Abstract
G protein–coupled receptors (GPCRs) play central roles in almost all physiological functions; mutations in GPCRs are responsible for more than 30 disorders. There is a great deal of information about GPCR structure but little information that directly relates structure to protein trafficking or to activation. The gonadotropin releasing hormone receptor, because of its small size among GPCRs, is amenable to preparation of mutants and was used in this study to establish the relation among a salt bridge, protein trafficking, and receptor activation. This bridge, between residues E90 [located in transmembrane segment (TM) 2] and K121 (TM3), is associated with correct trafficking to the plasma membrane. Agonists, but not antagonists, interact with residue K121, and destabilize the TM2–TM3 association of the receptor in the plasma membrane. The hGnRHR mutant E90K has a broken salt bridge, which also destabilizes the TM2–TM3 association and is typically retained in the endoplasmic reticulum. We show that this mutant, if rescued to the plasma membrane by either of two different means, has constitutive activity and shows modified ligand specificity, revealing a role for the salt bridge in receptor activation, ligand specificity, trafficking, and structure. The data indicate that destabilizing the TM2–TM3 relation for receptor activation, while requiring an intact salt bridge for correct trafficking, provides a mechanism that protects the cell from plasma membrane expression of constitutive activity.
Keywords: constitutive activity, hormone action, receptor, G-protein coupled receptor, receptor trafficking
Hundreds of gonadotropin releasing hormone receptor (GnRHR) mutants have been reported but none have constitutive activity (CA) (1). This observation is surprising, because many G protein coupled receptors (GPCRs) have mutants (2) or WT receptors (3) with CA. A highly conserved (4) structural feature of the GnRHR is a salt bridge between transmembrane segment (TM) 2 and TM3 (residues E90 and K121 in the human sequence) (5, 6). Gonadotropin releasing hormone (GnRH) agonists, but not peptide antagonists, bind receptor residues D98 and K121 (5, 6) and alter the relation between TM2 and TM3; this same relation is important for trafficking of the human GnRHR (hGnRHR) to the plasma membrane (4, 7). We considered that perturbing this relation is also a determinant of receptor activation.
Another way to perturb this relation, other than ligand binding, is to alter the charge of one of its constituents; mutant E90K would result in charge repulsion between K90 and K121. This mutant, which occurs in some cases of human hypogonadotropic hypogonadism, is typically recognized by the quality control system (QCS) as a misfolded protein (8), retained in the endoplasmic reticulum (ER) (9) and does not traffic to the plasma membrane.
E90K can be rescued by pharmacoperones, drugs that diffuse into cells and provide a folding template. This template enables (otherwise) misfolded mutants to fold or refold correctly (10), pass a quality control system (constituted of chemically heterogeneous chaperone proteins of the ER that either promote correct folding or retain misfolded proteins) (11), traffic to the plasma membrane, bind agonist, and produce a signal. Pharmacoperones of the hGnRHR rescue mutant E90K by creating a surrogate bridge between D98 and K121 that also stabilizes the relation between TM2 and TM3 (4). We took advantage of pharmacoperone rescue of mutants and a genetic modification that enhances trafficking to the plasma membrane to examine the relation among this bridge, receptor trafficking, and activation.
Results
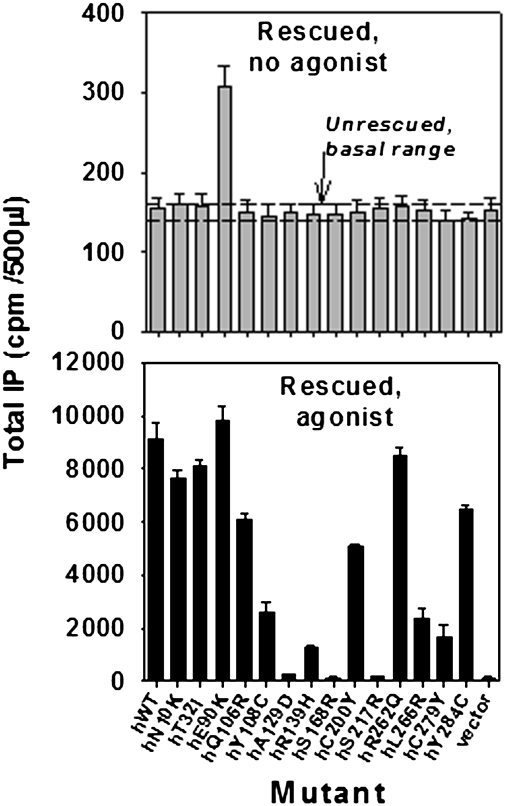
Mutations from patients with hypogonadotropic hypogonadism are widely distributed over the 328 residues of the hGnRHR structure. Most are misfolded and misrouted molecules (9) that are rescuable with pharmacoperones, such as In3 (12–16), and rerouted to the plasma membrane (10). In Fig. 1 Upper, the dashed lines show the range of basal activity (no agonist) of cells transfected with each of the mutants, but which have not been rescued. These dashed lines show that activity does not exceed the vector-only control. When rescued by pharmacoperone In3, only a single mutant, E90K, shows constitutive activity, producing inositol phosphate (IP) in the absence of agonist. Fig. 1 Lower shows pharmacoperone-rescued mutants subsequently incubated with the stable GnRHR agonist, 10−7 M Buserelin. This observation confirms that rescue occurs for most mutants and that they are able to couple to IP, but most exhibit no constitutive activity. S168R and S217R cannot be rescued for thermodynamic reasons (17), and A129D is minimally rescuable for unknown reasons.
Fig. 1.
Assessment of constitutive activity in IP production of 14 naturally occurring mutations of the hGnRHR after pharmacoperone rescue. COS-7 cells were transfected with 100 ng of WT or mutant cDNA as described in Materials and Methods. Mutants were incubated in media alone (shown as two dashed lines showing upper and lower level of IP production) or rescued with pharmacoperone (In3) as described in Materials and Methods; In3 was then washed out and IP production was measured in response to media alone (no agonist added) (Upper) or rescued with pharmacoperone (In3), then In3 was washed out and agonist added. In all figures, SEMs are shown for least three independent experiments performed in replicates of 4–6 (Lower).
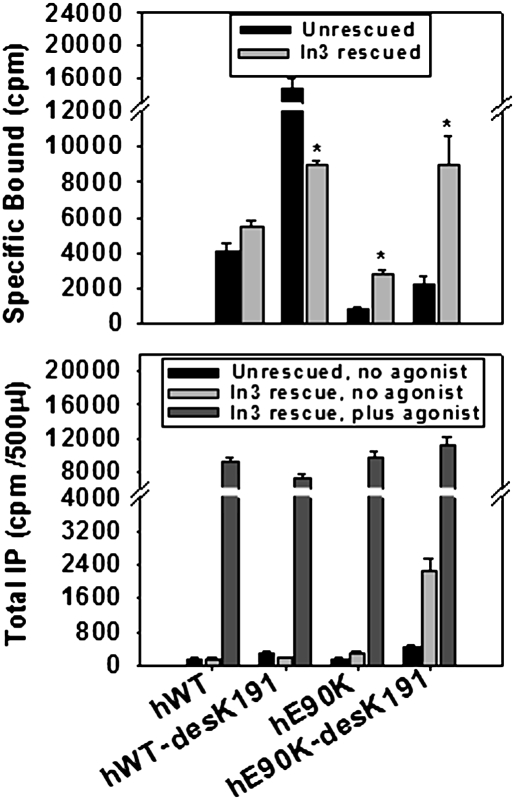
Fig. 2 Upper (radioligand binding) and Lower (IP production) show cells expressing WT hGnRHR, E90K, or sequences from which K191 was deleted, then either rescued by pharmacoperone In3 or unrescued. Like pharmacoperone rescue, deletion of residue K191 is known to rescue misrouted GnRHR mutants, including E90K (8). Like pharmacoperone rescue, deletion of K191 also reveals constitutive activation by E90K. When E90K-desK191 was subjected to pharmacoperone rescue, the constitutive activity (IP) was increased more dramatically than for E90K-desK191 alone, because routing to the plasma membrane is increased by both deletion of the K191 and pharmacoperone. Radioligand binding confirms that pharmacoperones and deletion of residue K191 increase the number of mutant receptors at the plasma membrane (Fig. 2 Upper). Because In3 was identified in a screen relying on rat GnRH (which does not contain K191), it is difficult to wash out of cells expressing hWT-desK191; accordingly, the specific binding after In3 rescue may not quantitatively reflect the number of receptors at the PM. The increased fold stimulation (and responsiveness) of E90K-desK191 to agonist, compared with constitutive activity shown by the mutant, suggests that the K90- K121 repulsion precludes attaining the optimal activation structure, but that this is corrected by the presence of the agonist (Fig. 2 Lower).
Fig. 2.
Effect on constitutive activity of deleting Lys191 on E90K mutant. Cells were transfected with 25 ng of WT or mutant (each with or without K191) cDNA and rescued with or without pharmacoperone (In3), as described in Materials and Methods for binding studies. The In3 was washed out and specific binding was determined by using 2 × 106 cpm/mL [125I-Buserelin] for 90 min at room temperature (Upper). The tracer was removed, cells were washed twice, and radioactivity was measured. *, P < 0.05 for the comparison of each mutant that is rescued or not. (Lower) Cells were transfected with 100 ng of WT or mutant (hE90K or hE90K-desK191) cDNA and rescued with or without pharmacoperone (In3), as described in Materials and Methods, and total IP production was measured in response to medium alone or the addition of agonist (10−7 M Buserelin). The values for hE90K and hE90K-desK191 are 156 ± 19 and 440 ± 50 (DMSO) and 292 ± 33 and 2,246 ± 300 (In3), respectively.
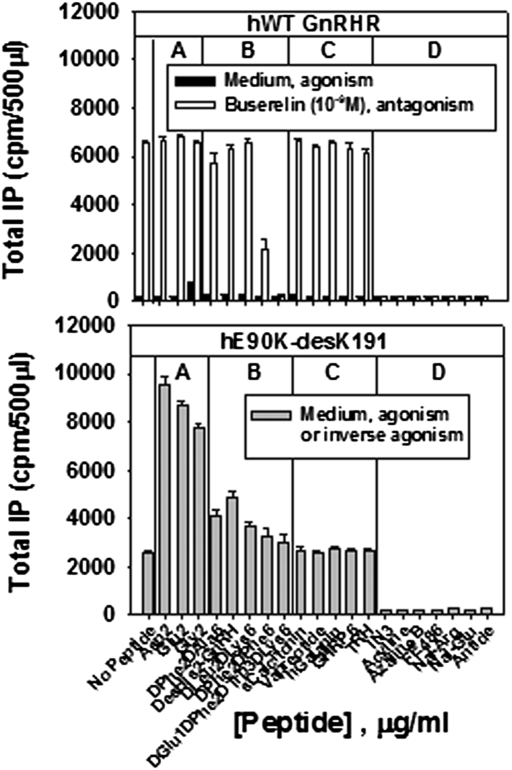
Because of the close relation of the agonist binding site and the salt bridge, we compared the ligand specificity of the CA mutant to that of the WT receptor. Fig. 3 shows the effects of GnRH peptide analogs (groups A, B, and D) and selected irrelevant compounds (group C) on signaling by the WT hGnRHR (Fig. 3 Upper) and by the constitutively active mutant, E90K-desK191 (Fig. 3 Lower). High affinity antagonists of the WT (Fig. 3 Upper, group D) inhibit responsiveness to the agonist, Buserelin; they are also inverse agonists of the mutant (that is, they inhibit constitutive activity). Irrelevant peptides that have no agonistic or antagonistic activity on the WT also have no activity on the rescued mutant (Fig. 3 Lower, group C). Replacement of the His2 in the natural ligand by Gly2, Asp2, or Glu2 results in little or no agonism with the WT receptor (i.e., in medium without Buserelin) (Fig. 3 Upper, group A; ref. 18) but strong agonism with the mutant (Fig. 3 Lower, group A). Deletion of His2 or its replacement by D-amino acid residues, along with D-amino acids at position 6, actions that stabilize the peptide against degradation, produce weak antagonists or no activity on the WT, but weak agonists of the mutant (group B). This observation suggests that the mutant possesses an altered ligand binding site compared with WT, has reduced specificity required for receptor activation by ligands and that both agonistic and antagonistic peptides, which bind near the active site of the mutant, can produce the configuration associated with activated receptor.
Fig. 3.
Assessment of GnRH peptides and irrelevant compounds on WT and hE90K-desK191 mutant IP production. (Upper) Cells were transfected with 100 ng of human WT GnRHR cDNA and left unrescued. Various GnRH peptides or irrelevant compounds were added in medium alone (1 μg/mL) or with 10−9 M Buserelin to show agonistic or antagonistic activity on total IP production. For comparison, a maximal concentration (10−9 M) of the GnRH agonist, Buserelin, produces a response of 6,517 ± 93 cpm. (Lower) Cells were transfected with 100 ng of hE90K-desK191 cDNA, then rescued with pharmacoperone (In3). The In3 was then washed out. After 18 h of preloading the cells with 3H-inositol, the cells were incubated with 1 μg/mL GnRH analogs or irrelevant compounds to assess agonistic or antagonistic activity on total IP production. Group A (Asp2, Glu2, Gly2); Group B (DPhe2DAla6, DGlu1DPhe2DTrp3DLys6, DesHis2-GnRH, DLeu2DLys6, DPhe2DPhe6); Group C (sCalcitonin, Vapreotide, hGalanin, GHRP6, TRH); Group D (In3, Acyline, Azaline B, FE486, Nal-Arg, Nal-Glu, Antide). For comparison, a maximal concentration (10−7 M) of the GnRH agonist, Buserelin, produces a response of 11,144 ± 1,098 cpm.
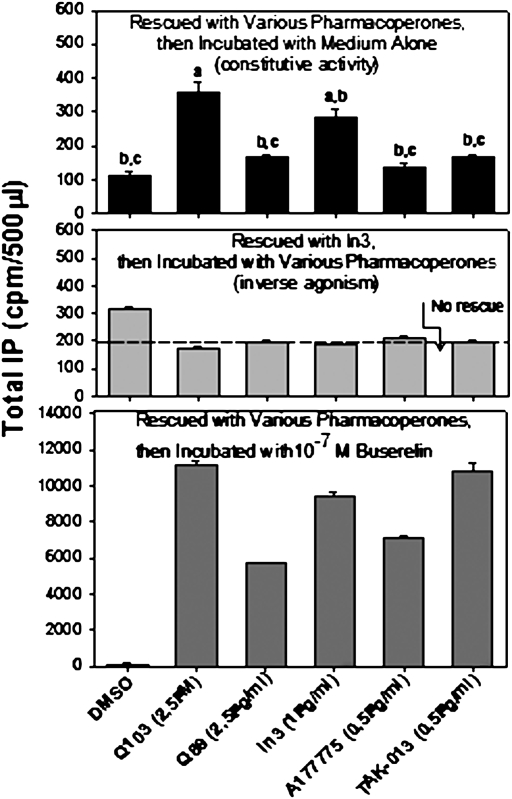
To determine whether the same chemical interactions of pharmacoperones with hE90K were involved in both mutant rescue and development of CA, we assessed (i) whether these agents had a similar efficacy order for the development of CA and for mutant rescue and (ii) whether pharmacoperones from different chemical classes acted as inverse agonists (inhibiting constitutive activity). In the protocols described thus far, once the mutant is rescued, In3 was removed so that the unoccupied receptor could be assessed for constitutive activity. Fig. 4 Top shows, using this approach, that the five different pharmacoperones tested (from four different chemical classes) rescue mutant E90K and lead to some degree of constitutive activity, although this is quite modest for A177775. The concentrations used were selected as the optimum for rescue from prior studies (4, 12). In Fig. 4 Middle, all cells were rescued by In3, then In3 was washed out and each of the five pharmacoperones was added to assess constitutive activation; this observation shows that the pharmacoperones also behave as inverse agonists, agents that inhibit constitutive activity (compare with dashed line, showing IP production by mutant E90K that was not rescued).
Fig. 4.
Comparison of constitutive activity of hE90K with four classes of pharmacoperones on rescue. All cells were transfected with 100 ng of cDNA of hE90K mutant, and rescue was attempted with each indicated pharmacoperone. The cells were then incubated with media alone to assess constitutive activity (Top). a, P < 0.05 compared with DMSO only; b, P < 0.05 compared with Q103; c, P < 0.05 compared with In3. (Middle) Rescued with In3 and then incubated with various pharmacoperones to assess their actions as inverse agonists. (Bottom) Stimulated with 10−7 M of the GnRH agonist, Buserelin to assess the ability to couple. All values are significant compared with DMSO only.
Because the pharmacoperones used in this study all form a bridge between receptor residues D98 and K121 (4, 11, 19), other interactions notwithstanding, they stabilize the TM2–TM3 relation, an event that allows correct trafficking but precludes constitutive activity, because of occupancy. When the pharmacoperones are washed out and the metabolically stable GnRH agonist, Buserelin, is present after rescue of E90K by each of the pharmacoperones, effector coupling is observed. It is interesting to note that In3 is more effective than TAK-013 in producing constitutive activity (Fig. 4 Top), while it is less effective in rescuing Buserelin-stimulated activity (Fig. 4 Bottom). This observation suggests that the binding or steric interactions required for production of constitutive activity and for mutant rescue may be subtly different between different classes of pharmacoperones.
Discussion
The concept of constitutive activity for GPCRs was introduced in 1989 (20), and it has been theorized that virtually all members of this class have mutants that couple to effectors in the absence of agonist (2). Inhibitors of constitutive activity, inverse agonists, are drug candidates for lowering unstimulated receptor activity. It was curious, in light of the clinical importance of the GnRHR and the many hundreds of mutants reported for this GPCR, that no mutant with CA was identified among either naturally occurring or designed mutants. The present work suggests that this constitutive activity has likely been missed because the cell recognizes a constitutively active mutant as misfolded and prevents it from being expressed at the plasma membrane.
We performed these studies having noted that hGnRHR residue D98 forms both an interhelical interaction with K121 and binds His2 in the naturally occurring agonist (5). It is likely that agonist binding interferes with the TM2 and TM3 interaction, both by breaking the interhelical interaction and because of the physical interpositioning of the peptide. In addition, nonconservative substitutions at K121 interfere with GnRH agonist but not antagonist binding (6), suggesting that antagonists, although competing for the binding site, are not candidates for altering the TM2–TM3 relation. For these reasons, we considered that positively charged residues at K90 (mutant E90K) and K121 would activate the receptor by a mechanism similar to that which occurs during agonist binding and leading to receptor activation. We knew that this mutant was recognized as incorrectly folded by the QCS and retained in the ER, so we examined two methods to rescue it.
Pharmacoperones of the hGnRHR rescue misfolded mutant E90K (which is unable to create the salt bridge between residues E90 and K121) by creating a surrogate bridge between D98 and K121 (4) that stabilizes the relation between TM2 and TM3. After rescue, the mutants were stabilized by hydrophobic interactions with the seven TMs, and pharmacoperones were removed to allow agonist occupancy without competition.
Mutant E90K was rescued in cells expressing it with pharmacoperone, and both constitutive activity and radioligand binding were assessed after removal of the drug. In other studies, mutant E90K was rescued by deleting amino acid K191, which promotes the formation of a C14–C200 bridge, a modification that decreases the requirement for the E90–K121 salt bridge (8, 17). It was not possible to quantitate receptors with immunological approaches because there is no known antibody for the hGnRHR that can be used for this purpose and tagging sequences, such as GFP and HA-antigen, alter the trafficking of this receptor (21).
All pharmacoperones examined were inverse agonists, blocking constitutive activity. Pharmacoperone structures associated with the highest amount of rescue were not those associated with production of the highest amount of constitutive activity. This observation suggests that the receptor structure associated with proper routing to the plasma membrane is distinct from the structure associated with receptor activation.
The present studies show that the cell protects itself against constitutive activity by requiring a bridge between TM2 and TM3 for correct routing to the plasma membrane. Because WT receptors are frequently only partially expressed at the plasma membrane (11, 19), these findings may explain why inverse agonists (in this case, pharmacoperones), which stabilize receptors in the configuration that is acceptable to the QCS, have been shown to produce greater receptor up-regulation in many systems than do neutral antagonists (22). This effect may occur by increasing the percentage of protein that passes the QCS. Agonist activation of the receptor is also associated with disruption of this bridge, making it attractive to consider that the cell may also use this structure as a signal for recognition of agonist occupancy before agonist-activated receptor down-regulation.
Materials and Methods
pcDNA3.1 (Invitrogen), GnRH analog, D-tert-butyl-Ser6-des-Gly10-Pro9-ethylamide-GnRH (Buserelin, Hoechst-Roussel Pharmaceuticals), myo-[2-3H(N)]-inositol (PerkinElmer; NET-114A), DMEM, OPTI-MEM, lipofectamine, PBS (GIBCO, Invitrogen), competent cells (Promega), and Endofree maxi-prep kits (Qiagen), were obtained as indicated. Mutant receptors: WT and mutant GnRHR cDNAs for transfection were prepared as reported (13); the purity and identity of plasmid DNAs were verified by dye terminator cycle sequencing (Applied Biosystems). GnRH analogs were obtained as indicated: DPhe2-DAla6-GnRH; DLeu2-DAla6 GnRH; Des-His2-GnRH; DPhe2, DPhe6-GnRH (Wyeth-Ayerst Laboratories); “Nal-Arg,” [Ac-DNal1-DCpa2-DPal3, Arg5-D-Arg6-D-Ala10]- GnRH; “Nal-Glu,” [Ac-DNal1, DCpa2, D3Pal3, Arg5, DGlu6, DAla10]-GnRH; acyline, [Ac-d-2Nal1, D4Cpa1,2-D3Pal3, Ser4Aph(Ac), d-4Aph(Ac)6-Leu7-ILys8-Pro9- D-Ala10-NH2]-GnRH; azaline B, [Ac-DNal1-DCpa2-DPal3-Aph5 (atz)-DAph6 (atz)-ILys8-DAla10]-GnRH; Asp2-GnRH, Glu2-GnRH, Gly2-GnRH (Jean Rivier, Salk Institute, La Jolla, CA); “FE486” [Ac-D2Nal1-D4Cpa2-D3Pal3-Ser4- 4Aph(l-hydroorotyl)3-D4Aph (carbamoyl)6-Leu7-ILys8-Pro9-DAla10]-GnRH (Ferring Research Institute); Antide, [Ac-(DNal) -(DpClPhe)-(DPal)-Ser-Lys(nicotinoyl)-[D-Lys(nicotinoyl)]-Leu-Lys(isopropyl)-Pro-[DAla]-NH2 (Serono Laboratories) and DpGlu1-DPhe2-DTrp3-DLys6-GnRH (John Stewart, University of Colorado, Denver). Irrelevant molecules were obtained as follows: GHRP6 (His1-DTrp2-Ala3-Trp4-DPhe5-Lys6-NH2), human galanin, salmon calcitonin, and TSH-releasing hormone (Phoenix Pharmaceuticals); vapreotide was obtained from Debiopharm. Pharmacoperones In3, Q89, Q103 (Merck), A177775 (Abbott Laboratories), and TAK-013 (Takeda Pharmaceuticals) were obtained as indicated (4, 11). The drugs are, respectively, from the following chemical classes: indoles, quinolones (both Q89 and Q103), erythromycin macrolides and (N–1-(2,6-difluorobenzyl)-2,4-dioxo-3-phenyl-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl]phenyl}-N’-methoxyurea). Chemical structures (11, 19) and the mechanism of action on the hGnRHR (4) have been reported.
Transient Transfection.
Cos-7 cells were cultured in growth medium (DMEM, 10% FCS, 20 μg/mL gentamicin) at 37 °C in a 5% CO2 humidified atmosphere. For transfection of WT or mutant receptors into cells, 5 × 104 cells were plated in 0.25 mL growth medium in 48-well Costar cell culture plates. Twenty-four hours after plating, the cells were washed with 0.5 mL of OPTI-MEM then transfected with WT or mutant receptor DNA with pcDNA3.1 (empty vector) to keep the total DNA constant (100 ng per well). Lipofectamine was used according to the manufacturer’s instructions. Five hours after transfection, 0.125 mL DMEM with 20% FCS and 20 μg/mL gentamicin was added. Twenty-three hours after transfection, the medium was replaced with 0.25 mL of fresh growth medium. Where indicated, pharmacoperones (indicated concentration) in 1% DMSO (“vehicle”) were added for 4 h in respective media to the cells and then removed 18 h before agonist treatment (16). In this study, we used Trypan blue exclusion to show cell viability after drug exposure.
IP Assays.
Twenty-seven hours after transfection, cells were washed twice with 0.50 mL DMEM/0.1% BSA/20 μg/mL gentamicin then “pre-loaded” for 18 h with 0.25 mL of 4 μCi/mL myo-[2-3H(N)]-inositol in inositol-free DMEM, then washed twice with 0.30 mL DMEM (inositol free) containing 5 mM LiCl and treated for 2 h with 0.25 mL of a saturating concentration of Buserelin (10−7 M) in the same medium. When constitutive activity was assessed, Buserelin was omitted from the assessment period. Total IP was then determined (23). This assay has been validated as a sensitive measure of PME for functional receptors when expressed at low amounts of DNA (<100 ng per well) and stimulated by excess agonist (12, 14, 16, 24–30).
Binding Assays.
Cells were cultured and plated in growth medium as described above, except 105 cells in 0.5 mL of growth medium were added to 24-well Costar cell culture plates (cell transfection and medium volumes were doubled accordingly). Twenty-three hours after transfection, the medium was replaced with 0.5 mL of fresh growth medium with or without pharmacoperone (1 μg/mL of In3). Twenty-seven hours after transfection, cells were washed twice with 0.5 mL of DMEM containing 0.1% BSA and 20 μg/mL gentamicin, then 0.5 mL of DMEM was added. After 18 h, cells were washed twice with 0.5 mL of DMEM/0.1% BSA/10 mM hepes, then 2 × 106 cpm/mL of [125I]-Buserelin, prepared in our laboratory (specific activity, 700–800 μCi/μg), was added to the cells in 0.5 mL of the same medium and allowed to incubate at room temperature for 90 min, consonant with maximum binding (31). New receptor synthesis during this period is negligible at room temperature. After 90 min, the media was removed and radioactivity was measured (22). To determine nonspecific binding, the same concentrations of radioligand were added to similarly transfected cells in the presence of 10 μg/mL unlabeled GnRH.
Statistics.
Data (n ≥ 3) were analyzed with one-way ANOVA and then Holm-Sidak test and paired with Student’s t test (SigmaStat 3.1, Jandel Scientific Software). SEMs are shown.
Acknowledgments
We thank Jo Ann Binkerd for formatting the manuscript and Drs. J. Fielding Hejtmancik, Jon Hennebold, and Richard Stouffer for commenting on a draft. This work was supported by National Institutes of Health Grants HD-19899, RR-00163, and HD-18185.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Tao YX. Constitutive activation of G protein-coupled receptors and diseases: Insights into mechanisms of activation and therapeutics. Pharmacol Ther. 2008;120:129–148. doi: 10.1016/j.pharmthera.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bond RA, Ijzerman AP. Recent developments in constitutive receptor activity and inverse agonism, and their potential for GPCR drug discovery. Trends Pharmacol Sci. 2006;27:92–96. doi: 10.1016/j.tips.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Seifert R, Wenzel-Seifert K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:381–416. doi: 10.1007/s00210-002-0588-0. [DOI] [PubMed] [Google Scholar]
- 4.Janovick JA, et al. Molecular mechanism of action of pharmacoperone rescue of misrouted GPCR mutants: The GnRH receptor. Mol Endocrinol. 2009;23:157–168. doi: 10.1210/me.2008-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flanagan CA, et al. Multiple interactions of the Asp(2.61(98)) side chain of the gonadotropin-releasing hormone receptor contribute differentially to ligand interaction. Biochemistry. 2000;39:8133–8141. doi: 10.1021/bi000085g. [DOI] [PubMed] [Google Scholar]
- 6.Zhou W, et al. A locus of the gonadotropin-releasing hormone receptor that differentiates agonist and antagonist binding sites. J Biol Chem. 1995;270:18853–18857. doi: 10.1074/jbc.270.32.18853. [DOI] [PubMed] [Google Scholar]
- 7.Blomenröhr M, et al. Proper receptor signalling in a mutant catfish gonadotropin-releasing hormone receptor lacking the highly conserved Asp(90) residue. FEBS Lett. 2001;501:131–134. doi: 10.1016/s0014-5793(01)02647-3. [DOI] [PubMed] [Google Scholar]
- 8.Maya-Núñez G, et al. Molecular basis of hypogonadotropic hypogonadism: restoration of mutant (E(90)K) GnRH receptor function by a deletion at a distant site. J Clin Endocrinol Metab. 2002;87:2144–2149. doi: 10.1210/jcem.87.5.8386. [DOI] [PubMed] [Google Scholar]
- 9.Brothers SP, Cornea A, Janovick JA, Conn PM. Human loss-of-function gonadotropin-releasing hormone receptor mutants retain wild-type receptors in the endoplasmic reticulum: Molecular basis of the dominant-negative effect. Mol Endocrinol. 2004;18:1787–1797. doi: 10.1210/me.2004-0091. [DOI] [PubMed] [Google Scholar]
- 10.Janovick JA, et al. Refolding of misfolded mutant GPCR: Post-translational pharmacoperone action in vitro. Mol Cell Endocrinol. 2007;272:77–85. doi: 10.1016/j.mce.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conn PM, Janovick JA. Drug development and the cellular quality control system. Trends Pharmacol Sci. 2009;30:228–233. doi: 10.1016/j.tips.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Janovick JA, et al. Structure-activity relations of successful pharmacologic chaperones for rescue of naturally occurring and manufactured mutants of the gonadotropin-releasing hormone receptor. J Pharmacol Exp Ther. 2003;305:608–614. doi: 10.1124/jpet.102.048454. [DOI] [PubMed] [Google Scholar]
- 13.Janovick JA, Maya-Nunez G, Conn PM. Rescue of hypogonadotropic hypogonadism-causing and manufactured GnRH receptor mutants by a specific protein-folding template: Misrouted proteins as a novel disease etiology and therapeutic target. J Clin Endocrinol Metab. 2002;87:3255–3262. doi: 10.1210/jcem.87.7.8582. [DOI] [PubMed] [Google Scholar]
- 14.Leaños-Miranda A, Janovick JA, Conn PM. Receptor-misrouting: an unexpectedly prevalent and rescuable etiology in gonadotropin-releasing hormone receptor-mediated hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2002;87:4825–4828. doi: 10.1210/jc.2002-020961. [DOI] [PubMed] [Google Scholar]
- 15.Leaños-Miranda A, Ulloa-Aguirre A, Janovick JA, Conn PM. In vitro coexpression and pharmacological rescue of mutant gonadotropin-releasing hormone receptors causing hypogonadotropic hypogonadism in humans expressing compound heterozygous alleles. J Clin Endocrinol Metab. 2005;90:3001–3008. doi: 10.1210/jc.2004-2071. [DOI] [PubMed] [Google Scholar]
- 16.Leaños-Miranda A, Ulloa-Aguirre A, Ji TH, Janovick JA, Conn PM. Dominant-negative action of disease-causing gonadotropin-releasing hormone receptor (GnRHR) mutants: a trait that potentially coevolved with decreased plasma membrane expression of GnRHR in humans. J Clin Endocrinol Metab. 2003;88:3360–3367. doi: 10.1210/jc.2003-030084. [DOI] [PubMed] [Google Scholar]
- 17.Janovick JA, et al. Regulation of G protein-coupled receptor trafficking by inefficient plasma membrane expression: molecular basis of an evolved strategy. J Biol Chem. 2006;281:8417–8425. doi: 10.1074/jbc.M510601200. [DOI] [PubMed] [Google Scholar]
- 18.Grant G, et al. In: Recent Studies of Hypothalamic Function Int. Symp. Lederis K, Cooper KE, editors. Calgary: Karger; 1973. pp. 180–195. [Google Scholar]
- 19.Conn PM, Janovick JA. Trafficking and quality control of the gonadotropin releasing hormone receptor in health and disease. Mol Cell Endocrinol. 2009;299:137–145. doi: 10.1016/j.mce.2008.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa T, Herz A. Antagonists with negative intrinsic activity at delta opioid receptors coupled to GTP-binding proteins. Proc Natl Acad Sci USA. 1989;86:7321–7325. doi: 10.1073/pnas.86.19.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brothers SP, Janovick JA, Conn PM. Unexpected effects of epitope and chimeric tags on gonadotropin-releasing hormone receptors: implications for understanding the molecular etiology of hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2003;88:6107–6112. doi: 10.1210/jc.2003-031047. [DOI] [PubMed] [Google Scholar]
- 22.Milligan G, Bond RA. Inverse agonism and the regulation of receptor number. Trends Pharmacol Sci. 1997;18:468–474. doi: 10.1016/s0165-6147(97)01139-5. [DOI] [PubMed] [Google Scholar]
- 23.Huckle WR, Conn PM. Use of lithium ion in measurement of stimulated pituitary inositol phospholipid turnover. Methods Enzymol. 1987;141:149–155. doi: 10.1016/0076-6879(87)41063-x. [DOI] [PubMed] [Google Scholar]
- 24.Conn PM, Leaños-Miranda A, Janovick JA. Protein origami: Therapeutic rescue of misfolded gene products. Mol Interv. 2002;2:308–316. doi: 10.1124/mi.2.5.308. [DOI] [PubMed] [Google Scholar]
- 25.Ulloa-Aguirre A, Janovick JA, Brothers SP, Conn PM. Pharmacologic rescue of conformationally-defective proteins: Implications for the treatment of human disease. Traffic. 2004;5:821–837. doi: 10.1111/j.1600-0854.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- 26.Castro-Fernández C, Maya-Núñez G, Conn PM. Beyond the signal sequence: protein routing in health and disease. Endocr Rev. 2005;26:479–503. doi: 10.1210/er.2004-0010. [DOI] [PubMed] [Google Scholar]
- 27.Cook JV, Eidne KA. An intramolecular disulfide bond between conserved extracellular cysteines in the gonadotropin-releasing hormone receptor is essential for binding and activation. Endocrinology. 1997;138:2800–2806. doi: 10.1210/endo.138.7.5233. [DOI] [PubMed] [Google Scholar]
- 28.Knollman PE, Janovick JA, Brothers SP, Conn PM. Parallel regulation of membrane trafficking and dominant-negative effects by misrouted gonadotropin-releasing hormone receptor mutants. J Biol Chem. 2005;280:24506–24514. doi: 10.1074/jbc.M501978200. [DOI] [PubMed] [Google Scholar]
- 29.Janovick JA, Ulloa-Aguirre A, Conn PM. Evolved regulation of gonadotropin-releasing hormone receptor cell surface expression. Endocrine. 2003;22:317–327. doi: 10.1385/ENDO:22:3:317. [DOI] [PubMed] [Google Scholar]
- 30.Ulloa-Aguirre A, Janovick JA, Leaños-Miranda A, Conn PM. Misrouted cell surface receptors as a novel disease aetiology and potential therapeutic target: the case of hypogonadotropic hypogonadism due to gonadotropin-releasing hormone resistance. Expert Opin Ther Targets. 2003;7:175–185. doi: 10.1517/14728222.7.2.175. [DOI] [PubMed] [Google Scholar]
- 31.Brothers SP, et al. Conserved mammalian gonadotropin-releasing hormone receptor carboxyl terminal amino acids regulate ligand binding, effector coupling and internalization. Mol Cell Endocrinol. 2002;190:19–27. doi: 10.1016/s0303-7207(02)00040-0. [DOI] [PubMed] [Google Scholar]