Abstract
It is known that SPARC gates VEGF-A signal transduction towards KDR, the primary angiogenic VEGF receptor. We sought to determine whether inhibition of SPARC activity using anti-SPARC peptide could inhibit laser-induced CNV by promoting binding of VEGF-A to FLT-1. We created anti-SPARC L-peptide and retro-inverso anti-SPARC D-peptide. Anti-SPARC peptides or PBS were injected intravitreally one day before or after laser induction. Intravitreal injection of anti-SPARC L-peptide one day before laser induction promotes Flt-1 phosphorylation and inhibited laser-induced CNV and anti-SPARC D-peptide had no effect. Injection one day after laser injury did not affect size of laser-induced CNV. Inhibition of SPARC activity could be complementary to existing anti–CNV therapy.
Keywords: Age-related macular degeneration (AMD), Choroidal neovascularization (CNV), SPARC, FLT-1, VEGF
Introduction
Neovascularization, the abnormal growth of new blood vessels, plays an important role in many diseases, including cancer and blindness (Bellner, Vitto, Patil, Dunn, Regan & Laniado-Schwartzman, 2008, Epstein, Stulting, Hendricks & Harris, 1987, Folkman, 1990). In patients with age-related macular degeneration (AMD) choroidal neovascularization (CNV) is a principal cause of blindness (Smith, Assink, Klein, Mitchell, Klaver, Klein, Hofman, Jensen, Wang & de Jong, 2001). CNV grows from the choroid into the subretinal space. These blood vessels may bleed into the subretinal space resulting in edema and tissue damage. Vascular endothelial growth factor (VEGF) has been found to be a potent stimulator of CNV (Amin, Puklin & Frank, 1994, Ng & Adamis, 2005). FLT-1 (also referred as VEGF receptor-1) and KDR (also referred as VEGF receptor-2) are the principal VEGF receptors and are critically involved in neovascularization and angiogenesis (de Vries, Escobedo, Ueno, Houck, Ferrara & Williams, 1992, Matsumoto, Bohman, Dixelius, Berge, Dimberg, Magnusson, Wang, Wikner, Qi, Wernstedt, Wu, Bruheim, Mugishima, Mukhopadhyay, Spurkland & Claesson-Welsh, 2005, Shibuya, 1995, Shibuya, 2006, Shibuya & Claesson-Welsh, 2006). Generally, activation of KDR by VEGF promotes angiogenesis. On the other hand, FLT-1 signaling has been reported to both promote (Carmeliet, Moons, Luttun, Vincenti, Compernolle, De Mol, Wu, Bono, Devy, Beck, Scholz, Acker, DiPalma, Dewerchin, Noel, Stalmans, Barra, Blacher, Vandendriessche, Ponten, Eriksson, Plate, Foidart, Schaper, Charnock-Jones, Hicklin, Herbert, Collen & Persico, 2001, Hiratsuka, Maru, Okada, Seiki, Noda & Shibuya, 2001) and suppress angiogenesis (Bussolati, Dunk, Grohman, Kontos, Mason & Ahmed, 2001, Kearney, Ambler, Monaco, Johnson, Rapoport & Bautch, 2002), depending on the tissue and context. In addition, due to alternative splicing, the FLT-1 gene produces membrane-bound FLT-1 and soluble FLT-1 isoforms. Soluble FLT-1 has a partial extracellular domain of membrane FLT-1 and a unique C-terminal peptide. Soluble FLT-1 binds VEGF-A as an extracellular antagonist and blocks its signaling. It is known that soluble FLT-1 is vital to corneal avascularity (Ambati, Nozaki, Singh, Takeda, Jani, Suthar, Albuquerque, Richter, Sakurai, Newcomb, Kleinman, Caldwell, Lin, Ogura, Orecchia, Samuelson, Agnew, St Leger, Green, Mahasreshti, Curiel, Kwan, Marsh, Ikeda, Leiper, Collinson, Bogdanovich, Khurana, Shibuya, Baldwin, Ferrara, Gerber, De Falco, Witta, Baffi, Raisler & Ambati, 2006). While VEGF is critical to angiogenesis, VEGF overexpression alone does not induce CNV in mice (Oshima, Oshima, Nambu, Kachi, Hackett, Melia, Kaleko, Connelly, Esumi, Zack & Campochiaro, 2004). This indicates other cellular or extracellular components may play key roles in the development of CNV.
In this study, we focused on the role of SPARC (secreted protein, acidic and rich in cysteine; also known as BM-40 and osteonectin) in laser-induced CNV, a well-established model for age-related macular degeneration. SPARC belongs to the matricellular family that mediates cell-matrix interactions, which affect biological functions including proliferation, survival, adhesion and migration (Bornstein & Sage, 2002). SPARC binds to VEGF and inhibits tyrosine phosphorylation of FLT-1 but not KDR (Kupprion, Motamed & Sage, 1998). Nozaki et al. demonstrated reduced SPARC temporarily after laser injury in the choroid, creating a window of permissibility for VEGF binding to FLT-1. FLT-1 activation inhibits signal transduction of KDR through SHP-1 and can decrease laser-induced CNV volume (Nozaki, Sakurai, Raisler, Baffi, Witta, Ogura, Brekken, Sage, Ambati & Ambati, 2006). Based on these earlier studies we hypothesized inhibition of SPARC activity could reduce or prevent laser-induced CNV. Based on this hypothesis we created anti-SPARC peptides to bind SPARC by phage screening and examined the effect of anti-SPARC peptides on a laser-induced CNV model.
Methods
Phage screening for anti-SPARC peptide
The Ph.D.™ Phage Display Peptide Library Kit (New England Biolabs, Ipswich, USA) was used for in vitro biopanning procedures. This ligand screening system is based on a library of random peptide 7-mers fused to a minor coat protein, pIII, of the filamentous coliphage M13. Phage selection in vitro was performed according to the manufacture’s protocol. SPARC recombinant proteins were coated on 96-well plates in 0.1M NaHCO3 coating buffer and incubated overnight at 4°C. The next day the plates were washed with 0.1% Tween-20 in TBS (TBST), followed by blocking in 3%BSA for 1 hour. In the first round of biopanning, 2 × 1011 phage (100 equivalents of original library) were added to each well with 0.1% bovine serum albumin (BSA) and incubated for 1 hour at room temperature. The plates were then washed ten times with TBST. After washing, 100µl of 0.2 M Glycine-HCl (pH 2.2) and 1 mg/ml BSA were added to the wells to elute recombinant protein bound phage. One microliter of eluted phage was tittered on LB/IPTG/Xgal plates and blue plaques were counted and phage titer was determined. The remaining phage was amplified using ER2738 E. Coli in LB-Tet according to the manufacture’s instructions. For the following steps 2 × 1011 pfu plaques were use. We performed 4 cycles of binding and amplification on the previously eluted and amplified phage to enrich the pool in favor of binding sequences.
Surface Plasmon Resonance
Interaction between anti-SPARC L- or D-peptide and SPARC was determined by Biacore 2000 (GE Healthcare/Biacore, Uppsala, Sweden). SPARC from PYS-2 cell (sigma) was immobilized using standard amine-coupling chemistry to a density of 2100 resonance units (RU). As a control, we also immobilized 2100 RU carbonic anhydrase. The peptides were tested in duplicate in a two-fold dilution series for binding to the protein surfaces. Fitting the responses to a simple binding isotherm yielded the affinities.
Intravitreal peptide injection
Wild-type C57/BL6 mice were purchased from Jackson Laboratory. Mice (6 to 8 weeks old) were anesthetized by intraperitoneal injection of Ketaset® (50mg/kg; Wyeth, Fort Dodge, USA) and xylazine (Tranqui Ved, 10mg/kg; VEDCO, St. Joseph, USA). Anti-SPARC L-peptide (20ng or 2µg), anti-SPARC D-peptide (20ng or 2µg), or PBS, as a control, was injected into the vitreous cavity in a total volume of 1µl with 33-gauge micro syringe (Hamilton Company, Reno, USA).
Laser induced choroidal neovascularization
Laser photocoagulation (532nm, 100mV, 100ms, 50µm; NIDEK MC-4000) was performed on both eyes (2 to 5 spots per eye) as described (Sakurai, Anand, Ambati, van Rooijen & Ambati, 2003, Sakurai, Taguchi, Anand, Ambati, Gragoudas, Miller, Adamis & Ambati, 2003). After enucleating the eyes, sclera/choroid/RPE complex were fixed in 4% paraformaldehyde for 2 hours at 4 °C. After blocking in 5% FBS/PBS with 0.02% tritonX-100 and 2mM MgCl2, samples were stained with 5µg/ml Alexa488 conjugated isolectin GS-IB4 (Invitrogen Corporation, Carlsbad, USA) overnight. After washing the samples were flat mounted on glass slides. CNV volume was measured by scanning laser confocal microscopy (Olympus America Inc., Center Valley, USA).
Immunoprecipitation and Western blot
After euthanizing the mice, RPE/choroid complex was separated from sclera under a stereomicroscope and placed in 200 µl of RIPA buffer (Sigma-Aldrich, St. Louis, USA) containing protease inhibitor cocktail (Roche Diagnostics Corporation, Indianapolis, USA) and Halt™ phosphatase inhibitor (Fisher Scientific, Pittsburg, USA). After homogenization with a sonic dismembrator (Fisher Scientific, Pittsburg, USA), 150 µl samples were subjected to immunoprecipitation for FLT-1 or KDR. For immunoprecipitation we used protein G or A coated magnetic beads (Invitrogen Corporation, Carlsbad, USA) and mouse anti-FLT-1 antibody or rabbit anti-KDR antibody (Abcam Inc., Cambridge, USA) following manufacture’s instructions. The proteins were eluted with Laemmli buffer for 10 minutes at 70°C and resolved by SDS-PAGE (8%) under reducing conditions. The protein was transferred to a nitrocellulose membrane and incubated for 1 hour with blocking buffer (3%BSA and 0.05% Tween20 in TBS) then incubated with anti-phospho-FLT-1(Tyr1213) antibody (1:1000, Millipore, Billerica, USA) or anti-tyrosine phosphorylation antibody in blocking buffer for 1 hour at room temperature. The membrane was washed once in TBST (0.05% Tween20/TBS) and twice in TBS. Finally, the membrane was incubated with the appropriate secondary HRP-linked antibody in blocking buffer for 30 minutes at room temperature. After washing once with TBST and thrice with TBS, the bands were illuminated by an ECL-PLUS Western Blot detection kit (Amersham Biosciences, Pittsburg, USA) and detected by FOTO/Analyst Electronic Imaging Systems (Fotodyne Inc., Hartland, USA).
Statistics
Data are shown as mean ± s.e.m or s.d. P values were calculated using Student’s t-test
Results
Generation of anti-SPARC peptide
SPARC is expressed in retinal pigment epithelium (RPE) (Hiscott, Hagan, Heathcote, Sheridan, Groenewald, Grierson, Wong & Paraoan, 2002). In addition, we detected SPARC in the cornea, lens and retina by immunohistochemistry, although the signal intensities less than RPE (data not shown). Laser-induced CNV occurs from the choroid to the retina through the RPE. The RPE is thought to play a key role in early and late stage AMD (Schmitz-Valckenberg, Fleckenstein, Scholl & Holz, 2009). We had previously determined the probable mode of VEGF-SPARC binding via computational molecular docking (Chandrasekaran, Ambati, Ambati & Taylor, 2007) as well as the relevance of that interaction in gating VEGF angiogenic vs. anti-angiogenic effects in a laser-induced CNV model (Nozaki et al., 2006). We therefore hypothesized that inhibiting SPARC binding of VEGF would inhibit laser-induced CNV.
To inhibit SPARC activity we employed a small peptide, as we hypothesized small molecules would enter the RPE more efficiently and induce less inflammation than antibodies. In addition we thought a small peptide would limit only SPARC activity to VEGF effectively. To create the peptide blocking SPARC activity we performed phage-screening against SPARC and obtained the L-peptide sequence SMPTYNK. There were two small regions of VEGF predicted to have significant protein-protein interactions with SPARC (one around residue 200, and another around 260–270; amino acid number according to Genbank file AAH65522) (Chandrasekaran et al., 2007). We compared L-peptide SMPTYNK with these regions of VEGF and found two possible matches (Fig.1). Results from X-ray crystallographic analysis and mutagenesis studies concluded FMDVYQR is a helix near the binding site of VEGF to FLT-1 and KDR (Keyt, Berleau, Nguyen, Chen, Heinsohn, Vandlen & Ferrara, 1996, Keyt, Nguyen, Berleau, Duarte, Park, Chen & Ferrara, 1996, Wiesmann, Fuh, Christinger, Eigenbrot, Wells & de Vos, 1997). From the 3-D docking model of VEGF-SPARC binding (Chandrasekaran et al., 2007) Met (M) and Tyr (Y) residues project on the same side of the helix forming an MXXY motif, a feature of SMPTYNK. We believe this part of VEGF-A binds to SPARC and inhibits SPARC activity. Based on these considerations we selected L-KFMDVYNR as anti-SPARC L-peptide. In addition to anti-SPARC L-peptide, we synthesized anti-SPARC D-peptide (retro-inverso peptide, DRNYVDMFK), sequenced based on anti-SPARC L-peptide. To confirm if anti-SPARC L-peptide and D-peptide bind to SPARC we measured the interaction by surface plasmon resonance (SPR; Table). We found the interaction between SPARC and anti-SPARC L-peptide or D-peptide exists although the interactions were weak.
Figure.1. Potential matches of phage display derived L-peptide SMPTYNK to the VEGF protein sequence.
Underlined residues in VEGF are predicted to be sites of interaction with SPARC (Tables 1 and the reference (Chandrasekaran et al., 2007)) by H-bonding or predicted hydrophobic interactions (e.g. FM near N-terminus shown). Exact matches are shown in bold, similarities in italics. Note that NK in the peptide is functionally similar to QR in VEGF. The partial VEGF-A sequence shown is numbered according to Genbank file AAH65522.
Table.
Interaction between anti-SPARC peptides and SPARC protein measured by surface plasmon resonance.
| Dissociation Constant (KD) |
|
|---|---|
| Anti-SPARC L-peptide | 2.0 mM |
| Anti-SPARC D-peptide | 1.6 mM |
Anti-SPARC L-peptide injection before laser photocoagulation inhibits laser CNV volume
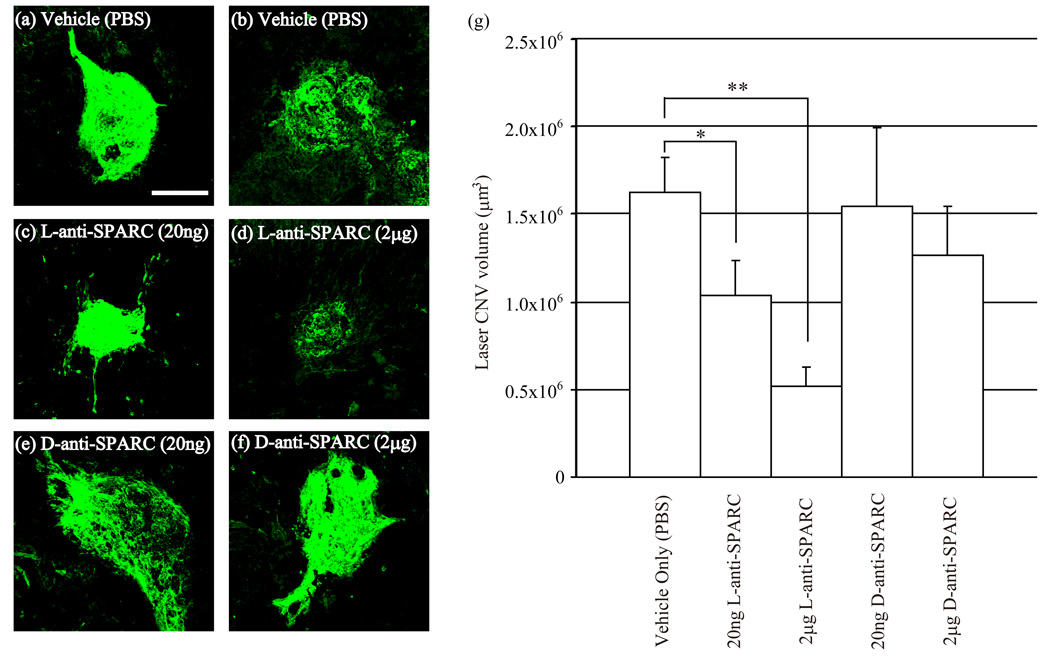
To determine if anti-SPARC peptide decreases laser CNV volume in mice we injected anti-SPARC L-peptide or D-peptide at 20ng and 2µg into the intravitreous cavity. One day after the injections we performed 2~5 spots of laser photocoagulation in each eye. After 10 days the volumes of laser CNV were measured by confocal microscopy. Figure 2 represents the laser induced CNV of PBS (a, b), anti-SPARC L-peptide 20ng(c), 2µg (d), and anti-SPARC D-peptide 20ng (e), and 2µg (f) injections. The anti-SPARC L-peptide injections decreased laser CNV volume in a dose-dependent manner compared with PBS injections, however anti-SPARC D-peptide did not affect the laser induced CNV volume (Fig.2 (g)). The sample number of each group was 10–15 laser-CNV spots.
Figure.2. Anti-SPARC L-peptide injection before laser inhibits CNV.
One day before laser injury, PBS, anti-SPARC L-peptide or D-peptide was injected into the intravitreal cavity. Representative fluorescence images are shown (a-f). PBS injection as a control (a, b). Anti-SPARC L-peptide injection, 20ng(c) and 2μg (d). Anti-SPARC L-peptide injection, 20ng(e) and 2µg(f). Scale bar: 200µm. Anti-SPARC L-peptide(g) reduced laser CNV volume, while anti-SPARC D-peptide showed no reduction compared with PBS,. P: risk factor by student t-test. *: p<0.01, **p<0.001. Error bar: s.e.m.
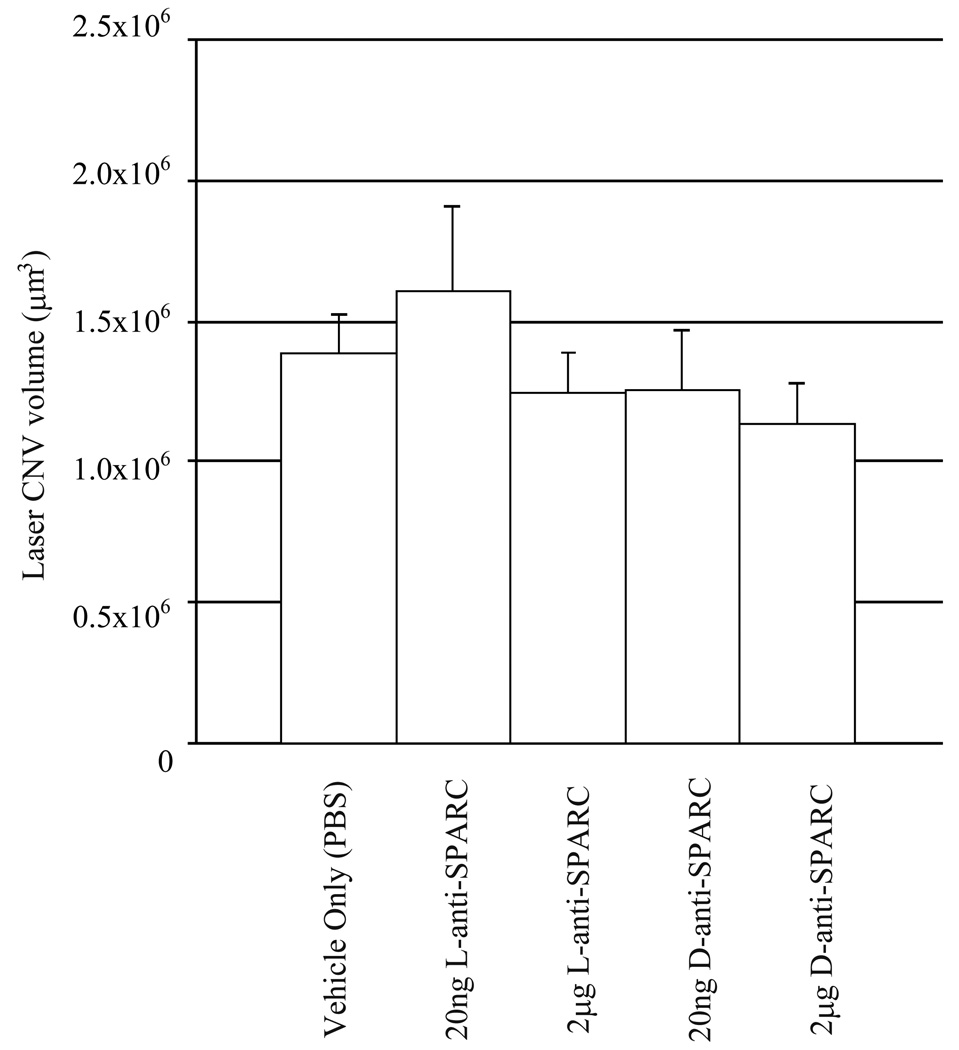
Anti-SPARC L-peptide injections after laser photocoagulation do not affect laser CNV volume
We injected anti-SPARC L- or D- peptide at 20ng or 2µg after one day of laser photocoagulation. After 10 days the volumes of laser CNV were measured (Fig.3). Unlike anti-SPARC L-peptide injection before laser photocoagulation, we could not detect a significant effect. The sample number of each group was 10–15 laser-CNV spots.
Figure.3. Anti-SPARC peptide injection after laser injury does not affect CNV.
One day after laser injury, PBS, anti-SPARC L-peptide and D-peptide were injected intravitreally. Significant differences were not found. Error bar: s.e.m.
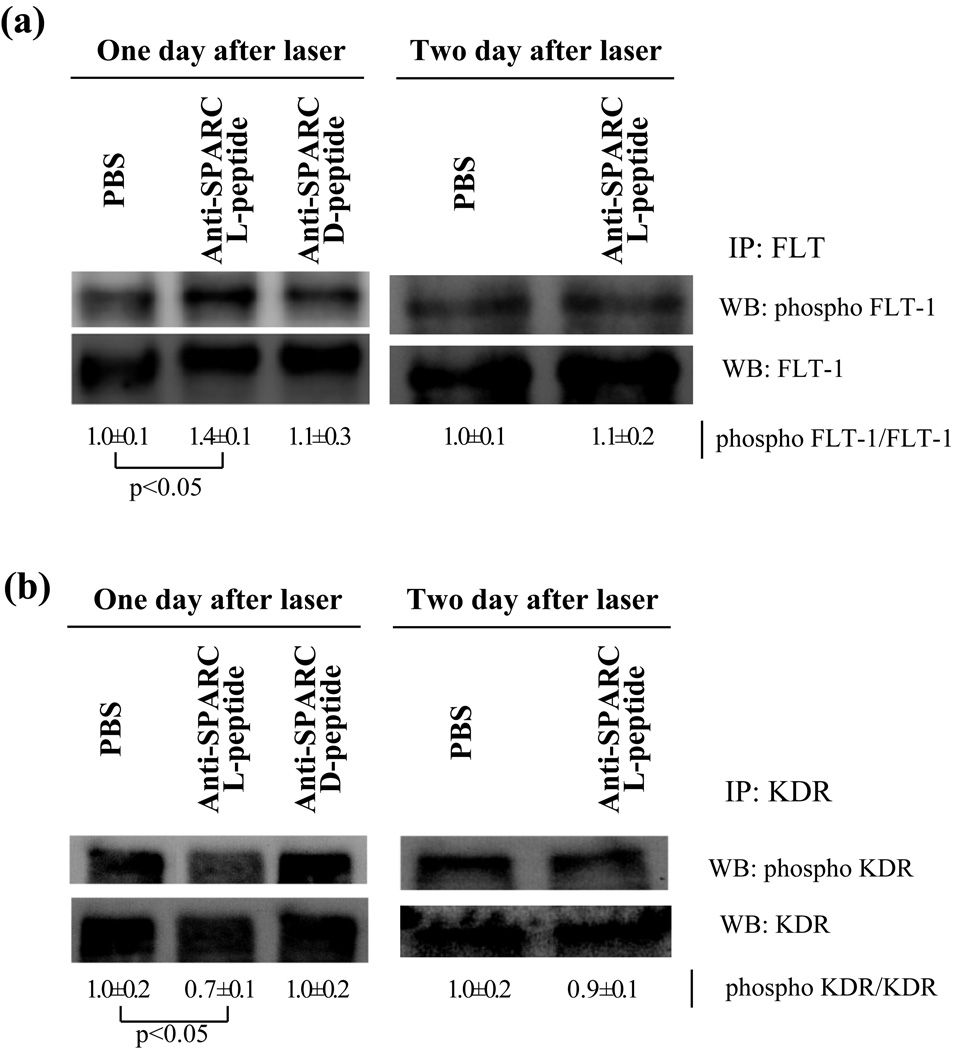
Anti-SPARC L-peptide increased phosphorylation of FLT-1 and decreased phosphorylation of KDR after one day of laser photocoagulation in vivo
Because it was reported that SPARC sequesters phosphorylation of FLT-1 by VEGF (Kupprion et al., 1998), we investigated whether anti-SPARC peptides would decrease SPARC activity in RPE/Choroid and eventually decrease phosphorylation of FLT-1. One day after anti-SPARC L-peptide, D-peptide or PBS injection we performed laser photocoagulation. One to two days later RPE/Choroid was carefully dissected from sclera and we examined phosphorylation of FLT-1 and KDR by western blot (Fig.4). Western blot showed anti-SPARC L-peptide increased phosphorylation of FLT-1 after one day of laser photocoagulation compared with PBS or anti-SPARC D-peptide injection. Two days after laser photocoagulation there were no significant differences in phosphorylation of FLT-1. Conversely, phosphorylation of KDR was decreased one day after but not two days after laser photocoagulation.
Figure.4. FLT phosphorylation is increased, but KDR phosphorylation is decreased by anti-SPARC L-peptide.
PBS, anti-SPARC L-peptide or D-peptide were injected to the intravitreous cavity one day before laser injury. One or two days after laser photocoagulation, the RPE/choroid was separated from sclera and immunoprecipitation for FLT-1 or KDR and western blot for phosphorylation of each protein were performed. (a) FLT-1, (b) KDR. Each figure is representation of three individual experiments. As a control, FLT-1 or KDR was examined in the same sample. Densitometric ratio of phosphoFLT-1 to FLT and phosphoKDR to KDR are shown as PBS treated one is 1.0. Error is s.d. P: risk factor by student t-test.
Discussion
VEGF, as a potent inducer of CNV, has been studied and its inhibition by anti-VEGF antibody (ranibizumab) has shown improvement of AMD patients’ vision (Chang, Bressler, Fine, Dolan, Ward & Klesert, 2007) (Rosenfeld, Brown, Heier, Boyer, Kaiser, Chung & Kim, 2006). Despite this discovery, its application has been limited to advanced stages. We believe treatment at earlier stages will be necessary to decrease CNV growth or prevent CNV. In addition, clinical trials of pegaptanib in AMD patients showed an inverse therapeutic dose response (Gragoudas, Adamis, Cunningham, Feinsod & Guyer, 2004). This indicates over-inhibition of VEGF may lead to an adverse effect and we hypothesized that the treatment of other targets related to VEGF may be helpful for AMD patients.
In addition to VEGF regulation, several other growth factors are affected by SPARC. For example, the stimulation of platelet-derived growth factor induced proliferation of human arterial vascular smooth muscle is inhibited in the presence of SPARC (Motamed, Funk, Koyama, Ross, Raines & Sage, 2002). Transforming growth factor β is also regulated by SPARC (Francki, McClure, Brekken, Motamed, Murri, Wang & Sage, 2004).
In this study we focused on SPARC activity as it relates to VEGF. We designed a small peptide to inhibit SPARC by phage-screening and previous computational analysis predictions (Chandrasekaran et al., 2007). Intravitreal injection of L-anti-SPARC peptide one day before laser photocoagulation prevented laser-induced CNV growth. However, we could not find a significant difference in laser induced CNV when we injected anti-SPARC peptide after laser injury. This may be due to two reasons. (1) Previous studies reported that one day after laser photocoagulation SPARC decreased in RPE/choroid temporarily, but recovered the next day (Nozaki et al., 2006). Hence, injection of anti-SPARC L-peptide after laser injury may not have a target as SPARC had already declined. (2) This may indicate SPARC regulation is operative in the initial phases of laser injury and determines the fate of cells in CNV through FLT-1 and KDR signaling. This concept is supported by western blot analysis of phosphorylation of FLT-1 and KDR (Fig 4). In eyes injected with anti-SPARC L-peptide one day before laser injury, phosphorylation of FLT-1 increased. Conversely, KDR phosphorylation decreased one day after laser photocoagulation. We could not detect differences in phosphorylation of FLT-1 and KDR in eyes treated two days after laser photocoagulation. Alternatively, it is possible that SPARC function may change in response to injury (Arnold & Brekken, 2009).
Although surface plasmon resonance showed low interaction between SPARC and anti-SPARC L-peptide, anti-SPARC L-peptide injection one day before laser photocoagulation reduced laser-induced CNV volume. It is possible that our dose of anti-SPARC L-peptide may overcome low binding affinity, further underlining the importance of SPARC at the initial stages of laser-induced CNV.
We successfully prevented laser-induced CNV using anti-SPARC L-peptide, but anti-SPARC D-peptide, which interacts with SPARC as much as anti-SPARC L-peptide did not inhibit CNV compared with PBS injection one day before or after laser photocoagulation. It is unclear why the D-peptide did not exert an inhibitory effect; one possibility is that there may be L-peptide specific transporters which enable transport of intravitreal peptides across the retina to sites of injury. Alternatively, there may be subtle steric interference points in the in vivo extracellular matrix blocking the anti-SPARC D-peptide activity. Figure 4 shows that anti-SPARC L-peptide, but not anti-SPARC D-peptide, promoted phosphorylation of FLT-1 and decreased phosphorylation of KDR. This suggests that anti-SPARC D-peptide may bind to SPARC but not inhibit SPARC-VEGF interactions in vivo.
The clinical relevance of finding anti-SPARC intervention to be most effective immediately prior to laser-injury in a murine model will depend on progress in assessing SPARC levels in macular degeneration patients. Patients with normal or above-normal SPARC levels may benefit from a combination of anti-SPARC plus anti-VEGF therapy.
In summary, we have shown that inhibition of SPARC by anti-SPARC L-peptide prior to laser photocoagulation reduces the volume of laser-induced CNV and that this is associated with increased phosphorylation of FLT-1 and decreased phosphorylation of KDR. Our results also confirm that SPARC plays a key role in the initial stages of laser-induced CNV. This may lay the foundation for anti-SPARC therapy to complement existing treatments for AMD.
Acknowledgements
This work was funded by RPB Physician-Scientist Award, Dept. of Defense PRMRP Award, and NEI 5R01EY017950.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, Albuquerque RJ, Richter E, Sakurai E, Newcomb MT, Kleinman ME, Caldwell RB, Lin Q, Ogura Y, Orecchia A, Samuelson DA, Agnew DW, St Leger J, Green WR, Mahasreshti PJ, Curiel DT, Kwan D, Marsh H, Ikeda S, Leiper LJ, Collinson JM, Bogdanovich S, Khurana TS, Shibuya M, Baldwin ME, Ferrara N, Gerber HP, De Falco S, Witta J, Baffi JZ, Raisler BJ, Ambati J. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443(7114):993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin R, Puklin JE, Frank RN. Growth factor localization in choroidal neovascular membranes of age-related macular degeneration. Invest Ophthalmol Vis Sci. 1994;35(8):3178–3188. [PubMed] [Google Scholar]
- Arnold SA, Brekken RA. SPARC: a matricellular regulator of tumorigenesis. J Cell Commun Signal. 2009 doi: 10.1007/s12079-009-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellner L, Vitto M, Patil KA, Dunn MW, Regan R, Laniado-Schwartzman M. Exacerbated corneal inflammation and neovascularization in the HO-2 null mice is ameliorated by biliverdin. Exp Eye Res. 2008;87(3):268–278. doi: 10.1016/j.exer.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14(5):608–616. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- Bussolati B, Dunk C, Grohman M, Kontos CD, Mason J, Ahmed A. Vascular endothelial growth factor receptor-1 modulates vascular endothelial growth factor-mediated angiogenesis via nitric oxide. Am J Pathol. 2001;159(3):993–1008. doi: 10.1016/S0002-9440(10)61775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, Vandendriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7(5):575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran V, Ambati J, Ambati BK, Taylor EW. Molecular docking and analysis of interactions between vascular endothelial growth factor (VEGF) and SPARC protein. J Mol Graph Model. 2007;26(4):775–782. doi: 10.1016/j.jmgm.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Chang TS, Bressler NM, Fine JT, Dolan CM, Ward J, Klesert TR. Improved vision-related function after ranibizumab treatment of neovascular age-related macular degeneration: results of a randomized clinical trial. Arch Ophthalmol. 2007;125(11):1460–1469. doi: 10.1001/archopht.125.11.1460. [DOI] [PubMed] [Google Scholar]
- de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255(5047):989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- Epstein RJ, Stulting RD, Hendricks RL, Harris DM. Corneal neovascularization. Pathogenesis and inhibition. Cornea. 1987;6(4):250–257. doi: 10.1097/00003226-198706040-00004. [DOI] [PubMed] [Google Scholar]
- Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82(1):4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- Francki A, McClure TD, Brekken RA, Motamed K, Murri C, Wang T, Sage EH. SPARC regulates TGF-beta1-dependent signaling in primary glomerular mesangial cells. J Cell Biochem. 2004;91(5):915–925. doi: 10.1002/jcb.20008. [DOI] [PubMed] [Google Scholar]
- Gragoudas ES, Adamis AP, Cunningham ET, Jr., Feinsod M, Guyer DR. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351(27):2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Maru Y, Okada A, Seiki M, Noda T, Shibuya M. Involvement of Flt-1 tyrosine kinase (vascular endothelial growth factor receptor-1) in pathological angiogenesis. Cancer Res. 2001;61(3):1207–1213. [PubMed] [Google Scholar]
- Hiscott P, Hagan S, Heathcote L, Sheridan CM, Groenewald CP, Grierson I, Wong D, Paraoan L. Pathobiology of epiretinal and subretinal membranes: possible roles for the matricellular proteins thrombospondin 1 and osteonectin (SPARC) Eye. 2002;16(4):393–403. doi: 10.1038/sj.eye.6700196. [DOI] [PubMed] [Google Scholar]
- Kearney JB, Ambler CA, Monaco KA, Johnson N, Rapoport RG, Bautch VL. Vascular endothelial growth factor receptor Flt-1 negatively regulates developmental blood vessel formation by modulating endothelial cell division. Blood. 2002;99(7):2397–2407. doi: 10.1182/blood.v99.7.2397. [DOI] [PubMed] [Google Scholar]
- Keyt BA, Berleau LT, Nguyen HV, Chen H, Heinsohn H, Vandlen R, Ferrara N. The carboxyl-terminal domain (111–165) of vascular endothelial growth factor is critical for its mitogenic potency. J Biol Chem. 1996;271(13):7788–7795. doi: 10.1074/jbc.271.13.7788. [DOI] [PubMed] [Google Scholar]
- Keyt BA, Nguyen HV, Berleau LT, Duarte CM, Park J, Chen H, Ferrara N. Identification of vascular endothelial growth factor determinants for binding KDR and FLT-1 receptors. Generation of receptor-selective VEGF variants by site-directed mutagenesis. J Biol Chem. 1996;271(10):5638–5646. doi: 10.1074/jbc.271.10.5638. [DOI] [PubMed] [Google Scholar]
- Kupprion C, Motamed K, Sage EH. SPARC (BM-40, osteonectin) inhibits the mitogenic effect of vascular endothelial growth factor on microvascular endothelial cells. J Biol Chem. 1998;273(45):29635–29640. doi: 10.1074/jbc.273.45.29635. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Bohman S, Dixelius J, Berge T, Dimberg A, Magnusson P, Wang L, Wikner C, Qi JH, Wernstedt C, Wu J, Bruheim S, Mugishima H, Mukhopadhyay D, Spurkland A, Claesson-Welsh L. VEGF receptor-2 Y951 signaling and a role for the adapter molecule TSAd in tumor angiogenesis. EMBO J. 2005;24(13):2342–2353. doi: 10.1038/sj.emboj.7600709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motamed K, Funk SE, Koyama H, Ross R, Raines EW, Sage EH. Inhibition of PDGF-stimulated and matrix-mediated proliferation of human vascular smooth muscle cells by SPARC is independent of changes in cell shape or cyclin-dependent kinase inhibitors. J Cell Biochem. 2002;84(4):759–771. doi: 10.1002/jcb.10095. [DOI] [PubMed] [Google Scholar]
- Ng EW, Adamis AP. Targeting angiogenesis, the underlying disorder in neovascular age-related macular degeneration. Can J Ophthalmol. 2005;40(3):352–368. doi: 10.1016/S0008-4182(05)80078-X. [DOI] [PubMed] [Google Scholar]
- Nozaki M, Sakurai E, Raisler BJ, Baffi JZ, Witta J, Ogura Y, Brekken RA, Sage EH, Ambati BK, Ambati J. Loss of SPARC-mediated VEGFR-1 suppression after injury reveals a novel antiangiogenic activity of VEGF-A. J Clin Invest. 2006;116(2):422–429. doi: 10.1172/JCI26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima Y, Oshima S, Nambu H, Kachi S, Hackett SF, Melia M, Kaleko M, Connelly S, Esumi N, Zack DJ, Campochiaro PA. Increased expression of VEGF in retinal pigmented epithelial cells is not sufficient to cause choroidal neovascularization. J Cell Physiol. 2004;201(3):393–400. doi: 10.1002/jcp.20110. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- Sakurai E, Anand A, Ambati BK, van Rooijen N, Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44(8):3578–3585. doi: 10.1167/iovs.03-0097. [DOI] [PubMed] [Google Scholar]
- Sakurai E, Taguchi H, Anand A, Ambati BK, Gragoudas ES, Miller JW, Adamis AP, Ambati J. Targeted disruption of the CD18 or ICAM-1 gene inhibits choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44(6):2743–2749. doi: 10.1167/iovs.02-1246. [DOI] [PubMed] [Google Scholar]
- Schmitz-Valckenberg S, Fleckenstein M, Scholl HP, Holz FG. Fundus autofluorescence and progression of age-related macular degeneration. Surv Ophthalmol. 2009;54(1):96–117. doi: 10.1016/j.survophthal.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Shibuya M. Role of VEGF-flt receptor system in normal and tumor angiogenesis. Adv Cancer Res. 1995;67:281–316. doi: 10.1016/s0065-230x(08)60716-2. [DOI] [PubMed] [Google Scholar]
- Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39(5):469–478. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312(5):549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Smith W, Assink J, Klein R, Mitchell P, Klaver CC, Klein BE, Hofman A, Jensen S, Wang JJ, de Jong PT. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology. 2001;108(4):697–704. doi: 10.1016/s0161-6420(00)00580-7. [DOI] [PubMed] [Google Scholar]
- Wiesmann C, Fuh G, Christinger HW, Eigenbrot C, Wells JA, de Vos AM. Crystal structure at 1.7 A resolution of VEGF in complex with domain 2 of the Flt-1 receptor. Cell. 1997;91(5):695–704. doi: 10.1016/s0092-8674(00)80456-0. [DOI] [PubMed] [Google Scholar]