Abstract
A membrane receptor, Fas (CD95), and its ligand FasL have been considered as key players in diabetes pathogenesis. They are known to mediate interactions between β cells and cytotoxic T cells, which results in apoptotic cell death. We hypothesized that the interruption of Fas-FasL interactions by suppressing Fas expression in β cells would affect the development of diabetes. The effect of Fas-silencing siRNA (Fas siRNA) on the diabetes development was evaluated in a cyclophosphamide (CY)-accelerated diabetes animal model after intravenous administration using a polymeric carrier, polyethylenimine (PEI). The systemic non-viral delivery of Fas siRNA showed significant delay in diabetes incidence up to 40 days, while the control mice treated with naked Fas siRNA, scrambled dsRNA, or PBS were afflicted with diabetes within 20 days. The retardation of diabetes incidence after the treatment of Fas siRNA may be due to the delayed progression of the pancreatic insulitis. In this study, the potential use of a non-viral carrier based siRNA gene therapy for the prevention of type-1 diabetes is demonstrated.
Keywords: type-1 diabetes, siRNA, Fas, gene delivery, non-viral vector
1. Introduction
Type-1 diabetes, one of the most prevalent autoimmune diseases, results from the destruction of insulin-producing β cells of the pancreatic islets, mostly leading to absolute insulin deficiency. Non-obese diabetes (NOD) mice have been popularly used as a model to study type-1 diabetes since the disease progress of the mice closely resembles that of humans [1, 2]. In this animal model, the disease is initiated by infiltration of mononuclear cells into the pancreatic islets [3, 4], which begins after 4–6 weeks of age and develops progressively. In this stage, only small portion of islet β cells is affected by the attack of the cellular immunity. This pre-diabetic stage is called insulitis and persists for months, after which overt onset of diabetes can be observed [5, 6]. Both CD4+ and CD8+ T cells play an important role in the initiation and progress of pathogenesis of type-1 diabetes [7–9]. The recruitment and activation of the lymphocytes stimulates further secretion of various inflammatory cytokines that could accelerate the destruction of islet β cells, resulting in systemic insulin depletion. The development of diabetes in NOD mice is dependent on animal gender, environmental conditions, diet and other features of each NOD sub-line. Cyclophosphamide (CY), an alkylating cytostatic drug, is known to promote susceptibility to type-1 diabetes in young pre-diabetic NOD mice [10]. Acceleration of type-1 diabetes in NOD mice by CY can provide a convenient synchronization of the disease process. Although the mechanism of action is not clear, several reports have suggested that CY may cause the destabilization of the local immune-regulatory balance by temporarily depleting suppressor cells [11, 12]. Like in unmanipulated NOD mice in which type-1 diabetes spontaneously occurs, the death of β cells caused by apoptosis in the CY-treated accelerated animal model [13, 14].
Death of β cells in type-1 diabetes involves multiple mechanisms. One of the major mechanisms of β cell death is a programmed cell death (apoptosis) by T cells, in which interactions between Fas (CD95) and its ligand (FasL) contributes significantly to the T cell-mediated β cell destruction [15, 16]. The Fas-FasL interactions activate multiple signaling pathways leading to apoptotic cell death [17]. The significance of Fas-FasL interactions in the disease development was initially observed in Fas-deficient NOD mice (lpr/lpr) which failed to induce the disease and are resistant to adoptive diabetes transfer [18, 19]. FasL-deficient NOD mice (gld/gld) were also protected from either insulitis or diabetes [20]. Prolonged survival of NOD islet graft in diabetic NOD mice was observed after administration of antibody against FasL (anti-FasL antibody) [21]. These results suggest that Fas-FasL interactions be involved in at least one (or more) critical steps in the diabetogenic process. Therefore, interfering Fas-FasL interactions could be a potential target for the prevention of diabetes.
Gene therapy based on RNA interference (RNAi) has attracted a lot of attention due to its enormous potential in clinical applications. RNAi, induced by a double-stranded RNA, is a naturally occurred phenomenon in a cell, by which a gene expression is regulated in a highly sequence-specific manner at a post-transcriptional level. Small interfering RNA (siRNA) that can induce a gene-specific RNAi, is a small double stranded RNA sequence composed of 21 to 25 base pairs [22–24]. A number of clinically active siRNA sequences have been screened by several research groups world-wide. However, the use of siRNA in clinical settings is limited mainly due to its highly negatively-charged backbone containing phosphodiester linkages, which are responsible for a poor intracellular uptake through a negatively-charged plasma membrane and a rapid degradation by extracellular enzymes, respectively [25]. A number of delivery systems designed to overcome such inherent drawbacks of siRNA has been suggested for the efficient delivery of therapeutic siRNAs [26]. Delivery systems based on cationic polymers have been popularly used for the delivery of synthetic siRNA since polymeric carriers are considered relatively safe from the unexpected adverse events, such as acute immune responses and systemic cytotoxicity, after repeated administrations for the treatment of a chronic disease, such as diabetes [25].
In this study, Fas siRNA was used to induce specific silencing of Fas gene expression in NOD mice treated with CY for accelerated induction of autoimmune diabetes. Polyethylenimine (PEI), one of the most widely used polymeric non-viral gene carriers, was employed as a carrier for delivery of the siRNA in vivo as well as in vitro. The PEI-based delivery of siRNA could effectively suppress the pancreatic expression of Fas, leading to delayed incidence of insulitis and CY-induced accelerated diabetes in NOD mice.
2. Materials and methods
2.1. Materials
The synthetic siRNA, annealed duplex of 21-nucleotide RNA modified with 3′-dTdT overhangs was synthesized and purified by Qiagen Inc (Valencia, CA). The target sequence for mouse Fas (CD95) and non-silencing negative control were GUGCAAGUGCAAACCAGAC and AATTCTCCGAACGTGTCACGT, respectively. Polyethylenimine (PEI, Mw 25,000) and cyclophosphamide (CY) was purchased from Sigma (St. Louis, MO). Fetal bovine serum (FBS), Dulbecco’s phosphate buffered saline (PBS) and Dulbecco’s Modified Eagle’s Medium (DMEM) were obtained from Invitrogen (Carlsbad, CA). Mouse interferon-γ(mIFN-γ) and mouse interleukin-1β (mIL-1β) were from Sigma (St. Louis, MO). Anti-mouse Fas antibody (Jo2) was purchased from BD PharMingen (San Jose, CA).
2.2. Formation, characterization and cellular uptake of siRNA/PEI complexes
The formation of siRNA/PEI complexes was confirmed by an electrophoretic mobility shift assay. Varying amount of PEI and a fixed amount of siRNA (0.3 μg) were separately diluted in PBS. Prior to use, the complexes were formed by mixing the diluted solutions and allowed to be stabilized for 20 min at a room temperature. Electrophoresis was performed in 1.5 % agarose gel with a current of 120 V for 20 min. The retardation of the complexes was visualized by a UV transilluminator equipped with a CCD image capturing unit (GelDoc, BioRad, Hercules, CA) after ethidium bromide staining. The size and surface charge of the complexes were determined by a dynamic light scattering device (ZetaPlus, Brookhaven Instrument Co. New York, NY) equipped with a He-Ne laser at a wavelength of 632 nm.
2.3. Cell culture and transfection
Mouse insulinoma cells (Min6) were cultured in DMEM supplemented with 15 % FBS and maintained at 37 °C in a humidified 5 % CO2 atmosphere. For transfection, the cells (5 × 104) were plated in a 35 mm culture dish. The cells were then incubated 37 °C in a humidified 5 % CO2 atmosphere for 24 h. The cell culture medium was replaced with serum-free medium prior to the addition of desired siRNA formulations. After 3 h incubation, the transfection medium was removed and supplemented with fresh growth medium containing 10 % FBS. The incubation was continued for 6 h and the medium was replaced with new growth medium containing 10 % FBS and cytokines (1000 U/ml mIFN-γ and 100 U/ml mIL-1β) to stimulate Fas expression. The cytokine treatment was continued for 18 h before RT-PCR analysis and induction of apoptosis. Apoptosis was induced by incubating the cytokine-treated cells with anti-mouse Fas antibody (Jo2, 5 μg/ml) for 24 h.
2.4. Flow cytometry
Flow cytometric method was used to observe cellular uptake of siRNA/PEI complexes. For flow cytometric analysis, Min6 cells grown in 100 mm dishes were transfected with FITC-labeled siRNA/PEI complexes as described above. After 4 h of incubation, the cells were trypsinized, washed three times with cold PBS, and fixed in 1 % paraformaldehyde in PBS at 4 °C for 30 min. The fixed cells were washed two times with cold PBS and subjected to flow cytometric analysis to determine cellular uptake (FACS Caliber, Becton-Dickinson, Mountain View, CA). At least 10,000 events were analyzed to generate each histogram.
2.5. Semi-quantitative RT-PCR and TUNEL assay
To determine the level of Fas expression, total RNA was isolated from the cells using RNeasy® RNA isolation kit (Qiagen, Valencia, CA), according to the manufacturer’s recommendation. Complementary DNA (cDNA) strand was synthesized from the RNA using oligo (dT)20 primer and reverse transcriptase (Invitrogen, Carlsbad, CA) at 50 °C for 30 min. Polymerase chain reaction was performed using Fas-specific primers (forward: 5′-GATGCACACTCTGCGATGAA-3′, reverse: 5′-CATGTCTTCAGCAATTCTCGG-3′) for 30 cycles at the following thermal cycling conditions: 94 °C for 30 sec, 60 °C for 30 sec, 72 °C for 60 sec. Primers for mouse β-actin were 5′-TGGAATCCTGTGGCATCCATGAAAC-3′ (forward) and 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′ (reverse). The PCR products were separated in a 1 % agarose gel by electrophoresis and visualized by staining with ethidum bromide under UV illumination.
To visualize apoptosis after the treatment of anti-mouse Fas antibody (Jo2), the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was carried out using colorimetric TUNEL assay kit (Promega, Madison, WI), according to the manufacturer’s instruction. Apoptotic cells stained with diaminobezidine were visualized under a microscope equipped with CCD camera unit (Olympus, Melville, NY).
2.6. Animals and administration of siRNA
NOD mice (Lt/Jax) were purchased from Jackson Laboratory (Bar Harbor, ME) and were kept under pathogen-free conditions. To accelerate diabetes, a single dose of CY (250 mg/kg body weight) was administered i.p. to 7-week-old NOD mice (Day 0). Desired siRNA formulations, including siRNA/PEI complex (3.3 nmol siRNA, N/P ratio of 10), were injected intravenously to NOD mice. Blood samples were collected weekly from a tail vein and analyzed by FreeStyle instant glucometer (Abbott Diabetes Care Inc., Alameda, CA) to determine blood glucose level. Animals with a blood glucose level above 250 mg/dL were considered diabetic.
2.7. Histological analysis
For histological experiments, mice were sacrificed by cervical dislocation following anesthetization with methoxyflurane (Schering-Plough Animal Health, Union, NJ) inhalation. The pancreas was harvested, fixed in 10 % phosphate-buffered formalin solution, and embedded in paraffin. Sections (5 μm) were cut and stained with hematoxylin and eosin. More than 20 islets from each pancreas (n=3) were examined using a double blind method. The grade of insulitis progression was determined by using following grading system [27]: grade 0, normal islets; grade 1, mononuclear cell infiltration in less than 25 % of the islet; grade 2, 25–50 % of the islet showing mononuclear cell infiltration; grade 3, over 50 % of the islet showing mononuclear cell infiltration. Apoptotic cells in the pancreatic islets were stained by TUNEL assay and visualized by microscopy.
3. Results and discussion
There have been a number of compelling evidences suggesting critical roles of autoreactive cellular immunity in the apoptotic cell death of islet β cells during the pathogenesis of type-1 diabetes [18, 28, 29]. Therefore, several strategies for the immunological intervention of the cellular immunity have been suggested. Diabetic NOD mice having syngeneic islet transplants could maintain normal blood glucose concentration after the administration of FasL antibody [21]. The infiltration of mononuclear cells into syngenic islet grafts was also significantly reduced in the NOD mice apparently due to the apoptotic death of T-cells mediated by the FasL antibody. Gene therapy-based immunological intervention for the prevention of diabetes was also suggested using a plasmid DNA encoding interleukin-4 (IL-4) and interleukin-10 (IL-10). Systemic administration of the IL-4/IL-10 plasmid DNA formulated with an analogue of poly(L-lysine), poly(α-[4-aminobutyl]-L-glycolic acid) (PAGA), could result in a significant reduction of the prevalence of both insulitis and diabetes in NOD mice [30, 31]. The overexpression of IL-4 and IL-10 provide a protective effect and counteract the effect of pathogenic cytokines such as interferon-γ. The cationic polymer, PAGA, can spontaneously form nano-size polyelectrolyte complexes with the plasmid DNA in an aqueous solution. In this study, we hypothesized that the intervention of death receptor-mediated apoptosis by RNA interference (RNAi) could prevent islet beta cells from apoptotic cell death. Fas siRNA was selected as a therapeutic candidate for the treatment of type-1 diabetes, since interactions between Fas and FasL has been considered as a critical event for the initiation of the disease in NOD mice.
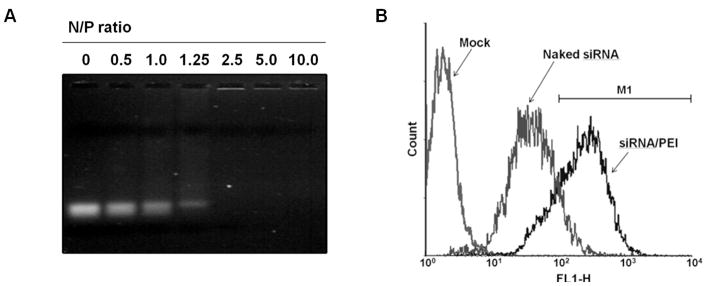
In this study, polyethylenimine (PEI 25 kDa) was employed as a cationic carrier for the delivery of siRNA. The backbone of PEI has primary, secondary, and tertiary amines with a high density, which gives the polymer a superior ability for nucleic acid condensation and a hypothetical proton buffering effect for efficient escape from acidic endosomal compartment [32]. For decades, PEI 25 kDa has been recognized as a gold standard in non-viral gene therapy due to its superior properties and reproducibility in transfection experiments. Systemic administration of PEI/DNA complexes successfully delivered a plasmid DNA to the major organs where transgene expression was observed [33–35]. In addition, a recent study showed the levels of inflammatory cytokines such as tumor necrosis factor-α (TNF-α) were much lower after intravenous administration of PEI/plasmid DNA complexes than the levels of the cytokines induced by cationic liposome-based lipoplexes, which was independent of the N/P ratio, the amount of plasmid DNA, or structure and molecular weight of PEI [36]. Similar result was also found in a study with siRNA/linear PEI complexes [37]. The polymer formed tight complexes with siRNA above the N/P ratio of 2.5, as observed in an electrophoretic band mobility shift assay (Figure 1A). Transfection and animal experiments were carried out at the N/P ratio of 10 at which the hydrodynamic diameter and surface zeta-potential determined by a light scattering method were 115 ± 23.2 nm and + 23 ± 3.5 mV, respectively. The particle size was significantly larger than shown in a previous study in which PEI/siRNA complexes prepared at the N/P ratio of 7.5 was 26 ± 8 nm, as determined by fluorescence correlation spectroscopy [38]. The significant difference in the particle size may be due to the difference in analytical methods used in the measurements.
Fig. 1.
Electrophoretic mobility shift assay of siRNA/PEI complexes at various polymer/siRNA N/P ratios (A) and cellular uptake of siRNA/PEI complexes by Min6 cells (B), determined by flow cytometry.
Cellular uptake of siRNA/PEI complexes by Min6 cells was monitored by flow cytometry. The percentage of the cells gated from an arbitrarily selected region M1 (102<FL1-H<104) for naked siRNA and siRNA/PEI complex was 11.1 % and 78.1 %, respectively (Figure 1B), suggesting that cellular uptake of siRNA could be significantly improved by forming complex with PEI.
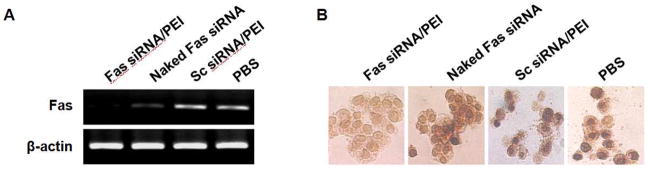
Significance of Fas siRNA as a specific RNA interference inducer was evaluated by the transfection of the siRNA into mouse insulinoma cells (Min6), followed by the addition of mIFN-γ and mIL-1β that stimulates Fas expression. It was reported that mIFN-γ and mIL-1β increase the level of Fas presented on the cell surface [39]. The formulation of Fas siRNA/PEI complexes effectively suppressed Fas mRNA transcripts, as determined by semi-quantitative RT-PCR (Figure 2A). In contrast, scrambled siRNA/PEI complexes did not show any silencing effect on Fas expression, suggesting the sequence specificity of the Fas siRNA. The anti-apoptotic effect of Fas siRNA was observed by using TUNEL assays (Figure 2B). Fas-mediated apoptosis was induced by incubating the cells with anti-Fas antibody (Jo2) after the cytokines treatment. The cells transfected with Fas siRNA/PEI complexes demonstrated significant suppression of anti-Fas antibody-mediated apoptosis, compared to control groups that include naked siRNA and scrambled siRNA/PEI complexes. The results suggest the anti-apoptotic effect of Fas siRNA comes from the specific silencing of Fas expression at the post-transcriptional level.
Fig. 2.
Suppression of Fas expression (A) and anti-apoptotic effect (B) of Fas siRNA/PEI complexes in Min6 cells. Fas expression was induced by treating the cells with 1000 U/ml mIFN-γ and 100 U/ml mIL-1β. Apoptosis was induced by incubating the cytokine-treated cells with anti-mouse Fas antibody (Jo2, 5 μg/ml).
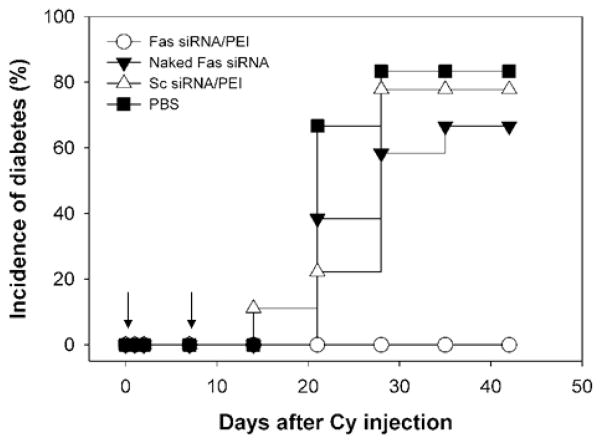
The effect of Fas gene silencing in the pancreas by Fas siRNA on the incidence of insulitis and diabetes was further studied in a CY-induced accelerated diabetes model. Diabetes could be induced in young pre-diabetic NOD mice within 4 weeks by a single intraperitoneal administration of CY (250 mg/kg). Apparently, CY triggers the acute diabetes by affecting immunoregulatory T cells. It was also reported that the intraperitoneal (i.p.) administration of CY to NOD mice makes islet infiltrating lymphocytes more pathogenic by increasing the number of lymphocytes producing IFN-γ [40]. In order to observe protective effect of Fas siRNA in vivo, Fas siRNA formulated with PEI was administered to the NOD mice through a tail vain concomitantly to CY i.p. injection (Day 0). An additional intravenous injection of the Fas siRNA/PEI formulation was given to the NOD mice on day 6. Glucose levels were monitored weekly using blood samples collected from the tail vain. As shown in Figure 3, the overt onset of diabetes was significantly delayed by the treatment of Fas siRNA up to 40 days after CY injection. In contrast, most of the control mice treated with naked Fas siRNA, scrambled siRNA (Sc siRNA), or PBS were afflicted with diabetes within 20 days. It should be noticed that blood glucose concentration of the Fas siRNA-treated mice also gradually increased with time, but the increment rate was much slower than the control groups (data not shown). In this study, animals with a blood glucose level above 250 mg/dL were considered diabetic. Naked Fas siRNA showed only limited preventive effect on the development of diabetes. This may be due to the inherent instability of siRNA in the biological fluid. The stability of the siRNA during systemic circulation could be significantly improved by forming tight polyelectrolyte complexes with PEI. Recent publication showed PEI could dramatically improve the in vivo stability of siRNA, leading to successful therapeutic effect of siRNA through systemic application [41].
Fig. 3.
Preventive effect of Fas siRNA/PEI ceomplexes on CY-induced diabetes in NOD mice. Diabetes was induced by injecting a single dose of CY (250 mg/kg body weight) (Day 0) prior to the administration of indicated formulations. Animals with a blood glucose level above 250 mg/dL were considered hyperglycemic (n = 15).
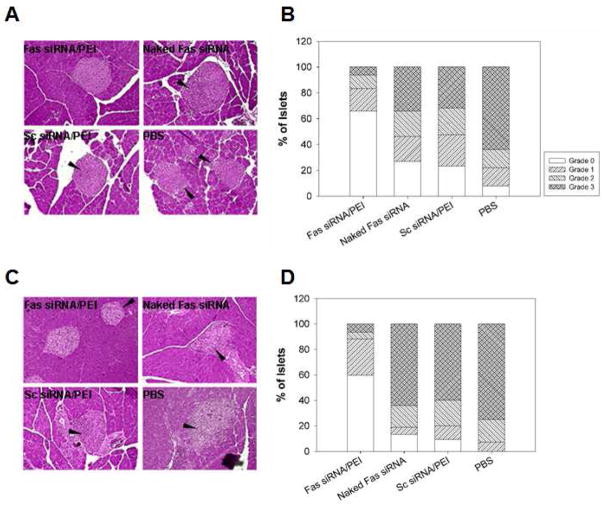
In order to investigate the effect of systemic Fas siRNA treatment on the progress of insulitis in the CY-treated NOD mice, the complexes formed from the interaction between Fas siRNA and PEI were intravenously administered (on day 0 and 6) to 7-week old NOD mice that had been treated with CY (i.p.) on day 0. After 7 and 14 days, the mice were sacrificed. The pancreata were harvested and stained with hematoxylin and eosin for the evaluation of insulitis. The result showed that 60 % of the observed islets were intact in the group treated with Fas siRNA/PEI complexes after 14 days (Figure 4C and D). In contrast, less than 15 % remained intact in control groups treated with naked siRNA. Furthermore, in the Fas siRNA treatment group, the majority of the islets infiltrated by mononuclear cells were scored as peripheral insulitis (grade 1). However, over 60 % of islets in the control groups showed severe insulitis (grade 3) after 14 days. Only a few mononuclear cell infiltrations into the islets was detected in all test groups before the day of CY treatment (data not shown). This result suggests that the administration of Fas siRNA/PEI complexes could efficiently suppress the recruitment and infiltration of mononuclear cells.
Fig. 4.
Suppression of insulitis after systemic administration of Fas siRNA/PEI complexes in CY-treated NOD mice. The mouse pancreata were harvested on day 7 (A, B) and day 14 (C, D) and stained with hematoxylin and eosin. Arrows indicate mononuclear cell infiltrations. More than 20 islets from three animals (n=3) were examined to determine insulitis grade (B, D) based on the grading system described in Materials and Methods.
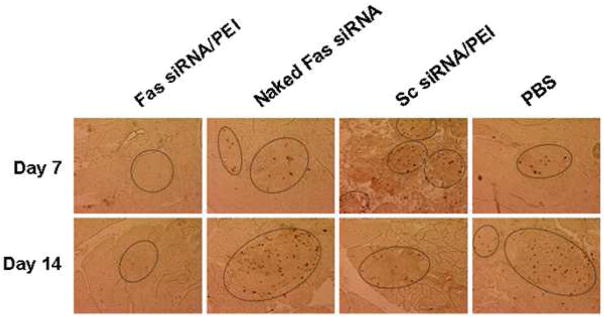
TUNEL staining of the histological sections demonstrated that most of the islets did not undergo apoptosis in the Fas siRNA/PEI treatment group after 14 days (Figure 5). Together with the H&E staining result, this suggests systemic administration of Fas siRNA/PEI complexes could effectively suppress the incidence of diabetes by reducing insulitis and apoptosis of islet cells.
Fig. 5.
Prevention of apoptosis in pancreatic islets after intravenous injection of Fas siRNA/PEI complexes. Cellular apoptotic event in pancreatic islets (inside the marked boundary) was visualized by TUNEL staining.
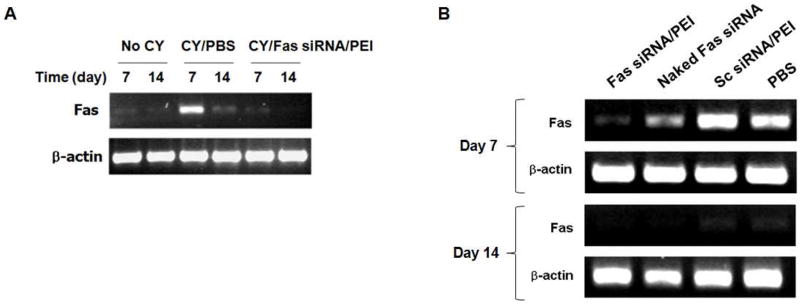
Pancreatic expression profiles of Fas mRNA transcript in the CY-treated NOD mice were observed using RT-PCR. The Fas siRNA/PEI complexes were administered to the CY-treated NOD mice via tail vein (on day 0 and 6). After 7 and 14 days, mRNA was isolated from the pancreas and analyzed by RT-PCR using Fas-specific primers. The pancreatic expression of Fas transcript was only detected in CY-treated mice and not in the mock (PBS) treated mice (Figure 6A). Significant suppression of Fas expression was observed in the mice treated with Fas siRNA/PEI complexes on day 7 (Figure 6A and 6B). However, the control groups treated with sc siRNA/PEI and PBS failed to silence Fas expression. Naked Fas siRNA exhibited much lower suppression of Fas expression, compared to the Fas siRNA/PEI formulation. Interestingly, on day 14, the Fas mRNA band disappeared in both treatment and control groups (Figure 6B).
Fig. 6.
(A) Effect of CY treatment on pancreatic Fas expression. (B) Suppression of Fas expression after the intravenous administration of Fas siRNA/PEI complexes in CY-treated NOD mice (n=3).
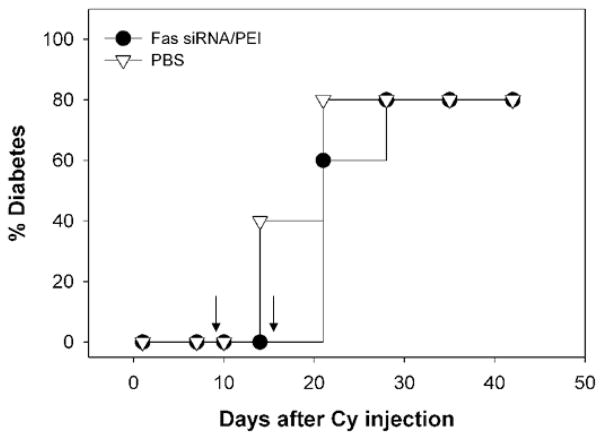
One possible reason of this result could be the rapid clearance of Fas-expressing cells via interactions with mononuclear cells having FasL on their membrane. Another possible explanation is that it may take a while for the restoration of the immune-regulatory balance between suppressor and pathogenic regulatory cells, which was temporarily disrupted by the CY treatment. Although the mechanism of CY-induced diabetes in NOD mice is not fully understood, a previous result observed dramatic increase in an IFN-γ secretion level in NOD spleenocytes after CY injection [40]. The over-expression of IFN-γ can be supported by another study using a microarray analysis, in which a marked increase in expression of IFN-γ and its related genes was observed 3 days after CY administration [42]. The acute increase of IFN-γ expression may induce Fas expression on islet cells [15, 43]. The suppressor cells such as Th2-like cells secreting protective IL-4 and IL-10 seems to be either more sensitive to CY (therefore, preferentially eliminated) or regenerated at a slower rate [11], while regulatory cells secreting pathogenic IFN-γ are relatively resistant to CY-induced depletion [40]. The results suggest the potential role of Fas in the early stage of diabetes. In addition, when Fas siRNA/PEI complexes were administered to CY-treated NOD mice in the late phase of the disease development (on day 9 and 15), the retardation of diabetes onset was not observed (Figure 7). This could support the hypothetical role of Fas in the early stage of diabetic pathogenesis. Critical role of Fas-FasL interactions for the initiation of type-1 diabetes was discussed in a previous report [44].
Fig. 7.
Effect of intravenous administration of Fas siRNA/PEI complexes in the late phase of diabetes development on diabetes incidence in CY-treated NOD mice (n = 10).
In this study, PEI-mediated delivery of Fas siRNA could successfully silence the target gene expression, leading to the delayed incidence of type-1 diabetes in CY-treated NOD mice. However, the biodistribution of siRNA/PEI complexes in the pancreatic islets could not be clearly observed. Recent studies also failed to clearly show the pancreatic distribution of radio isotope-labeled siRNA/PEI complexes following intravenous administration [45, 46]. This may be due to the characteristics of pancreatic islets. Pancreatic islet is highly vascularized since the islets attract endothelial cells by secreting vascular endothelial growth factor (VEGF) to induce neo-angiogenesis, which promotes β-cell proliferation and islet growth [47]. In addition, islet blood flow is very high and islet capillaries are fenestrated, which allows rapid glucose uptake and metabolite exchange by islet cells [48]. The high blood flow and the fenestrated structure of islet capillaries may facilitate the localization of siRNA/PEI complexes in the islets. However, the average number of islets in mouse pancreas is only 500 ~ 800, which only make up 1~2 % of the total pancreas cells [49]. Thus, despite the potential accumulation of probe-labeled siRNA/PEI complexes in the islets, the probe signal may not be high enough to reach a detection limit of a device. Therefore, it should be considered as a next step to design a gene delivery system which can specifically target islet beta-cells, which may provide the enhanced therapeutic effect of the siRNA and the opportunity of resolving the pancreatic biodistribution of siRNA/carrier complexes.
4. Conclusion
Although polymer-based non-viral gene carriers still have a room for improvement in terms of gene transduction efficiency, they have an advantage over its viral counterparts for the treatment of chronic diseases, such as diabetes. Synthetic carriers can be repeatedly administered for an extended period of time without safety concerns, such as a host immune stimulation and a risk of unexpected genomic insertion. In this study, we demonstrated that non-viral gene therapy based on RNAi-mediated gene silencing could effectively retard the progress of insulitis and type-1 diabetes in CY-treated NOD mice. Further studies including the development of a gene delivery system that can target pancreatic islet β-cells, Fas siRNA gene therapy in a spontaneous diabetes model in NOD mice, and the elucidation of detailed mechanisms concerning the role of Fas in early phase of diabetic pathogenesis should be carried out as next steps for the diabetes gene therapy.
Acknowledgments
This work is supported by the grant from NIH (NIDDK-DK77703), the Ministry of Education, Science and Technology, Republic of Korea (R01-2008-000-11229-0), and Korea Research Foundation (D00756 (I01778)).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kikutani H, Makino S. The murine autoimmune diabetes model: NOD and related strains. Adv Immunol. 1992;51:285–322. doi: 10.1016/s0065-2776(08)60490-3. [DOI] [PubMed] [Google Scholar]
- 2.Castano L, Eisenbarth GS. Type-I diabetes: a chronic autoimmune disease of human, mouse, and rat. Annu Rev Immunol. 1990;8:647–679. doi: 10.1146/annurev.iy.08.040190.003243. [DOI] [PubMed] [Google Scholar]
- 3.Harrison LC, Chu SX, DeAizpurua HJ, Graham M, Honeyman MC, Colman PG. Islet-reactive T cells are a marker of preclinical insulin-dependent diabetes. J Clin Invest. 1992;89(4):1161–1165. doi: 10.1172/JCI115698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker R, Bone AJ, Cooke A, Baird JD. Distinct macrophage subpopulations in pancreas of prediabetic BB/Erats. Possible role for macrophages in pathogenesis of IDDM. Diabetes. 1988;37(9):1301–1304. doi: 10.2337/diab.37.9.1301. [DOI] [PubMed] [Google Scholar]
- 5.Debussche X, Lormeau B, Boitard C, Toublanc M, Assan R. Course of pancreatic beta cell destruction in prediabetic NOD mice: a histomorphometric evaluation. Diabete Metab. 1994;20(3):282–290. [PubMed] [Google Scholar]
- 6.Shimada A, Charlton B, Taylor-Edwards C, Fathman CG. Beta-cell destruction may be a late consequence of the autoimmune process in non obese diabetic mice. Diabetes. 1996;45(8):1063–1067. doi: 10.2337/diab.45.8.1063. [DOI] [PubMed] [Google Scholar]
- 7.Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986;314(21):1360–1368. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 8.Atkinson MA, Maclaren NK. The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med. 1994;331(21):1428–1436. doi: 10.1056/NEJM199411243312107. [DOI] [PubMed] [Google Scholar]
- 9.Wong FS, Janeway CA., Jr The role of CD4 vs. CD8 T cells in IDDM. J Autoimmun. 1999;13(3):290–295. doi: 10.1006/jaut.1999.0322. [DOI] [PubMed] [Google Scholar]
- 10.Harada M, Makino S. Promotion of spontaneous diabetes in non-obese diabetes-prone mice by cyclophosphamide. Diabetologia. 1984;27(6):604–606. doi: 10.1007/BF00276978. [DOI] [PubMed] [Google Scholar]
- 11.Yasunami R, Bach JF. Anti-suppressor effect of cyclophosphamide on the development of spontaneous diabetes in NOD mice. Eur J Immunol. 1988;18(3):481–484. doi: 10.1002/eji.1830180325. [DOI] [PubMed] [Google Scholar]
- 12.Charlton B, Mandel TE. Progression from insulitis to beta-cell destruction in NOD mouse requires L3T4+ T-lymphocytes. Diabetes. 1988;37(8):1108–1112. doi: 10.2337/diab.37.8.1108. [DOI] [PubMed] [Google Scholar]
- 13.Augstein P, Elefanty AG, Allison J, Harrison LC. Apoptosis and beta-cell destruction in pancreatic islets of NOD mice with spontaneous and cyclophosphamide-accelerated diabetes. Diabetologia. 1998;41(11):1381–1388. doi: 10.1007/s001250051080. [DOI] [PubMed] [Google Scholar]
- 14.Kim YH, Kim S, Kim KA, Yagita H, Kayagaki N, Kim KW, Lee MS. Apoptosis of pancreatic beta-cells detected in accelerated diabetes of NOD mice: no role of Fas-Fas ligand interaction in autoimmune diabetes. Eur J Immunol. 1999;29(2):455–465. doi: 10.1002/(SICI)1521-4141(199902)29:02<455::AID-IMMU455>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 15.Suarez-Pinzon W, Sorensen O, Bleackley RC, Elliott JF, Rajotte RV, Rabinovitch A. Beta-cell destruction in NOD mice correlates with Fas (CD95) expression on beta-cells and proinflammatory cytokine expression in islets. Diabetes. 1999;48(1):21–28. doi: 10.2337/diabetes.48.1.21. [DOI] [PubMed] [Google Scholar]
- 16.Savinov AY, Tcherepanov A, Green EA, Flavell RA, Chervonsky AV. Contribution of Fas to diabetes development. Proc Natl Acad Sci USA. 2003;100(2):628–632. doi: 10.1073/pnas.0237359100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kagi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and per for in pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265(5171):528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 18.Chervonsky AV, Wang Y, Wong FS, Visintin I, Flavell RA, Janeway CA, Jr, Matis LA. The role of Fas in autoimmune diabetes. Cell. 1997;89(1):17–24. doi: 10.1016/s0092-8674(00)80178-6. [DOI] [PubMed] [Google Scholar]
- 19.Itoh N, Imagawa A, Hanafusa T, Waguri M, Yamamoto K, Iwahashi H, Moriwaki M, Nakajima H, Miyagawa J, Namba M, Makino S, Nagata S, Kono N, Matsuzawa Y. Requirement of Fas for the development of autoimmune diabetes in non obese diabetic mice. J Exp Med. 1997;186(4):613–618. doi: 10.1084/jem.186.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su X, Hu Q, Kristan JM, Costa C, Shen Y, Gero D, Matis LA, Wang Y. Significant role for Fas in the pathogenesis of autoimmune diabetes. J Immunol. 2000;164(5):2523–2532. doi: 10.4049/jimmunol.164.5.2523. [DOI] [PubMed] [Google Scholar]
- 21.Suarez-Pinzon WL, Power RF, Rabinovitch A. Fas ligand-mediated mechanisms are involved in autoimmune destruction of islet beta cells in non-obese diabetic mice. Diabetologia. 2000;43(9):1149–1156. doi: 10.1007/s001250051506. [DOI] [PubMed] [Google Scholar]
- 22.McManus MT, Sharp PA. Gene silencing in mammals by small interfering RNAs. Nat Rev Genet. 2002;3(10):737–747. doi: 10.1038/nrg908. [DOI] [PubMed] [Google Scholar]
- 23.Hannon GJ, Rossi JJ. Unlocking the potential of the human genome with RNA interference. Nature. 2004;431(7006):371–378. doi: 10.1038/nature02870. [DOI] [PubMed] [Google Scholar]
- 24.Caplen NJ, Parrish S, Imani F, Fire A, Morgan RA. Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate systems. Proc Natl Acad Sci USA. 2001;98(17):9742–9747. doi: 10.1073/pnas.171251798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeong JH, Kim SW, Park TG. Molecular design of functional polymers for gene therapy. Progress in Polymer Science. 2007;32:1239–1274. [Google Scholar]
- 26.Jeong JH, Mok H, Oh YK, Park TG. siRNA conjugate delivery systems. Bioconjug Chem. 2009;20(1):5–14. doi: 10.1021/bc800278e. [DOI] [PubMed] [Google Scholar]
- 27.Lee M, Ko KS, Oh S, Kim SW. Prevention of autoimmune insulitis by delivery of a chimeric plasmid encoding interleukin-4 and interleukin-10. J Control Release. 2003;88(2):333–342. doi: 10.1016/s0168-3659(03)00031-2. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu J, Kanagawa O, Unanue ER. Presentation of beta-cell antigens to CD4+ and CD8+ T cells of non-obese diabetic mice. J Immunol. 1993;151(3):1723–1730. [PubMed] [Google Scholar]
- 29.Kurrer MO, Pakala SV, Hanson HL, Katz JD. Beta cell apoptosis in T cell-mediated autoimmune diabetes. Proc Natl Acad Sci USA. 1997;94(1):213–218. doi: 10.1073/pnas.94.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koh JJ, Ko KS, Lee M, Han S, Park JS, Kim SW. Degradable polymeric carrier for the delivery of IL-10 plasmid DNA to prevent autoimmune insulitis of NOD mice. Gene Ther. 2000;7(24):2099–2104. doi: 10.1038/sj.gt.3301334. [DOI] [PubMed] [Google Scholar]
- 31.Ko KS, Lee M, Koh JJ, Kim SW. Combined administration of plasmids encoding IL-4 and IL-10 prevents the development of autoimmune diabetes in nonobese diabetic mice. Mol Ther. 2001;4(4):313–316. doi: 10.1006/mthe.2001.0459. [DOI] [PubMed] [Google Scholar]
- 32.Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92(16):7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdallah B, Hassan A, Benoist C, Goula D, Behr JP, Demeneix BA. A powerful nonviral vector for in vivo gene transfer into the adult mammalian brain: polyethylenimine. Hum Gene Ther. 1996;7(16):1947–1954. doi: 10.1089/hum.1996.7.16-1947. [DOI] [PubMed] [Google Scholar]
- 34.Koshkina NV, Agoulnik IY, Melton SL, Densmore CL, Knight V. Biodistribution and pharmacokinetics of aerosol and intravenously administered DNA-polyethyleneimine complexes: optimization of pulmonary delivery and retention. Mol Ther. 2003;8(2):249–254. doi: 10.1016/s1525-0016(03)00177-1. [DOI] [PubMed] [Google Scholar]
- 35.Bragonzi A, Dina G, Villa A, Calori G, Biffi A, Bordignon C, Assael BM, Conese M. Biodistribution and transgene expression with nonviral cationic vector/DNA complexes in the lungs. Gene Ther. 2000;7(20):1753–1760. doi: 10.1038/sj.gt.3301282. [DOI] [PubMed] [Google Scholar]
- 36.Kawakami S, Ito Y, Charoensit P, Yamashita F, Hashida M. Evaluation of proinflammatory cytokine production induced by linear and branched polyethylenimine/plasmid DNA complexes in mice. J Pharmacol Exp Ther. 2006;317(3):1382–1390. doi: 10.1124/jpet.105.100669. [DOI] [PubMed] [Google Scholar]
- 37.Bonnet ME, Erbacher P, Bolcato-Bellemin AL. Systemic delivery of DNA or siRNA mediated by linear polyethylenimine (L-PEI) does not induce an inflammatory response. Pharm Res. 2008;25(12):2972–2982. doi: 10.1007/s11095-008-9693-1. [DOI] [PubMed] [Google Scholar]
- 38.Meyer M, Philipp A, Oskuee R, Schmidt C, Wagner E. Breathing life into polycations: functionalization with pH-responsive endosomolytic peptides and polyethyleneglycol enables siRNA delivery. J Am Chem Soc. 2008;130(11):3272–3273. doi: 10.1021/ja710344v. [DOI] [PubMed] [Google Scholar]
- 39.Augstein P, Dunger A, Salzsieder C, Heinke P, Kubernath R, Bahr J, Fischer U, Rettig R, Salzsieder E. Cell surface trafficking of Fas in NIT-1 cells and dissection of surface and total Fas expression. Biochem Biophys Res Commun. 2002;290(1):443–451. doi: 10.1006/bbrc.2001.6215. [DOI] [PubMed] [Google Scholar]
- 40.Ablamunits V, Quintana F, Reshef T, Elias D, Cohen IR. Acceleration of autoimmune diabetes by cyclophosphamide is associated with an enhanced IFN-gamma secretion pathway. J Autoimmun. 1999;13(4):383–392. doi: 10.1006/jaut.1999.0331. [DOI] [PubMed] [Google Scholar]
- 41.Urban-Klein B, Werth S, Abuharbeid S, Czubayko F, Aigner A. RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)- complexed siRNA in vivo. Gene Ther. 2005;12(5):461–466. doi: 10.1038/sj.gt.3302425. [DOI] [PubMed] [Google Scholar]
- 42.Matos M, Park R, Mathis D, Benoist C. Progression to islet destruction in a cyclophosphamide-induced transgenic model: a microarray overview. Diabetes. 2004;53(9):2310–2321. doi: 10.2337/diabetes.53.9.2310. [DOI] [PubMed] [Google Scholar]
- 43.Nagata S, Golstein P. The Fas death factor. Science. 1995;267(5203):1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 44.Nakayama M, Nagata M, Yasuda H, Arisawa K, Kotani R, Yamada K, Chowdhury SA, Chakrabarty S, Jin ZZ, Yagita H, Yokono K, Kasuga M. Fas/Fas ligand interactions play an essential role in the initiation of murine autoimmune diabetes. Diabetes. 2002;51(5):1391–1397. doi: 10.2337/diabetes.51.5.1391. [DOI] [PubMed] [Google Scholar]
- 45.Malek A, Merkel O, Fink L, Czubayko F, Kissel T, Aigner A. In vivo pharmacokinetics, tissue distribution and underlying mechanisms of various PEI (-PEG)/siRNA complexes. Toxicol Appl Pharmacol. 2009;236(1):97–108. doi: 10.1016/j.taap.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 46.Merkel OM, Librizzi D, Pfestroff A, Schurrat T, Behe M, Kissel T. In vivo SPECT and real-time gamma camera imaging of biodistribution and pharmacokinetics of siRNA delivery using an optimized radiolabeling and purification procedure. Bioconjug Chem. 2009;20(1):174–182. doi: 10.1021/bc800408g. [DOI] [PubMed] [Google Scholar]
- 47.Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fassler R, Gu G, Gerber HP, Ferrara N, Melton DA, Lammert E. The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev Cell. 2006;10(3):397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 48.Van Schaftingen E, Schuit F. Advances in Molecular and Cellular Biology: The Biology of the Pancreatic beta-cell. JAI Press; Stamford, CT: 1999. [Google Scholar]
- 49.Haschek WM, Rousseaux CG. Fundamentals of Toxicologic Pathology: Part II. Endocrine Pancreas. Academic Press; San Diego, CA: 1998. [Google Scholar]