Abstract
Glioblastoma multiforme (GBM) is the most aggressive brain tumor that, by virtue of its resistance to chemo- and radiotherapy, is currently incurable. Identification of molecules whose targeting may eliminate GBM cells and/or sensitize glioblastoma cells to cytotoxic drugs is therefore urgently needed. CD44 is a major cell surface hyaluronan receptor and cancer stem cell marker that has been implicated in the progression of a variety of cancer types. However, the major downstream signaling pathways that mediate its pro-tumor effects and the role of CD44 in the progression and GBM chemoresponse of GBM have not been established. Here we show that CD44 is up-regulated in GBM and that its depletion blocks GBM growth and sensitizes GBM cells to cytotoxic drugs in vivo. Consistent with this observation, CD44 antagonists potently inhibit glioma growth in preclinical mouse models. We provide the first evidence that CD44 functions upstream of the mammalian Hippo signaling pathway and that CD44 promotes tumor cell resistance to reactive oxygen species- and cytotoxic agent-induced stress by attenuating activation of the Hippo signaling pathway. Together, our results identify CD44 as a prime therapeutic target for GBM, establish potent anti-glioma efficacy of CD44 antagonists, uncover a novel CD44 signaling pathway, and provide a first mechanistic explanation as to how up-regulation of CD44 may constitute a key event in leading to cancer cell resistance to stresses of different origins. Finally, our results provide a rational explanation for the observation that functional inhibition of CD44 augments the efficacy of chemo- and radiation therapy.
Keywords: CD44, glioblastoma, mammalian Hippo signaling pathway, chemosensitizer, therapeutic target
Introduction
It is now well established that the host tissue microenvironment provides an essential contribution to cancer progression and modulates the response of cancer cells to treatments, implying that elements within the tumor microenvironment constitute potentially important targets for anti-cancer therapy (1–4). The tumor microenvironment consists of resident and infiltrating host cells as well the components of the extracellular matrix (ECM). Physical interactions and functional cross-talk between tumor cells and their microenvironment are mediated primarily by cell surface receptors that are responsible for the cell-cell and cell-ECM adhesion thereby providing potentially attractive therapeutic targets (5).
Malignant gliomas is the most common type of primary brain cancer and the most malignant being grade IV astrocytoma, also known as glioblastoma multiforme (GBM) (6). Despite aggressive multimodal therapy, GBM remains incurable, with an estimated median survival of less than 1 year and with less than 3% of patients surviving longer than 5 years (6–7). Identification of new therapeutic targets and means to improve the efficacy of existing forms of therapy are therefore urgently needed. The central nervous system contains elevated levels of glycosaminoglycan hyaluronan (HA) (8) and glioma cells express the major cell surface HA receptor, CD44 (9), which is implicated in diverse cellular functions (10–12). CD44 is up regulated in a broad range of malignant tumors and its elevated expression correlates with poor prognosis of some cancer types (9–12). CD44 plays an important role in melanoma (13–14) and breast cancer (15–17) growth and progression but little is known about its contribution to the progression of GBM and the response of GBM cells to chemotherapy. CD44 has been associated with numerous signaling events and serves as a co-receptor of several receptor tyrosine kinases (RTKs) (9–12) but no single major downstream signaling pathway of CD44 has been defined to date.
The cytoplasmic domain of CD44 interacts with ezrin-radixin-moesin (ERM) family proteins (18) and merlin (19–20), which serve as linkers between cortical actin filaments and the plasma membrane (21–22). In Drosophila, merlin functions upstream of the Hippo (Hpo) signaling pathway that plays an important role in restraining cell proliferation and promoting apoptosis (23–26). Although still incompletely characterized, the Hippo pathway is believed to be conserved in mammals (27–28). Mammalian homologs of Hpo, Warts (Wts), Yorkie (Yki), and dIAP are, respectively, Mammalian Sterile Twenty-like (MST) kinase1 and 2 (MST1/2) (26, 29), Large tumor suppressor homolog 1 and 2 (Lats1 and 2) (30), Yes-Associated Protein (YAP) (31), and cellular Inhibitor of Apoptosis (cIAP1/2)(28). However, the upstream components of the mammalian Hippo signaling pathway have not been identified.
We have shown recently that merlin activates the Lats2 signaling pathway in human glioblastoma cells, sensitizes the response of GBM cells to chemotherapeutic agents, and inhibits GBM growth in vivo (27) and that merlin exerts its tumor suppressor function by inhibiting the hyaluronan-CD44 interaction (32). We therefore addressed the possibility that CD44 may play a potentially important role in malignant glioma growth, progression, and response to chemotherapy by regulating the mammalian Hippo signaling pathway. Our results demonstrate that CD44 is up-regulated in human GBM and that knockdown of CD44 expression inhibits GBM cell growth and/or sensitizes GBM cells to cytotoxic drugs in vivo. Moreover, we provide the first evidence that CD44 functions upstream of mammalian Hippo signaling pathway and protects GBM cells from reactive oxygen species (ROS) and cytotoxic agent-induced stress and apoptosis by attenuating the activity of the entire Hippo signaling pathway and that of the downstream JNK/p38 kinases and by decreasing induction of p53 expression. We further demonstrate that purified soluble CD44-Fc fusion proteins potently inhibit growth of pre-established intracranial GBM in mice and significantly extend survival of the animals. Our results thus identify CD44 as an important therapeutic target for GBM and help establish CD44 antagonists as potent anti-GBM agents. They also uncover the first candidate signaling pathway whose activation appears to be directly dependent on CD44 dysfunction, and provide a mechanistic basis for CD44 involvement in GBM progression and chemoresistance.
Materials and Methods
Patient Glioma Samples and Reagents
The glioma tissues were obtained from Cooperative Human Tissue Network (CHTN). Anti -MST1/2, -Lats1/2 (Bethyl Lab), -CD44, -Erk1/2, -AKT, -JNK, -p21, -p38, -p53, -cIAP1/2, and -merlin (Santa Cruz), -actin (Sigma), -v5 epitope, -phospho-merlin, -puma (Invitrogen), -cleaved caspase 3, -phospho-Erk1/2, -phospho-AKT, -phospho-JNK, -phospho-p38, -phospho-MST1/2, -phospho-Lats1, -phospho-YAP (Cell signaling), -YAP, -phospho-Lats2 (Abnova), and –heparan sulfate (HS, CalBiochem) antibodies were used in the experiments. Apoptag kit was from Chemicon and anti-Brdu from Roche.
CD44-Fc Fusion Expression Constructs, Mutagenesis, and Knockdown Constructions
Three human soluble CD44-Fc (hsCD44) fusion proteins were generated by fusing the extracellular domain of human CD44v3-v10, CD44V8-v10, or CD44s to the constant region (Fc) of human IgG1. R41A mutants of these CD44-Fc fusion proteins were generated using the QuikChange™ mutagenesis kits (Stratagene) as described (16). Several shRNAmir and TRC constructs against human CD44 and a non-targeting shRNAmir and a non-targeting TRC control shRNA were obtained from Open Biosystems and Addgene.
Production and Purification of Soluble CD44-Fc and Soluble CD44R41A-Fc Fusion Proteins
COS-7 cells infected with the retroviruses carrying hsCD44v3-v10-Fc, hsCD44v8-v10-Fc, hsCD44s-Fc, hsCD44v3-v10R41A-Fc, hsCD44v8-v10R41A-Fc, and hsCD44sR41A-Fc constructs were cultured in RPMI medium containing 10% fetal bovine serum (FBS) until confluence and were then switched to serum free RPMI medium (SFM) for an additional three days of culture. The conditioned cell culture medium was retrieved and the fusion proteins purified on protein A columns (GE Healthcare Biosciences). Before elution from protein A column, some of preparations of the bound CD44-Fc fusion proteins were treated heparinase I (10 units/ml) and heparinase III (2 unit/ml) at 37 °C for 4 h.
Western Blot Analysis, Immunocytochemistry, and Fluorescein-labeled HA (FL-HA) Binding Assay
Western blots and immunocytochemistry were performed as described (27, 32). To assess FL-HA binding, GBM cells were cultured in 35-mm dishes for 24–48 h; 20 µ;g/ml FL-HA was added to the cultured cells for an additional 12 h. The FL-HA binding assay was performed as described previously (33).
Subcutaneous and Intracranial Tumor Growth Experiments, and Bioluminescence Imaging Analysis of the Intracranial Gliomas
Mice were used in accordance with the approved IACUC Protocol. Pooled populations of transduced U87MG and U251 glioma cells were used in subcutaneous and intracranial tumor growth experiments as described (27). Briefly, 2 or 5 ×106 glioma cells were injected subcutaneously into each immunocompromised B6.129S7-Rag1tmMom (Rag1, Jackson Lab) mouse. For the intracranial tumor growth experiments, U87MG (4×105cells in 10µl HBSS/Rag1 mouse)/U251 cells (2×105cells in 10µl HBSS/Rag1 mouse) were injected as described (27). Following the injections, mice were monitored closely and the duration of their survival was recorded. Mice that showed signs of distress and morbidity were euthanized and considered as if they had died on that day. Survival rates were calculated as follows: survival rate (%) = (number of mice still alive/total number of experimental mice) × 100%. Bioluminescence-imaging approach is used to monitor the growth of intracranial gliomas in live animal as described (27) using IVIS-200 imaging system (Xenogen). Images were obtained 12min after injection of D-luciferin using the same intensity scaling.
Statistics
One-way ANOVA statistical analyses were performed to analyze differences of the tumor volumes and growth rates between the control and experimental groups. LogRank analyses were performed for the survival experiments. Differences were considered statistically significant at p<0.05.
Results
CD44 is up regulated in human GBM
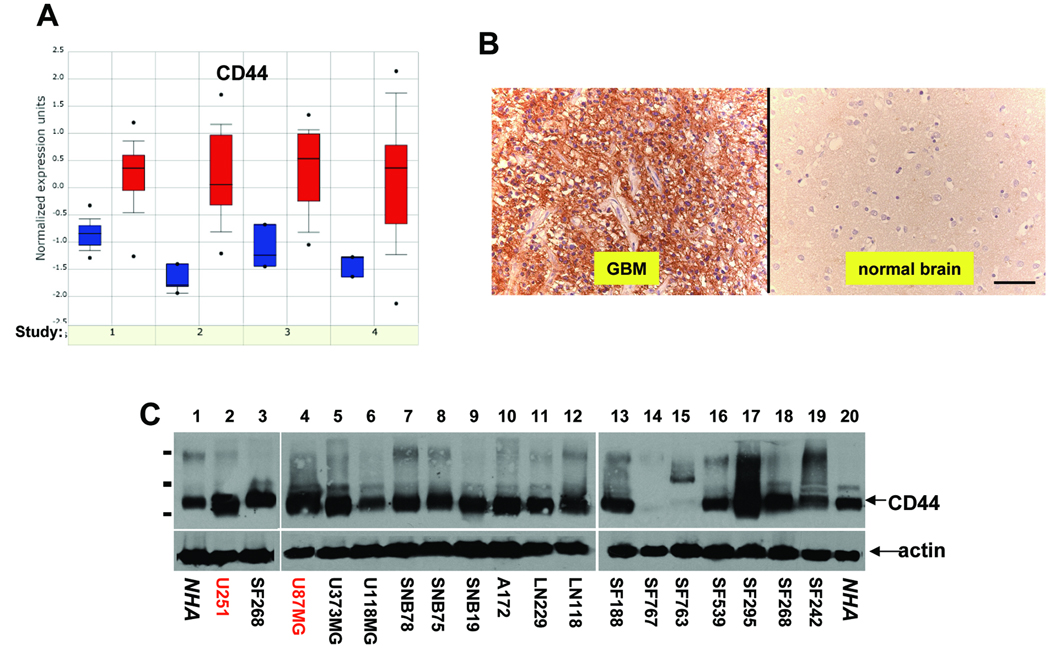
To determine CD44 expression level in GBM, we mined available gene expression datasets at www.oncomine.org (34). In all four independent datasets, CD44 transcripts were consistently up regulated in human GBM compared to either normal brain (Fig 1A, studies 1, 2, and 4) (35–37) or normal white matter (study 3)(38). In addition, immunohistochemistry analysis of paraffin sections of primary tumors showed that CD44 was up regulated in all 14 GBM cases analyzed compared to eight cases of normal human brain (Fig 1B and data not shown).
Figure 1. CD44 is up-regulated in human glioma.
A, Microarray data sets form oncomine were analyzed for CD44 transcript levels in multiple glioma tissues compared to normal human brain tissues (study 1, 2, and 4) or to normal white matter (study 3). B, Representative pictures of CD44 immunohistochemistry performed on 14 GBM tissues and 8 normal human brain samples using anti-CD44 antibody (Santa Cruz). Bar, 50µm. C, Expression of endogenous CD44 in a panel of human glioma cells and normal human astrocytes (NHAs, ALLCELLS, Inc.) was determined by Western blotting using anti-CD44 antibody (Santa Cruz). 50µg of total proteins were loaded in each lane. Actin was included as an internal control for loading (lower panels). The molecular weight bars correspond to 197kDa, 110kDa, and 72kDa.
To address the role of CD44 in GBM growth and progression, we analyzed CD44 protein levels in a variety of human glioma cell lines. We found that the majority of the glioma cells tested express higher levels of CD44 than normal human astrocytes (NHAs) and that the standard 85–90 kDa form (CD44s, Fig 1C) is the predominant isoform expressed. Based on their high CD44 expression level and their tumorigenicity in immunocompromised mice, U87MG and U251 glioma cells were selected to investigate how CD44 contributes to GBM growth and progression.
Lenti-viral based shRNAs effectively knock down CD44 expression in human GBM cells
To knock down endogenous CD44 expression in GBM cells, we screened a set of human CD44-specific shRNA constructs (shRNA-TRC-CD44#1-#5 and shRNAmir-CD44#1-#3, Open Biosystems) Non-targeting (NT) control shRNAs (shRNA-TRC-NT and shRNAmir-NT) were included as the negative controls. We found that two out of three shRNAmirs (shRNAmir-CD44#1 and #3) and 1–2 TRC-shRNA (shRNA-TRC-CD44#3 and/or #4) knocked down CD44 expression efficiently in the U87MG and U251 GBM cells, as assessed by real-time qPCRs (data not shown), western blot analysis (supplemental Fig 1A), and immunocytochemistry (supplemental Fig 1B) whereas non-targeting controls displayed no effect. Furthermore, effective knockdown of CD44 expression dramatically reduced binding and endocytosis of FL-HA, whereas non-targeting shRNAs had no effect (supplemental Fig 1C).
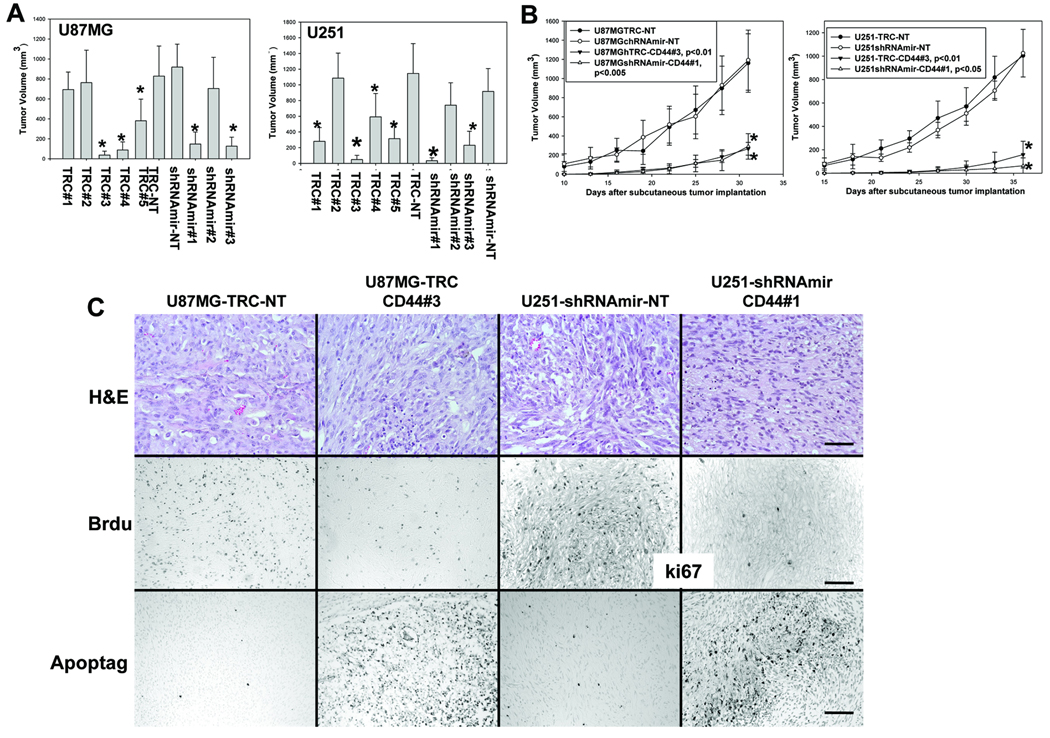
Knockdown of CD44 expression inhibits subcutaneous growth of GBM cells by inhibiting their proliferation and promoting their apoptosis in vivo
Pooled populations of the transduced U87MG and U251 cells with different degrees of CD44 depletion were used in subcutaneous (s.c.) tumor growth experiments. Decreased CD44 expression in these cells correlated with reduced tumor volume (Fig 2A) and significantly reduced growth rates in vivo (Fig 2B). We observed that the shRNAs that knocked down CD44 expression, but not the non-targeting shRNAs, inhibited glioma cell proliferation (Fig 2C, middle panels) and promoted apoptosis in vivo (Fig 2C, bottom panels), consistent with the notion that CD44 plays an important role in regulating GBM cell growth and survival.
Figure 2. Knockdown of CD44 expression inhibits subcutaneous growth of U87MG and U251 glioma cells by inhibiting proliferation and promoting apoptosis of the cells in vivo.
5 ×106 glioma cells were injected subcutaneously per mouse. A, The effects of CD44 knockdown on subcutaneous growth of U87MG (left) and U251 (right) cells infected with different shRNA constructs were assessed by measuring tumor volume 5 weeks after tumor cell implantation. B, Growth rates of the subcutaneous tumors derived from U87MG (left) and U251 (right) cells infected with different shRNA constructs were determined and expressed as the mean of tumor volumes (mm3) +/− SD. Six mice were used for each type of transduced glioma cells in panel A and B. C, Morphology, proliferation and apoptosis status of subcutaneous gliomas were assessed. Tumor sections were stained with H&E for histology (C, top panels). In vivo glioma cell proliferation was detected using an anti-BrdU (Roche, C, middle, first two panels) and an anti-k67 antibody (C, middle, last two panels). Apoptotic GBM cells in situ were detected using Apoptag kit (Chemicon, C, bottom panels). Bar, in a-d, 50µm and in e-l, 100 µm.
Knockdown of CD44 expression inhibits intracranial GBM growth
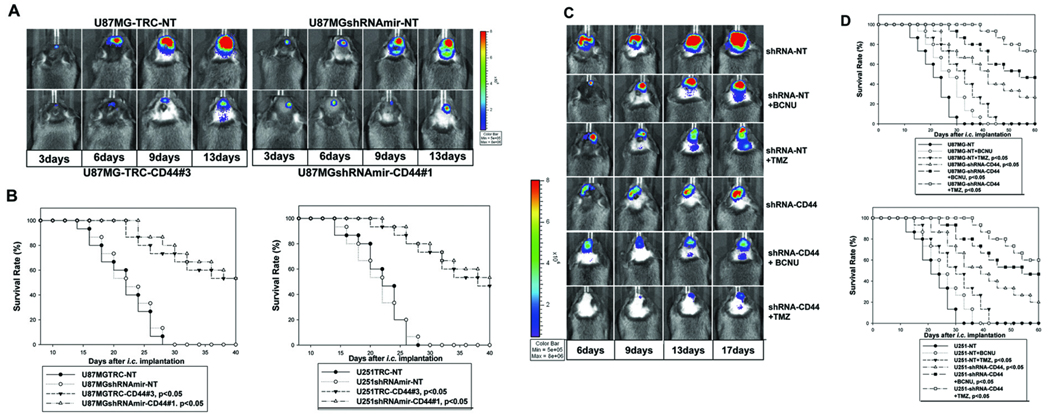
To determine how CD44 knockdown affects intracranial glioma growth, the double drug-resistant pooled populations of U87MG-Luc/U251-Luc cells that express luciferase and display efficient CD44 knockdown were injected intracranially into Rag-1 mice. We found that suppression of CD44 expression significantly inhibited intracranial tumor growth and extended the survival of the experimental mice compared to the animals injected with U87MG/U251-Luc cells transduced with non-targeting shRNAs (Fig 3A–B).
Figure 3. Knockdown of CD44 expression inhibits intracranial growth of U87MG and U251 gliomas and sensitizes responses of the glioma cells to cytotoxic drugs in vivo.
A, Bio-luminescence imaging analysis of mice 3, 6, 9, and 13 days following the intracranial injection of U87MG-TRC-NT, U87MGshRNAmir-NT controls (upper panels), and U87MG-TRC-CD44#3 and U87MGshRNAmir-CD44#1 (shRNAs effectively knock down CD44 expression, bottom panels). B, Survival rates of mice following intracranial injections of the transduced U87MG (B, left) and U251 (B, right) cells as detailed in the panels. 15 mice were used for each type of transduced GBM cells. C, Bioluminescence imaging analysis of mice 6, 9, 13, and 17 days following intracranial injection of U87MG-NT (shRNA controls), or U87MGshRNA-CD44 (a mixture of shRNAs effectively knocks down CD44 expression). Mice were injected with or without a single dose of BCNU (10 mg/kg) or TMZ (5mg/kg) 3 days after intracranial injection of the tumor cells. D, Survival rates of mice following intracranial injections of the transduced U87MG (top) and U251 (bottom) cells are shown in the panels. 15 mice were used for each treatment.
Reduced CD44 expression sensitizes GBM cells to cytotoxic drugs in vivo
The first-line cytotoxic drugs for GBM are temozolomide (TMZ) and carmustine (BCNU). We therefore investigated whether reduced CD44 expression sensitizes glioma cells to BCNU and TMZ treatment in vivo. We observed that BCNU and TMZ displayed a weak and a moderate inhibitory effect on the glioma growth, respectively, when used as single agents (Fig 3C). However, CD44 depletion sensitized the response of U87MG and U251 GBM cells to BCNU and TMZ and the combination of CD44 knockdown and treatment with BCNU or TMZ resulted in a synergistic inhibition of intracranial glioma progression as determined by markedly prolonged median survival of the experimental mice (Fig 3D).
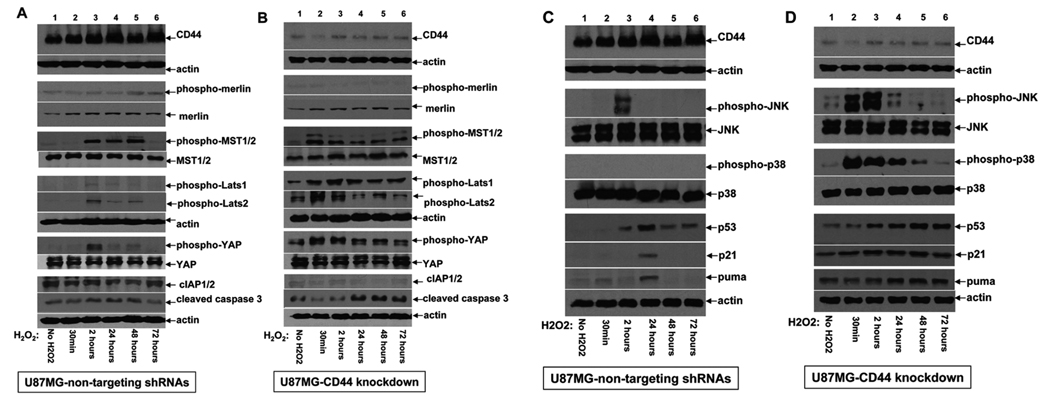
CD44 attenuates activation of the mammalian equivalent of Hippo signaling pathway and plays a key role in regulating stress and apoptotic responses of human GBM cells
Radiation therapy and some cytotoxic agents generate reactive oxygen species (ROS) that constitute a major inducer of cell death and underlie their anti-glioma effects. To address the molecular mechanisms that underlie the observed chemosensitizing effect of CD44 depletion on glioma cells, we investigated how reduced CD44 expression affects GBM cell response to oxidative stress induced by H2O2 and cytotoxic stress induced by TMZ. Our results showed that the GBM cells depleted of CD44 responded to oxidative stress with robust and sustained phosphorylation/activation of MST1/2 and Lats1/2, phosphorylation/inactivation of YAP, and reduced expression of cIAP1/2 (Fig 4A–B). These effects correlated with reduced phosphorylation/activation of merlin, increased levels of cleaved caspase-3, and reduced cell viability (Fig 4B and 2C, and data not shown). By contrast, high expression of endogenous CD44 promoted phosphorylation/inactivation of merlin, attenuated stress induced activation of entire mammalian equivalent of Hippo signaling pathway, and up-regulated cIAP1/2 leading to inhibition of caspase-3 cleavage and apoptosis (Fig 4A, 2C, data not shown). Together, for the first time, these results place CD44 upstream of the mammalian Hippo signaling pathway (merlin-MST1/2-Lats1/2-YAP-cIAP1/2) and suggest a functional role of CD44 in attenuating tumor cell responses to stress and stress-induced apoptosis.
Figure 4. CD44 functions upstream of the Hippo signaling pathway and plays an essential role in attenuating the apoptotic response of glioma cells induced by oxidative stress.
Western blots were performed using the cell lysates derived from U87MG-NT cells infected with a mixture lentiviruses carrying non-targeting shRNAs (A and C), and U87MGshRNA-CD44 cells infected with a mixture of lentiviruses carrying CD44-specific shRNAs (B and D). These cells were treated with or without 60µm H2O2 for 30min, 2h, 24h, 48h, and 72h as indicated in the panels. 100µg of total protein were loaded in each lane. Actin was included as an internal control for protein loading. The antibodies used against the different signaling mediators are indicated in the panels.
Because MST1/2 kinases have multiple downstream effectors, we investigated whether known effectors of MST1/2 also function downstream of the newly discovered CD44-MST1/2 signaling axis. Knockdown of CD44 was observed to result in elevated and sustained activation of JNK and p38 stress kinases in U87MG cells exposed to oxidative stress, as well as sustained up-regulation of p53, a known downstream effector of JNK/p38, and its target genes, p21 and puma (Fig 4D). By contrast, GBM cells with high levels of endogenous CD44 displayed transient and attenuated activation of JNK/p38 and induction of p53, p21, and puma (Fig 4C).
To address the mechanism whereby CD44 depletion sensitizes glioma cells to cytotoxic drugs in vivo (Fig 3C–D), we performed experiments similar to those outlined in Figure 4 but using TMZ instead of H2O2 to induce cytotoxic stress in U87MG cells expressing a high or low level of CD44. Similar to their response to oxidative stress, GBM cells depleted of CD44 mounted more robust and sustained activation of MST1/2 upon exposure to TMZ, along with phosphorylation/inactivation of YAP that correlates with reduced levels of cIAPs, activation of p38 but not JNK, and up-regulation p53 and its target gene p21 (Supplemental Fig 2). Together, these results establish a novel CD44 signaling pathway and a novel role of CD44 in inhibiting stress/apoptotic responses of tumor cells by attenuating activation of the mammalian Hippo signaling pathway, and provide a first mechanistic explanation as to how up-regulation of CD44 in advanced/malignant cancers may constitute a key event in leading to their resistance to stress of various origins, including host defense and therapeutic intervention.
CD44 modulates ErbB and c-Met receptor tyrosine kinase (RTK) mediated growth-signaling pathways in glioma cells
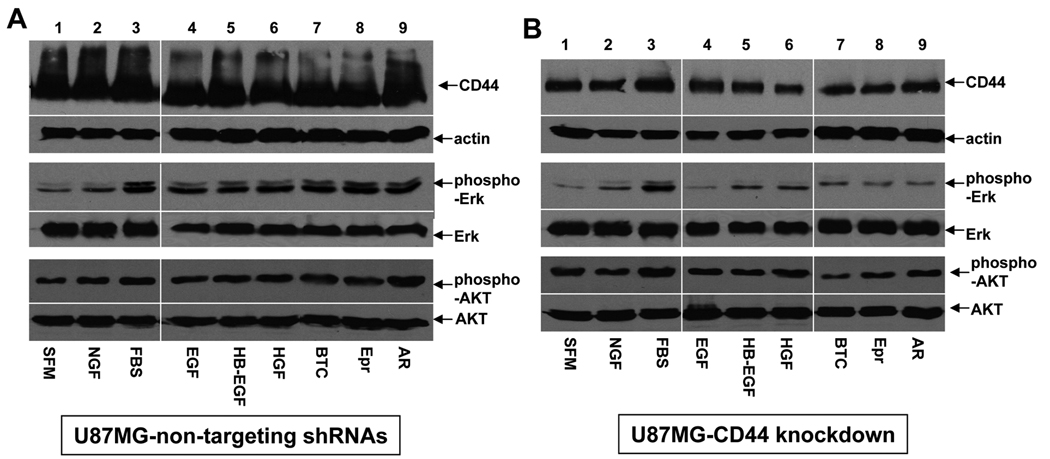
Our in vivo results have shown that CD44 knockdown inhibits proliferation of GBM cells in vivo (Fig 2C). Previous studies have shown that CD44 is a co-stimulator of ErbB and c-Met RTK signaling pathways (12, 39–40). To determine whether CD44 knockdown diminishes the activation of downstream signaling pathways induced by EGF family ligand- and HGF in GBM cells, we treated serum starved CD44-high or -low U87MG cells with EGF family ligands, HGF, NGF, and 10% FBS. Our results showed that reduced CD44 expression diminished EGF family ligand- and HGF- but not NGF- and FBS-induced phosphorylation of Erk1/2 kinase but not that of AKT kinase (Fig 5), suggesting that CD44 preferentially modulates proliferation but not survival signaling pathways activated by these growth factors.
Figure 5. CD44 enhances the ErbB and c-Met receptor tyrosine kinase (RTK) mediated activation of Erk1/2 kinase.
Western blots were performed using the cell lysates derived from U87MG-TN (A) and U87MGshRNA-CD44 cells (B). The serum starved transduced U87MG cells were treated with or without FBS, NGF (10ng/ml), EGF (2ng/ml), HB-EGF (5ng/ml), betacellulin (BTC, 5ng/ml), epiregulin (Epr, 5ng/ml), amphiregulin (AR, 5ng/ml), or HGF (20ng/ml) for 12h as indicated in the panels. The growth factors and antigens to which corresponding antibodies were used are indicated. 100µg of total protein were loaded in each lane. Actin was included as an internal control for protein loading.
Antagonists of CD44, hsCD44v3-v10-Fc, hsCD44v8-v10-Fc, and hsCD44s-Fc, serve as effective therapeutic agents against human GBM in mouse models
To determine whether CD44 antagonists can be used to inhibit GBM progression in preclinical mouse models, we developed several fusion proteins composed of the constant region of human IgG1 (Fc) fused to the extracellular domain of human CD44v3-v10, CD44v8-v10, or CD44s. Successful attempts to generate antagonists of RTKs and CD44 using this approach have been reported (41–42). Receptor-Fc fusion proteins are believed to function by trapping ligands and/or interfering with endogenous receptor functions.
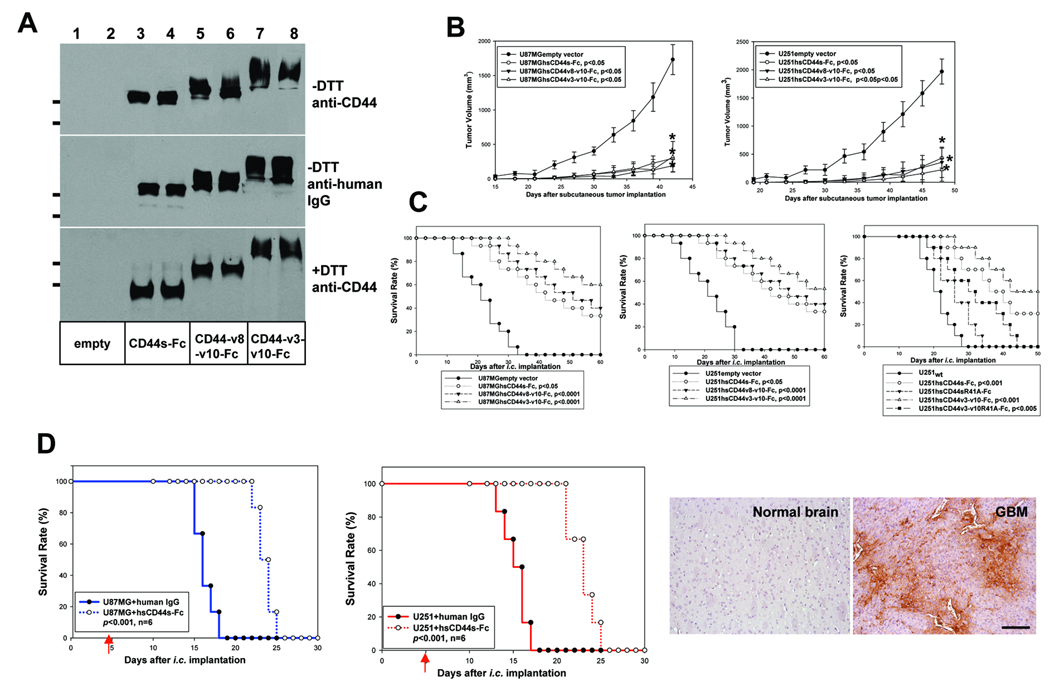
U87MG and U251 cells were transduced with retroviruses carrying the expression constructs encoding these CD44-Fc fusion proteins or empty expression vector. Pooled puromycin resistance cells expressed high levels of hsCD44v3-v10-Fc, hsCD44v8-v10-Fc, and hsCD44s-Fc fusion proteins (Fig 6A, supplemental Fig 3A). We first assessed whether the soluble CD44-Fc fusion proteins are capable of altering FL-HA binding to endogenous GBM cell surface CD44 and found that expression of the fusion proteins reduced binding of FL-HA to the GBM cells (Supplemental Fig 3B). These cells were then compared to empty vector-transfected cells for subcutaneous and intracranial growth in Rag-1 mice. hsCD44v3-v10-Fc, hsCD44v8-v10-Fc, and hsCD44s-Fc expression markedly inhibited subcutaneous and intracranial growth of U87MG and U251 cells and significantly extended survival of mice bearing the intracranial tumors. The hsCD44v3-v10-Fc fusion protein displayed the most profound inhibitory effect (Fig 6B and C).
Figure 6. Antagonists of CD44, hsCD44v3-v10-Fc, hsCD44v8-v10-Fc, and hsCD44s-Fc, are effective therapeutic agents against human GBM in mouse models.
A, U87MG and U251 cells were transduced with the retroviruses carrying empty vector and the expression constructs of hsCD44s-Fc, hsCD44v8-v10-Fc, and hsCD44v3-v10 and their serum free cell culture supernatants were collected and analyzed on Western blots using anti-CD44 antibody (Santa Cruz) or anti-human IgG antibody. The molecular weight bars correspond to 199kDa and 116 kDa. B, 2 ×106 of the transduced U87MG and U251 cells were injected subcutaneously per mouse. Growth rates of the subcutaneous tumors were determined and expressed as the mean of tumor volume (mm3) +/− SD. Six mice were used for each construct. C, Survival rates of mice following the intracranial injections of transduced U87MG (C, left) and U251 (C, right) cells. 15 mice were used for each type of transduced glioma cells in first two panels and ten mice were used in the last panel. D, Treatment of pre-established intracranial U87MG and U251 gliomas with intravenous delivery of 5mg/kg purified hsCD44s-Fc fusion proteins or human IgG every third day. The results show that hsCD44s-Fc but not human IgG significantly extended the survival of the experimental mice (p<0.001). Six Rag-1 mice were used for each treatment. Distribution of hsCD44s-Fc fusion proteins in intracranial gliomas and normal adjacent brain tissues are shown. The fusion proteins were detected using anti-human IgG antibody. Bar, 60 µm.
CD44 plays an important role in regulating immune and inflammatory responses, which affect tumor progression (43). Our experiments thus far were performed in Rag-1 mice that have innate immunity but no mature T or B cells. To determine whether CD44-Fc fusion proteins display similar anti-GBM activity in the mice with functional adaptive immunity, we assessed their effect on intracranial growth of a mouse glioma cell, GL261, in immunocompetent mice. GL261 cells form aggressive invasive intracranial tumors in syngeneic C57BL/6 mice that have intact adaptive immunity. Overexpression of hsCD44-Fc proteins in GL261 cells inhibited their intracranial growth in C57BL/6 mice similar to their effect on U87MG/U251 human GBM cells in Rag-1 mice (data not shown), suggesting that the results obtained were not significantly affected by lack of the adaptive immunity in Rag-1 mice and are broadly applicable.
The v3 exon of CD44 contains a Ser-Gly-Ser-Gly motif for covalent attachment of heparan sulfate (HS) side chains (44). To assess whether hsCD44v3-v10-Fc proteins are modified by HS, purified hsCD44s-Fc, hsCD44v8-v10-Fc, and hsCD44v3-v10-Fc fusion proteins were treated with or without heparinase I/III before elution from protein A columns. These proteins were then coated on Elisa plates in triplicate. After blocking with BSA, the coated proteins were tested for reactivity with anti-HS antibody. The intensity of the reaction, as assessed by a colorimetric assay, was normalized by the reactivity with anti-CD44 antibody, which provides relative quantity of the coated fusion proteins on the plates. Our results showed that only hsCD44v3-v10-Fc was modified by HS and stained positively with anti-HS antibody. The observed reactivity was sensitive to heparinase I/III treatment (supplemental Figure 3C).
CD44 has multiple ligands including HA, osteopontin, heparin binding growth factors, fibronectin, serglycin, laminin, MMP-9, and fibrin (9–12, 44) and cooperates with several RTKs and other cell surface receptors (9, 11, 40). Many of CD44 functions are mediated through its interaction with HA (12) that is abolished by the single R41A mutation (45). To determine whether the CD44-HA interaction alone is responsible for the GBM promoting activity of CD44, we generated pooled populations of U87MG and U251 cells expressing hsCD44sR41A-Fc or hsCD44v3-v10R41A-Fc and compared their anti-GBM effects with that of the wild type counterparts. We showed that unlike wild type CD44-Fc fusion proteins, CD44R41A-Fc proteins are incapable of inhibiting FL-HA binding to the GBM cells (supplemental Fig 3B and data not shown). However, whereas hsCD44sR41A-Fc displayed a weak anti-GBM effect, hsCD44v3-v10R41A-Fc retained a substantial level of anti-GBM activity (Fig 6 C right panel and data not shown), consistent with our earlier observations that hsCD44v3-v10-Fc fusion protein exerts the most potent anti-GBM effect of the three CD44-Fc fusion proteins tested, suggesting a mechanism of action in addition to trapping HA. Together, these results suggest that CD44, and especially CD44 variants, promote tumor progression both in a HA dependent and HA-independent fashion.
Finally, we assessed the anti-GBM efficacy of purified hsCD44s-Fc fusion proteins in pre-established intracranial gliomas resulting from injection of 5–105 U87MG or U251 cells into Rag-1 mice. Intracranial tumors were grown for 5 days before the mice were treated by intravenous injection of 0.9% NaCl containing 5mg/kg human IgG or purified hsCD44s-Fc fusion proteins every third day until completion of the experiments. Systemic delivery of hsCD44s-Fc fusion proteins but not human IgG markedly inhibited intracranial growth of U87MG and U251 cells and significantly (p<0.001) extended median survival of the experimental mice (Fig 6D left and middle panels). GBM and brain issue were collected at the time of mouse euthanasia, sectioned, and stained with anti-human IgG antibody to assess the bio-distribution of hsCD44-Fc fusion proteins. Our results showed that hsCD44-Fc fusion proteins readily penetrated tumor blood vessels and displayed a remarkable intra-glioma distribution pattern (Fig 6D, right panel) whereas negligible fusion proteins were observed in normal adjacent brain tissue, most likely due to the presence of an intact blood-brain-barrier (Fig 6D). These results suggest CD44-Fc proteins preferentially accumulate within the tumor tissue, which contains leaky blood vessels. Together, our results demonstrate that hsCD44-Fc fusion proteins are potentially attractive therapeutic agents for GBM.
Discussion
CD44 promotes malignant glioma progression and is a prime target for anti-glioma therapy
We have demonstrated that CD44 is up-regulated in human GBM and that knockdown of CD44 reduces GBM growth in vivo by inhibiting glioma cell proliferation and promoting apoptosis. Furthermore, we established for the first time that depletion of CD44 sensitizes GBM cells to chemotherapeutic agents in vivo and that CD44 antagonists in the form of CD44-specific shRNAs and CD44-Fc fusion proteins serve as effective GBM growth inhibitory agents in mouse models.
Current anti-cancer therapeutic strategies and target selection are heavily concentrated on frequently mutated kinases whose activity cancer cells become addicted to (46). Although these approaches are supported by notable successes, they are hampered by emergence of resistant tumor cells capable of bypassing the targeted signaling pathways through mechanisms that may be related to the frequently mutated nature of the targets themselves. It is now well accepted that therapeutic interventions targeting only a single signaling pathway, no matter how seemingly important, are relatively easily evaded by cancer cells as they acquire new genetic and epigenetic alterations. An alternative strategy may therefore be to identify versatile molecules that, unlike the key drivers of oncogenesis, are not central to any single tumor cell property but participate in multiple functions, including modulation of diverse signaling pathways and regulation of interactions between tumor cells and the host tissue microenvironment and tumor cell responses to various forms of stress. Based on their obvious contributions to tumor growth and progression, such molecules are likely to be up regulated in malignant tumors but unlikely to be frequently mutated. Selective inhibition of these types of broad-spectrum functional regulators, especially in combination with chemo- and radiation therapy as well as other targeted therapies, may therefore achieve more efficacious and/or long lasting clinical benefits. Our present observations indicate that CD44 may be one such target in GBM and our preclinical results provide strong support for therapeutic potential of targeting CD44 and potential usage of CD44 antagonists against malignant glioma.
CD44 functions upstream of the mammalian Hippo signaling pathway and attenuates its activation induced by oxidative and cytotoxic stress
CD44 has been implicated in the modulation of several signaling pathways (9–12), but no single intact core pathway that mediates CD44-derived signals has been established thus far. In Drosophila, merlin functions upstream of the Hippo signaling pathway, but a definitive link between merlin and the mammalian Hippo pathway homologs has not been established. The present study demonstrates for the first time that CD44 functions upstream of merlin-MST1/2-Lats1/2-YAP-cIAP1/2 and two other downstream stress kinases, JNK and/or p38, along with their effectors, p53, and caspases (Fig 4, S2). It also provides evidence that CD44 plays an essential novel role in attenuating activation of these stress and apoptotic signaling pathways induced by chemotherapeutic agents and ROS whereas loss of CD44 function leads to their sustained activation that promotes apoptosis of GBM cells, which constitutes a plausible mechanism underlying the chemosensitizing effect of CD44 antagonists.
Resistance to cytotoxic agents and radiation constitutes the major obstacle to successful treatment of GBM. Increasing evidence suggests the existence of glioma cancer stem cells (GBM CSC) that are highly resistant to chemo- and radiation therapy and my be responsible for GBM recurrence following therapeutic intervention (47–48). Although the implication of CD44 in GBM CSC remains to be elucidated, CD44 is a major cell surface CSC marker in numerous tumors (9). We show in the present studies that CD44 attenuates the activation of the Hippo stress/apoptotic signaling pathway in GBM cells and provides a chemoprotective function in vivo. It will be of interest to determine whether CD44 exerts the same effects in GBM CSCs.
CD44-Fc fusion proteins and CD44-specific shRNAs are effective therapeutic agents against malignant glioma
CD44 antagonists, shRNAs against CD44 and hsCD44-Fc fusion proteins when expressed by GBM cells or delivered systemically, displayed potent anti-GBM activity in mouse models. Human sCD44-Fc fusion proteins may not only interfere with the function of CD44 expressed by GBM cells but also with that expressed by host cells infiltrating the tumors, including immune, inflammatory, and stromal cells, which are known to regulate tumor progression (43, 49). The host inflammatory response can exert both anti- and pro-tumor effects and CD44 plays an important role in limiting and resolving inflammation (43, 50). We found that overexpression of hsCD44-Fc proteins in GL261 mouse GBM cells inhibited their intracranial growth in C57BL/6 mice that have intact immunity (data not shown), suggesting the anti-GBM effect of CD44-Fc fusion proteins were not significantly affected by lack of the adaptive immunity in Rag-1 mice and are broadly applicable. Furthermore, we have shown that CD44v3-v10-Fc fusion proteins can be produced as heparan sulfate proteoglycans (S3). It is not surprising that this fusion protein, which is likely capable of sequestering heparin-binding growth factors in addition to trapping other CD44 ligands, is the most efficacious one among the fusion proteins tested and that CD44v3-v10R41A-Fc, which is incapable of binding to HA, still retained a certain degree of anti-GBM activity (Fig 6C).
Currently available first line treatment options for human GBM are chemo- and radiation therapy, although both are largely palliative. One hope for a better clinical outcome is to identify targets that play essential roles in mediating the microenvironment-derived survival signal and -mediated drug-resistance and that their antagonists sensitize the response of GBM cells to radiation and chemotherapeutic drugs. Our observation that CD44 plays an important role in protecting cancer cells from oxidative and cytotoxic stress-induced apoptotic signaling while enhancing RTK signaling (supplemental Fig 4) suggests that it may serve as an ideal therapeutic target to sensitize malignant glioma to radiation and chemotherapy, and to the inhibitors of these RTKs.
Supplementary Material
Acknowledgments
We thank Ms. Rong Lu for excellent technical support, the Cooperative Human Tissue Network (CHTN) for providing human glioma tissues, and Dr. Yu Zhou for helping with imaging analysis of the mice. This work was supported by the grants from the DOD-U.S. Army Medical Research and NIH (W81XWH-06-1-0246 and R01CA135158A1 to QY), and Swiss National Science Foundation and NCCR Molecular Oncology (FNS 31003A-105833 to IS).
Footnotes
The authors have no conflicts of financial interest to declare.
References
- 1.Araujo RP, Liotta LA, Petricoin EF. Proteins, drug targets and the mechanisms they control: the simple truth about complex networks. Nat Rev Drug Discov. 2007;6:871–880. doi: 10.1038/nrd2381. [DOI] [PubMed] [Google Scholar]
- 2.Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585–598. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- 3.Gilbertson RJ, Rich JN. Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 4.Hoelzinger DB, Demuth T, Berens ME. Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. J Natl Cancer Inst. 2007;99:1583–1593. doi: 10.1093/jnci/djm187. [DOI] [PubMed] [Google Scholar]
- 5.Marastoni S, Ligresti G, Lorenzon E, Colombatti A, Mongiat M. Extracellular matrix: a matter of life and death. Connect Tissue Res. 2008;49:203–206. doi: 10.1080/03008200802143190. [DOI] [PubMed] [Google Scholar]
- 6.Maher EA, Furnari FB, Bachoo RM, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15:1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 7.Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21:1624–1636. doi: 10.1200/JCO.2003.05.063. [DOI] [PubMed] [Google Scholar]
- 8.Park JB, Kwak HJ, Lee SH. Role of hyaluronan in glioma invasion. Cell Adh Migr. 2008;2:202–207. doi: 10.4161/cam.2.3.6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stamenkovic I, Yu Q. Shedding light on proteolytic cleavage of CD44: the responsible sheddase and functional significance of shedding. J Invest Dermatol. 2009;129:1321–1324. doi: 10.1038/jid.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 11.Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin Cancer Biol. 2000;10:415–433. doi: 10.1006/scbi.2000.0379. [DOI] [PubMed] [Google Scholar]
- 12.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 13.Guo Y, Ma J, Wang J, et al. Inhibition of human melanoma growth and metastasis in vivo by anti-CD44 monoclonal antibody. Cancer Res. 1994;54:1561–1565. [PubMed] [Google Scholar]
- 14.Ahrens T, Sleeman JP, Schempp CM, et al. Soluble CD44 inhibits melanoma tumor growth by blocking cell surface CD44 binding to hyaluronic acid. Oncogene. 2001;20:3399–3408. doi: 10.1038/sj.onc.1204435. [DOI] [PubMed] [Google Scholar]
- 15.Yu Q, Toole BP, Stamenkovic I. Induction of apoptosis of metastatic mammary carcinoma cells in vivo by disruption of tumor cell surface CD44 function. J Exp Med. 1997;186:1985–1996. doi: 10.1084/jem.186.12.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu Q, Stamenkovic I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev. 1999;13:35–48. doi: 10.1101/gad.13.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 18.Tsukita S, Yonemura S. ERM (ezrin/radixin/moesin) family: from cytoskeleton to signal transduction. Curr Opin Cell Biol. 1997;9:70–75. doi: 10.1016/s0955-0674(97)80154-8. [DOI] [PubMed] [Google Scholar]
- 19.Sainio M, Zhao F, Heiska L, et al. Neurofibromatosis 2 tumor suppressor protein colocalizes with ezrin and CD44 and associates with actin-containing cytoskeleton. J Cell Sci. 1997;110(Pt 18):2249–2260. doi: 10.1242/jcs.110.18.2249. [DOI] [PubMed] [Google Scholar]
- 20.Morrison H, Sherman LS, Legg J, et al. The NF2 tumor suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes Dev. 2001;15:968–980. doi: 10.1101/gad.189601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClatchey AI, Giovannini M. Membrane organization and tumorigenesis--the NF2 tumor suppressor, Merlin. Genes Dev. 2005;19:2265–2277. doi: 10.1101/gad.1335605. [DOI] [PubMed] [Google Scholar]
- 22.Okada T, You L, Giancotti FG. Shedding light on Merlin's wizardry. Trends Cell Biol. 2007;17:222–229. doi: 10.1016/j.tcb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Hamaratoglu F, Willecke M, Kango-Singh M, et al. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- 25.Pellock BJ, Buff E, White K, Hariharan IK. The Drosophila tumor suppressors Expanded and Merlin differentially regulate cell cycle exit, apoptosis, and Wingless signaling. Dev Biol. 2007;304:102–115. doi: 10.1016/j.ydbio.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matallanas D, Romano D, Hamilton G, Kolch W, O'Neill E. A Hippo in the ointment: MST signalling beyond the fly. Cell Cycle. 2008;7:879–884. doi: 10.4161/cc.7.7.5630. [DOI] [PubMed] [Google Scholar]
- 27.Lau YK, Murray LB, Houshmandi SS, Xu Y, Gutmann DH, Yu Q. Merlin is a potent inhibitor of glioma growth. Cancer Res. 2008;68:5733–5742. doi: 10.1158/0008-5472.CAN-08-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng Q, Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell. 2008;13:188–192. doi: 10.1016/j.ccr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Lehtinen MK, Yuan Z, Boag PR, et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi Y, Miyoshi Y, Takahata C, et al. Down-regulation of LATS1 and LATS2 mRNA expression by promoter hypermethylation and its association with biologically aggressive phenotype in human breast cancers. Clin Cancer Res. 2005;11:1380–1385. doi: 10.1158/1078-0432.CCR-04-1773. [DOI] [PubMed] [Google Scholar]
- 31.Overholtzer M, Zhang J, Smolen GA, et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci U S A. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bai Y, Liu YJ, Wang H, Xu Y, Stamenkovic I, Yu Q. Inhibition of the hyaluronan-CD44 interaction by merlin contributes to the tumor-suppressor activity of merlin. Oncogene. 2007;26:836–850. doi: 10.1038/sj.onc.1209849. [DOI] [PubMed] [Google Scholar]
- 33.Xu Y, Yu Q. E-cadherin negatively regulates CD44-hyaluronan interaction and CD44-mediated tumor invasion and branching morphogenesis. J Biol Chem. 2003;278:8661–8668. doi: 10.1074/jbc.M208181200. [DOI] [PubMed] [Google Scholar]
- 34. cited; Available from: www.oncomine.org.
- 35.Sun L, Hui AM, Su Q, et al. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell. 2006;9:287–300. doi: 10.1016/j.ccr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Bredel M, Bredel C, Juric D, et al. Functional network analysis reveals extended gliomagenesis pathway maps and three novel MYC-interacting genes in human gliomas. Cancer Res. 2005;65:8679–8689. doi: 10.1158/0008-5472.CAN-05-1204. [DOI] [PubMed] [Google Scholar]
- 37.Liang Y, Diehn M, Watson N, et al. Gene expression profiling reveals molecularly and clinically distinct subtypes of glioblastoma multiforme. Proc Natl Acad Sci U S A. 2005;102:5814–5819. doi: 10.1073/pnas.0402870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shai R, Shi T, Kremen TJ, et al. Gene expression profiling identifies molecular subtypes of gliomas. Oncogene. 2003;22:4918–4923. doi: 10.1038/sj.onc.1206753. [DOI] [PubMed] [Google Scholar]
- 39.van der Voort R, Taher TE, Wielenga VJ, et al. Heparan sulfate-modified CD44 promotes hepatocyte growth factor/scatter factor-induced signal transduction through the receptor tyrosine kinase c-Met. J Biol Chem. 1999;274:6499–6506. doi: 10.1074/jbc.274.10.6499. [DOI] [PubMed] [Google Scholar]
- 40.Orian-Rousseau V, Chen L, Sleeman JP, Herrlich P, Ponta H. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev. 2002;16:3074–3086. doi: 10.1101/gad.242602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sy MS, Guo YJ, Stamenkovic I. Inhibition of tumor growth in vivo with a soluble CD44-immunoglobulin fusion protein. J Exp Med. 1992;176:623–627. doi: 10.1084/jem.176.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le Bitoux MA, Stamenkovic I. Tumor-host interactions: the role of inflammation. Histochem Cell Biol. 2008;130:1079–1090. doi: 10.1007/s00418-008-0527-3. [DOI] [PubMed] [Google Scholar]
- 44.Bennett KL, Jackson DG, Simon JC, et al. CD44 isoforms containing exon V3 are responsible for the presentation of heparin-binding growth factor. J Cell Biol. 1995;128:687–698. doi: 10.1083/jcb.128.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peach RJ, Hollenbaugh D, Stamenkovic I, Aruffo A. Identification of hyaluronic acid binding sites in the extracellular domain of CD44. J Cell Biol. 1993;122:257–264. doi: 10.1083/jcb.122.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma SV, Gajowniczek P, Way IP, et al. A common signaling cascade may underlie "addiction" to the Src, BCR-ABL, and EGF receptor oncogenes. Cancer Cell. 2006;10:425–435. doi: 10.1016/j.ccr.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hambardzumyan D, Squatrito M, Carbajal E, Holland EC. Glioma formation, cancer stem cells, and akt signaling. Stem Cell Rev. 2008;4:203–210. doi: 10.1007/s12015-008-9021-5. [DOI] [PubMed] [Google Scholar]
- 48.Suva ML, Riggi N, Janiszewska M, et al. EZH2 Is Essential for Glioblastoma Cancer Stem Cell Maintenance. Cancer Res. 2009 doi: 10.1158/0008-5472.CAN-09-1622. [DOI] [PubMed] [Google Scholar]
- 49.Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 50.Johnson P, Ruffell B. CD44 and its role in inflammation and inflammatory diseases. Inflamm Allergy Drug Targets. 2009;8:208–220. doi: 10.2174/187152809788680994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.