Abstract
Purpose
Although most children with B-lineage acute lymphoblastic leukemia (ALL) and non-Hodgkin lymphoma (NHL) are cured, new agents are needed to overcome drug resistance and reduce toxicities of chemotherapy. We hypothesized that the novel anti-CD22 immunotoxin RFB4(dsFv)-PE38 (BL22, CAT-3888) would be active and have limited non-specific side effects in children with CD22-expressing hematologic malignancies. We conducted the first pre-clinical and Phase I clinical studies of BL22 in that setting.
Experimental Design
Lymphoblasts from children with B-lineage ALL were assessed for CD22 expression by flow cytometry and for BL22 sensitivity by in vitro cytotoxicity assay. BL22 was evaluated in a human ALL murine xenograft model. A Phase I clinical trial was conducted for pediatric subjects with CD22+ ALL and NHL.
Results
All samples screened were CD22+. BL22 was cytotoxic to blasts in vitro (median IC50 9.8 ng/mL) and prolonged leukemia free survival of murine xenografts. Phase I trial cohorts were treated at escalating doses and schedules ranging from 10 to 40 µg/kg every other day × 3 to 6 doses repeated every 21 to 28 days. Treatment was associated with an acceptable safety profile, adverse events were rapidly reversible, and no maximum tolerated dose was defined. Pharmacokinetics were influenced by disease burden consistent with rapid drug binding by CD22+ blasts. Although no responses were observed, transient clinical activity was seen in most subjects.
Conclusions
CD22 represents an excellent target and anti-CD22 immunotoxins offer therapeutic promise in B-lineage hematologic malignancies of childhood.
Keywords: acute lymphoblastic leukemia, non-Hodgkin lymphoma, childhood cancer, CD22, immunotoxin
Introduction
There has been great progress in the curative treatment of hematologic malignancies in childhood (1). Acute lymphoblastic leukemia (ALL), the most common pediatric cancer, is highly curable and 80% of children with B-precursor ALL (pre-B ALL) achieve long term relapse free survival (2). However, the outlook remains guarded for individuals with certain high-risk features at diagnosis and for those who relapse and hematologic malignancies remain a leading cause of cancer-related mortality in pediatrics (3, 4). Additionally, current therapies carry risks of treatment-associated morbidity and mortality (5, 6). Thus, novel approaches that can overcome chemotherapy resistance and decrease non-specific toxicities are needed to improve the outcome for children with hematologic malignancies.
CD22 is a B-lineage restricted surface molecule that modulates B cell receptor signaling and mediates cellular adhesion (7). Immunotoxins are proteins that consist of two primary components: a targeting moiety responsible for cell binding, and a bacterial or plant toxin that induces cell death upon internalization (8). The recombinant immunotoxin RFB4(dsFv)-PE38 (BL22, CAT-3888) contains the variable domains of the anti-CD22 monoclonal antibody (MoAb) RFB4 fused to a 38 kDa fragment of Pseudomonas aeruginosa exotoxin A (PE) (9, 10). BL22 is cytotoxic towards CD22+ cell lines and malignant cells from patients, and it is active in murine xenograft models (11–13). In Phase I and II human clinical trials, BL22 induced complete remissions in adults with hairy cell leukemia resistant to purine analog therapy and exhibited a safety profile conducive to continued development (14–16). We hypothesized that this novel anti-CD22 immunotoxin would be active and have limited non-specific side effects in children with CD22-expressing hematologic malignancies. We conducted the first pre-clinical studies and Phase I clinical trial of BL22 for pediatric ALL and non-Hodgkin lymphoma (NHL).
Materials and Methods
Patient samples
Fresh bone marrow or peripheral blood blasts were collected from children with B-lineage ALL.
In vitro cytotoxicity
Seventy-two h in vitro cytotoxicity assays were performed using protein synthesis inhibition ([3H]-leucine incorporation) and colorimetric viability (WST-1). Results were expressed as the 50% inhibitory concentration (IC50) value (concentration of BL22 required to reduce viability/protein synthesis by 50% in comparison to untreated controls) as previously described (12).
Flow cytometry and antigen binding site determination
CD22 antigen expression and absolute peripheral blast counts were determined by flow cytometry. Antigen site density was quantified by determining the anti-CD22 antibody binding capacity per cell (17) using the BD Biosciences QuantiBRITE system for fluorescence quantitation.
Murine xenografts
Cells from the human ALL line EU-1 were used for xenograft studies. This cell line was established and authenticated as previously described (18) and phenotype was re-confirmed by serial flow cytometric analyses including at the time of the xenograft studies. EU-1 cells were injected by tail vein into 5-week-old female C.B-17 severe combined immunodeficient −/− mice (107 cells/mouse). Seventy-two h after injection, cohorts of 10 (treatment) or 5 (control) xenografts were treated with BL22 at dose levels of 1.5 µg, 3 µg, or 4.5 µg/dose, or control agents via intraperitoneal injection every other day for 9 doses. Xenograft-recipients were euthanized at hind-limb paralysis and evaluated for the presence of human leukemia by histopathology.
BL22 and control agents
Recombinant immunotoxins were produced as previously described (11, 12, 19). Clinical grade BL22 for human use was produced by the Monoclonal Antibody and Recombinant Protein Facility of the NCI (Frederick, MD) and provided by MedImmune Cambridge, formerly Cambridge Antibody Technology, Inc, a subsidiary of MedImmune, LLC. Control reagents for pre-clinical studies included phosphate-buffered saline (PBS), anti-CD22 MoAb (RFB4-IgG provided by the Developmental Therapeutics Program of the NCI), and the anti-CD25-PE immunotoxin anti-Tac(Fv)-PE38 (LMB2).
Phase I clinical trial
A Phase I trial was conducted at the NIH Clinical Center, Bethesda, MD. (ClinicalTrials.gov number: NCT00077493)
Eligibility
Individuals between 6 mon and 24 yr of age with relapsed or refractory ALL or non-Hodgkin's lymphoma who had exhausted available curative therapies were eligible. Measurable or evaluable disease that was CD22+ by flow cytometry (>30%) or immunohistochemistry (>15%) was required. Subjects must have been off other investigational agents for at least 30 days and systemic chemotherapy for at least 14 days. Individuals with isolated testicular relapse and active central nervous system malignancy were excluded, but concurrent prophylactic intrathecal chemotherapy was permitted. Concurrent corticosteroids were allowed for patients who had been previously treated with such to reduce the likelihood of rapid disease progression during the time required to travel to the NIH and undergo eligibility screening. The doses of corticosteroids could not have been increased for at least 7 days prior to trial enrollment and patients were required to have persistent or progressive (i.e., not decreasing) disease burden. Eligibility required aspartate aminotransferase and alanine aminotransferase (ALT) ≤ 5-times the normal upper limit, total bilirubin ≤ 2 mg/dL, and age adjusted normal creatinine.
BL22 administration
BL22 was administered i.v. over 30 min every other day for 3 or 6 doses. I.V. hydration was initiated 6 h prior to BL22 using 5% dextrose 0.45% sodium chloride at a rate of 90 ml/m2/h. Pre-medication consisted of acetaminophen, diphenhydramine, and ranitidine.
Study design
Cohorts of 3 to 6 subjects were treated at doses of 10, 20, 25, 30, and 40 µg/kg/dose. During the course of the trial the escalation scheme was modified to shorten the treatment interval from 28 to 21 days and to increase the number of doses per cycle from 3 to 6 (Table 1). If dose-limiting toxicity (DLT) was encountered in 1 subject in a cohort of 3, an additional 3 patients were entered at that dose level. If DLT occurred in ≥ 2 patients at any given dose level, the maximum tolerated dose was considered to have been exceeded. Retreatment required recovery of BL22-related toxicity to < Grade 2 and the absence of DLT, high-titer neutralizing antibodies, and disease progression.
Table 1.
Subject characteristics
| ID | Dx | Age (years) |
Disease Status | Steroids | CD22 Expression |
CD22 Site Density (/cell) |
PB Blast Count (/µL) |
BM Blasts |
Cycles | Dose Level | Clinical Activity | Response | Nab | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre/ Peak |
Post/ Nadir |
Pre | Post | ||||||||||||
| 1 | ALL | 9 | Relapse #5, refractory 9 prior regimens |
100% | 6,478 | 43 | 17 | >90% | >90% | 1 | 1 (10 µg/kg QOD × 3 Q 28 days) |
PD | − | ||
| 2 | ALL/ LBL |
14 | Relapse #6, refractory 8 prior regimens, allo-SCT, extramedullary chloromas |
100% | 1,661 | 0 | 0 | <5% | <5% | 1 | 1 (10 µg/kg QOD × 3 Q 28 days) |
PD | − | ||
| 3 | ALL | 13 | Relapse #1, refractory 7 prior regimens |
+ | 100% | 4,341 | 2,401 | 3,160 | >90% | ND | 1 | 1 (10 µg/kg QOD × 3 Q 28 days) |
⇓ pain | PD | − |
| 4 | ALL | 22 | Relapse #1, refractory 4 prior regimens |
100% | 2,303 | 96,660 | 69,540 | >90% | ND | 1 | 2 (20 µg/kg QOD × 3 Q 28 days) |
⇓ PB blasts | PD | − | |
| 5 | ALL | 19 | Relapse #1, refractory 6 prior regimens |
100% | 1,835 | 61,661 | 26,358 | >90% | ND | 1 | 2 (20 µg/kg QOD × 3 Q 28 days) |
⇓ PB blasts | PD | − | |
| 6 | ALL | 9 | Relapse #2 2 prior regimens |
100% | 7,978 | 33 | 29 | 72% | 90% | 1 | 2 (20 µg/kg QOD × 3 Q 28 days) |
PD | − | ||
| 7 | ALL | 16 | Relapse #1, refractory 4 prior regimens |
+ | 100% | 5,978 | 27 | 4 | >60% | 63% | 1 | 3 (25 µg/kg QOD × 3 Q 28 days) |
PD (CNS) | + | |
| 8 | ALL | 3 | Relapse #1, refractory 4 prior regimens |
100% | 4,546 | 67,815 | 187,000 | >90% | ND | 1 | 3 (25 µg/kg QOD × 3 Q 28 days) |
PD | − | ||
| 9 | ALL | 17 | Relapse #4 6 prior regimens, allo-SCT × 2 |
100% | 1,448 | 876 | 401 | 79% | >90% | 1 | 3 (25 µg/kg QOD × 3 Q 28 days) |
⇓ PB blasts | PD | − | |
| 10 | LBL | 14 | Relapse #1, refractory 3 prior regimens |
100% | 3,856 | 4 | 3 | 30 – 70% |
23 – 90% |
3 | 4 (30 µg/kg QOD × 3 Q 28 days) |
Improved PET scan (skeletal sites), platelet count normalization |
No response ➔ PD (post#3) |
+ (post #3) |
|
| 11 | ALL | 5 | Relapse #2, refractory 6 prior regimens, allo-SCT |
+ | 100% | 3,053 | 5,568 | 37,474 | 78% | ND | 1 | 4 (30 µg/kg QOD × 3 Q 28 days) |
PD | − | |
| 12 | ALL | 16 | Relapse #1, refractory 4 prior regimens |
100% | 4,371 | 46,540 | 26,640 | >90% | ND | 1 | 4 (30 µg/kg QOD × 3 Q 28 days) |
⇓ PB blasts | PD | − | |
| 13 | BL | 17 | Refractory 4 prior regimens, auto-SCT |
100% | ND | 0 | 0 | <5% | ND | 1 | 5 (30 µg/kg QOD × 3 Q 21 days) |
⇓ pain | PD | − | |
| 14 | ALL | 4 | Relapse #2, refractory 5 prior regimens |
100% | 3,402 | 1 | 588 | >90% | 90% | 1 | 5 (30 µg/kg QOD × 3 Q 21 days) |
⇓ BM cellularity (50– 70%) |
PD | − | |
| 15 | ALL | 11 | Relapse #2, refractory 7 prior regimens |
+ | 75–82% | 5,640 | 34 | 2,385 | 25% | >90% | 1 | 6 (30 µg/kg QOD × 6 Q 21 days) |
PD | − | |
| 16 | ALL | 18 | Relapse #2, refractory 5 prior regimens |
+ | 100% | 9,380 | 12,880 | 33 | >90% | 60– 90% |
1 | 6 (30 µg/kg QOD × 6 Q 21 days) |
⇓ PB and BM blasts, BM cellularity (0 –85%) LDH, hepatosplenomegaly, renal infiltration |
No response |
+ |
| 17 | ALL | 19 | Relapse #2, refractory 4 prior regimens, allo-SCT |
+ | 100% | 7,070 | 276 | 2 | >90% | 30– 50% |
3 | 6 (30 µg/kg QOD × 6 Q 21 days) |
⇓ PB and BM blasts, uric acid, ⇑ platelet and reticulocyte counts |
No response | − |
| 18 | ALL | 4 | Relapse #1, refractory 5 prior regimens |
+ | 100% | 6,577 | 2 | 0 | >90% | Hypo >90% |
2 | 7 (40 µg/kg QOD × 6 Q 21 days) |
⇓ BM cellularity (5– 20%) splenomegaly, ⇑ neutrophil count |
No response |
− |
| 19 | ALL | 11 | Relapse #2, refractory 6 prior regimens |
+ | 100% | 4,721 | 164 | 136 | >90% | >90% | 1 (3 doses) |
7 (40 µg/kg QOD × 6 Q 21 days) |
⇓ BM cellularity (focal) |
No response |
− |
| 20 | ALL | 9 | Relapse #2, refractory 4 prior regimens |
100% | 7,549 | 6 | 2 | 80– 90% |
70– 80% |
2 | 7 (40 µg/kg QOD × 6 Q 21 days) |
⇓ BM blasts (focal), regenerating trilineage hematopoiesis, ANC and platelet count normalization |
No response ➔ PD (post#2) |
− | |
| 21 | ALL | 8 | Relapse #2, refractory 4 prior regimens |
100% | 3,849 | 988 | 268 | >90% | >90% | 1 | 7 (40 µg/kg QOD × 6 Q 21 days) |
⇓ PB blasts | PD | − | |
| 22 | ALL | 10 | Relapse #2, refractory 3 prior regimens |
100% | 731 | 22,735 | 200 | >90% | >90% | 2 | 7 (40 µg/kg QOD × 6 Q 21 days) |
⇓ PB blasts, LDH, splenomegaly, pain, testicular masses, adenopathy |
PD* | − | |
| 23 | ALL | 18 | Relapse #2 4 prior regimens, allo-SCT |
100% | 7,585 | 1,398 | 10 | >90% | >90% | 1 | 7 (40 µg/kg QOD × 6 Q 21 days) |
⇓ PB blasts | No response |
− | |
ALL: acute lymphoblastic leukemia; allo-SCT: allogeneic stem cell transplant; ANC: absolute neutrophil count; auto-SCT: autologous stem cell transplant BL: Burkitt lymphoma; BM: bone marrow; CNS: central nervous system; Dx: Diagnosis; LBL: lymphoblastic lymphoma; Hypo: hypocellular; ID: identification number; Nab: neutralizing antibody development; ND: not done; PB: peripheral blood; PD: progressive disease; Post: Post-treatment; Pre: Pre-treatment; Q: every; QOD: every other day.
Progressive extramedullary disease (renal, intraorbital masses) determined retrospectively after cycle #2.
Toxicity grading and definition
Version 3.0 of the NCI Common Terminology Criteria for Adverse Events1 was utilized for toxicity and adverse event reporting. DLT was defined as non-hematologic toxicity ≥ grade 3 with the following exceptions: tumor lysis syndrome, abnormal electrolytes responding to supplementation, Grade 3 hepatic dysfunction with resolution prior to the next cycle, and Grade 3 fever, hypertriglyceridemia, hypercholesterolemia, and hypoalbuminemia in the absence of vascular leak syndrome. Hematological DLT was defined as Grade 4 hematologic toxicity (with the exception of lymphocytes) lasting more than 5 days or any platelet transfusion. Subjects with abnormal blood counts due to bone marrow infiltration were not evaluable for hematologic toxicity.
Pharmacokinetics
Plasma levels of BL22 were determined by incubating dilutions of plasma with Raji cells and comparing cytotoxicity as assessed by [3H]-leucine incorporation to that obtained by a BL22 standard as previously described (15). Samples were obtained before, immediately after, and at ½, 1, 1½, 2, 4, 8, 12, and 18 h after each day 1 dose, and before and immediately after subsequent doses. Area under the curve was calculated on the first dose of the cycle from either a mono- or bi-exponential model based on Aikake’s rule (20). For biexponential kinetics, the β-half-life was used in analyses.
Neutralizing antibody assay
To assay for the presence of neutralizing antibodies, mixtures containing 90% serum and 10% BL22 (final BL22 concentration 1,000 ng/mL) were incubated at 37°C for 15 min, diluted, and cultured with Raji cells and the percent inhibition in the presence of subject serum was calculated as previously described (15). High-titer neutralizing antibodies were defined as levels that resulted in > 75% neutralization of 1,000 ng/mL of BL22 in this assay.
Response criteria
Standard disease specific clinical and laboratory response criteria were employed (21, 22).
Statistical analyses
For mice evaluated in pre-clinical studies, the probability of survival as a function of time, according to treatment administered, was determined by the Kaplan-Meier method with a log-rank test used to determine the difference of the probability of survival between the three pooled control groups and the combined BL22 treated groups. For the dose effect, pooled data from the two higher dose level groups were analyzed in comparison to the lowest dose. Results were not adjusted for multiple comparisons.
The relationship between clearance and peripheral blast count/µL in subjects treated on the clinical trial was determined using Spearman rank correlation, with |r| > 0.70 indicating a strong correlation, and the p-value indicating a result from a test of whether r = 0. Changes in peak BL22 level between first and last dose were evaluated using a paired t-test after verifying that the differences followed a normal probability distribution. Although patients received varying numbers of doses, the difference is meant to illustrate the effect associated with maximal dosing on the trial. All p-values are two-tailed and are reported without adjustment for multiple comparisons.
Human subjects protections
All studies were approved by the Investigational Review Boards of the NCI (pre-clinical studies, clinical trial NCT00077493) or Emory University School of Medicine (pre-clinical studies).
Results
CD22 expression
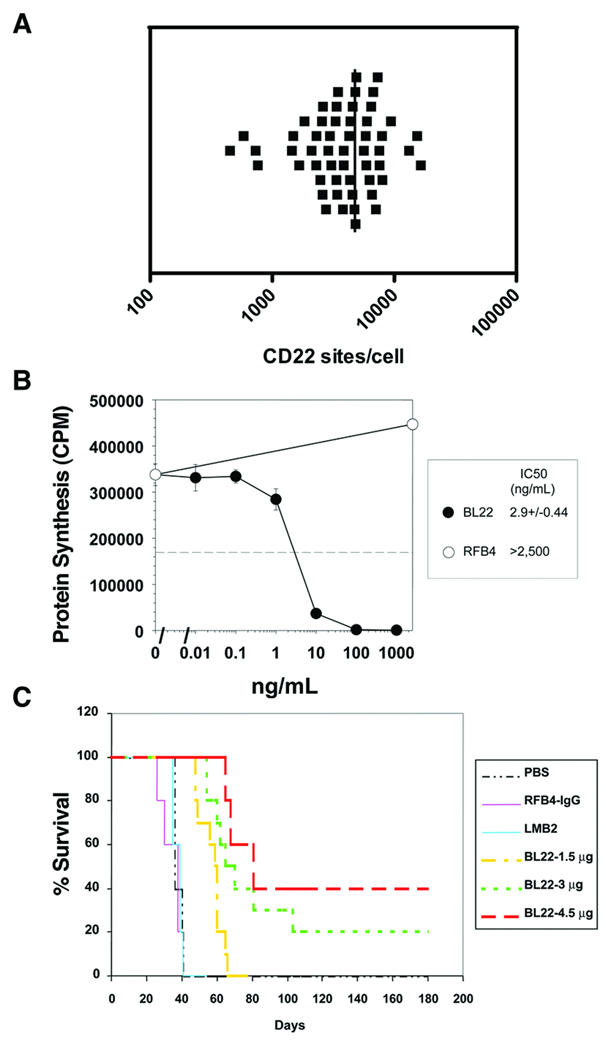
To assess the frequency of CD22 expression in B-lineage ALL, blasts from 93 children with pre-B or Burkitt-type ALL were evaluated by flow cytometry. All cases were CD22+. CD22 expression distribution and density were further quantified in 54 of these cases. One-hundred percent of the blasts within individual cases expressed CD22 in 52 of 54 cases. Minor populations of blasts without demonstrable CD22 expression were detected in two individuals (approximately 80% and 90% CD22+). Determination of antibody binding capacity per cell revealed that the average CD22 site density within cases ranged from 451 to 16,523 sites per blast (median, 4,062), and only four cases demonstrated less than 1,000 CD22 sites per cell (Fig. 1).
Fig. 1.
Activity of BL22 against CD22-expressing human ALL blasts. (A) CD22 was expressed by primary patient blasts. Anti-CD22 antibody binding capacity per cell quantified in 54 primary patient samples revealed an average CD22 density of 451 – 16,523 sites per cell (median, 4,062). (B) BL22 was cytotoxic against primary patient samples in vitro in a dose responsive manner. A representative cytotoxicity curve is presented. (C) Murine xenografts treated with BL22 had significant prolongation of leukemia free survival in a dose responsive fashion (p < 0.05). PBS, phosphate-buffered saline; RFB4, anti-CD22 MoAb; LMB2, anti-CD25-PE immunotoxin
Cytotoxicity assays
In vitro cytotoxicity assays were performed on blast samples obtained from 42 children with pre-B ALL (Fig. 1). BL22-induced killing was observed in all samples, with IC50’s that ranged from 0.5 to 100 ng/mL (median, 9.8).
Xenograft studies
Murine xenografts treated with BL22 had significant prolongation of leukemia free survival (Fig. 1). Treatment cohorts were analyzed against the controls (using grouped data for both), and differences (treatment vs. control) were significant (p < 0.001). To evaluate dose response, 3 and 4.5 µg cohorts were combined after determining that they had similar survival, and analyzed in comparison to the 1.5 µg cohort. The difference was significant (p < 0.05).
Phase I trial
Twenty-three subjects ranging in age from 3 to 22 yr (median, 13) were treated on the Phase I trial. Twenty had ALL with marrow relapse, and one each had ALL with extramedullary relapse, Stage 4 lymphoblastic lymphoma, and Burkitt lymphoma. All had been heavily pretreated having received a median of 4 prior regimens (range, 2 to 9) and 20 were refractory to chemotherapy at the time of enrollment (Table 1). Twenty-two subjects completed at least 1 cycle of BL22 and 1 subject at dose level 7 received 3 of 6 doses. All subjects were evaluable through the DLT evaluation period for the primary study endpoints (i.e., toxicity, pharmacokinetics, immunogenicity). During the course of the trial the treatment schedule was amended to escalate the dose intensity (Table 1) based on safety and activity data from preceding cohorts. Cohort 5 was terminated early in order to increase the number of doses administered per cycle from 3 to 6.
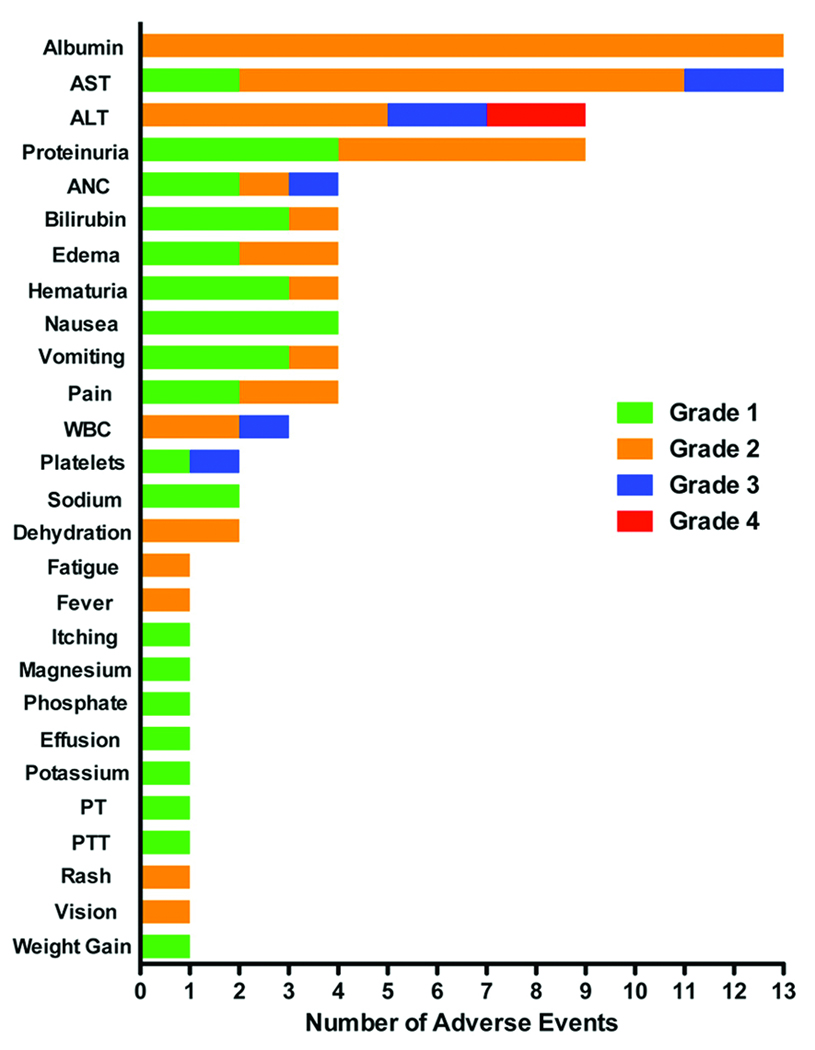
Toxicity
BL22 treatment was associated with an acceptable safety profile. No subject experienced infusion reactions, allergic events, vascular leak syndrome, or hemolytic uremic syndrome. All adverse events were self-limited and most were Grade 1 and 2 (Fig. 2). Grade 3 and 4 events were rapidly reversible. Two subjects treated at dose level 7 experienced Grade 4 ALT elevation of 1 to 2 days in duration, which met the original protocol definition of DLT. However, both of these resolved to levels required for ongoing treatment as scheduled supporting a revision in the DLT definition.
Fig. 2.
Adverse events attributed to BL22. Maximum toxicity grade per patient per cycle (N=23 subjects, 30 cycles). AST, aspartate aminotransferase; ALT, alanine aminotransferase; ANC, absolute neutrophil count; WBC, white blood count; PT, prothrombin time; PTT, partial thromboplastin time
Pharmacokinetics
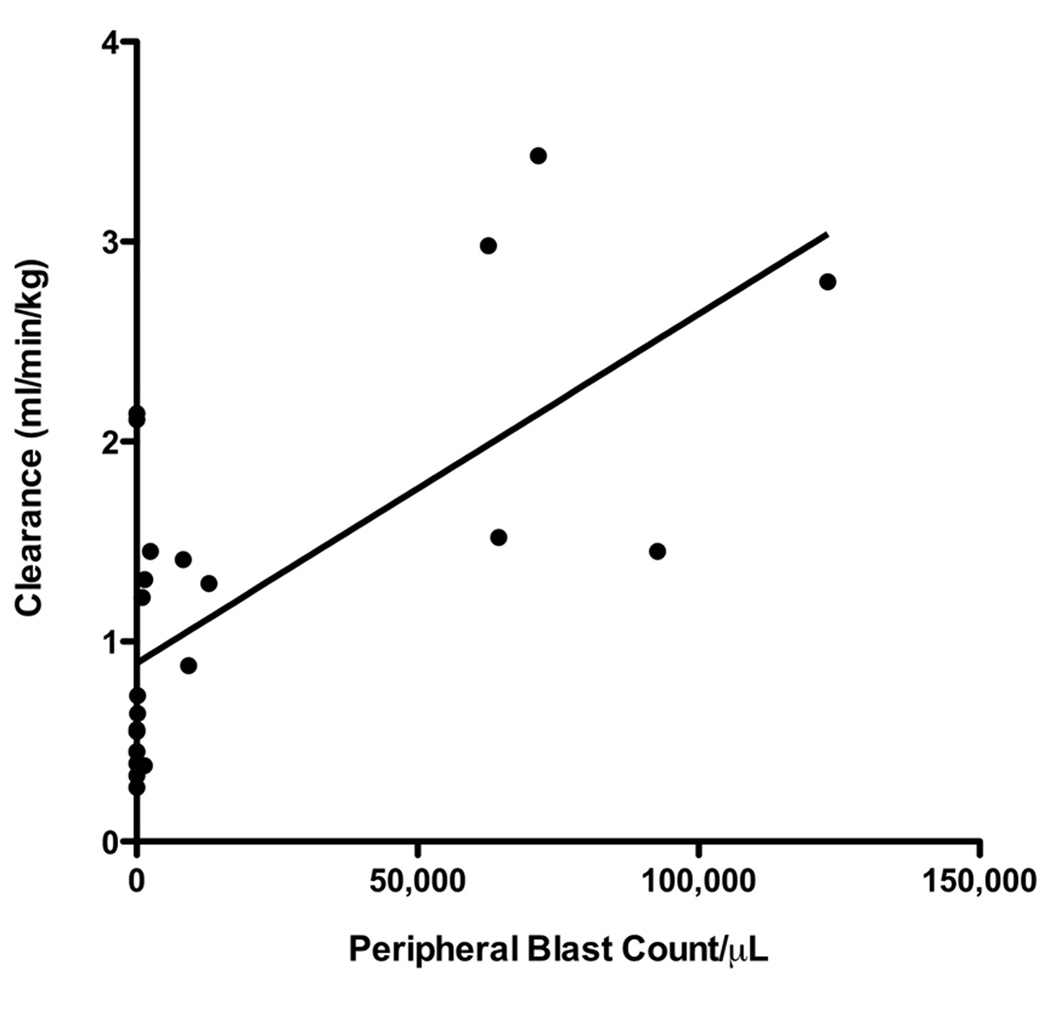
There was a dose related increase in the peak plasma levels of BL22 with wide inter-patient variation in pharmacokinetic parameters (Table 2). BL22 was rapidly cleared from circulation with a plasma half-life on cycle 1 that ranged from 35 to 248 min (median, 106). Cycle 1 clearance correlated with increased peripheral blast count (Spearman correlation r = 0.73, 95% confidence interval, 0.45 – 0.88; p < 0.0001) (Fig. 3). Peak plasma levels increased with progressive dosing in individuals who experienced blast reduction treated at the highest doses (30 and 40 µg/kg) (p = 0.01) (data not shown). This was in contrast to subjects without peripheral blast count reduction. Thus, pharmacokinetics appeared to be influenced by disease burden, consistent with rapid BL22 binding by CD22+ blasts.
Table 2.
Cycle 1 pharmacokinetic parameters
| Dose (µg/kg) |
Subjects | Peak Day 1 (ng/mL) |
T1/2 (minutes) |
AUC (mcg*min/mL) |
Vd (L/kg) |
Clearance (mL/min/kg) |
|---|---|---|---|---|---|---|
| 10 | 3 | 65 (61–107) | 68 (40–146) | 7 (4–22) | 0.12 (0.10–0.14) | 1.45 (0.45–2.11) |
| 20 | 3 | 139 (64–341) | 85 (35–120) | 11 (7–36) | 0.09 (0.02–0.14) | 2.13 (0.55–2.98) |
| 25 | 3 | 186 (112–413) | 110 (101–136) | 17 (12–66) | 0.06 (0.02–0.08) | 1.52 (0.38–2.14) |
| 30 | 8 | 356 (85–500) | 118 (43–248) | 52 (5–116) | 0.11 (0.03–0.28) | 0.60 (0.27–343) |
| 40 | 6 | 469 (287–589) | 108 (78–167) | 53 (26–84) | 0.10 (0.06–0.58) | 0.81 (0.39–1.31) |
All values represent median (range).
Fig. 3.
The impact of disease burden on BL22 pharmacokinetics. Cycle 1 BL22 clearance correlated with the absolute peripheral blood blast count (Spearman correlation r = 0.73, 95% confidence interval, 0.45 – 0.88; p < 0.0001)
Immunogenicity
Only 3 of 23 subjects (13%) developed neutralizing antibodies. One had pre-existing low-titer antibodies that increased to 93% after cycle 1. Two developed de novo antibodies with 78% neutralization after cycle 1 and 65% after cycle 3.
Clinical activity
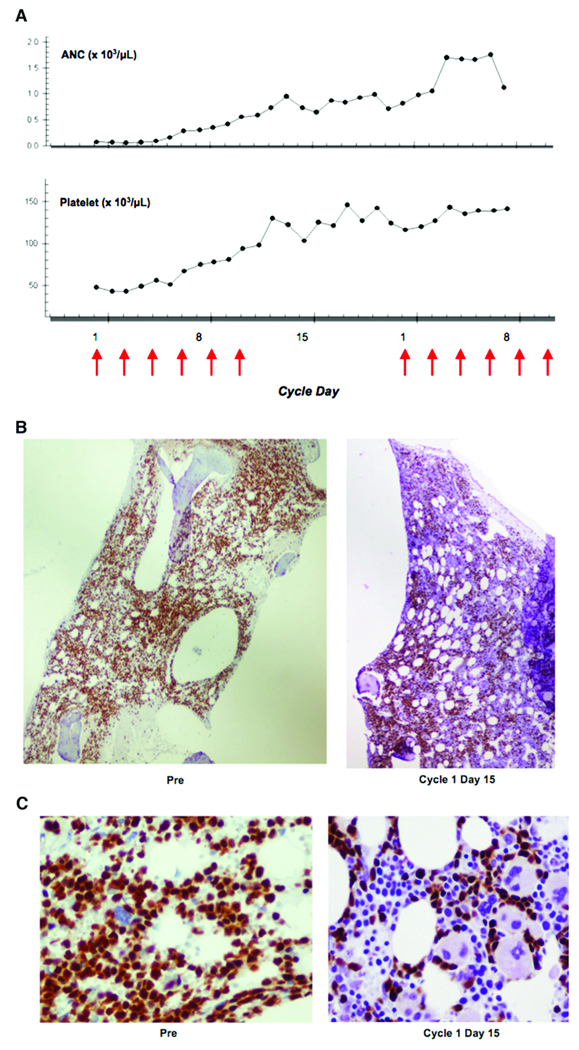
Most subjects had high disease burden and rapidly progressive disease at the time of protocol enrollment. No responses were observed, however, transient clinical activity was seen in 16 of 23 subjects (70%), which in some cases was dramatic and clinically significant (Table 1; Fig. 4). For example, four subjects had > 2-log10 reduction in circulating blasts and four had recovery of normal blood counts. Decreased blast infiltration of bone marrow [6] and extramedullary sites [3] was also observed. Notably, the most significant clinical activity was seen at the highest dose levels, including the four individuals with the largest reduction in peripheral blast counts (two each treated at dose levels 6 and 7) and all those with normalization of blood counts (treated at doses of 30 µg/kg or higher). Of the seven subjects without disease progression, two were ineligible to remain on the study (neutralizing antibodies, Grade 4 ALT elevation); two chose to discontinue treatment after 2 to 3 cycles; and three developed progressive disease after 2 to 3 cycles, one associated with the development of neutralizing antibodies.
Fig. 4.
Hematologic improvement after BL22. (A) Improvement in the absolute neutrophil count (ANC) and platelet count during therapy. Arrows represent BL22 treatment days. (B) [Olympus BX51 100× magnification] and (C) [Olympus BX51 1,000× oil magnification]: Bone marrow biopsies reveal decrease in blast infiltration and increase in normal hematopoietic precursors. TdT (terminal deoxynucleotidyl transferase) immunohistochemistry (blasts stain brown). (Subject #20)
Discussion
Despite the significant progress in the curative treatment of childhood hematologic malignancies, relapse remains one of the greatest challenges in pediatric oncology (3). Further, survivors have life-long risks of treatment-associated morbidity and mortality (6). New therapeutic approaches are needed to overcome chemotherapy resistance and to reduce side effects (23).
CD22 is rapidly internalized upon antibody or immunotoxin binding (24). As demonstrated, this antigen is expressed in high frequency in childhood ALL. Unconjugated MoAbs can induce cytotoxicity by direct and indirect (e.g., immune-mediated) mechanisms, the latter of which are expected to be defective in individuals with ALL (25). Unconjugated MoAb against CD22 (epratuzumab) has recently been studied in childhood ALL and its activity as a single agent in the setting of relapsed ALL appears to be limited (26). Notably, anti-RFB4 control showed no activity against ALL xenografts or in cytotoxicity assays with primary samples from children with ALL (Fig. 1).
The activity of MoAbs can be dramatically increased by linkage to toxic moieties. Plant and bacterial toxins cause cellular cytotoxicity via inhibition of protein synthesis after internalization. These are highly potent and active in minute quantities, such that even a single molecule in the cytoplasm is sufficient to kill a cell (27). There have been limited studies of immunotoxins in childhood hematologic malignancies and previously evaluated agents have been associated with severe adverse events and a high incidence of immunogenicity (28, 29). Clinical development of immunotoxins in general has been hampered by non-specific toxicities, immunogenicity, and production complexities. Serial modifications in the Pseudomonas-based immunotoxin constructs utilized at the NCI have reduced non-specific toxicities, increased stability, enhanced tissue penetration, and improved targeted cellular killing (30).
BL22 is a potent immunotoxin that targets CD22, which as shown, is expressed in relatively high density on the surface of 100% of the blasts in the vast majority (96%) of cases of childhood B-lineage ALL. BL22 was demonstrated to have clinical activity with acceptable toxicity in adults with relapsed and refractory hairy cell leukemia, where a maximum tolerated dose of 40 µg/kg every other day × 3 every 28 days was defined (15). This pediatric Phase I trial extends those observations and establishes that activity can be achieved in highly resistant childhood ALL with acceptable toxicity. Notably, BL22 was tolerated at greater dose intensity (i.e., every other day for 6 doses every 21 days) in comparison to adults, and hemolytic uremic syndrome, which was the DLT in adults, was not observed. Importantly, anti-leukemia activity was seen at all dose levels, however, clinical benefits in this highly refractory population were modest and transient at the doses tested. There are a number of possible explanations for the limited observed activity. Higher doses are likely required to achieve maximal benefit. Further, while peak levels at the upper doses exceeded concentrations required for in vitro cytotoxicity, drug exposure was limited in most subjects due to rapid clearance associated with large disease burden. CD22 expression has been demonstrated to be a determinant of response to BL22 in vitro (12), although there was no obvious influence of antigen density on clinical activity in this trial (Table 1). However, small numbers preclude definitive conclusions in this regard, and notably, all subjects without progressive disease had site densities that exceeded 3,000 sites/cell, whereas 6 of 16 with progressive disease had lower levels of expression.
This trial shows that BL22 can be administered at doses up to 40 µg/kg/dose every other day × 6 in children with ALL. No maximum tolerated dose was defined. Although two subjects treated at the highest dose level developed brief Grade 4 ALT elevations, this was not dose limiting given the short duration (1 to 2 days). We subsequently chose to close the trial and apply the schedule developed in this study (every other day × 6 every 21 days) to Phase I testing of a modified BL22 construct with higher affinity for the CD22 antigen. This second-generation agent, HA22 or CAT-8015, was engineered to replace three amino acid residues in the heavy chain complementary determining region 3 of the BL22 binding domain. This modification increased the binding affinity for CD22 by 14-fold, which resulted in approximately a 1-log10 improvement in cytotoxicity against a variety of CD22+ malignancies (31, 32).
In summary, CD22 represents an excellent target for pediatric B-lineage hematologic malignancies and these studies offer proof-of-principle that anti-CD22 Pseudomonas-based immunotoxins can be administered to children and have the potential to overcome chemotherapy resistance and induce cytotoxicity of CD22+ blasts refractory to standard therapy. Anti-CD22 immunotoxins hold therapeutic promise in this common subtype of pediatric cancer.
Statement of Translational Relevance
B-lineage hematologic malignancies remain a leading cause of cancer-related mortality in pediatrics and current therapies are associated with a wide array of toxicities. New agents are needed to overcome drug resistance and reduce non-specific adverse effects. We report results of the first pre-clinical studies and Phase I clinical trial of a novel anti-CD22 immunotoxin, RFB4(dsFv)-PE38, in the setting of childhood hematologic malignancies. An acceptable toxicity profile and transient clinical activity were observed. This Phase I clinical trial serves as proof-of-principle that this immunotoxin construct is cytotoxic to chemotherapy-resistant CD22+ blasts and that it can be administered to children in doses that achieve serum levels that exceed the expected in vitro IC50. The trial established a dose and schedule for subsequent testing of RFB4(dsFv)-PE38 with a modified Fv sequence that confers higher binding affinity for CD22. Future trials are planned in combination with standard chemotherapy agents.
Acknowledgements
We gratefully acknowledge the clinical trial participants and their families, referring physicians, David Waters, Ph.D., Lubing Gu, M.D., CURE Childhood Cancer, Inc., and the staffs of the NCI, NIH Clinical Center, Afla c Cancer Center and Blood Disorders Service, Emory University/Children's Healthcare of Atlanta, Genencor, Inc., and MedImmune, LLC.
Footnotes
Research Support: This research was supported in part by the Intramural Research Program of the National Institutes of Health (NIH), National Cancer Institute (NCI), Center for Cancer Research and a portion of this research was conducted through a Cooperative Research and Development Agreement between the NIH, NCI and MedImmune, LLC.
Disclosure of Potential Conflict of Interest
Robert J. Kreitman, David J. FitzGerald, and Ira Pastan are co-inventors on patents assigned to the NIH for the investigational product used in this research.
References
- 1.Pulte D, Gondos A, Brenner H. Trends in 5- and 10-year survival after diagnosis with childhood hematologic malignancies in the United States, 1990–2004. J Natl Cancer Inst. 2008;100:1301–1309. doi: 10.1093/jnci/djn276. [DOI] [PubMed] [Google Scholar]
- 2.Smith MA, Gloeckler Ries LA, Gurney JG, Ross JA. Reis LAG, Smith MA, Gurney JG, et al., editors. Leukemia. Bethesda: MD: Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995, National Cancer Institute, SEER Program, 1999. 1999:17–34. NIH Pub No 99-4649.
- 3.Gaynon PS. Childhood acute lymphoblastic leukaemia and relapse. Br J Haematol. 2005;131:579–587. doi: 10.1111/j.1365-2141.2005.05773.x. [DOI] [PubMed] [Google Scholar]
- 4.Gloeckler Ries LA. Reis LAG, Smith MA, Gurney JG, et al., editors. Childhood cancer mortality. Bethesda: MD: Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995, National Cancer Institute, SEER Program, 1999. 1999:165–170. NIH Pub No 99-4649.
- 5.Pui C-H, Cheng C, Leung W, et al. Extended follow-up of long-term survivors of childhood acute lymphoblastic leukemia. N Engl J Med. 2003;349:640–649. doi: 10.1056/NEJMoa035091. [DOI] [PubMed] [Google Scholar]
- 6.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 7.Nitschke L. CD22 and Siglec-G: B-cell inhibitory receptors with distinct functions. Immunol Rev. 2009;230:128–143. doi: 10.1111/j.1600-065X.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- 8.Pastan I, Hassan R, FitzGerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev. 2006;6:559–565. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 9.Li JL, Shen GL, Ghetie MA, et al. The epitope specificity and tissue reactivity of four murine monoclonal anti-CD22 antibodies. Cell Immunol. 1989;118:85–99. doi: 10.1016/0008-8749(89)90359-6. [DOI] [PubMed] [Google Scholar]
- 10.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin treatment of cancer. Annu Rev Med. 2007;58:221–237. doi: 10.1146/annurev.med.58.070605.115320. [DOI] [PubMed] [Google Scholar]
- 11.Mansfield E, Amlot P, Pastan I, FitzGerald D. Recombinant RFB4 immunotoxins exhibit potent cytotoxic activity for CD22-bearing cells and tumors. Blood. 1997;90:2020–2026. [PubMed] [Google Scholar]
- 12.Kreitman RJ, Margulies I, Stetler-Stevenson M, Wang QC, FitzGerald DJP, Pastan I. Cytotoxic activity of disulfide-stabilized recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) towards fresh malignant cells from patients with B-cell leukemias. Clin Cancer Res. 2000;6:1476–1487. [PubMed] [Google Scholar]
- 13.Kreitman RJ, Wang QC, FitzGerald DJP, Pastan I. Complete regression of human B-cell lymphoma xenografts in mice treated with recombinant anti-CD22 immunotoxin RFB4(dsFv)-PE38 at doses tolerated by cynomolgus monkeys. Int J Cancer. 1999;81:148–155. doi: 10.1002/(sici)1097-0215(19990331)81:1<148::aid-ijc24>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 14.Kreitman RJ, Wilson WH, Bergeron K, et al. Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N Eng l J Med. 2001;345:241–247. doi: 10.1056/NEJM200107263450402. [DOI] [PubMed] [Google Scholar]
- 15.Kreitman RJ, Squires DR, Steler-Stevenson M, et al. Phase I trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with B-cell malignancies. J Clin Oncol. 2005;23:6719–6729. doi: 10.1200/JCO.2005.11.437. [DOI] [PubMed] [Google Scholar]
- 16.Kreitman RJ, Stetler-Stevenson M, Margulies M, et al. Phase II trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with hairy cell leukemia. J Clin Oncol. 2009;27:2983–2990. doi: 10.1200/JCO.2008.20.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz A, Marti GE, Poon R, Gratama JW, Fernández-Repollet E. Standardizing flow cytometry: a classification system of fluorescence standards used for flow cytometry. Cytometry. 1998;33:106–114. [PubMed] [Google Scholar]
- 18.Zhou MX, Findley HW, Ma LH, et al. Effect of tumor necrosis factor-alpha on the proliferation of leukemic cells from children with B-cell precursor-acute lymphoblastic leukemia (BCP-ALL): studies of primary leukemic cells and BCP-ALL cell lines. Blood. 1991;77:2002–2007. [PubMed] [Google Scholar]
- 19.Pastan I, Beers R, Bera TK. Recombinant immunotoxins in the treatment of cancer. Methods Mol Biol. 2004;248:503–518. doi: 10.1385/1-59259-666-5:503. [DOI] [PubMed] [Google Scholar]
- 20.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19:716–723. [Google Scholar]
- 21.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 22.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 23.Wayne AS, Reaman G, Helman LJ. Progress in the curative treatment of childhood hematologic malignancies. J Natl Cancer Inst. 2008;100:1271–1273. doi: 10.1093/jnci/djn306. [DOI] [PubMed] [Google Scholar]
- 24.Du X, Beers R, Fitzgerald DJ, Pastan I. Differential cellular internalization of anti-CD19 and-CD22 immunotoxins results in different cytotoxic activity. Cancer Res. 2008;68:6300–6305. doi: 10.1158/0008-5472.CAN-08-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haining WN, Cardoso AA, Keczkemethy HL, et al. Failure to define window of time for autologous tumor vaccination in patients with newly diagnosed or relapsed acute lymphoblastic leukemia. Exp Hematol. 2005;33:286–294. doi: 10.1016/j.exphem.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Raetz EA, Cairo MS, Borowitz MJ, et al. Chemoimmunotherapy reinduction with epratuzumab in children with acute lymphoblastic leukemia in marrow relapse: a Children’s Oncology Group pilot study. J Clin Oncol. 2008;26:3756–3762. doi: 10.1200/JCO.2007.15.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaizumi M, Mekada E, Uchida T, Okada Y. One molecule of diphtheria toxin fragment A introduced into a cell can kill the cell. Cell. 1978;15:245–250. doi: 10.1016/0092-8674(78)90099-5. [DOI] [PubMed] [Google Scholar]
- 28.Dinndorf P, Krailo M, Liu-Mares W, Frierdich S, Sondel P, Reaman G. Phase I trial of anti-B4-blocked ricin in pediatric patients with leukemia and lymphoma. J Immunother. 2001;24:511–516. doi: 10.1097/00002371-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Herrera L, Bostrom B, Gore L, et al. A Phase I study of combotox in pediatric patients with refractory B-lineage acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2009 Oct 27; doi: 10.1097/MPH.0b013e3181bdf211. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Pastan I. Immunotoxins containing Pseudomonas exotoxin A: a short history. Cancer Immunol Immunother. 2003;52:338–341. doi: 10.1007/s00262-002-0353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salvatore G, Beers R, Margulies I, Kreitman RJ, Pastan I. Improved cytotoxic activity toward cell lines and fresh leukemia cells of a mutant anti-CD22 immunotoxin obtained by antibody phage display. Clin Cancer Res. 2002;8:995–1002. [PubMed] [Google Scholar]
- 32.Decker T, Oelsner M, Kreitman RJ, et al. Induction of caspase-dependent programmed cell death in B-cell chronic lymphocytic leukemia by anti-CD22 immunotoxins. Blood. 2004;103:2718–2726. doi: 10.1182/blood-2003-04-1317. [DOI] [PubMed] [Google Scholar]