1999;96(26):15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltseva IV, Maslobev YP. Alpha rhythm parameters and short-term memory span. Int J Psychophysiol. 1997;26(1–3):369–380. doi: 10.1016/s0167-8760(97)00776-9. [DOI] [PubMed] [Google Scholar]
- Meltzer JA, Zaveri HP, Goncharova II, Distasio MM, Papademetris X, et al. Effects of working memory load on oscillatory power in human intracranial EEG. Cereb Cortex. 2008;18:1843–1855. doi: 10.1093/cercor/bhm213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltner WH, Braun C, Arnold M, Whitte H, Taub E. Coherence of gamma-band EEG activity as a basis for associative learning. Nature. 1999;397(6718):391–393. doi: 10.1038/17126. [DOI] [PubMed] [Google Scholar]
- Mima T, Oluwatimilehin T, Hiaoka T, Hallet M. Transient interhemispheric neuronal synchrony correlates with object recognition. J Neurosci. 2001;21:3942–3948. doi: 10.1523/JNEUROSCI.21-11-03942.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murias M, Webb SJ, Greenson J, Dawson G. Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biol Psychiatry. 2007;62(3):270–273. doi: 10.1016/j.biopsych.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva S, Palva JM. New vistas for alpha-frequency band oscillations. Trends Neurosci. 2007;30:150–158. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Palva JM, Palva S, Kaila K. Phase synchrony among neuronal oscillations in the human cortex. J Neurosci. 2005;25(15):3962–3972. doi: 10.1523/JNEUROSCI.4250-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L, Kounios J. Coherent oscillatory networks supporting short-term memory retention. Brain Res. 2009;1247:126–132. doi: 10.1016/j.brainres.2008.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancak A, Jr, Neuper C. Event-related synchronization (ERS) in the alpha-band—an electrophysiological correlate of cortical idling: a review. Int J Psychophysiol. 1996;24:39–46. doi: 10.1016/s0167-8760(96)00066-9. [DOI] [PubMed] [Google Scholar]
- Sarnthein J, Petsche H, Rappelsberger P, et al. Synchronization between prefrontal and posterior association cortex during human working memory. Proc Natl Acad Sci USA. 1998;95:7092–7096. doi: 10.1073/pnas.95.12.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Stadler W, et al. A shift of visual spatial attention is selectively associated with human EEG alpha activity. Eur J Neurosci. 2005a;22:2917–2926. doi: 10.1111/j.1460-9568.2005.04482.x. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Doppelmayr S, et al. EEG alpha synchronization and functional coupling during top-down processing in a working memory task. Hum Brain Mapp. 2005b;26:148–155. doi: 10.1002/hbm.20150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schack B, Klimesch W. Frequency characteristics of evoked and oscillatory electroencephalic activity in a human memory scanning task. Neurosci Lett. 2002;331:107–110. doi: 10.1016/s0304-3940(02)00846-7. [DOI] [PubMed] [Google Scholar]
- Schack B, Weiss S, Rappelsberger P. Cerebral information transfer during word processing: where and when does it occur and how fast is it? Hum Brain Mapp. 2003;19:18–36. doi: 10.1002/hbm.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelen JM, Gross J. Source connectivity analysis with MEG and EEG. Hum Brain Mapp. 2009;30(6):1857–1865. doi: 10.1002/hbm.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipacek A, Grabner RH, Neuper A, et al. Sensitivity of human EEG alpha band desynchronization to different working memory components and increasing levels of memory load. Neurosci Lett. 2003;353:193–196. doi: 10.1016/j.neulet.2003.09.044. [DOI] [PubMed] [Google Scholar]
- Supp GG, Schlögl A, Trujillo-Barreto N, Müller MM, Gruber T. Directed cortical information flow during human object recognition: analyzing induced EEG gamma-band responses in brain’s source space. PLoS One. 2007;2(1):e684. doi: 10.1371/journal.pone.0000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Fischer C. Oscillatory synchrony between human extrastriate areas during visual short-term memory maintenance. J Neurosci. 2001;21(RC177):1–5. doi: 10.1523/JNEUROSCI.21-20-j0008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. α-Band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2(4):229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Ward LM. Synchronous neural oscillations and cognitive processes. Trends Cogn Sci. 2003;7(12):553–559. doi: 10.1016/j.tics.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Wilson H, Moiseev A, Podin S, Quraan M. Continuous head localization and data correction in MEG. Int Congr Ser. 2007;1300:623–626. [Google Scholar]
- Worden MS, Foxe JJ, Wang N, Simpson GV. Anticipatory biasing of visuospatial attention indexed by retinotopically specific α-band electroencephalography increases over occipital cortex. J Neurosci. 2000;20(RC63):1–6. doi: 10.1523/JNEUROSCI.20-06-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanova JY, Kolev VN. Developmental changes in the alpha response system. Electroencephalogr Clin Neurophysiol. 1996;99(6):527–538. doi: 10.1016/s0013-4694(96)95562-5. [DOI] [PubMed] [Google Scholar]
- Yordanova JY, Kolev VN. Alpha response system in children: changes with age. Int J Psychophysiol. 1997;26(1–3):411–430. doi: 10.1016/s0167-8760(97)00779-4. [DOI] [PubMed] [Google Scholar]