Abstract
Vaccination with irradiated B16 melanoma cells expressing either GM-CSF (Gvax) or Flt3-ligand (Fvax) combined with antibody blockade of the negative T-cell costimulatory receptor cytotoxic T-lymphocyte antigen-4 (CTLA-4) promotes rejection of preimplanted tumors. Despite CTLA-4 blockade, T-cell proliferation and cytokine production can be inhibited by the interaction of programmed death-1 (PD-1) with its ligands PD-L1 and PD-L2 or by the interaction of PD-L1 with B7-1. Here, we show that the combination of CTLA-4 and PD-1 blockade is more than twice as effective as either alone in promoting the rejection of B16 melanomas in conjunction with Fvax. Adding αPD-L1 to this regimen results in rejection of 65% of preimplanted tumors vs. 10% with CTLA-4 blockade alone. Combination PD-1 and CTLA-4 blockade increases effector T-cell (Teff) infiltration, resulting in highly advantageous Teff-to-regulatory T-cell ratios with the tumor. The fraction of tumor-infiltrating Teffs expressing CTLA-4 and PD-1 increases, reflecting the proliferation and accumulation of cells that would otherwise be anergized. Combination blockade also synergistically increases Teff-to-myeloid-derived suppressor cell ratios within B16 melanomas. IFN-γ production increases in both the tumor and vaccine draining lymph nodes, as does the frequency of IFN-γ/TNF-α double-producing CD8+ T cells within the tumor. These results suggest that combination blockade of the PD-1/PD-L1- and CTLA-4-negative costimulatory pathways allows tumor-specific T cells that would otherwise be inactivated to continue to expand and carry out effector functions, thereby shifting the tumor microenvironment from suppressive to inflammatory.
Keywords: Flt3-ligand, melanoma, PD-L1, immunotherapy, vaccine
The interaction between the T-cell receptor complex (TCR) and antigenic peptides bound in surface MHC molecules provides the specificity that defines adaptive T-cell immunity. In addition to TCR activation, costimulation via ligation of the coreceptor CD28 on T cells by B7 molecules on antigen-presenting cells (APCs) is required for optimal T-cell activation (1). Once mobilized, however, T cells begin to express other members of the CD28/B7 receptor family that attenuate the immune response through inhibition of proliferation and cytokine production (2). Cytotoxic T-lymphocyte antigen-4 (CTLA-4) is rapidly up-regulated following T-cell activation and binds to B7 molecules with a higher affinity than does CD28. The receptor programmed death-1 (PD-1) is also expressed on T cells following activation, where, on binding to its ligands PD-L1 and PD-L2, it promotes T-cell anergy, apoptosis, and exhaustion. Recently, an additional coinhibitory ligand/receptor interaction has been described that involves binding of PD-L1 on T cells to B7-1 on APCs or vice versa (3). Although the existence of so many layers of T-cell inhibition may seem surprising, the severe and sometimes fatal autoimmunity that results when even one of these pathways is disrupted attests to their necessity.
Malignant transformation was classically defined by the ability to avoid the normal regulatory mechanisms of cell growth, division, and death. Another common feature uniting all lineages of tumor is the ability to evade destruction by the host immune system. Ample evidence now exists that T cells infiltrating tumors can be inhibited by both CTLA-4 and PD-1 coinhibitory signals. Myeloid-derived suppressor cells (MDSCs) populating the tumor microenvironment can express both B7-1 and PD-L1 (4). In addition, tumors themselves sometimes express PD-L1, which can negatively signal T cells through both the PD-1 and B7-1 molecules on their surface (5). Antibody blockade of CTLA-4 or PD-1 has been shown to enhance antitumor immune responses in both murine preclinical models and clinical trials (2, 5).
Previously, we have shown that vaccination with B16-GM-CSF (Gvax) or B16-Flt3-ligand (Fvax) promotes the rejection of preimplanted B16-BL6 melanomas when combined with antibody blockade of CTLA-4 (6, 7). Although CTLA-4 and PD-1 belong to the same family of molecules and are both coinhibitory, evidence suggests they use distinct nonredundant mechanisms to inhibit T-cell activation. Most strikingly, CTLA-4 knockout mice die by 4 weeks of age from a lethal lymphoproliferative disorder, whereas some colonies of PD-1 knockout BL6 mice live over a year before manifesting lupus-like symptoms with ∼50% penetrance (8, 9). Although the phosphatases Src homology 2-containing tyrosine phosphatase (SHP)-1 and SHP-2 have been implicated in both CTLA-4 and PD-1 negative signaling, the phosphatase PP2A binds only CTLA-4 and leads to inhibition of Akt phosphorylation through a pathway that is distinct from that of PD-1 (10). Also, both PD-1/PD-L1 and CTLA-4/B7 interactions help to maintain peripheral tolerance through modifications in T-cell motility; however, they seem to do so through distinct mechanisms (11, 12). Because the CTLA-4 and PD-1 inhibitory pathways appeared to be nonredundant, we investigated whether PD-1 blockade in addition to CTLA-4 blockade could further enhance rejection of B16 melanoma.
Here, we show that in a B16 melanoma treatment setting in which Fvax + αCTLA-4 cures only 10% of animals, Fvax + αPD-1 promotes tumor rejection in almost 25% of animals. More strikingly, combination blockade of PD-1 and CTLA-4 leads to rejection of 50% of melanomas, and the further addition of αPD-L1 allows 65% of mice to survive tumor-free. We show that blockade of single negative costimulatory pathways often leads to enhanced effector T-cell (Teff) infiltration of tumors but that these T cells accumulate high levels of the unblocked negative coreceptors that eventually limit their expansion. Blockade of multiple coinhibitory pathways allows CD8+ and CD4+ T cells to continue to survive, proliferate, and carry out effector functions within the tumor. Combination blockade of the CTLA-4 and PD-1 pathways also cooperates to increase the ratio of Teffs to both regulatory T cells (Tregs) and MDSCs, thereby reducing suppression and promoting inflammation in the tumor microenvironment. Combination blockade further enhances this inflammatory state by increasing the frequency of IFN-γ and TNF-α double-producing T cells within the tumor and by increasing the proliferation of both CD8+ and CD4+ Teffs relative to Fvax treatment alone. Combination blockade of the PD-1, CTLA-4, and PD-L1 coinhibitory molecules coupled with Fvax vaccination initiates an inflammatory cascade in the tumor microenvironment leading to enhanced Teff infiltration, activation, and cytokine production, thereby antagonizing tumor-induced immune suppression and promoting tumor rejection.
Results
PD-1 Blockade Cooperates with CTLA-4 Blockade and Fvax Vaccination to Promote B16 Melanoma Rejection.
Combination vaccination with either Gvax or Fvax and antibody blockade of the coinhibitory receptor CTLA-4 prevent the outgrowth of a majority of 3-day preimplanted B16-BL6 melanomas (6). If vaccination is delayed until 5 days following implantation, the efficacy of this therapy declines substantially. When combined with Fvax, PD-1 blockade alone was more effective than CTLA-4 blockade alone and combination blockade of both coinhibitory pathways was synergistic, promoting full rejection of B16 melanoma in half of the animals (Fig. S1A). When combined with Gvax + αCTLA-4 therapy, we found that the addition of PD-1 blockade failed to increase survival or slow tumor growth (Fig. S1B).
Because higher levels of B7-1 expression have been reported following vaccination with GM-CSF relative to Flt3-ligand, we asked whether increased B7-1/PD-L1 inhibition might be responsible for the observed discrepancy between Gvax and Fvax (13).
With Fvax, Blockade of CTLA-4/B7, PD-1/PD-L1, and B7-1/PD-L1 Interactions Each Promotes Rejection of B16 Melanoma and Is Additive when Used in Combination.
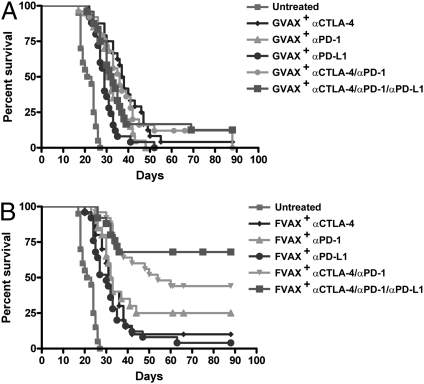
To test the contribution of B7-1/PD-L1 inhibition, we used a high B16 challenge (5 × 104 cells) and vaccination on days 3, 6, and 9. In this setting, no antibody regimen without a cellular vaccine was curative (Fig. S2). Also, neither blockade of any single coinhibitory receptor nor of any combination resulted in greater than 20% survival when combined with Gvax (Fig. 1A). In the context of Fvax, however, blockade of either the PD-L1 (8% survival), CTLA-4 (10% survival), or PD-1 (25% survival) receptor demonstrated modest therapeutical benefit (Fig. 1B). Combination blockade of CTLA-4 and PD-1 promoted tumor rejection in 50% of mice, whereas the further addition of αPD-L1 elevated the rate of tumor-free survival to 65%.
Fig. 1.
Survival of mice challenged with 5 × 104 B16-BL6 cells and vaccinated on days 3, 6, and 9 with 1 × 106 Gvax (A) or Fvax (B) intradermally and the indicated antibody or combination i.p.. Lack of survival was defined as death or tumor size >1,000 mm3. Each curve represents three to four independent experiments of 5–15 mice per group.
Combination Blockade of the CTLA-4 and PD-1 Pathways Strongly Increases Both CD8+ T-Cell/Treg and CD4+ Teff/Treg Ratios Within the Tumor.
Because there was a clear hierarchy of effect of each costimulatory blocking therapy as well as of the combinations when paired with Fvax, we sought to dissect the effects of each therapy on T-cell infiltration of tumor in this background. Both CTLA-4 and PD-1 blockade strongly increased infiltration of tumor by CD8+ T cells, whereas αPD-L1 blockade alone did so only modestly (Fig. 2A and Fig. S3A). The combination of CTLA-4 and PD-1 blockade increased CD8+ T-cell infiltration more than CTLA-4 blockade alone. In contrast to CTLA-4 and PD-L1 blockade, PD-1 blockade had little effect on the percentage of tumor-infiltrating lymphocytes (TILs) composed of CD4+ Teffs (Fig. 2B); however, PD-1 and CTLA-4 increased absolute numbers of infiltrating CD4+ Teffs to a similar degree (Fig. S3B). Combination PD-1/CTLA-4 blockade with or without αPD-L1 increased the percentage of CD4 Teff infiltration to a similar degree to that of αCTLA-4 alone; however, absolute numbers of CD4 Teffs appear to be increased to a greater extent by combination therapy. Anti-CTLA-4 treatment alone or in combination with other coinhibitory blockade decreased the fraction of TILs composed of Tregs (Fig. 2C); however, the number of Tregs per cubic millimeter of tumor was not diminished (Fig. S3C). PD-1 blockade alone, in contrast, did not decrease the TIL fraction of Tregs and seemed to promote an increase in their absolute numbers.
Fig. 2.
Lymphocyte infiltration of B16 melanoma. Mice were challenged with 1.5 × 105 B16-BL6 cells; treated with Fvax and the indicated antibody on days 3, 6, and 9; and killed on day 16. Percentages of CD45+ TILs of CD8+ T cells (A), CD4+ Teffs (B), and Tregs (C) are shown. The ratios of CD8+ T cells to Tregs (D) and CD4+ Teffs to Tregs (E) are shown. Values shown are for individually analyzed mice and are the sum of four to seven independent experiments with 5–15 mice per group. To determine statistical significance between samples, t tests were performed. *P ≤ 0.05; **P ≤ 0.01; ***P < 0.001.
CTLA-4, PD-1, or PD-L1 blockade increases CD8+ T-cell-to-Treg ratios within the tumor, which we have previously described as being predictive of therapeutical efficacy in the B16 melanoma model (14) (Fig. 2D). Combination therapy with CTLA-4 and PD-1 blockade increases CD8/Treg ratios significantly more than αCTLA-4 alone, whereas triple coinhibitory blockade is statistically superior to any individual antibody therapy.
Of the single coinhibitory antibodies, only αCTLA-4 significantly improves CD4 Teff/Treg ratios within the tumor (Fig. 2E). The combination of αCTLA-4, αPD-1, and αPD-L1 is superior to any other single or combination therapy in terms of promoting advantageous CD4 Teff/Treg ratios in TILs.
Combination Coinhibitory Pathway Blockade Allows Accumulation of High Percentages of Active T Cells Expressing CTLA-4 and PD-1 that Would Otherwise Be Anergized.
We hoped to learn more about the activation state of the tumor-infiltrating T cells by measuring their expression of relevant costimulatory molecules. In the CD4 Teff population, the fraction of CTLA-4-expressing cells was increased by each costimulatory blocking antibody alone and cooperatively by the double- and triple-antibody combinations (Fig. 3A). The frequency of PD-1 expression by these cells also increased in response to blockade of each coinhibitory pathway, but this elevation in PD-1 frequency was strongest in response to αCTLA-4 alone or in combination with other agents. The sum of these effects more than doubled the frequency of CTLA-4/PD-1 double-positive CD4+ Teffs in the combination-treated vs. untreated mice.
Fig. 3.
CTLA-4 and PD-1 expression by TILs. Mice challenged with 1.5 × 105 B16-BL6 cells and treated on days 3, 6, and 9 were killed on day 16. TILs were fixed and stained for lymphocyte lineage and activation markers using the FoxP3 fixation kit. CTLA-4 and PD-1 were stained for surface and intracellular expression. Percentages of CD8+ T cells (A) and CD4+ Teffs (B) expressing PD-1, CTLA-4, or both are shown. Values shown are for individually analyzed mice and are the sum of three to six independent experiments with 5–15 mice per group. To determine statistical significance between samples, t tests were performed. *P ≤ 0.05; **P ≤ 0.01; ***P < 0.001.
In the case of CD8+ T cells, CTLA-4 was again up-regulated by blockade of any coinhibitory pathway; however, in this case, αPD-1 alone or in combination with other antibodies produced the greatest increase in CTLA-4 expression (Fig. 3B). Blockade of CTLA-4, PD-1, or PD-L1 alone resulted in an increased fraction of CD8+ T cells expressing PD-1. Simultaneous blockade of all three receptors resulted in an even higher frequency of PD-1 expression, with nearly every CD8+ T cell staining positive for PD-1. Vaccination with Fvax combined with any of these antibodies increases the frequency of CTLA-4/PD-1 double-positive CD8+ T cells; however, αPD-1 has a significantly stronger effect than either αCTLA-4 or αPD-L1. Similar to CD4 Teffs, combination costimulatory blockade results in over 75% of CD8+ T cells coexpressing PD-1 and CTLA-4, whereas double-expressing cells make up less than 30% of the CD8+ T cells infiltrating untreated tumors.
Levels of coinhibitory receptor expression were also measured and further demonstrate that blockade of one coinhibitory pathway leads to compensatory up-regulation of others (Fig. S4 A and B).
Combination Coinhibitory Receptor Blockade Decreases Expression of Treg Activation Markers Except Inducible T-Cell Costimulator (ICOS).
CTLA-4 and PD-1, although inhibitory for Teffs, have been implicated in the formation of inducible Tregs as well as in Treg suppressive function, respectively (15, 16). Treatment with αCTLA-4 diminished the rate of CTLA-4 expression by CD4+FoxP3+ Treg cells, whereas both PD-1 and CTLA-4 blockade reduced the frequency of PD-1 expression (Fig. S5). Combined blockade of PD-1, CTLA-4, and PD-L1 reduced the fraction of intratumoral Tregs expressing CTLA-4 and PD-1 individually as well as the fraction of double-positive cells. In more than a third of triple-combination blockade-treated mice, less than 50% of the Tregs in TILs expressed both CTLA-4 and PD-1, a phenotype not observed in any untreated or Fvax alone-treated animals. Although αCTLA-4 and αPD-1 increased Treg expression levels of the unblocked receptor, both depressed PD-L1 levels when used individually (Fig. S4C).
In addition to CTLA-4 and PD-1, expression of 4-1BB and KLRG1 has been associated with enhanced Treg function (17, 18). In each case, we found that combination blockade of the CTLA-4 and PD-1 pathways significantly decreased the fraction of Tregs expressing the relevant activation marker (Fig. S6 A and B). ICOS expression has also been associated with enhanced Treg function; however, in contrast to the other activation molecules under study, we found that ICOS levels increased on Tregs following treatment with αCTLA-4 or αPD-1 (19) (Fig. S6C). Further, simultaneous blockade of both receptors resulted in significantly increased ICOS levels relative to αCTLA-4 alone. Interestingly, αPD-L1 did not promote an increased frequency of ICOShi CD4+ Tregs when used alone (Fig. S6C).
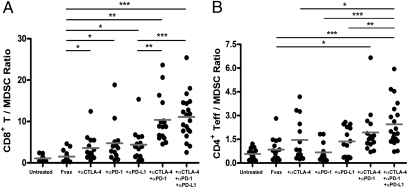
Combination Coinhibitory Receptor Blockade Synergistically Increases the Ratio of Teffs to MDSCs in the Tumor.
The activity of the enzyme arginase-1 is an important mechanism by which MDSCs dampen T-cell activity within tumors (4). We assessed the effects of our therapy on MDSCs using both the traditional CD11b+GR-1+ gating and a functional CD11b+arginase-1+ stain. Blockade of either CTLA-4, PD-1, or PD-L1 in the context of Fvax vaccination increased the ratio of CD8+ T cells to MDSCs within the tumor (Fig. 4A). Combination blockade of these negative costimulatory receptors synergistically increased CD8/MDSC ratios to more than double those achieved with single coinhibitory blockade and more than 10 times the levels in untreated tumors. Similar results were obtained if MDSCs were defined as CD11b+GR-1+ cells (Fig. S7A).
Fig. 4.
Ratios of Teffs to MDSCs within tumors. Mice challenged with 1.5 × 105 B16-BL6 cells and treated on days 3, 6, and 9, were killed on day 16. TILs were fixed and stained for lymphocyte and myeloid lineage and activation markers using the FoxP3 fixation kit. Arginase-1 was stained using the 8C9 clone from Santa Cruz. The ratios of CD8+ T cells to CD11b+arginase-1+ MDSCs (A) and CD4+ Teffs to CD11b+arginase-1+ cells (B) within CD45+ melanoma TILs are shown for mice receiving the indicated therapy. Values shown are for individually analyzed mice and are the sum of three independent experiments with 5–15 mice per group. To determine statistical significance between samples, t tests were performed. *P ≤ 0.05; **P ≤ 0.01; ***P < 0.001.
CD4 Teff-to-MDSC (CD11b+arginase-1+) ratios were increased relative to untreated tumor by Fvax + αCTLA-4 vaccination (Fig. 4B). As with CD8+ T cells, both double and triple coinhibitory blockade regimens significantly increased the ratio of CD4+ Teffs relative to MDSCs in tumors compared with Fvax treatment alone. Only triple coinhibitory blockade was statistically superior to any single-antibody therapy in this setting. Similar results were obtained if MDSCs were defined as CD11b+GR-1+ cells (Fig. S7B).
Combination Coinhibitory Receptor Blockade Increases Inflammatory Cytokine Production in the Vaccine and Tumor Draining Lymph Nodes and in the Tumor Itself.
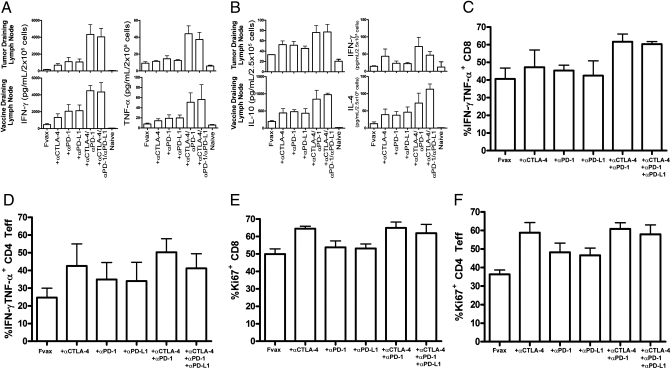
To define the functional effects of each therapy on effector cytokine production clearly, we elected to use the B16-ovalbumin (B16-Ova) model antigen system to measure cytokine responses. Mice were challenged with 2.5 × 105 B16-Ova cells in 30% Matrigel (BD Bioscience) and vaccinated on days 6, 9, and 12. Combination costimulatory blockade significantly increased IFN-γ and TNF-α production from CD8 cells in both the vaccine and tumor draining lymph nodes relative to blockade of any single receptor alone (Fig. 5A). The addition of PD-L1 blockade to αCTLA-4/αPD-1 combination therapy did not significantly alter inflammatory cytokine production.
Fig. 5.
Changes in cytokine production in response to coinhibitory blockade. Mice challenged with 2.5 × 105 B16-Ova cells and treated on days 6, 9, and 12 were killed on day 14. T cells were purified from tumor-draining lymph nodes and vaccine-draining lymph nodes, stained with antibodies, and sorted by flow cytometry into CD4+ and CD8+ subsets. Cytokine production was measured after 36 h using the TH1/TH2/TH17 CBA Kit and is shown for 2 × 105 CD8 T cells restimulated on 1 × 105 Ova 257–264 peptide-pulsed DCs (A) and for 2.5 × 105 CD4 T cells restimulated on 1 × 105 Ova 323–339 peptide-pulsed DCs (B). Data are shown for three to four independent experiments with five pooled mice per group. (C and D) TILs were purified from 5–10 pooled tumors per group and enriched using the Miltenyi T-cell purification kit. A total of 2 × 106 TILs were restimulated with 7.5 × 105 DCs [1:1 mix of Ova(257) and Ova(323) pulsed DCs] per well for 8 h in the presence of GolgiPlug. Cells were fixed using the FoxP3 kit and analyzed by flow cytometry for lymphocyte markers and intracellular IFN-γ and TNF-α production for CD8 cells (C) and CD4 Teffs (D) and also for Ki67 expression in CD8 cells (E) and CD4 Teffs (F). TIL data are from four independent experiments. All means shown are ± SEM.
CD4+ T cells in the vaccine draining lymph node demonstrated increased IL-10 and IL-4 production with combination costimulatory blockade (Fig. 5B). Although this increase reflects the increased activation resulting from PD-1 and CTLA-4 combination blockade, the apparent T helper (Th) 2 polarization likely reflects the known Th2 bias of Ova-specific CD4 responses in this background (20). Despite this Th2 bias, we still observed a significant increase in IFN-γ production in the tumor draining lymph node following CTLA-4 and CTLA-4/PD-1 combination coinhibitory blockade (Fig. 5B).
To investigate changes in cytokine expression resulting from combination coinhibitory blockade within the tumor itself further, we restimulated tumor-infiltrating T cells for 8 h with both Ova- and B16 peptide-pulsed dendritic cells (DCs) and measured intracellular cytokine accumulation by flow cytometry. Combination CTLA-4 and PD-1 blockade has previously been associated with increased IFN-γ and TNF-α expression by reactivated CD8+ T cells responding to hepatitis C virus infection (21). Similarly, combination coinhibitory blockade produced high frequencies of CD8+ T cells in TILs producing both IFN-γ and TNF-α (Fig. 5C and Fig. S8). Much as we observed in the lymph nodes, addition of αPD-L1 to dual PD-1/CTLA-4 blockade did not significantly enhance inflammatory cytokine production. In the CD4+ Teff population of TILs, the highest fraction of IFN-γ and TNF-α double-producing cells was induced by CTLA-4 blockade alone or in combination with other antibodies (Fig. 5D). We also assayed IL-10 production but did not observe substantial levels from any population of B16-Ova infiltrating T cells.
Combination coinhibitory receptor blockade increases Teff infiltration of tumors, and those cells appear to be producing high levels of inflammatory cytokines. We assayed Ki67 expression of tumor-infiltrating T cells in an effort to determine the extent of proliferation of these cells in response to each therapy. CTLA-4 blockade was the strongest driver of intratumoral T-cell proliferation of any single agent tested, and combining it with PD-1 blockade did not further increase division in CD4 or CD8 cells (Fig. 5 E and F). Intratumoral Treg proliferation was also highest with CTLA-4 blockade alone but was very similar among all samples (Fig. S9).
Discussion
Here, we show that in the context of Fvax vaccination, combination blockade of the CTLA-4- and PD-1-negative costimulatory receptors leads to synergistic levels of tumor rejection. Underlying this effect, we find dramatically enhanced levels of Teff infiltration of tumors and inflammatory cytokine production. The accumulation of CTLA-4/PD-1 double-positive Teffs within B16 melanomas in the context of combination blockade suggests that T cells that would otherwise be functionally and proliferatively repressed are instead able to continue expanding and carrying out effector functions. Addition of PD-L1 blockade to αCTLA-4/αPD-1 combination therapy increased mean survival from 50 to 65% in mice challenged with 5 × 104 B16-BL6 cells. It is unlikely that this represents a direct antitumor effect, because B16 melanoma expresses little to no PD-L1 in vivo (22). The primary benefit of blocking the additional PD-L1/B7-1 inhibitory pathway in this context appeared to be in augmenting the CD4/Treg and CD4/MDSC ratios within B16 melanomas.
It has been shown previously that both CTLA-4 and PD-1 blockade individually augments the capacity of Gvax to promote rejection of B16 melanomas (6, 7, 23). We purposely chose the less immunogenic B16-BL6 model (as opposed to B16-F10) and settings of high tumor challenge or delayed vaccination in hopes of observing cooperative effects between blockade of multiple coinhibitory pathways. Still, we were surprised that even with triple coinhibitory blockade, we were unable to observe any significant additive effects of these antibodies in the context of Gvax. Although we did not pursue the roots of the differences between Fvax and Gvax further, the inability of any T-cell-potentiating antibody combination to promote cure of more than 20% of mice with Gvax suggests the presence of a dominant suppressive mechanism. We have observed that Gvax, even when administered on the opposite flank from the tumor site, leads to increased myeloid suppressor accumulation in the tumor, although Fvax can have the opposite effect (6). Gvax may also increase elaboration of tolerogenic cytokines such as TGF-β (24). Additionally, Fvax has been shown to elicit greater CD8 infiltration of both vaccine and tumor sites relative to Gvax, which may also be partially responsible for the increased synergy with therapies such as αPD-1, which interact strongly with CD8 T cells. It is also possible that the optimal timing of coinhibitory blockade may differ in the context of Gvax vs. Fvax. In the end, further study will be necessary to define the reasons underlying the lack of cooperativity between CTLA-4/PD-1 combination blockade and Gvax.
Although we observe enhanced infiltration, activation marker expression, and inflammatory cytokine production following simultaneous blockade of the CTLA-4 and PD-1 pathways, the greatest difference between single and multiple coinhibitory blockade was in enhancing the ratio of CD8+ T cells relative to Tregs and MDSCs in tumors. In each case, ratios following combination antibody treatment were more than double those from mice receiving any single antibody and ≈10-fold higher than those found in untreated mice. In the case of CD8/Treg ratios, the cooperativity we observe likely results from increased CD8 infiltration and expansion, impaired conversion of CD4 T cells into Tregs resulting from CTLA-4 blockade, and reduced Treg suppression through PD-1 blockade (15, 25). In the case of MDSCs, enhanced production of Th1-type cytokines by the increased fraction of tumor-infiltrating T cells likely antagonizes further MDSC formation, although converting a portion of the tumor-infiltrating myeloid cells into functional APCs at the same time. Supporting this model, we found that combination coinhibitory blockade significantly reduced the fraction of TILs composed of MDSCs (Fig. S10). Direct antagonism of MDSC function through coinhibitory blockade has been reported in ovarian cancer; however, we observed minimal expression of PD-1 and CTLA-4 by these cells in untreated B16 tumors (26) (Fig. S11). PD-L1, in contrast, was expressed by a substantial portion of MDSCs, making it impossible for us to exclude direct effects on these cells in mice receiving αPD-L1 antibody. Anti-PD-L1 alone, however, did not appear to have a substantially greater impact on MDSC-to-T-cell ratios than other coinhibitory blocking antibodies (Fig. 4). This striking reversal in the ratios of CD8+ T cells to suppressor T and myeloid cells within the tumor is largely responsible for shifting the microenvironment from one of dominant suppression to an inflammatory milieu permissive for immune-mediated tumor rejection.
In clinical trials, antibodies that block CTLA-4 have shown efficacy in treating advanced melanoma and prostate cancer but at the price of autoimmune complications that are often severe. Human studies of αPD-1 and αPD-L1 antibodies are at a much earlier stage yet suggest that these agents may be efficacious while engendering fewer autoimmune complications (27). Our preclinical data in the B16 melanoma model strongly suggest that combining these agents may have synergistic effects in driving tumor rejection. Although combining multiple coinhibitory blocking antibodies in the clinic should be approached with great caution, our studies suggest that blocking a single inhibitory receptor only leads to up-regulation of the unblocked pathway. To allow tumor-infiltrating T cells the capacity to expand, kill, and produce inflammatory cytokines within the tumor microenvironment appears to require simultaneous blockade of multiple negative costimulatory receptors.
Materials and Methods
Mice.
C57BL/6 mice (4–6-week-old male; Jackson Laboratories) were cared for accordance with National Institutes of Health and American Association for the Accreditation of Laboratory Animal Care International regulations. Experiments were all approved by the Memorial Sloan-Kettering Cancer Center Institutional Animal Care and Use Committee. B6.SJL mice (6-week-old male) were obtained from Taconic.
Antibodies.
Anti-CTLA-4 (9D9), αPD-1 (RMP1-14) (28), αPD-L1 (9G2), Rat Ig, and mIgG2b used in vivo were obtained from Bioxcell. Dosing per injection was 100 μg of 9D9, 250 μg of RMP114, 100 μg of 9G2, 250 μg of Rat Ig, and 100 μg of mIgG2b.
Staining antibodies included CD4-Q605, CD8-Pacific Orange, and CD3-APCAlexa750 (Invitrogen); CD4-APC, FoxP3-Pacific Blue, KLRG1-APC, PD-1-FITC, 4-1BB-biotin, GR-1-phycoerythrin (PE) Cy7, and CD11b (eBioscience); CD8-PE, PD-L1-PE, IFN-γ-PE-CY7, Ki67-FITC, and TNF-α-APC (BD Bioscience); ICOS and CTLA-4 (4F10) (Bioxcell); CD45.1 peridinin chlorophyll protein (Biolegend); and arginase-1 (Santa Cruz). Some clones were conjugated using Invitrogen monoclonal antibody conjugation kits to Alexa 532, Alexa 594, or Qdot 655.
Cell Lines.
B16/BL6 cells as well as B16-sFlt3L-Ig (Fvax) and B16-GM-CSF (Gvax) have been described previously.
Peptides.
Ova 257–264 (SIINFEKL) and 323–339 (ISQAVHAAHAFINEAGR) (American Peptide Company) were used at a final concentration of 5 μM. GP100 (25), Trp-1 (455, 481, and 522), and Trp-2 (181) peptides were obtained from Biosynthesis, Inc. and used at a final concentration of 10 μm.
B16 Melanoma Treatment Experiments.
Mice were injected in the flank intradermally at day 0 with 5 × 104 B16-BL6 cells and treated on days 3, 6, and 9, with 1 × 106 irradiated (150 Gy) gene-modified B16 cells on the contralateral flank and the indicated therapeutical antibody i.p.
Tumor Infiltration/Activation Marker Analysis.
Mice receiving a 1.5 × 105 B16-BL6 challenge were vaccinated as discussed in the previous section on days 3, 6, and 9 and were killed on day 16. Tumors were measured immediately before the mice were killed. Excised tumors were digested in Liberase (Roche) and DNase (Roche), cells were counted, and lymphocytes were enriched on a Ficoll gradient (Histopaque 1119; Sigma). Cells were stained using a FoxP3 staining kit (eBioscience); CTLA-4 and PD-1 were stained for total intracellular and extracellular protein. Stained samples were run completely on an LSRII (BD Bioscience) cytometer.
Analysis of Cytokine Production.
Mice receiving a 2.5 × 105 B16-Ova challenge in 30% collagen matrix (Matrigel) were vaccinated as discussed in the previous section on days 6, 9, and 12 and killed on day 14. Vaccine and tumor draining lymph nodes as well as tumors were pooled from five mice per group. Lymph node cells were stained with DAPI and antibodies to CD4 and CD8 and sorted on a MoFlo cell sorter (Beckman Coulter). Tumor cells were purified as discussed previously except that before restimulation, the T-cell fraction was enriched using a Miltenyi T-cell purification kit. A total of 2 × 105 CD8 cells and 2.5 × 105 CD4 cells per well were restimulated in 96-well round-bottom plates with 1 × 105 Ova peptide-pulsed DCs purified from B6.SJL mouse spleens using CD11c+ selection beads (Miltenyi) for 36 h, and cytokine production was then assessed using a TH1/TH2/TH17 CBA kit from BD Bioscience. For tumor samples, 2 × 106 T-enriched TILs were restimulated with 7.5 × 105 Ova and B16 melanoma peptide-pulsed DCs for 8 h in the presence of GolgiPlug (BD Bioscience) and then fixed and stained for intracellular cytokine production using a FoxP3 kit.
Supplementary Material
Footnotes
Conflict of interest statement: J.P.A. is a paid consultant for Bristol-Myers Squibb and is the primary inventor on the patent “Blockade of T lymphocyte down-regulation associated with CTLA-4 signaling.”
This article contains supporting information online at www.pnas.org/cgi/content/full/0915174107/DCSupplemental.
References
- 1.Linsley PS, et al. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991;173:721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peggs KS, Quezada SA, Allison JP. Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy. Immunol Rev. 2008;224:141–165. doi: 10.1111/j.1600-065X.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 3.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okazaki T, Honjo T. PD-1 and PD-1 ligands: From discovery to clinical application. Int Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 6.Curran MA, Allison JP. Tumor vaccines expressing Flt3 ligand synergize with CTLA-4 blockade to reject preimplanted tumors. Cancer Res. 2009;69:7747–7755. doi: 10.1158/0008-5472.CAN-08-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 9.Tivol EA, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 10.Parry RV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fife BT, et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10:1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudd CE. The reverse stop-signal model for CTLA4 function. Nat Rev Immunol. 2008;8:153–160. doi: 10.1038/nri2253. [DOI] [PubMed] [Google Scholar]
- 13.Mach N, et al. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 2000;60:3239–3246. [PubMed] [Google Scholar]
- 14.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W, et al. PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4+ CD25(Hi) regulatory T cells. Int Immunol. 2009;21:1065–1077. doi: 10.1093/intimm/dxp072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng SG, et al. TGF-beta requires CTLA-4 early after T cell activation to induce FoxP3 and generate adaptive CD4+CD25+ regulatory cells. J Immunol. 2006;176:3321–3329. doi: 10.4049/jimmunol.176.6.3321. [DOI] [PubMed] [Google Scholar]
- 17.Beyersdorf N, Ding X, Tietze JK, Hanke T. Characterization of mouse CD4 T cell subsets defined by expression of KLRG1. Eur J Immunol. 2007;37:3445–3454. doi: 10.1002/eji.200737126. [DOI] [PubMed] [Google Scholar]
- 18.Zhang P, et al. Agonistic anti-4-1BB antibody promotes the expansion of natural regulatory T cells while maintaining Foxp3 expression. Scand J Immunol. 2007;66:435–440. doi: 10.1111/j.1365-3083.2007.01994.x. [DOI] [PubMed] [Google Scholar]
- 19.Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199:1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linton PJ, et al. Costimulation via OX40L expressed by B cells is sufficient to determine the extent of primary CD4 cell expansion and Th2 cytokine secretion in vivo. J Exp Med. 2003;197:875–883. doi: 10.1084/jem.20021290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamoto N, et al. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 2009;5:e1000313. doi: 10.1371/journal.ppat.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwai Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li B, et al. Anti-programmed death-1 synergizes with granulocyte macrophage colony-stimulating factor–secreting tumor cell immunotherapy providing therapeutic benefit to mice with established tumors. Clin Cancer Res. 2009;15:1623–1634. doi: 10.1158/1078-0432.CCR-08-1825. [DOI] [PubMed] [Google Scholar]
- 24.Jinushi M, et al. MFG-E8-mediated uptake of apoptotic cells by APCs links the pro- and antiinflammatory activities of GM-CSF. J Clin Invest. 2007;117:1902–1913. doi: 10.1172/JCI30966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, et al. Regulation of arginase I activity and expression by both PD-1 and CTLA-4 on the myeloid-derived suppressor cells. Cancer Immunol Immunother. 2009;58:687–697. doi: 10.1007/s00262-008-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berger R, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 28.Yamazaki T, et al. Blockade of B7-H1 on macrophages suppresses CD4+ T cell proliferation by augmenting IFN-gamma-induced nitric oxide production. J Immunol. 2005;175:1586–1592. doi: 10.4049/jimmunol.175.3.1586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.