Abstract
This study describes the previously unreported intrinsic capacity of poly-L-lysine (PLL) sixth generation (G6) dendrimer molecules to exhibit systemic antiangiogenic activity that could lead to solid tumor growth arrest. The PLL-dendrimer-inhibited tubule formation of SVEC4-10 murine endothelial cells and neovascularization in the chick embryo chick chorioallantoic membrane (CAM) assay. Intravenous administration of the PLL-dendrimer molecules into C57BL/6 mice inhibited vascularisation in Matrigel plugs implanted subcutaneously. Antiangiogenic activity was further evidenced using intravital microscopy of tumors grown within dorsal skinfold window chambers. Reduced vascularization of P22 rat sarcoma implanted in the dorsal window chamber of SCID mice was observed following tail vein administration (i.v.) of the PLL dendrimers. Also, the in vivo toxicological profile of the PLL-dendrimer molecules was shown to be safe at the dose regime studied. The antiangiogenic activity of the PLL dendrimer was further shown to be associated with significant suppression of B16F10 solid tumor volume and delayed tumor growth. Enhanced apoptosis/necrosis within tumors of PLL-dendrimer-treated animals only and reduction in the number of CD31 positive cells were observed in comparison to protamine treatment. This study suggests that PLL-dendrimer molecules can exhibit a systemic antiangiogenic activity that may be used for therapy of solid tumors, and in combination with their capacity to carry other therapeutic or diagnostic agents may potentially offer capabilities for the design of theranostic systems.
Keywords: angiogenesis, cancer, nanoparticle
Tumor growth is largely dependent on angiogenesis, the biological process leading to the growth of new capillary blood vessels (1). In healthy tissues angiogenesis is kept at a balance between endogenous proangiogenic and antiangiogenic factors (2). However, in growing tumors angiogenesis is initiated for the creation of a new vascular network that will provide adequate blood supply and facilitate growth (1). It has been hypothesized that angiogenesis inhibitors can be used to control tumor growth (3), and the development of antiangiogenic tumor therapeutics has become an area of intense research interest for treatment of many cancer types (1, 4–6).
The process of angiogenesis is stimulated by various cytokines, such as the vascular endothelial growth factor (VEGF) and the basic fibroblast growth factor (bFGF). The interaction of cytokines with their endothelial receptors depends on the presence of the extracellular macromolecule heparin or heparan sulphate proteoglycan (7). It has also been recognized that heparin potentiates the activity of angiogenic growth factors although this mechanism is not yet clearly understood (8). Azizkhan and coworkers reported that heparin released by mast cells accumulates at the tumor site, enhancing the migration of capillary endothelial cells prior to ingrowth of new blood capillaries (7). Heparin has been validated previously as a potential target for antiangiogenesis therapy by binding to protamine, an arginine-rich basic protein of 4,300 Da, that leads to inhibition of angiogenic growth factor activity (9).
Dendrimers are three-dimensional nanocontainers synthesized in a stepwise manner by attaching branching units to an emanating core (10). Their size, molecular weight, and surface functionalities can be easily controlled (10). The use of dendrimers in relation to angiogenesis has been restricted to applications such as MRI contrast agents of neovascularized areas and as carriers for antiangiogenic genes (11–14). Most of these studies have focused on local administration of dendrimer-nucleic acid complexes (14, 15).
We hypothesized that the competition of cationic dendrimers with in situ angiogenic factors for binding sites on heparin (16) may lead to antiangiogenic effects. Only two studies have previously associated dendrimers with antiangiogenic activity (16, 17). Kasai et al. (16) synthesized a cationic poly arginine dendrimer with 16-surface arginine residues to resemble the structure of the endogenous angiogenesis inhibitor endostatin (16), and reported only preliminary data on its antiangiogenic activity in ovo using the CAM assay. The second study to report an intrinsic antiangiogenic activity of dendrimers was that of the anionic, polyamidoamine (PAMAM) generation 3.5 (G3.5) dendrimer conjugated to D(+)-glucosamine and D(+)-glucosamine 6-sulfate in a clinically relevant rabbit model after local administration. In both these previous studies the dendrimer antiangiogenic activity was either not demonstrated in vivo at all (16) or reported in vivo only by local (eye) administration (17). There have not been any previous reports of dendrimer-mediated systemic antiangiogenic activity in any disease model in vivo.
The present study is based on the hypothesis that sixth generation cationic poly-L-lysine (PLL) dendrimers Gly-Lys63(NH2)64 (18) may have the capability to exhibit antiangiogenic activity within solid tumors after systemic (i.v.) administration. We tested this hypothesis by using multiple in vitro and in vivo angiogenesis assays and tumor models. The antiangiogenic activity of the PLL dendrimer could lead to biological activity and therapeutic efficacy (tumor growth delay) in the absence of any other therapeutic agent.
Results
PLL Dendrimer Inhibits Tubule Formation by Murine Endothelial Cells (SVEC4-10) in Vitro at Nontoxic Concentrations.
In an attempt to explore synthetic mimics of heparin-binding molecules for therapeutic purposes, different generations of cationic polylysine (PLL) dendrimers have been synthesized (18). Based on results obtained from heparin-binding assays (unpublished), molecular modelling, and the physicochemical characterisation of the dendrimers (Fig. S1), the molecular architecture of the G6PLL dendrimer was considered the best candidate molecule due to its compact globular structure, mimicking the endogenous angiogenic inhibitor endostatin that also possesses a highly positively charged surface.
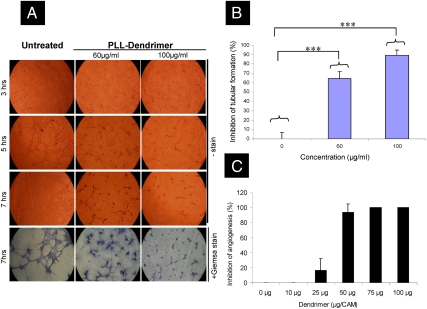
An initial study was performed to explore the ability of the PLL dendrimer to interfere with the migration and differentiation of endothelial cells and thus the inhibition in the formation of capillary-like structures by murine lymph node endothelial (SVEC4-10) cell cultures (19) on Matrigel. SVEC4-10 cells were incubated with PLL dendrimer (60–100 μg/mL) and placed on Matrigel. In vitro cytotoxicity studies showed that the PLL dendrimer was not toxic at concentrations up to 100 μg/mL (Fig. S1). Quantification of inhibition of tubule formation was performed by averaging the number of branch points in an area of interest. Fig. 1A shows the inhibition of tubule formation of SVEC4-10 by PLL dendrimer in a dose-dependent manner. After 5 h of incubation, nontreated cells migrated and started to form tubules (Fig. 1A). In contrast, SVEC4-10 treated with PLL dendrimer exhibited delayed tubule formation. After 7 h of incubation, tubule formation by nontreated cells was complete, contrary to a significant reduction in tubule formation for 60 μg/mL and 100 μg/mL PLL-dendrimer-treated cell cultures leading to 64.5% ± 7.3 and 89.0 ± 5.5% inhibition (% of control untreated cells) respectively (Fig. 1B). This was considered an indication that the PLL dendrimer can interfere with the mechanism of capillary formation, known to be involved in the angiogenesis process, at nontoxic doses.
Fig. 1.
PLL-dendrimer inhibits tubular formation of SVEC4-10 and neovascularization in the CAM assay (A) Morphology of formed tubules of SVEC4-10 in the presence of different concentrations PLL dendrimer for up to 7 h. All images were captured by 10x lens. (B) Percent inhibition of tubular formation of SVEC4-10 by the PLL dendrimer after 7 h incubation. Data were expressed as means ± SD from 4 different fields of view (∗∗∗P < 0.001). (C) Dose-dependent inhibition angiogenesis in CAM by PLL dendrimer. Antiangiogenic response was considered by the formation of an avascular 3 mm diameter region, and became obvious at doses ≥25 μg/CAM. Data were expressed as a mean ± SD (n = 7–10) from three experiments.
To further elucidate any effects on the endothelial cell cytoskeleton, cells were stained for microtubules and actin filaments after incubation with the PLL dendrimer for 1 h. Immunofluorescence staining of cytoskeletal structures (Fig. S2) showed that microtubules were thinner and partially disrupted in cells exposed to ≥30 μg/mL PLL dendrimer. Actin structures were also altered in cells exposed to ≥100 μg/mL PLL dendrimer. In these cells there was a distinct indication of actin remodeling into contractile stress fibers.
PLL Dendrimer Inhibits Neovascularization in the CAM Assay.
To further explore the observed capacity of the PLL-dendrimer molecules to inhibit endothelial cell tubule formation, a more complex angiogenesis assay was used. The ability of PLL dendrimers to inhibit formation of new blood vessels (neovascularization) in the developing chick embryo (CAM assay) in ovo was investigated. PLL dendrimer was applied into a silicon ring that was always placed in the same area of the CAM, distant from the embryo. PLL dendrimer was used at doses neither toxic to the embryo nor to the CAM (0–100 μg/CAM). The dose-dependent inhibition in neovascularization at PLL-dendrimer doses between 25 and 50 μg/CAM (of 16.3% ± 16.0 and 93.5% ± 11.4, respectively) is depicted in Fig. 1C. Higher doses resulted in new vessel growth inhibition in 100% of the eggs. Furthermore, this effect was also time dependent (Fig. S3). Overall, these results indicated that the PLL dendrimer was interfering with the angiogenesis process in vitro and in ovo at nontoxic doses that warranted further study using animal models.
PLL Dendrimer Inhibits bFGF-Induced Angiogenesis in Matrigel Plug Assay After Aystemic (I.V.) Administration.
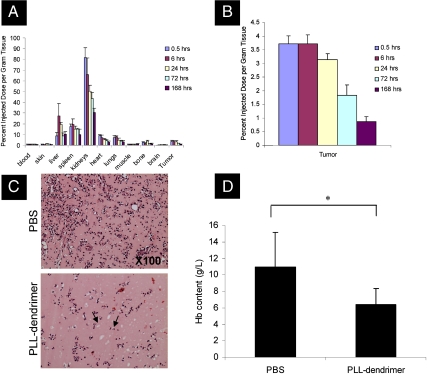
The fundamental aim of this study was to investigate PLL dendrimers as synthetic antiangiogenic agents for systemic administration. To do that, the pharmacokinetic, tissue distribution, and toxicological profile of these molecules needed to be established. Fig. 2A depicts this profile following a single intravenous administration of 25 mg/kg PLL dendrimer radiolabelled with 3H in tumor-bearing mice. Kidney was the organ with the highest percentage of accumulated injected dose per gram tissue followed by liver and spleen (Fig. 2A). PLL dendrimers accumulated at the tumor site rapidly with 3.5–4% of injected dose per gram tissue detected within the first 30 min after administration (Fig. 2B). The same level of PLL dendrimer was detected after 6 h with gradual reduction to < 1% after one week (Fig. 2B). That result indicated that the PLL-dendrimer molecules that reached the tumor site remained there for a prolonged period of time. In parallel, non-tumor-bearing animals were monitored for signs of toxicity by histological examination of major organs (Fig. S4), changes in body weight, water consumption, urine and fecal excretion, and urinalysis (Fig. S5) indicating no toxicological side effects at this dosage regimen. The hematological profile of the non-tumor-bearing animals was also obtained and is shown in Table S1.
Fig. 2.
PLL dendrimer inhibits bFGF-induced angiogenesis in Matrigel plug assay after systemic (i.v.) administration in vivo biodistribution of PLL dendrimer after i.v. administration of 25 mg/kg as a single dose in tumor-bearing C57Bl/6 mice. (A) Percent injected dose per gram tissue at different time points and (B) percent injected dose per gram tissue in B16F10 tumors. Data expressed as the mean ± SD (n = 4) using tumor-bearing mice. (C) H&E-stained Matrigel plugs with arrows pointing at the microvessels and are significantly lower in PLL-dendrimer treated plugs. (D) Hemoglobin content in Matrigel plugs mixed with heparin and bFGF and implanted subcutaneously for 7 days in the abdominal region of C57/BL6 mice. Animals were injected via tail vein with 50 mg/kg/day PLL dendrimer (once daily, on days 1 and 2 postimplantation) or with PBS alone. Data expressed as a mean ± SD (n = 4) (∗P < 0.05).
To study the antiangiogenic activity of the PLL dendrimers after systemic administration (i.v) a variety of animal models were established and used. With the understanding that the PLL dendrimers can reach the tumor site after systemic administration, the first in vivo angiogenesis model used was the Matrigel plug assay, that has been increasingly become a method of choice in many studies involving angiogenesis in vivo (20). In this assay, the angiogenesis-inducing growth factor bFGF was incorporated within cold liquid Matrigel, a basement-membrane extract from an Engelbreth–Holm–Swarn tumor, and the mixture was injected subcutaneously into the midventral area of C57/BL6 mice and allowed to solidify. The Matrigel allows penetration of endothelial host cells that induce vascularization. The PLL dendrimer was injected via the tail vein at 50 mg/kg/day (once daily for two consecutive days) 24 h after Matrigel implantation. Seven days later, plugs were excised and fixed for subsequent H&E staining of microvessels formed within the Matrigel. Quantitative assessment of angiogenesis was achieved by the determination of hemoglobin content in the plugs after lysis. Fewer microvessels were formed within the Matrigel in mice injected with the PLL dendrimer (Fig. 2C). This was quantitatively represented by a statistically significant reduction in hemoglobin content from 11.0 ± 4.1 g/L in PLL-dendrimer-treated plugs compared to 6.4 ± 1.8 g/L in untreated plugs (p < 0.05) (Fig 2D). This indicated that the PLL-dendrimer molecules exhibited antiangiogenic activity in vivo against bFGF-induced Matrigel vascularization after systemic (i.v.) administration.
Reduction in Tumor Vascularization After I.V. Administration of PLL Dendrimer.
The effect of PLL-dendrimer treatment by systemic administration on tumor vascularization was then investigated. P22 rat sarcoma tumors were grown in dorsal skin flap window chambers in SCID mice. Tumor vascular morphology was monitored on a daily basis after treatment with PLL dendrimer or PBS (negative control) using intravital microscopy.
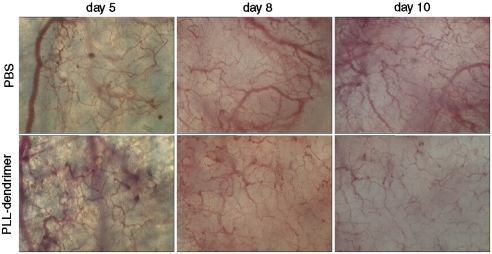
Fig. 3 illustrates the vascularization of P22 tumors at early and late phases of growth in a window chamber. Fragments of P22 rat sarcoma at a size of 2.2–2.8 mm2 were implanted into the window chamber (day 0). In control (PBS-treated) animals, 4 d after implantation tumors began to vascularize when their size reached an area of 6.2–6.8 mm2 (Fig. 3, top panel on day 5). Tumor vascularization became more evident after 10–14 d (10–12 mm2 tumor surface area) postsurgery. Blood vessels in the control group were typically characterized by irregularity in width, mainly dilated, and chaotic in their architecture (Fig. 3, top panel on day 10). PLL dendrimer was injected via the mouse tail vein at a dose of 50 mg/kg/day, initially on day 4 postsurgery when vascularization began to occur, followed by a second dose on day 7 postsurgery. Transmitted light images of tumors at 8 and 10 d after surgery showed a well-vascularized center in the P22 tumors injected with PBS. There was clear reduction in vascularization in PLL-dendrimer treated animals compared to the PBS-treated group, manifested by narrower vessels and reduction in the total number of neovasculature formed with the effect being more profound on day 10 postsurgery. Reproducible results were obtained from at least three different tumors implanted in three independent dorsal window chambers (Fig. S6). These results indicated the capacity of intravenously administrered PLL dendrimer to inhibit vascularization in vivo in both a tumor (P22 rat sarcoma) and a nontumor vascularization model (Matrigel).
Fig. 3.
Tumor microvessels are sparser in a window chamber model after i.v. administration of PLL dendrimer transmitted light images of tumors at days 5, 8, and 10 after tumor transplantation. Mice injected via tail vein with 50 mg/kg/day dendrimer (once daily, on days 4 and 7 after tumor transplantation) or with PBS alone.
Systemic Administration of PLL Dendrimer Leads to Antiangiogenic Activity, Apoptosis, and Delayed Solid Tumor Growth in Vivo.
To investigate whether the biological activity of intravenously administered PLL dendrimer alone could have an effect on tumor vascularization and growth, a solid tumor model of murine melanoma B16F10 (1 × 106) was established subcutaneously in C57/BL6 mice. Palpable tumors began to form within one week after subcutaneous implantation and PLL dendrimers were injected intravenously at 50 mg/kg/day (once daily) on days 1 and 2 posttumor inoculation. Protamine was used in comparison because it was previously shown to inhibit tumor angiogenesis via interaction with heparin leading subsequently to tumor growth when locally applied into the tumor (9). Protamine was injected subcutaneously every 12 h at a dose of 60 mg/kg as reported previously and tumor growth was monitored daily by measurement of tumor volume using calipers.
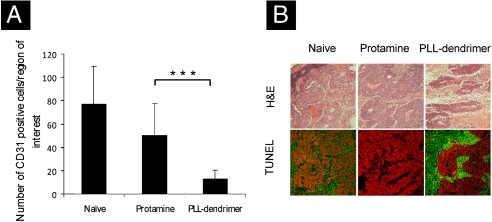
Immunohistochemical staining for CD31 was performed on tumor sections from the B16F10 tumor-bearing mice. CD31 expression is a well-established marker for endothelial cells used as an indicator of the degree of vascularization. Staining with anti-CD31 antibody showed more CD31 + cells among the naïve and protamine-treated tumor sections, whereas fewer number of CD31 + cells were found in the tumors treated with PLL dendrimer (Fig. S7). The number of CD31 + cells were counted in twelve random images from each tumor in all the groups and the average was plotted in Fig. 4A. This illustrated that the number of CD31 + cells was significantly lower in the PLL-dendrimer treated tumors compared to protamine-treated or naïve tumors.
Fig. 4.
PLL dendrimer reduces CD31 + cells and enhances apoptosis in tumors after i.v. administration. Murine B16F10 melanoma tumors were implanted s.c. PLL dendrimer (50 mg/kg/day) or PBS alone were injected i.v. twice on day 1 and 2 after tumor inoculation. Tumors were excised on day 10 and stained for CD31 or TUNEL. (A) Number of CD31 + cells per region of interest. Data were expressed as a mean ± SD from 12 different fields of view (∗∗∗P < 0.001). (B) Tumor sections H&E stained (top row) or TUNEL and propidium iodide nuclear counterstained to identify apoptotic (green) cells from the total cell population (red) in the bottom row.
H&E histology and TUNEL staining of the excised tumor sections were carried out to investigate the occurrence of apoptosis and necrosis in the tumor tissue. H&E staining indicated that much more extensive necrosis occurred in PLL-dendrimer treated tumors (light pink patches) compared to the viable regions in the other two treatment groups (Fig. 4B Upper). In the TUNEL assay of the tumor sections apoptotic cells were stained green and propidium iodide was used to counterstain all nuclei red (Fig. 4B Lower). Both naïve and protamine-treated groups showed predominantly healthy cells with only limited areas stained positive for apoptosis (green). PLL-dendrimer treated tumor sections indicated much more extensive apoptotic areas. After two tail vein administrations with the PLL dendrimer, significant reduction in the number of CD31 + cells could be observed that resulted in extensive apoptosis and necrosis at the tumor site. Interestingly, the antiagiogenic activity of the PLL-dendrimer treatment also had an effect on tumor growth, leading to statistically significant inhibition compared to control and protamine-treated groups (early time-point tumor growth curves and images of the excised tumors are shown in Fig. S8).
Overall, systemic administration of two daily i.v. doses at 50 mg/kg PLL dendrimer resulted in reduced vascularization, extensive apoptosis/necrosis within the tumor tissue, and statistically significant but moderate reduction in tumor volume, in the absence of any remarkable histological or physiological abnormality due to major toxicity to other critical organs, such as liver and kidneys (Fig. S9).
Discussion
Dendrimers have been proposed as delivery agents for chemotherapeutic drugs to solid tumors. Malik et al. (21) showed that conjugates of cisplatin with the negatively charged PAMAM dendrimer exhibited antitumor activity against B16F10 solid tumors. PEGylation has shown to prolong the blood circulation of dendrimers, such as polyester (22) and G6 lysine dendrimers (23), improving tumor accumulation. Methotrexate (MTX) conjugated to PEGylated PLL dendrimers (G5, PEG1100) have also shown to accumulate in solid Walker 256 and HT-1080 tumors in rats and mice (24). However, no therapeutic efficacy was assessed in any of these studies. Recently, a study by Fox et al. (25) reported therapeutic efficacy of PEGylated PLL dendrimer-camptothecin conjugates in C26 and HT-29 tumor models (25). For targeting purposes, Kukowska–Latallo et al. (26) performed studies on folate-conjugated MTX dendrimers administered intravenously, showing 10 times more effective growth delay of KB tumors in mice (26). Also, cationic polypropylenimine dendrimers systemically administered for gene delivery purposes exhibited moderate tumor growth delay, however no mechanism for such effects was shown (27). Despite the fact that previous reports have demonstrated that antiangiogenic activity may be an intrinsic property of some dendrimer molecules, to our knowledge no study has illustrated antiangiogenesis from systemic dendrimer administrations that can be utilized against tumor-induced neovascularization. This work shows that G6 PLL dendrimer (MW 8149 Da) has the ability to accumulate and persist in solid tumor sites after systemic administration and exhibit antiangiogenic activity in the absence of cytotoxicity.
The in vitro endothelial cell tubule-formation assay (28) was used and the presence of PLL dendrimer at noncytotoxic concentrations was found to inhibit endothelial cell migration and three-dimensional tubule formation. Further evidence of the PLL-dendrimer antiangiogenic activity in a dose-dependent manner was obtained using the more complex CAM assay. A previous study has shown that molecules resembling protamine in structure and charge density, such as PLL, poly-L-arginine, and poly-L-glutamic acid were not able to exhibit antiangiogenic effects in the CAM at equivalent doses to protamine (9). Kasai et al. (16) synthesized poly-L-arginine dendrimers with 8 and 16 surface amines to mimic the structure of endostatin, an endogenous angiogenesis inhibitor. They reported antiangiogenic activity for these arginine dendrimers reaching 100% inhibition of angiogenesis in the CAM at 10–20 μg/CAM, compared to 10–20 μg/CAM and 100 μg/CAM needed to achieve the same effect by endostatin and angiostatin, respectively. However, such observations were not shown using any in vivo model.
The tissue distribution and systemic toxicity profile of G6 PLL dendrimer were evaluated herein at 25 mg/kg and 50 mg/kg after intravenous administration via tail vein injection in mice. Boyd et al. (29) have recently studied the biodistribution of G4 and G5 (16 and 32 amines) 3H-labeled PLL dendrimers in rats at the much lower dose of 5 mg/kg injected via the jugular vein (29). They reported blood half-lives of < 10 min, proposing rapid accumulation of the dendrimers in the vascular endothelium, a process driven by electrostatic interactions as previously described for other cationic nanocarriers (30, 31). This mechanism—even though speculative—seems in agreement with the G6 cationic PLL dendrimer used in this study, that also showed rapid blood clearance with 50–55% of the injected dose detected in the body (mainly in kidneys, liver, and spleen) and only 5% excreted in the first 24 h following administration. The PLL dendrimer reached the tumor at 4% of the i.d. per gram tissue within the first 30 min. This further supports the hypothesis that the PLL-dendrimer accumulation at the tumor site takes place via strong electrostatic interactions between the cationic PLL-dendrimer surface and the negatively charged, heparin-rich walls of the tumor neovasculature. It has been previously reported using an ex vivo perfusion model that high molecular weight PLL (32) and PAMAM dendrimers (33) accumulated and adhered tightly on artery walls. Importantly, the PLL-dendrimer systemic toxicity profile in this study indicated that no adverse histological, physiological, or hematological effects were caused by these treatments.
Antiangiogenic activity in vivo following systemic administration of PLL dendrimers was first obtained using the Matrigel plug assay. The capability of systemically administered PLL dendrimer to inhibit microvessel formation in vivo was further shown using the dorsal skinfold window chamber model. The degree of vascularization and significant alteration in the developing microvascular architecture were monitored by intravital microscopy (34, 35). The PLL-dendrimer-treated group showed reduced numbers of microvessels after treatment with PLL dendrimer compared to the control group. A further indication of the PLL-dendrimer antiangiogenic activity was provided by the significant reduction of the number of CD31 + cells in the tumor sections of the treated animals compared to untreated controls. The increase in TUNEL staining of solid tumors in C57/BL6 mice further indicated that the PLL-dendrimer induced apoptosis in a large tumor cell population. Protamine has been shown previously to inhibit angiogenesis and to delay tumor growth of melanoma tumors, when injected subcutaneously at 60 mg/kg every 12 h (9). In that study no effect of protamine was observed on growth of Lewis lung carcinoma tumors or B16 tumors established from fragments obtained by passaging into mice. In the present work, protamine also showed no growth delay effects on B16F10 tumors.
To determine whether a therapeutic effect could be obtained solely on the grounds of the PLL-dendrimer antiangiogenic activity, the B16F10 murine melanoma tumor model was used. It has been established by others that reduction of microvessel density in B16F10 tumors can alter the tumor cell proliferation and lead to tumor cell death by apoptosis or necrosis (36). In this study, only two intravenous administrations of the PLL dendrimer were able to lead to delayed tumor growth. Numerous studies using antiVEGF agents, like bevacizumab (Avastin®) have indicated that the addition of antiangiogenic therapeutics along with chemotherapy or radiotherapy (37) can synergistically improve tumor responses to treatment. Dings et al. (38) have shown that the time to volume quadrupling for the B16F10 melanoma tumors was 4.0 ± 0.4 days when treated with Avastin® (10 mg/kg i.v., single administration) compared to 3.6 ± 0.4 days when treated with vehicle alone, so the quadrupling time was delayed by 0.4 day. In comparison, PLL-dendrimer treatment led to a delay in tumor volume quadruple time of 4.7 ± 2.5 days compared to 3.7 ± 1.6 days when treated with vehicle alone. Therefore, the PLL dendrimer seems comparable in delaying B16F10 tumor growth to Avastin®.
Many molecules have been previously described to exhibit antiangiogenic activities in vitro, however most have not been successful in vivo, either due to the lack of an optimum pharmacokinetic profile or due to systemic toxicity at biologically active doses. Protamine is such an example, shown to inhibit tumor angiogenesis after subcutaneous administration (9) but after intravenous administration severe toxicity prohibited its further clinical use. Moreover, we believe that this work indicates significant opportunities for the design of unique therapeutic modalities from utilization of dendrimer molecules as “antiangiogenic nanocontainers” that may potentially act in combination with other cytotoxic agents simultaneously delivered to tumors using PLL dendrimers.
Experimental Procedures
Cell Cultures.
SVEC 4-10 cells or B16F10 cells were maintained in DMEM or Advanced RPMI medium 1640, respectively, supplemented with 10% FBS, 50 U/mL penicillin, 50 μg/mL streptomycin, 1% L-glutamine and 1% nonessential amino acids at 37 °C in 5% CO2. Cells were passaged when they reached 80% confluence to maintain exponential growth. B16F10 cells used for tumor inoculation were passaged twice in antibiotic-free media to ensure the line was free of contaminants prior to implantation.
Tubule Formation Assay.
In vitro endothelial tubular formation assay was performed as described previously with minor modifications (39). Percent inhibition of tubular formation was measured by counting and averaging the branch points from 4 different fields (× 100). Data were analyzed by Student’s t test (one-tailed) on Excel program and P < 0.05 was considered significant. Details are described in SI Text.
CAM Angiogenesis Assay.
Antiangiogenic activity in CAM was assayed as described in ref. 16. Angiogenic inhibition was indicated by the formation of an avascular zone around the ring of 3 mm diameter. The results were expressed as the percentage of embryos showing inhibition (n = 7–10) from three different experiments. Details are described in SI Text.
Matrigel Plug in Vivo Assay.
The assay was performed as described in details by Passaniti (20). Data were analyzed by Student’s t test (one-tailed) on Excel program and P < 0.05 was considered significant. For histological analysis, other halves of matrigel plugs were fixed in 10% buffered formalin and processed for routine histology with H&E stain as described in Heamatoxylin/Eosin Tissue Histology. Details are described in SI Text.
Window Chamber Assay and Tumor Implantation.
Female severe combined immunodeficiency (SCID) mice (12–16 weeks old, 28–32 g) were anesthetized using fentanyl-fluanisone and midazolam i.p., as described previously (40). SCID mice received 200 μL of dendrimer in PBS (50 mg/kg/day/i.v.) once daily on day 4 and 7 posttumor implantation or the same volume of PBS. Tumors were approximately 3–5 mm in diameter when used. For the assessment of tumor vascularization in treated and untreated tumors, transmitted light images were captured at various magnifications once per day for 10–12 d after tumor transplantation.
Tumor Growth Delay Study.
Mice were inoculated subcutaneously with 1 × 106 B16F10 Murine melanoma cells in 100 μL PBS on the left flank. The tumor volume was estimated by bilateral Vernier caliper measurement once daily and calculated using the formula (width × width) × (length) × (π/6), where length was taken to be the longest diameter across the tumor. Details are described in SI Text.
Tumor Necrosis and Apoptosis Assays.
Tissue sections were deparaffinized in Histoclear and rehydrated through graded ethanol. The DeadEnd™ Fluorometric TUNEL System (Promega) was used to label nicked DNA through incorporation of fluorescein-12-dUTP. Samples were incubated with recombinant Terminal Deoxynucleotidyl Transferase as per manufacturer’s instructions and fluorescein labelling was visualised using confocal microscopy (LSM 510, Zeiss). Propidium iodide was used to counterstain nuclei. Areas of necrosis and apoptosis were assessed qualitatively by examination of H&E and TUNEL processed sections respectively.
Immunohistochemistry Staining for CD31.
The rat polyclonal antimouse CD31 antibody (SantaCruz Biotechnology) was used at 1∶100 dilution as a primary antibody. Sections were covered with the primary antibody and left in a humid chamber for 1 h. After washing, sections were incubated with biotinylated antirat IgG secondary antibody (IgG ABC kit, Vectastain) for 45 min. Details are described in SI Text.
Statistical Analysis.
Data was expressed as mean + SEM where indicated. Statistical differences were analyzed using the Student’s t test and p values < 0.05 were taken to be statistically significant.
Supplementary Material
Acknowledgments.
K.T.A.-J. is a recipient of the postdoctoral Maplethorpe Fellowship from the University of London. W.T.A.-J. is a recipient of the Overseas Research Student Award Scheme from the University of London.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908401107/DCSupplemental.
References
- 1.Folkman J. Clinical applications of research on angiogenesis. N Engl J Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- 2.Kerbel RS. Tumour angiogenesis: Past, present and the near future. Carcinogenesis. 2000;21:505–515. doi: 10.1093/carcin/21.3.505. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Anti-angiogenesis: New concept for therapy of solid tumors. Ann Surg. 1972;175:409–416. doi: 10.1097/00000658-197203000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Folkman J. Angiogenesis inhibitors: A new class of drugs. Cancer Biol Ther. 2003;2:S127–S133. [PubMed] [Google Scholar]
- 5.Folkman J. Angiogenesis: An organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 6.Ma J, Waxman DJ. Combination of antiangiogenesis with chemotherapy for more effective cancer treatment. Mol Cancer Ther. 2008;7:3670–3684. doi: 10.1158/1535-7163.MCT-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azizkhan RG, Azizkhan JC, Zetter BR, Folkman J. Mast cell heparin stimulates migration of capillary endothelial cells in vitro. J Exp Med. 1980;152:931–944. doi: 10.1084/jem.152.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235:442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- 9.Taylor S, Folkman J. Protamine is an inhibitor of angiogenesis. Nature. 1982;297:307–312. doi: 10.1038/297307a0. [DOI] [PubMed] [Google Scholar]
- 10.Tomalia DA, Naylor AM, Goddard WA. Starburst dendrimers: Molecular-level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angew Chem Int Edit. 1990;29:138–175. [Google Scholar]
- 11.Knopp MV, von Tengg-Kobligk H, Choyke PL. Functional magnetic resonance imaging in oncology for diagnosis and therapy monitoring. Mol Cancer Ther. 2003;2:419–426. [PubMed] [Google Scholar]
- 12.Roberts TP, et al. Tumor microvascular changes to anti-angiogenic treatment assessed by MR contrast media of different molecular weights. Acad Radiol. 2002;9(Suppl 2):S511–S513. doi: 10.1016/s1076-6332(03)80279-9. [DOI] [PubMed] [Google Scholar]
- 13.Marano RJ, et al. Dendrimer delivery of an anti-VEGF oligonucleotide into the eye: A long-term study into inhibition of laser-induced CNV, distribution, uptake and toxicity. Gene Ther. 2005;12:1544–1550. doi: 10.1038/sj.gt.3302579. [DOI] [PubMed] [Google Scholar]
- 14.Vincent L, et al. Efficacy of dendrimer-mediated angiostatin and TIMP-2 gene delivery on inhibition of tumor growth and angiogenesis: In vitro and in vivo studies. Int J Cancer. 2003;105:419–429. doi: 10.1002/ijc.11105. [DOI] [PubMed] [Google Scholar]
- 15.Marano RJ, et al. Inhibition of in vitro VEGF expression and choroidal neovascularization by synthetic dendrimer peptide mediated delivery of a sense oligonucleotide. Exp Eye Res. 2004;79:525–535. doi: 10.1016/j.exer.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 16.Kasai S, et al. Design and synthesis of antiangiogenic/heparin-binding arginine dendrimer mimicking the surface of endostatin. Bioorg Med Chem Lett. 2002;12:951–954. doi: 10.1016/s0960-894x(02)00066-5. [DOI] [PubMed] [Google Scholar]
- 17.Shaunak S, et al. Polyvalent dendrimer glucosamine conjugates prevent scar tissue formation. Nat Biotechnol. 2004;22:977–984. doi: 10.1038/nbt995. [DOI] [PubMed] [Google Scholar]
- 18.Al-Jamal KT, Ruenraroengsak P, Hartell N, Florence AT. An intrinsically fluorescent dendrimer as a nanoprobe of cell transport. J Drug Target. 2006;14:405–412. doi: 10.1080/10611860600834441. [DOI] [PubMed] [Google Scholar]
- 19.Walter-Yohrling J, et al. Murine endothelial cell lines as models of tumor endothelial cells. Clin Cancer Res. 2004;10:2179–2189. doi: 10.1158/1078-0432.ccr-03-1013. [DOI] [PubMed] [Google Scholar]
- 20.Passaniti A, et al. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest. 1992;67:519–528. [PubMed] [Google Scholar]
- 21.Malik N, Evagorou EG, Duncan R. Dendrimer-platinate: A novel approach to cancer chemotherapy. Anticancer Res. 1999;10:767–776. [PubMed] [Google Scholar]
- 22.Gillies ER, Frechet JM. Designing macromolecules for therapeutic applications: polyester dendrimer-poly(ethylene oxide) “bow-tie” hybrids with tunable molecular weight and architecture. J Am Chem Soc. 2002;124:14137–14146. doi: 10.1021/ja028100n. [DOI] [PubMed] [Google Scholar]
- 23.Okuda T, et al. PEGylated lysine dendrimers for tumor-selective targeting after intravenous injection in tumor-bearing mice. J Control Release. 2006;116:330–336. doi: 10.1016/j.jconrel.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Kaminskas LM, et al. Pharmacokinetics and tumor disposition of PEGylated, methotrexate conjugated poly-l-lysine dendrimers. Mol Pharm. 2009;6:1190–1204. doi: 10.1021/mp900049a. [DOI] [PubMed] [Google Scholar]
- 25.Fox ME, et al. Synthesis and In vivo antitumor efficacy of PEGylated poly(l-lysine) dendrimer-camptothecin conjugates. Mol Pharm. 2009;6:1562–1572. doi: 10.1021/mp9001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kukowska-Latallo JF, et al. Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res. 2005;65:5317–5324. doi: 10.1158/0008-5472.CAN-04-3921. [DOI] [PubMed] [Google Scholar]
- 27.Dufes C, Uchegbu IF, Schatzlein AG. Dendrimers in gene delivery. Adv Drug Deliv Rev. 2005;57:2177–2202. doi: 10.1016/j.addr.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Lawley TJ, Kubota Y. Induction of morphologic differentiation of endothelial cells in culture. J Invest Dermatol. 1989;93:59S–61S. doi: 10.1111/1523-1747.ep12581070. [DOI] [PubMed] [Google Scholar]
- 29.Boyd BJ, et al. Cationic poly-L-lysine dendrimers: Pharmacokinetics, biodistribution, and evidence for metabolism and bioresorption after intravenous administration to rats. Mol Pharm. 2006;3:614–627. doi: 10.1021/mp060032e. [DOI] [PubMed] [Google Scholar]
- 30.Campbell RB, et al. Cationic charge determines the distribution of liposomes between the vascular and extravascular compartments of tumors. Cancer Res. 2002;62:6831–6836. [PubMed] [Google Scholar]
- 31.Eichhom M, et al. Protamine enhances uptake of cationic liposomes in angiogenic microvessels of solid tumours. Angiogenesis. 2004;7:133–141. doi: 10.1007/s10456-004-1428-2. [DOI] [PubMed] [Google Scholar]
- 32.Sakharov DV, et al. Polylysine as a vehicle for extracellular matrix-targeted local drug delivery, providing high accumulation and long-term retention within the vascular wall. Arterioscl Throm Vasc. 2001;21:943–948. doi: 10.1161/01.atv.21.6.943. [DOI] [PubMed] [Google Scholar]
- 33.Tomalia DA, Reyna LA, Svenson S. Dendrimers as multi-purpose nanodevices for oncology drug delivery and diagnostic imaging. Biochem Soc T. 2007;35:61–67. doi: 10.1042/BST0350061. [DOI] [PubMed] [Google Scholar]
- 34.Dellian M, et al. Quantitation and physiological characterization of angiogenic vessels in mice: Effect of basic fibroblast growth factor, vascular endothelial growth factor/vascular permeability factor, and host microenvironment. Am J Pathol. 1996;149:59–71. [PMC free article] [PubMed] [Google Scholar]
- 35.Leunig M, et al. Angiogenesis, microvascular architecture, microhemodynamics, and interstitial fluid pressure during early growth of human adenocarcinoma LS174T in SCID mice. Cancer Res. 1992;52:6553–6560. [PubMed] [Google Scholar]
- 36.Ren B, et al. The antiangiogenic and therapeutic implications of endostatin. Method Find Exp Clin. 2003;25:215–224. doi: 10.1358/mf.2003.25.3.769643. [DOI] [PubMed] [Google Scholar]
- 37.Hurwitz H, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 38.Dings RP, et al. Scheduling of radiation with angiogenesis inhibitors anginex and Avastin improves therapeutic outcome via vessel normalization. Clin Cancer Res. 2007;13:3395–3402. doi: 10.1158/1078-0432.CCR-06-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medhora M, et al. Epoxygenase-driven angiogenesis in human lung microvascular endothelial cells. Am J Physiol Heart Circ Physiol. 2002;284:H215–H224. doi: 10.1152/ajpheart.01118.2001. [DOI] [PubMed] [Google Scholar]
- 40.Tozer GM, et al. Intravital imaging of tumour vascular networks using multiphoton fluorescence microscopy. Adv Drug Deliver Rev. 2005;57:135–152. doi: 10.1016/j.addr.2004.07.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.