Abstract
Recently we described a novel cell penetrating peptide, POD (peptide for ocular delivery) that could deliver small molecules including fluorescent dyes into retinal cells. The objective of the current study was to examine whether biologically relevant macromolecules such as proteins, genetically fused with POD could also be delivered into retinal tissues in vivo. We generated a POD-GFP fusion protein and examined its cell and tissue penetrating properties. We found that endogenously expressed POD-GFP fusion protein localized to the nucleus, suggesting that POD acts as a nuclear localization signal. Adenovirus (Ad) vectors expressing POD-GFP fusion protein were constructed and the recombinant protein was purified from Ad-infected human embryonic retinoblasts (HER). Exogenously supplied POD-GFP fusion protein rapidly transduced A549 and HER cells and colocalized in part with markers of late endosomes, from which it could escape. Following subretinal delivery, POD-GFP localized to the retinal pigment epithelium and the photoreceptor cell bodies. When injected into the vitreous, POD-GFP localized to the ganglion cells and the inner nuclear layer of the retina as well as the lens capsule. Topical application of POD-GFP to ocular surfaces resulted in uptake by the corneal epithelium. POD-GFP also transduced non-ocular tissues, including the epidermis of the skin following topical application.
Keywords: POD, Protein Transduction, Cell Penetrating Peptide, Protein Therapy, Retinal Degeneration
INTRODUCTION
According to public opinion polls, blindness is the second most feared condition amongst Americans after cancer. Almost 200 different loci and 130 different genes responsible for inherited retinal degeneration have thus far been identified, making retinal degeneration one of the most heterogeneous genetic disorders in humans (www.retnet.org). A large number of these genes are expressed exclusively in the photoreceptors or retinal neurons. Efficient delivery of therapeutic proteins into retinal cells is currently limited to the use of recombinant viruses for delivery of DNA expression cassettes- an approach that in some tissues can be deleterious (Donsante, Miller, Li, Vogler, Brunt, Russell & Sands, 2007). Hence, relatively intensive and significantly protracted preclinical studies need to be performed prior to the use of gene delivery in humans. Given the large number of genes involved in retinal degeneration, preclinical testing of gene therapy approaches for every gene is prohibitive. Hence, one may envisage that progress towards the development of therapies for many retinal degenerative diseases may be accelerated by developing the theoretically less risky approach of protein delivery- administration of which can be readily terminated upon the initial observation of any adverse events. For example, although viral-mediated gene transfer of ciliary neurotrophic factor (CNTF) to animal models of retinal degeneration has been documented to be deleterious (Buch, MacLaren, Duran, Balaggan, MacNeil, Schlichtenbrede, Smith & Ali, 2006), phase I trials have been safely completed by use of encapsulated CNTF-producing cells that could be readily removed from the ocular compartment of retinitis pigmentosa patients (Sieving, Caruso, Tao, Coleman, Thompson, Fullmer & Bush, 2006).
For some retinal degenerative diseases such as rhodopsin or peripherin/RDS-associated retinitis pigmentosa (RP), over-expression of therapeutic gene product may be deleterious, as has been observed with both viral gene transfer (Sarra, Stephens, de Alwis, Bainbridge, Smith, Thrasher & Ali, 2001) and in transgenic mice (Tan, Wang, Quiambao, Xu, Qtaishat, Peachey, Lem, Fliesler, Pepperberg, Naash & Al-Ubaidi, 2001). Hence, therapies for RP patients could theoretically be safer by delivery of protein instead of DNA. In the context of retinal degeneration, which is typically a slow progressive disease, one may envisage the use of slow release formulations or depots in the intraocular environment for long-term delivery of therapeutic proteins as has been achieved previously in some clinical studies (Sieving et al., 2006). However, macromolecules do not readily cross the plasma membrane and do not penetrate neural tissues such as the retina.
Previously, several proteins exhibiting the unusual property of traversing lipid bilayers have been identified. These molecules contain protein transduction domains (PTDs) that can be chemically cross-linked to heterologous proteins, antibodies and enzymes and facilitate their transport across the plasma membrane (Anderson, Nichols, Manger, Woodle, Barry & Fritzberg, 1993, Fawell, Seery, Daikh, Moore, Chen, Pepinsky & Barsoum, 1994). Protein transduction was first reported over a decade ago by Green and Frankel who independently demonstrated that the Human Immunodeficiency Virus (HIV) TAT protein was able to enter cells when added to the surrounding media (Frankel & Pabo, 1988, Green & Loewenstein, 1988). Subsequently, several other proteins with transducing capabilities have been identified, the most intensively studied of which are the Drosophila homeotic transcription factor ANTP (encoded by the antennapedia gene) and the Herpes Simplex Virus (HSV) VP22 (Elliott & O’Hare, 1997, Joliot, Pernelle, Deagostini-Bazin & Prochiantz, 1991, Joliot, Triller, Volovitch, Pernelle & Prochiantz, 1991). A variety of additional cell penetrating peptides have since been discovered (Vives, Schmidt & Pelegrin, 2008). Apart from HIV TAT, there is very limited information available on the performance of PTDs in the retina (Barnett, Elangovan, Bullok & Piwnica-Worms, 2006, Cashman, Morris & Kumar-Singh, 2003, Dietz, Kilic & Bahr, 2002, Schorderet, Manzi, Canola, Bonny, Arsenijevic, Munier & Maurer, 2005).
Previously, we examined the potential use of HIV TAT (Cashman et al., 2003) and HSV VP22 (Cashman, Sadowski, Morris, Frederick & Kumar-Singh, 2002) in delivering recombinant proteins to human embryonic retinoblasts in culture and to retinal tissues in vivo. We found that whereas both of these PTDs acted efficiently in vitro, their performance in the retina in vivo was limited. Hence, we recently developed and described a novel cell and tissue penetrating peptide referred to as POD – (peptide for ocular delivery; GGG[ARKKAAKA]4) that could be used to efficiently deliver fluorescent dyes or other similarly small molecules into the retina in vivo.
The objective of the current study was to examine the potential of genetically fusing biologically relevant macromolecules such as whole proteins to POD in order to determine whether novel transduction properties are conferred upon the recombinant fusion protein in terms of delivery into the retinal photoreceptors and interneurons. For proof-of-principle, here we examine delivery of a POD-GFP fusion protein to retinal cells in vitro, ex vivo and in vivo. Our results indicate that POD-fusion proteins allow the penetration and dispersion of macromolecules into a variety of cells and tissues and hence may have significant therapeutic applications.
RESULTS
De novo synthesized POD-GFP localizes to the nucleus
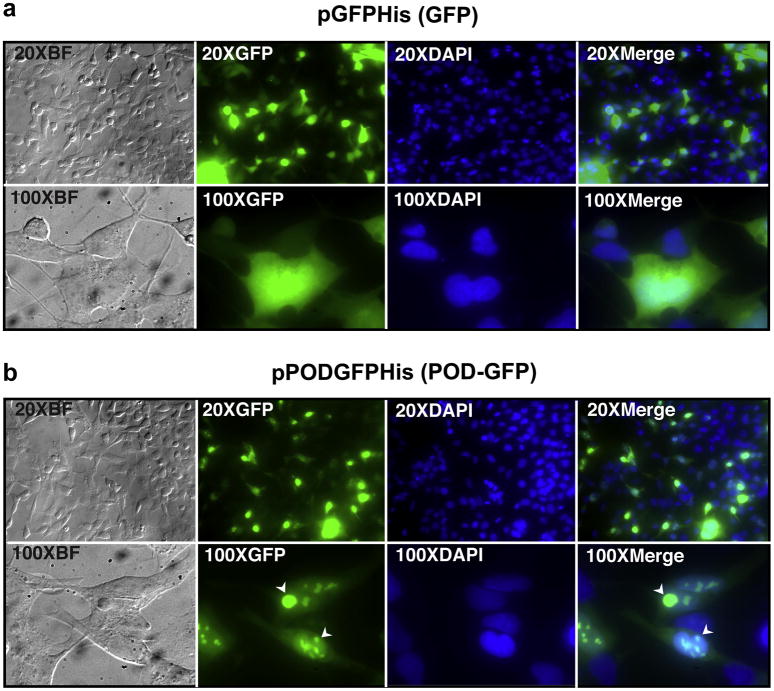
Transfection of human embryonic retinoblast (HER) cells (Fallaux, Kranenburg, Cramer, Houweling, Van Ormondt, Hoeben & van der Eb, 1996) with pGFPHis, a plasmid expressing a His-tagged GFP, resulted in relatively diffuse cytoplasmic and nuclear localization of recombinant GFP (Figure 1a). In contrast, transfection of HER cells with pPODGFPHis, a plasmid expressing a His-tagged POD-GFP fusion protein, resulted in relatively weak cytoplasmic and intense nuclear localization (Figure 1b). Furthermore, POD-GFP appeared to concentrate in sub nuclear compartments (Figure 1b, arrowheads). This pattern of localization associated with POD-GFP contrasts with that of exogenously added lissamine-conjugated POD peptide (L-POD), which we have previously shown to localize primarily to the cytoplasm in a punctate pattern- reminiscent of endocytosis (Johnson, Cashman & Kumar-Singh, 2007). We conclude that endogenously expressed POD can act as a nuclear localization signal for POD fusion proteins.
Figure 1. De novo synthesized POD-GFP fusion protein localizes to the nucleus.
HER cells were transfected with either pGFPHis (a) or pPODGFPHis (b), plasmids expressing His-tagged GFP or POD-GFP fusion proteins respectively. Whereas GFP localizes to the cytoplasm and nucleus (a), POD-GFP localizes primarily to the nucleus (b). Furthermore, POD-GFP appears to be concentrated in sub nuclear compartments (b, arrowheads). BF, brightfield. Color version of this figure appears online.
Purification of POD-GFP from adenovirus-infected HER cells
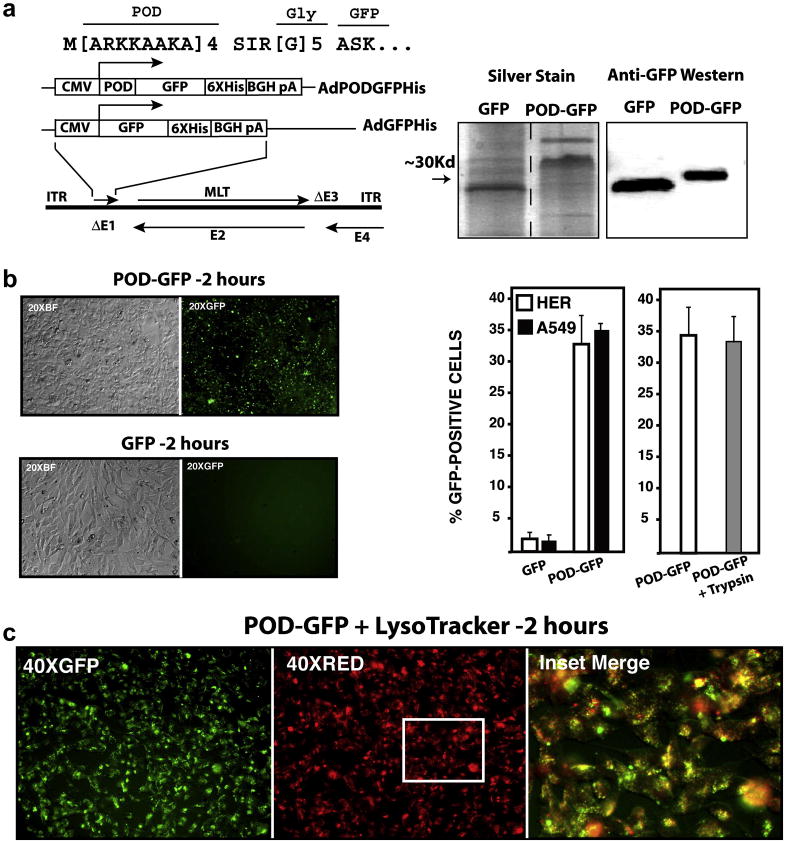
In order to examine protein transduction by exogenously supplied POD-GFP, expression cassettes coding for His-tagged POD-GFP or GFP, regulated by a CMV promoter, were cloned into an E1/E3-deleted human adenovirus serotype 5 vector- AdPODGFPHis and AdGFPHis respectively (Figure 2a). POD-GFP and GFP were purified from adenovirus infected HER cells and electrophoresed on a Tris-HCl acrylamide gel. Following silver staining, major bands were identified at approximately 32 and 28 Kd, corresponding to roughly the expected molecular weight of POD-GFP and GFP respectively (Figure 2a). Minor bands of slightly greater molecular weight were also detected for each of the proteins by silver staining. Western blot analyses of the purified protein fractions using a monoclonal antibody against GFP confirmed that the major bands corresponded to GFP and POD-GFP respectively (Figure 2a). We conclude that recombinant adenovirus vectors can be used to generate highly enriched preparations of POD-fusion proteins from human cells.
Figure 2. Recombinant adenovirus vectors expressing POD-GFP and uptake of POD-GFP into cells.
Expression cassettes coding for POD-GFP or GFP were cloned into the deleted E1 region (ΔE1) of a first generation adenovirus vector. POD was fused to GFP via a flexible polyglycine linker (a). Silver staining of purified protein indicated major bands of the anticipated molecular weights for POD-GFP and GFP. Anti-GFP western blot confirmed that these fusion proteins contained GFP (a). Purified POD-GFP or GFP protein was incubated with HER cells for 2 hours and GFP-positive cells were counted by FACS (b). POD-GFP entered cells and colocalized in part with Lysotracker, a marker of late endosomes (c). CMV, cytomegalovirus; 6XHis, His tag; BGH pA, bovine growth hormone polyadenylation signal; ITR, inverted terminal repeat; MLT, major late transcription unit; E1–E4; early regions 1–4 respectively.
Transduction Properties of POD-GFP in vitro
Approximately 0.2×106 human lung carcinoma epithelial (A549) or HER cells were incubated with 10μg purified recombinant POD-GFP or GFP protein for 2 hours and the number of GFP-positive cells counted by FACS. A total of 35.3±0.9% and 1.4±0.4% of A549 cells were GFP-positive when incubated with POD-GFP or GFP respectively. Similarly, a total of 34.2±3.4% and 1.8±0.4% of HER cells were GFP-positive when incubated with POD-GFP or GFP respectively (Figure 2b). As determined previously for the short peptide C-POD (Johnson et al., 2007), here we re examined whether POD-GFP was also internalized and not simply membrane associated by incubation of transduced cells with trypsin. A total of 33.1±4.1% of cells were GFP-positive following treatment with trypsin- not significantly different to the number of GFP-positive cells observed in the absence of trypsin, suggesting that POD-GFP is not membrane associated, but rather, internalized (Figure 2b). Toxicity to cells during protein transduction was measured by uptake of propidium iodide (PI). A total of 2.8±3.8% or 3.2±2.7% of HER cells were PI-positive when incubated with POD-GFP or GFP respectively, similar to that of untransduced cells (3.6±3.0%). In contrast to endogenous gene expression, POD-GFP now appeared in the cytoplasm of HER cells in a punctate pattern (Figure 2c), similar to our previous observations with L-POD peptide (Johnson et al., 2007) and reminiscent of an endocytic mechanism of uptake. POD-GFP failed to colocalize with ER tracker or DAPI but did colocalize in part with Lysotracker (Figure 2c), a marker of late endosomes- suggesting that POD-GFP enters cells by more than one mechanism. We conclude that POD-GFP can transduce cells in culture, does not significantly damage the plasma membrane during entry and in contrast to endogenous expression, colocalizes in part with late endosomes.
Transduction Properties of POD-GFP in vivo and ex vivo
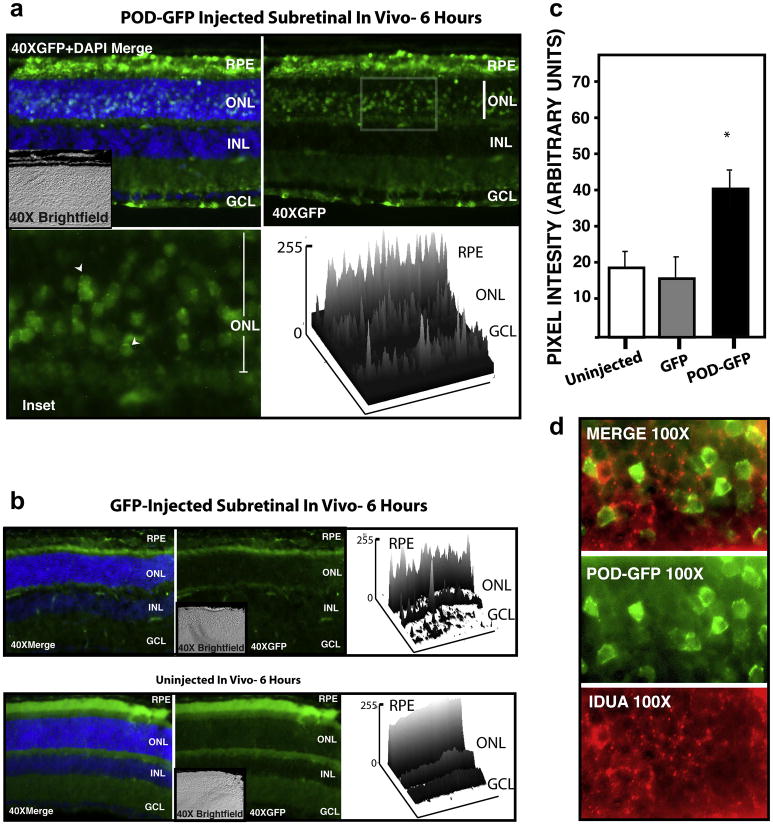
Investigation of the transduction properties of POD-GFP in vivo was performed by subretinal and intravitreal injections in mice. A total of 8.5μg purified POD-GFP or GFP protein was injected into the subretinal space, i.e. between the retinal pigment epithelium (RPE) and photoreceptors of 6 week-old male C57BL6/J mice. Eyes were harvested after 6 hours and frozen sections examined by fluorescence microscopy (Figure 3). POD-GFP was found to localize to the subretinal space as expected, but it was also detected in the RPE and abundantly present in the outer nuclear layer (ONL), i.e. the location of the photoreceptor cell bodies (Figure 3a). Depending upon the extent of retinal detachment that was created during the injection procedure, transduction of the ONL was found in up to 40% of the retinal surface (data not shown). Occasional GFP-positive cells were also noted in the nerve fiber layer following subretinal injection. Closer examination of the photoreceptor cell bodies in the ONL showed POD-GFP distributed in a diffuse pattern throughout the cytoplasm, with some punctate fluorescence in the perinuclear space (Figure 3a, arrowheads). In contrast, GFP-fluorescence associated with injection of GFP protein was detectable in the subretinal space as expected, but was not significantly greater than background auto-fluorescence associated with uninjected eyes (Figure 3b). Furthermore, there was no evidence of GFP in any other part of the retina exceeding that of auto-fluorescence (Figure 3b). Total GFP-associated fluorescence intensity determined from two dimensional surface plots indicated a significant (p < 0.05) difference in the fluorescence intensity of the GFP-injected eyes relative to POD-GFP injected eyes (15.9 ±4.2 versus 37.4 ±4.4). The fluorescence intensity of uninjected eyes was 18.3 ±3.6 and not significantly different from GFP injected eyes (Figure 3c).
Figure 3. Transduction Properties of POD-GFP following subretinal injection in vivo.
POD-GFP or GFP protein was injected into the subretinal space of adult mice. POD-GFP entered the RPE and photoreceptor cell bodies in the ONL (a) and localized to photoreceptor cell body in a perinuclear, punctate and cytoplasmic manner (arrowheads). Surface plots of 40× retina indicate relative GFP-fluorescence units across the tissue. GFP-associated signal in GFP-injected eyes was not significantly above background auto fluorescence associated with uninjected eyes, except in the subretinal space as expected (b). Total fluorescence intensity associated with each experiment is quantified in (c). Lysosomal marker IDUA (red) indicated that POD-GFP (green) may not be sequestered in the lysosomes in vivo (d). ONL, Outer nuclear Layer; INL, Inner Nuclear Layer; GCL, Ganglion Cell Layer; RPE, Retinal Pigment Epithelium. Uninjected, n=3, GFP and POD-GFP, n = 4.
To investigate the potential for POD-GFP to escape endosomes in vivo, retinal sections were stained with an antibody to α-L-iduronidase (IDUA), an enzyme involved in the catabolism of glycosoaminoglycans (GAGs) and known to localize to lysosomes (von Figura & Weber, 1978). IDUA was localized almost exclusively in a punctate pattern in the perinuclear region of the photoreceptor cell bodies, consistent with a lysosomal localization (Figure 3d). In contrast, POD-GFP was dispersed throughout the cell body of the photoreceptors in the ONL, strongly suggestive of its ability to escape the endosomal compartment.
A total of 6.6 μg purified POD-GFP or GFP protein was injected into the intravitreal space, i.e. between the lens and neural retina of 6 week-old male C57BL6/J mice and eyes were harvested after 6 hours and frozen sections examined by fluorescence microscopy. POD-GFP could be detected in ganglion cells (Figure 4a) and in a subpopulation of cells in the inner nuclear layer (INL) (Figure 4a, arrowhead). In contrast to subretinal injection, there was no POD-GFP signal detectable in the ONL or RPE beyond that of background auto-fluorescence (Figure 4a) and the overall number of GFP-positive cells was significantly less than that achieved by subretinal injection. Closer examination of the inner retina revealed that a large number of dendrites also contained POD-GFP (Figure 4a, arrow). Following intravitreal injection, GFP-fluorescence associated with injection of GFP protein was not greater than autofluorescence associated with uninjected eyes (Figure 4b). Quantitation of the areas of transduction by compiling data from two-dimensional surface plots indicated that the GFP fluorescence intensity was 20.8±2.9 and 21.4±2.5 arbitrary units for uninjected and GFP-injected eyes respectively. In contrast, the GFP-fluorescence intensity associated with POD-GFP injected eyes was 54.5±7.6, a significant (p < 0.01) increase over both the GFP and uninjected eyes (Figure 4c).
Figure 4. Transduction Properties of POD-GFP following intravitreal injection in vivo.
POD-GFP or GFP protein was injected into the intravitreal space of adult mice and eyes harvested after 6 hours. POD-GFP was detected (a) in the ganglion cells and a subset of cells in the inner nuclear layer (INL) (arrowhead) and dendrites in the inner retina (arrow). Significant GFP-associated fluorescence could not be detected above background auto fluorescence associated with uninjected or GFP-injected eyes (b). Total GFP intensity associated with each experiment is quantified in (c). Apparent GFP-signal in uninjected eyes is auto fluorescence of the outer segments (OS), which was not typically observed in GFP-injected eyes. Significant binding of POD-GFP to the lens capsule was noted (d). ONL, Outer nuclear Layer; INL, Inner Nuclear Layer; GCL, Ganglion Cell Layer; RPE, Retinal Pigment Epithelium. Uninjected, n = 3, GFP and POD-GFP, n = 4. Color version of this figure appears online.
Following intravitreal injection, we noted that the lens capsule had a very strong affinity for POD-GFP but not for GFP (Figure 4d). The strong affinity of POD-GFP for the lens may have reduced the amount of protein available for retinal transduction. This may account in part for the poorer transduction of the neural retina (in terms of number of cells) via the intravitreal route relative to the subretinal route. Our efforts to inject higher amounts of protein into the vitreous were thwarted by the limited volume of the mouse vitreous and a propensity for POD-GFP to aggregate at high concentrations. Hence, we removed the lens and vitreous and examined the uptake of POD-GFP or GFP when administered directly into eyecups ex vivo.
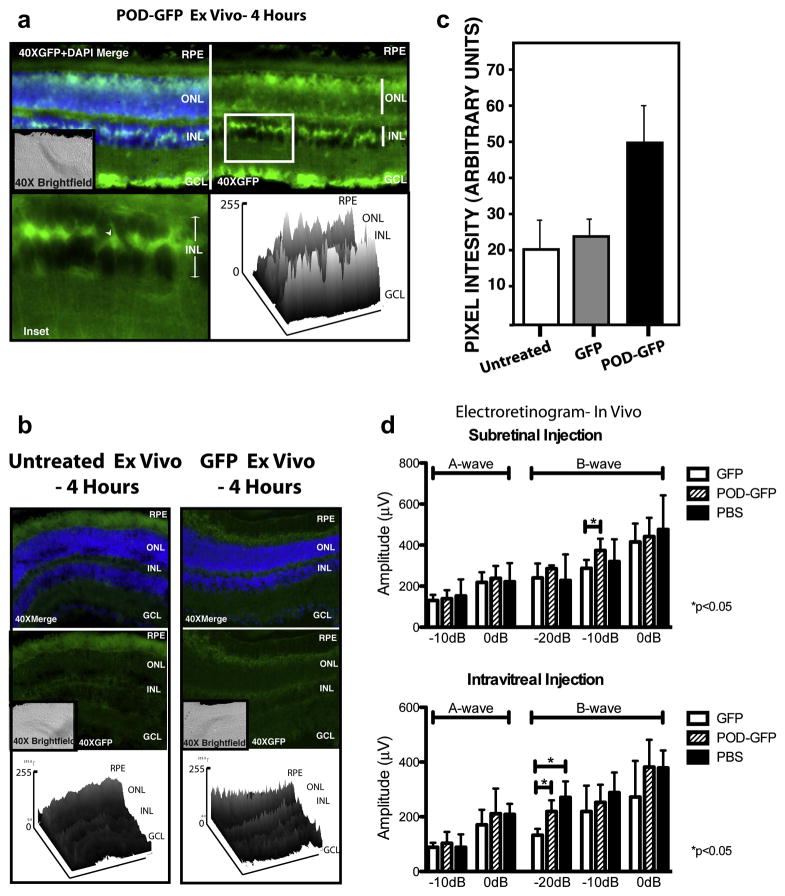
The posterior chamber of the mouse eye was incubated for 4 hours ex vivo with 8.5 μg of POD-GFP or GFP prior to fixing and sectioning. GFP-associated signal was detected in the ganglion cell layer (GCL), a subset of cells in the INL (Figure 5a, arrowhead) and a relatively weak GFP signal in the ONL of the POD-GFP-treated eyes (Figure 5a). There appeared to be a gradient of GFP expression with the most robust signal located in the GCL and diminishing towards the ONL (Figure 5a). No significant GFP-associated signal was observed in GFP or untreated eyecups (Figure 5b). Quantitative analysis from two-dimensional surface plots revealed that the fluorescence intensity of the POD-GFP eyes was significantly greater than GFP treated eyes (Figure 5c); 49.2 ±10.6 versus 23.5 ±2.7, (p < 0.01) suggestive of an effective POD-mediated uptake of GFP ex vivo.
Figure 5. Transduction properties of POD-GFP ex vivo and electroretinograms of POD-GFP injected eyes in vivo.
POD-GFP or GFP protein was placed in the eyecups of adult mice for 4 hours. Significant levels of GFP were detected in the GCL, a subset of cells in the INL (arrowhead) and potentially the ONL (a). Relatively lower levels of GFP were detected in the eyecups of GFP-injected or uninjected animals (b), as confirmed in the accompanying surface plots. (c) Total GFP-intensity for each fusion protein. POD-GFP, GFP n=4, untreated, n=3. Electroretinograms (ERGs) were performed 48 hours following subretinal or intravitreal injections of POD-GFP, GFP, or PBS (d). Graphical representation of the amplitude of A and B-waves at the indicated flash intensities following subretinal injection or intravitreal injection indicate no significant difference (p > 0.05) between POD-GFP and PBS for all light intensities examined. * p < 0.05. n=4 for each protein. GCL, ganglion cell layer, INL, inner nuclear layer, ONL, outer nuclear layer, RPE, retinal pigment epithelium. Color version of this figure appears online.
In summary, we conclude that POD-GFP delivered to the subretinal space transduces the RPE and photoreceptor cells. In contrast, delivery of POD-GFP to the intravitreal space transduces the ganglion cells and a limited number of cells in the INL, as well as the lens capsule.
POD-GFP does not cause functional toxicity in vivo
To examine the toxicity of POD-GFP or GFP in vivo, we injected 8.5μg of either protein into the subretinal space of adult mice. Similarly, 6.6μg of either protein was injected intravitreally, followed by measurement of the electroretinogram (ERG) after 48 hours. As a negative control we examined the effects of PBS injected in the same volumes used for POD-GFP or GFP. A and B-wave amplitudes (Figure 5d) indicated that there was no significant difference between the POD-GFP and the PBS under any of the conditions or injections tested (p>0.05). The GFP fusion protein showed a significant slight decrease in amplitude of the B-wave compared to POD-GFP by injection into the subretinal space at −10dB (p<0.05) (Figure 5d) and compared to both the POD-GFP and PBS intravitreal injections at −20dB (p<0.05) (Figure 5d). We conclude that the ERG is not significantly altered after 48 hours in POD-GFP injected eyes relative to GFP or PBS injected eyes.
Topical Application of POD-GFP to cornea in vivo
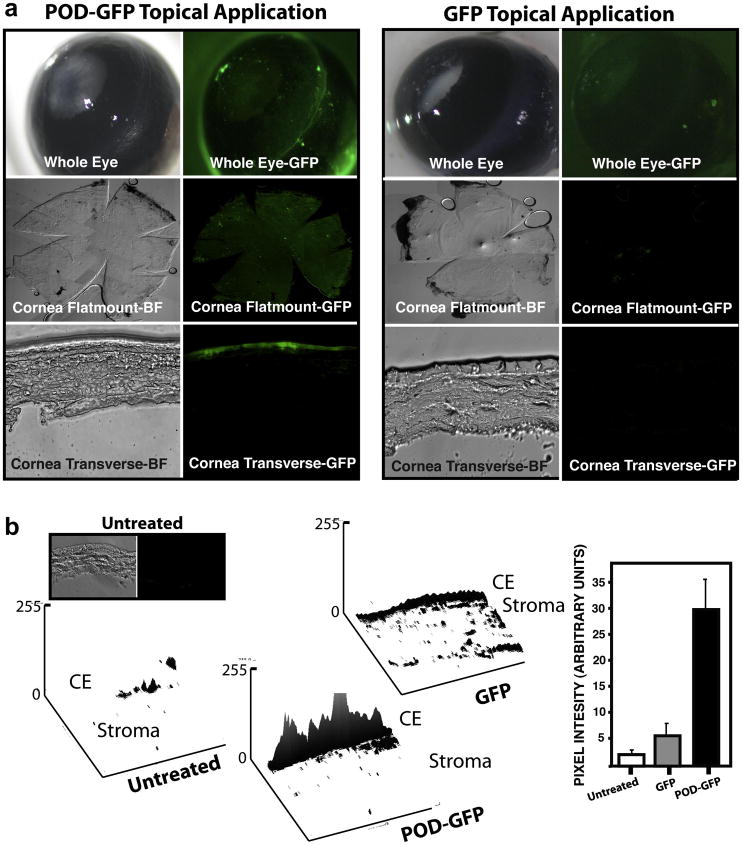
A total of 40μg purified POD-GFP or GFP protein was topically applied to the cornea of an anesthetized 6 week-old male C57BL6/J mouse and eyes harvested 45 minutes later for sectioning. Excitation of whole mouse eyes with 474 nm light revealed that whereas GFP was washed away and therefore absent from the outer surface of the eye, POD-GFP could be detected on most of the ocular surface (Figure 6a). To further examine localization of POD-GFP, the anterior ocular tissues were dissected and corneas flat mounted or cross-sectioned and re examined by fluorescence microscopy. Again, whereas GFP-fluorescence was not detectable in flat mounts that had been prepared from mice with topical application of GFP, GFP-fluorescence could be readily detected on both flat mounts and cross sections of corneas from mice that received topical application of POD-GFP (Figure 6b). Transverse sections revealed that the majority of the GFP-fluorescence associated with POD-GFP emanated from the corneal epithelium and minimally from the stroma (Figure 6a). No GFP-signal was detected in corneal cross-sections prepared from mice that received topical application of GFP. Quantitation using two-dimensional surface plots indicated substantially more GFP signal associated with tissue following treatment with POD-GFP (28.4 ±5.9) than with GFP (3.5 ±3.8) or no treatment (1.8 ±0.7) (Figure 6b). Again, the difference between GFP and untreated corneas was not significant (p > 0.5) whereas the difference between POD-GFP and the other treatments was significant (p < 0.01). Surface plots of corneal application indicated minimal auto fluorescence in the GFP or untreated eyes. The signal from the POD-GFP treatment varied in intensity throughout the epithelium but was generally the most intense at the center of the section (Figure 6). We conclude that topical application of POD-GFP to ocular tissues permits binding to the corneal epithelium.
Figure 6. Topical application of POD-GFP on murine cornea in vivo.
Recombinant POD-GFP or GFP proteins were applied to the eyes of anesthetized adult mice and eyes washed and harvested 45 minutes later. Whereas GFP does not substantially bind to ocular tissues and is not internalized, POD-GFP binds to the entire corneal surface and can be readily detected in corneal wholemounts. Transverse sections of cornea indicates that POD-GFP localizes to the corneal epithelium. Surface plots of cross-sections indicates that the majority of the signal emanates from the corneal epithelium (b). CE, Corneal Epithelium; Untreated, n = 3, PODGFP and GFP, n = 4. Color version of this figure appears online.
Topical Application of POD-GFP to skin in vivo
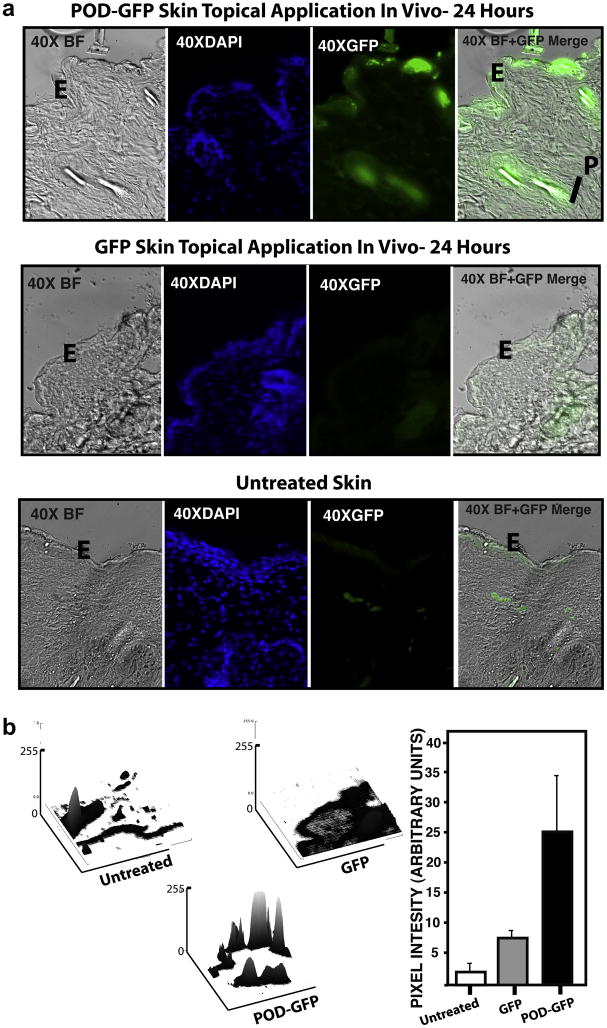
All of the above data and our previous report describing the properties of POD focused on retinal or ocular tissues. The success of the corneal topical application, as well as the potential of this mode of delivery for therapeutic proteins, prompted us to investigate this type of application to other tissues. To begin to extend these observations to other organ systems, we examined the potential of trans dermal delivery of POD-fusion protein. We topically applied 40μg of POD-GFP or GFP to the shaved skin of adult C57BL6/J mice. The animals were sacrificed 24 hours later and frozen sections of the treated region were examined by fluorescence microscopy (Figure 7a). We noted that hair follicles and hair within tissues was auto fluorescent and resulted in an overall GFP-fluorescence signal of 2.3 ±1.5 (Figure 7b). Similar to the untreated tissue, GFP-fluorescence associated with topical application of GFP was 6.6 ±3.3 (Figure 7b) whereas the epidermis of skin prepared from mice that received POD-GFP was significantly GFP-positive (23.4 ±8.8, p < 0.05) (Figure 7b). Uptake of GFP appeared punctate and diffuse but was almost exclusively associated with the epidermis and not the deeper layers of the skin. Binding was also observed around the hair follicle but this could not be definitively separated from auto fluorescence. We conclude that while GFP does not significantly bind to mouse skin, POD-GFP not only binds for at least 24 hours, but may also potentially be taken up by the epidermis. Surface plots of transdermal sections indicated that POD-GFP appeared to cluster at specific regions on the epidermis, resulting in very prominent peaks coupled with lower neighboring peaks, suggesting that the transduction, while effective, was not uniform (Figure 7b).
Figure 7. Topical Application of POD-GFP to shaved skin in vivo.
POD-GFP or GFP protein was applied to the skin for 24 hours. Whereas GFP does not bind skin beyond that of auto fluorescence, POD-GFP binds to the epidermis (a). Although hair (arrowhead) and papilla of the hair are also GFP-positive in sections exposed to POD-GFP, a significant portion of this signal is due to auto fluorescence (b). E, Epidermis; P, Papilla. Untreated, n = 3, POD-GFP and GFP, n = 4. Color version of this figure appears online.
DISCUSSION
Previously, we examined the potential use of HIV TAT (Cashman et al., 2003) and HSV VP22 (Cashman et al., 2002) in delivering recombinant GFP to retinal tissues in vivo. These studies were the first examples of the use of PTDs in the retina and amongst the first examples of the use of PTDs in vivo. We found that whereas both of these PTDs acted efficiently in vitro, their performance in vivo was limited. Hence, recently we turned our attention to identifying alternative PTDs that may have superior performance in neuronal tissues such as the retina in vivo. Through the use of molecular modeling, we previously designed and tested a novel cell and tissue penetrating peptide -POD (Johnson et al., 2007). Previously we demonstrated that POD could deliver inert molecules such as fluorophores and quantum dots across the plasma membrane of retinal neurons in vivo. The objective of the current study was to examine the potential of utilizing POD to deliver biologically relevant macromolecules such as proteins into retinal neurons including photoreceptors and ganglion cells, two principle target cells for retinal degeneration.
For proof-of-principle, we selected to deliver GFP- primarily because it is endogenously fluorescent and hence permits facile localization in vivo and also because it is not known to be associated with any facilitated transport systems. Furthermore, GFP is an unremarkable protein in terms of its molecular weight (~27 Kd), allowing us to reach some general conclusions regarding the relevance of our data to potentially therapeutic applications. Supporting our previous observations with the short POD peptide, we found that POD-GFP was internalized into cells and not simply membrane associated. We also observed co localization in part with late endosomes in vitro. POD-GFP did not co-localize with the lysosomal enzyme IDUA in vivo, indicating that POD-GFP was not sequestered in lysosomes at the time point measured here. This is a significant finding since endosomal/lysosomal escape is a critical obstacle for drug and/or DNA delivery by cell penetrating peptides. We were surprised to observe that endogenously expressed POD-GFP protein localized to the nucleus. Since the new observations presented here suggest that POD can also act as a nuclear localization signal, one may envisage use for such a protein transfer system that targets the nucleus for delivery of for example custom zinc finger nucleases for gene correction in vivo (Urnov, Miller, Lee, Beausejour, Rock, Augustus, Jamieson, Porteus, Gregory & Holmes, 2005) or other proteins whose function resides in the nucleus.
In the current study, we demonstrated delivery of a macromolecule to the RPE, retinal photoreceptors and neurons in the inner nuclear layer. To the best of our knowledge, this is the first demonstration of direct transduction of photoreceptors by a recombinant protein in vivo. Injection of the POD-GFP fusion protein into either the subretinal or intravitreal space did not result in any significant toxicity as measured by ERG. Transduction of photoreceptors with POD-fusion proteins may have potential applications in the treatment of diseases such as RP, Leber’s congenital amaurosis (CEP290) etc. whereas transduction of the RPE will have potential applications in the treatment of diseases such as age related macular degeneration (AMD). One may also envisage use of POD fusion proteins to achieve enhanced penetration of antibodies. For example, lucentis or avastin can block the activity of vascular endothelial growth factor (VEGF)- an approach found to be efficacious in the treatment of AMD (Pieramici & Avery, 2006). Perhaps a POD-lucentis or POD-avastin fusion protein or a POD-single chain antibody may be more efficacious than an antibody alone.
Intravitreal injection allowed targeting of the ganglion cells, degeneration of which is associated with glaucoma- one of the leading causes of blindness in the working population (Sample, Bosworth & Weinreb, 2000). Degeneration of ganglion cells is also a well-established model for testing CNS-targeted therapies. For example, in a model of traumatic injury in the CNS, optic nerve axotomy, TAT-GDNF reduced the number of activated caspase-3-positive cells and increased the survival of retinal ganglion cells relative to GDNF alone (Kilic, Kilic, Dietz & Bahr, 2004). Perhaps POD-GDNF fusion protein will have potential use in the treatment of disorders of the central nervous system (CNS). Efficacy of GDNF is limited in part due to the inability of this macromolecule to disperse in tissues (Salvatore, Ai, Fischer, Zhang, Grondin, Zhang, Gerhardt & Gash, 2006)- a problem that may potentially be addressed by use a POD-GDNF fusion protein.
We observed relatively strong binding of POD-GFP to the lens capsule. This is not surprising given that the lens capsule contains abundant levels of heparan sulphate proteoglycans (Rossi, Morita, Sormunen, Airenne, Kreivi, Wang, Fukai, Olsen, Tryggvason & Soininen, 2003, Winkler, Wirbelauer, Frank & Laqua, 2001), a putative receptor for POD (Johnson et al., 2007). Accordingly, studies performed ex vivo using murine eyecups indicated superior transduction of photoreceptor cell bodies in the absence of vitreous and lens.
Delivery of POD-GFP to the corneal epithelium represents a potential means to improve efficacy and delivery of drugs or proteins for the treatment of diseases of this tissue, including dry eye and infections such as Herpes Zoster. Delivery of drugs to ocular tissues via topical administration to the cornea is common practice, however, drainage of the majority of the drug occurs via the lacrimal ducts and hence reduces drug potency and activity. Prolonged binding of drugs to the ocular surface may increase their bioavailability, but this remains to be determined.
In the past we have speculated on the usefulness of POD and associated fusion proteins in areas beyond ophthalmology. Here we described a potential role for POD-mediated protein delivery to the integumentary system. Application of POD-GFP to the skin produced intriguing results that suggested POD fusion proteins might be useful in treating diseases of the skin. However, while POD-GFP successfully localized to the epidermis, it failed to penetrate into the dermal layer. Nonetheless, we are encouraged by these initial results because the binding and possible internalization of POD-GFP persisted for at least 24 hours. Additional studies will be necessary to determine if longer treatment times and multiple applications may result in deeper penetration of POD-GFP into the skin.
In summary, we have designed and constructed a POD fusion protein and shown, in support of our original hypothesis that a POD fusion protein may be an effective transducer of cells and ocular tissues in vivo. Importantly, we demonstrated localization of POD-GFP to the photoreceptor cell bodies and ganglion cells, paving the way for future studies to examine use of POD fusion proteins in animal models of retinal degeneration. Also, we showed for the first time that POD has applications outside of the eye by performing trans dermal topical application. The success of POD-GFP binding to the skin coupled with the promising results obtained from ocular injections suggest that there are numerous organ systems and diseases in which POD may be a useful delivery agent. Although initial studies are very promising, further studies will need to be performed to for example, achieve cellular targeting specificity and determine how long the delivered protein can remain in the target cell as well as more clearly define any toxicity associated with POD-GFP.
MATERIALS AND METHODS
Cell culture and transfection
Human embryonic retinoblast (HER) cells were cultured using previously described methods (Fallaux et al., 1996). Carcinoma human alveolar basal epithelial cells (A549, American Type Culture Collection, Manassas, VA) were grown to 70–80 % confluence in DMEM media supplemented with 10% fetal bovine serum (FBS). All experimental cell culture procedures were performed in DMEM supplemented with 2% FBS. Transfection of plasmids was carried out using 2:1 μl Lipofectamine: μg plasmid in Opti-MEM media. Forty-eight hours post-transfection, the cells were fixed and stained with 4′,6-diamidino-2-phenylindole (DAPI).
Plasmid and adenovirus construction
Oligonucleotides POD-upper and POD-lower coding for the POD protein transduction domain were cloned into SacI/EcoRI digested pQBI25fA1 (Qbiogene, Carlsbad, CA) to generate pPODGFP. pPODGFPHis and pGFPHis were cloned by inserting the oligonucleotides His-upper and His-lower into ClaI/MluI digested pPODGFP and pQBI25fA1, respectively. pPODGFPHis and pGFPHis were digested with BglII and XmnI and ligated with BglII/EcoRV-digested pShuttle. The corresponding pShuttle plasmids were linearized with PmeI, gel purified, and cotransfected with pAdEasy-1into E. coli BJ5183 cells. Recombinant plasmids were digested with PacI and transfected into HER cells. AdPODGFPHis and AdGFPHis were isolated using a virus purification kit (Puresyn, Malvern, PA).
POD-upper: GCCACCATGGCTCGTAAGAAGGCTGCTAAGGCTGCCCGCAAGAAGGCTGCCAAGGCCGCACGAAAGAAGGC AGCAAAGGCGGCTCGTAAGAAGGCTGCCAAGGCGTC
POD-lower: AATTGACGCCTTGGCAGCCTTCTTACGAGCCGCCTTTGCTGCCTTCTTTCGTGCGGCCTTGGCAGCCTTCT TGCGGGCAGCCTTAGCAGCCTTCTTACGAGCCATGGTGGCGC
His tag-upper: CGATCATCATCACCATCACCATTGA
His tag-lower: CGCGTCAATGGTGATGGTGATGATGAT
POD and His tag sequences are indicated in bold.
Protein isolation and purification
HER cells were infected with either AdPODGFPHis or AdGFPHis at a multiplicity of infection (MOI) of 200 viral particles/cell. Cells were harvested when greater than 90% of cells were GFP-positive as determined by fluorescence microscopy which occurred approximately 96 hours post-infection. The cytoplasm and nucleus were isolated and lysed for protein purification using the NuCLEAR Extraction Kit (Sigma, St. Louis, MO). Fusion proteins were isolated using the His-Select kit (Sigma) and 250 mM imidazole according to manufacturer’s instructions. The final protein eluates were dialyzed in PBS using a 10K Ultrafree column (Millipore, Billerica, MA). Protein concentrations were measured using a Bradford assay (Bio-Rad, Hercules, CA). To eliminate the potential of virus contamination in the protein prep, fusion proteins were heated to 70°C for five minutes to inactivate any residual virus and tested for cytopathic effect in HER cells.
Cellular uptake and localization of PODGFPHis
To evaluate cellular penetration of POD fusion proteins, HER cells were seeded on Lab Tek-II chamber slides and incubated with 10 μg of POD-GFP or GFP for two hours. Following fixation, cells were visualized by light and fluorescence microscopy using an Olympus BX51 microscope with differential interference contrast (DIC) and the appropriate fluorescence filters. Images were captured using a Retiga 2000R FAST camera and QCapture Pro 5.0 (QImaging, British Columbia, Canada). To quantitate protein uptake, 0.2×106 of either HER or A549 cells were incubated with 10 μg POD-GFP or GFP for 2 hours. Cells were washed with PBS, pelleted and resuspended in PBS for FACS analysis. Protein internalization was differentiated from membrane binding as described previously (Johnson et al., 2007). Briefly, HER cells were treated with POD-GFP as above followed by trypsin (2.5 mg/ml) for 12 minutes prior to FACS analysis. Cell membrane integrity was evaluated by measuring the percentage of Propidium Iodide (1 μM) positive cells in the presence of fusion protein. FACS analysis of protein uptake, internalization and toxicity was performed using a FACSCAlibur (Becton Dickinson, Franklin Lakes, NJ) and results analyzed using CellQuest Pro (Becton Dickinson). 10,000 events were counted for each experiment, which was performed in triplicate and repeated.
HER and A549 cells were treated as above before the addition of LysoTracker, ER Tracker (Molecular Probes, Carlsbad, CA), or DAPI for one hour. These reagents were removed and cells fixed with formalin for imaging.
Silver staining and Western blotting
1 μg of POD-GFP and 250 ng of recombinant GFP were loaded onto a 12 % Tris-HCl gel (Bio-Rad). Silver staining was carried out using Silver Stain Plus Kit (BioRad, Hercules, CA). For Western blotting, proteins were transferred to PVDF membranes, which were probed for GFP expression using anti-AFP 5001 11E5 monoclonal antibody (MP Biomedicals, Solon, OH) and HRP-conjugated goat anti-mouse secondary antibody. The signal was detected using SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL)
Delivery of PODGFPHis to ocular tissues ex vivo and in vivo
The use of animals in this study was in accordance with the ARVO Statement for the Use of Animals in Opthalmic and Vision Research. Ex vivo applications were performed as described previously (Johnson et al., 2007). Briefly, C57BL/6 mice were sacrificed and the eyes were enucleated. The cornea and lens were removed and the posterior chamber was washed with PBS prior to incubation with 8.5 μg of PODGFPHis or GFPHis in 2% DMEM for 4 hours at 37°C. Eyes were washed with PBS, fixed in 4% paraformadehyde and prepared for sectioning.
Subretinal and intravitreal injections of the fusion proteins were performed using previously described techniques (Cashman, Bowman, Christofferson & Kumar-Singh, 2006). Briefly, 8.5 μg of POD-GFP or 6.6 μg of GFP protein was injected into the subretinal or intravitreal space respectively, of 6–8 week old C57BL/6J mice. Four independent injections were performed and yielded nearly identical results. Six hours post-injection, mice were sacrificed by CO2 inhalation and cervical dislocation. For topical delivery of the PODGFPHis or GFPHis, C57BL/6J mice were anesthetized with xylazine/ketamine and 2 μl containing 40 μg of protein were dropped onto the corneal surface. Forty-five minutes following administration, mice were sacrificed as above and eyes enucleated, washed twice in PBS, and fixed overnight in 4% paraformaldehyde. Eyes were embedded in Optimal Cutting Temperature Compound (Sakura Finetek, Torrance, CA) and 14 μm sections were collected using a Microm 550 cryostat.
Immunocytochemistry
Lysosomal identification was carried out by staining eye sections with a rabbit polyclonal antibody to IDUA (BP13, BioMarin Pharmaceutical Inc.) at a dilution of 1:100. Sections were blocked with 5% non-fat milk in PBS with 0.25% Triton X-100 before overnight incubation at 4°C with the primary antibody. The sections were washed three times with PBS and incubated with a Cy3 goat anti-rabbit secondary antibody (Jackson ImmunoResearch) for 1.5 hours. The sections were again washed three times with PBS and allowed to dry prior to imaging. To examine antibody specificity and non-specific binding of the secondary, additional sections were treated as described above in the absence of primary antibody. No fluorescence was detected in these sections.
Electroretinography
Mice were injected with either the fusion proteins POD-GFP, GFP or an equal volume of PBS into the subretinal (8.5μg) or intravitreal (6.6μg) space and analyzed by electroretinography (ERG) 48-hours after injection. Mice were dark-adapted overnight, anaesthetized as described above and pupils dilated with 1% Tropicamide (Akorn, Inc., Lake Forest, IL). Scotopic ERGs were recorded at 3 different flash intensities (−20, −10 and 0 dB) using contact lens electrodes and the UTAS system with BigShot ganzfeld (LKC Technologies, Inc, Gaithersburg, MD). 5–10 flashes were averaged for the high-low intensities. A and B-waves were measured at −10 and 0dB and the B-wave at −20 dB. Significance was analyzed using a student’s t-test. For all conditions n=4, except in the intravitreal GFP fusion protein condition for which n=3.
Transdermal application
Adult mice were anesthetized as described above and the abdomen shaved to reveal a 2.25 cm2 area of skin. 40 μg of protein was applied to the skin for 24 hours. Upon completion of the treatment, animals were sacrificed and the treated skin dissected and fixed overnight in 4% paraformaldehyde and transverse sections were obtained as described above.
Quantitation of protein transduction
Images of untreated tissue or tissue treated with POD-GFP or GFP were captured as above. Untreated tissue was included to assess autofluorescence. Image J (National Institutes of Health) converts image pixels into brightness values, which are reported here for quantitation and comparison purposes. Identical regions of each image were measured for each application and plotted as arbitrary units of brightness. Significant differences in brightness were determined using the student’s t-test with a p-value of less than 0.05 being recorded as significant. Surface plots were generated using Image J.
Acknowledgments
We would like to thank Jay Duker and Elias Reichel (Department of Ophthalmology, Tufts University) for valuable discussions throughout the course of this study. This study was supported by funding from NIH/NEI (EY014991 & EY013887), The Foundation Fighting Blindness, The Ellison Foundation, Lions Eye Foundation and grants to the Department of Ophthalmology at Tufts University from Research To Prevent Blindness.
References
- Anderson DC, Nichols E, Manger R, Woodle D, Barry M, Fritzberg AR. Tumor cell retention of antibody Fab fragments is enhanced by an attached HIV TAT protein-derived peptide. Biochem Biophys Res Commun. 1993;194(2):876–884. doi: 10.1006/bbrc.1993.1903. [DOI] [PubMed] [Google Scholar]
- Barnett EM, Elangovan B, Bullok KE, Piwnica-Worms D. Selective cell uptake of modified Tat peptide-fluorophore conjugates in rat retina in ex vivo and in vivo models. Invest Ophthalmol Vis Sci. 2006;47(6):2589–2595. doi: 10.1167/iovs.05-1470. [DOI] [PubMed] [Google Scholar]
- Buch PK, MacLaren RE, Duran Y, Balaggan KS, MacNeil A, Schlichtenbrede FC, Smith AJ, Ali RR. In contrast to AAV-mediated Cntf expression, AAV-mediated Gdnf expression enhances gene replacement therapy in rodent models of retinal degeneration. Mol Ther. 2006;14(5):700–709. doi: 10.1016/j.ymthe.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Cashman SM, Bowman L, Christofferson J, Kumar-Singh R. Inhibition of choroidal neovascularization by adenovirus-mediated delivery of short hairpin RNAs targeting VEGF as a potential therapy for AMD. Invest Ophthalmol Vis Sci. 2006;47(8):3496–3504. doi: 10.1167/iovs.05-1610. [DOI] [PubMed] [Google Scholar]
- Cashman SM, Morris DJ, Kumar-Singh R. Evidence of protein transduction but not intercellular transport by proteins fused to HIV tat in retinal cell culture and in vivo. Mol Ther. 2003;8(1):130–142. doi: 10.1016/s1525-0016(03)00131-x. [DOI] [PubMed] [Google Scholar]
- Cashman SM, Sadowski SL, Morris DJ, Frederick J, Kumar-Singh R. Intercellular Trafficking of Adenovirus-Delivered HSV VP22 from the Retinal Pigment Epithelium to the Photoreceptors-Implications for Gene Therapy. Mol Ther. 2002;6(6):813–823. doi: 10.1006/mthe.2002.0806. [DOI] [PubMed] [Google Scholar]
- Dietz G, Kilic E, Bahr M. Inhibition of Neuronal Apoptosis in Vitro and in Vivo Using TAT-Mediated Protein Transduction. Mol Cell Neurosci. 2002;21(1):29. doi: 10.1006/mcne.2002.1165. [DOI] [PubMed] [Google Scholar]
- Donsante A, Miller DG, Li Y, Vogler C, Brunt EM, Russell DW, Sands MS. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317(5837):477. doi: 10.1126/science.1142658. [DOI] [PubMed] [Google Scholar]
- Elliott G, O’Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88(2):223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- Fallaux FJ, Kranenburg O, Cramer SJ, Houweling A, Van Ormondt H, Hoeben RC, van der Eb AJ. Characterization of 911: a new helper cell line for the titration and propagation of early region 1-deleted adenoviral vectors. Hum Gene Ther. 1996;7:215–222. doi: 10.1089/hum.1996.7.2-215. [DOI] [PubMed] [Google Scholar]
- Fawell S, Seery J, Daikh Y, Moore C, Chen LL, Pepinsky B, Barsoum J. Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci U S A. 1994;91(2):664–668. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55(6):1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- Green M, Loewenstein PM. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell. 1988;55(6):1179–1188. doi: 10.1016/0092-8674(88)90262-0. [DOI] [PubMed] [Google Scholar]
- Johnson LN, Cashman SM, Kumar-Singh R. Cell-penetrating Peptide for Enhanced Delivery of Nucleic Acids and Drugs to Ocular Tissues Including Retina and Cornea. Mol Ther. 2007;16(1):107–114. doi: 10.1038/sj.mt.6300324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliot A, Pernelle C, Deagostini-Bazin H, Prochiantz A. Antennapedia homeobox peptide regulates neural morphogenesis. Proc Natl Acad Sci U S A. 1991;88(5):1864–1868. doi: 10.1073/pnas.88.5.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliot AH, Triller A, Volovitch M, Pernelle C, Prochiantz A. alpha-2,8-Polysialic acid is the neuronal surface receptor of antennapedia homeobox peptide. New Biol. 1991;3(11):1121–1134. [PubMed] [Google Scholar]
- Kilic U, Kilic E, Dietz GP, Bahr M. The TAT protein transduction domain enhances the neuroprotective effect of glial-cell-line-derived neurotrophic factor after optic nerve transection. Neurodegener Dis. 2004;1(1):44–49. doi: 10.1159/000076669. [DOI] [PubMed] [Google Scholar]
- Pieramici DJ, Avery RL. Ranibizumab: treatment in patients with neovascular age-related macular degeneration. Expert Opin Biol Ther. 2006;6(11):1237–1245. doi: 10.1517/14712598.6.11.1237. [DOI] [PubMed] [Google Scholar]
- Rossi M, Morita H, Sormunen R, Airenne S, Kreivi M, Wang L, Fukai N, Olsen BR, Tryggvason K, Soininen R. Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. Embo J. 2003;22(2):236–245. doi: 10.1093/emboj/cdg019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore MF, Ai Y, Fischer B, Zhang AM, Grondin RC, Zhang Z, Gerhardt GA, Gash DM. Point source concentration of GDNF may explain failure of phase II clinical trial. Exp Neurol. 2006;202(2):497–505. doi: 10.1016/j.expneurol.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Sample PA, Bosworth CF, Weinreb RN. The loss of visual function in glaucoma. Semin Ophthalmol. 2000;15(4):182–193. doi: 10.3109/08820530009037870. [DOI] [PubMed] [Google Scholar]
- Sarra GM, Stephens C, de Alwis M, Bainbridge JW, Smith AJ, Thrasher AJ, Ali RR. Gene replacement therapy in the retinal degeneration slow (rds) mouse: the effect on retinal degeneration following partial transduction of the retina. Hum Mol Genet. 2001;10(21):2353–2361. doi: 10.1093/hmg/10.21.2353. [DOI] [PubMed] [Google Scholar]
- Schorderet DF, Manzi V, Canola K, Bonny C, Arsenijevic Y, Munier FL, Maurer F. D-TAT transporter as an ocular peptide delivery system. Clin Experiment Ophthalmol. 2005;33(6):628–635. doi: 10.1111/j.1442-9071.2005.01108.x. [DOI] [PubMed] [Google Scholar]
- Sieving PA, Caruso RC, Tao W, Coleman HR, Thompson DJ, Fullmer KR, Bush RA. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci U S A. 2006;103(10):3896–3901. doi: 10.1073/pnas.0600236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E, Wang Q, Quiambao AB, Xu X, Qtaishat NM, Peachey NS, Lem J, Fliesler SJ, Pepperberg DR, Naash MI, Al-Ubaidi MR. The relationship between opsin overexpression and photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2001;42(3):589–600. [PubMed] [Google Scholar]
- Urnov FD, Miller JC, Lee YL, Beausejour CM, Rock JM, Augustus S, Jamieson AC, Porteus MH, Gregory PD, Holmes MC. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature. 2005;435(7042):646–651. doi: 10.1038/nature03556. [DOI] [PubMed] [Google Scholar]
- Vives E, Schmidt J, Pelegrin A. Cell-penetrating and cell-targeting peptides in drug delivery. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbcan.2008.03.001. [DOI] [PubMed] [Google Scholar]
- von Figura K, Weber E. An alternative hypothesis of cellular transport of lysosomal enzymes in fibroblasts. Effect of inhibitors of lysosomal enzyme endocytosis on intra- and extra-cellular lysosomal enzyme activities. Biochem J. 1978;176(3):943–950. doi: 10.1042/bj1760943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler J, Wirbelauer C, Frank V, Laqua H. Quantitative distribution of glycosaminoglycans in young and senile (cataractous) anterior lens capsules. Exp Eye Res. 2001;72(3):311–318. doi: 10.1006/exer.2000.0952. [DOI] [PubMed] [Google Scholar]