Abstract
To improve the efficacy of T cell–based vaccination, we pursued the principle that CD4+ T cells provide help for functional CD8+ T cell immunity. To do so, we administered HIV gag to mice successively as protein and DNA vaccines. To achieve strong CD4+ T cell immunity, the protein vaccine was targeted selectively to DEC-205, a receptor for antigen presentation on dendritic cells. This targeting helped CD8+ T cell immunity develop to a subsequent DNA vaccine and improved protection to intranasal challenge with recombinant vaccinia gag virus, including more rapid accumulation of CD8+ T cells in the lung. The helper effect of dendritic cell-targeted protein vaccine was mimicked by immunization with specific MHC II binding HIV gag peptides but not peptides from a disparate Yersinia pestis microbe. CD4+ helper cells upon adoptive transfer allowed wild-type, but not CD40−/−, recipient mice to respond better to the DNA vaccine. The transfer also enabled recipients to more rapidly accumulate gag-specific CD8+ T cells in the lung following challenge with vaccinia gag virus. Thus, complementary prime boost vaccination, in which prime and boost favor distinct types of T cell immunity, improves plasmid DNA immunization, including mobilization of CD8+ T cells to sites of infection.
Keywords: complementary, prime, boost, vaccination, helper
To improve the efficacy of safe vaccines against global pathogens like HIV-1, “heterologous prime-boost” strategies are being tested, in particular priming with a DNA vaccine and boosting with a recombinant viral vector (1, 2). During heterologous prime-boosting, vaccine antigens are introduced in different vectors to reduce the risk of anti-vector immunity; e.g., adenoviral vectors quickly induce neutralizing antibodies minimizing the response to multiple doses. Here, we have pursued a “complementary” prime boost vaccination strategy, in which the two vaccines induce different types of immunity and no microbial vectors are required.
We previously reported that a protein-based vaccine becomes more immunogenic for mice when it is directly targeted to dendritic cells (DCs), the principal antigen-presenting cells for initiating T cell immunity (3), along with a suitable adjuvant to stimulate DC maturation. Selective and efficient antigen targeting to DCs was achieved by introducing HIV gag (4, 5) into a monoclonal antibody (mAb) to a DC-restricted, antigen uptake receptor, DEC-205 or CD205. Synthetic double-stranded RNA, polyIC, proved to be an effective adjuvant for this protein vaccine (5). Nevertheless, the achieved immunity was primarily comprised of Th1-type CD4+ T cells whereas, in contrast, DNA- and adenoviral vector-based vaccines induced higher CD8+ T cell frequencies (4).
It is known that CD4+ T cells provide essential help for generating CD8+ T cell responses (6–11). Here, we will show that protective CD8+ T cell immunity to a DNA vaccine is improved by priming a helper T cell response with a DC-targeted protein vaccine. One component of the improved protection is a more rapid accumulation of gag-specific CD8+ T cells to a mucosal infection challenge site.
Results
DC-Targeted Protein Enhances Protection Afforded by a DNA Vaccine.
To test the protection afforded by protein and DNA vaccines, we first compared two doses of DC-targeted, DEC-gag protein vaccine to two doses of gag plasmid DNA vaccine, given 1 month apart, and we used a higher intranasal (i.n.) dose of challenge virus than our prior studies (4, 5). Both protein and DNA forms of vaccination induced protection against weight loss (Fig. S1A) and an ∼100-fold reduction to challenge recombinant vaccinia virus (Fig. 1A; compare orange and green with black).
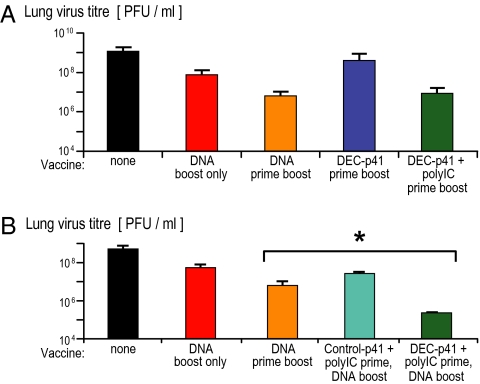
Fig. 1.
DC-targeted protein vaccine enhances protection from DNA vaccine. (A) Groups of five female 6- to 10-week-old CxB6 F1 mice were primed and boosted with protein (5 μg of anti-DEC HIV gag p41 plus 50 μg of polyIC) or DNA vaccine (10 μg of HIV gag p41 DNA) i.m. 8 weeks apart. Twelve weeks after the boost, a lethal dose (105 PFU) of recombinant vaccinia gag was given i.n., and vaccinia virus titers in the lung (PFU/lung) were measured 7 days later. (B) As in (A), but complementary protein-prime DNA-boost vaccination (blue) was evaluated to induce protection as monitored by lung vaccinia virus titers (mean ± SD of three experiments).
Next, to try to improve protective immunity, we primed mice with a single dose of DEC-targeted gag protein vaccine followed by a boost with gag plasmid DNA 4–8 weeks later. Twelve weeks after boosting, mice were challenged with recombinant vaccinia gag, whereupon weight loss was monitored daily and lung virus titers determined as described (4, 5, 12). Again, mice receiving two doses of DNA exhibited some protection against weight loss (Fig. S1B, orange) and also an ∼100-fold reduction in virus titers (Fig. 1B, orange). A single dose of DNA vaccine did not protect against weight loss and reduced virus titers only by 10-fold (Fig. 1B, red). However, priming with DEC-gag plus polyIC protein vaccine followed by plasmid DNA vaccine provided superior protection against weight loss to two DNA vaccines (Fig. 1B, *) and reduced virus titers in the lung by an average of 5,000-fold in three experiments (Fig. 1B, dark blue), which titers were significantly lower than mice vaccinated twice with DNA (P < 0.005). The control for this and the following experiments was a control Ig (not binding to DCs) gag protein vaccine followed by a single DNA boost, but this control prime boost strategy offered only 1 log of protection (Fig. 1B, turquoise). We will use the term “complementary” prime boost to describe DEC-gag protein prime plus DNA boost, because we will show that each part of the vaccine induces a distinct type of immune response.
T Cells Provide Protection After a Complementary Protein Prime-DNA Boost Vaccine.
To assess the contribution of T cells to protective immunity, we treated mice with either rat IgG control mAb or with depleting mAbs to CD4 and CD8 before the i.n. challenge with recombinant vaccinia gag virus. We verified these mAbs selectively depleted CD4+ and CD8+ CD3+ T cells. In addition, we found that treatment of mice with control mAb did not interfere with the observed 3 logs of protection afforded by the complementary prime boost vaccine to vaccinia gag challenge (Fig. 2A Upper Left, dark blue bar). However, depletion of either CD4+ or CD8+ T cells reduced protection significantly (10- to 30-fold) for the mice vaccinated with the DEC-gag prime DNA boost regimen but did not completely eliminate protection (Fig. 2A, two right bars). We then treated vaccinated mice with either control Ig or a combination of mAbs to CD4 and CD8 (Fig. 2B, Left and Right). Depletion of both CD4+ and CD8+ T cells before challenge eliminated protection from weight loss (Fig. S1 C and D) and increased lung virus titers (Fig. 2B, compare dark blue bars in Left and Right). Therefore, the superior protection from complementary prime boost requires both CD4+ and CD8+ T cells.
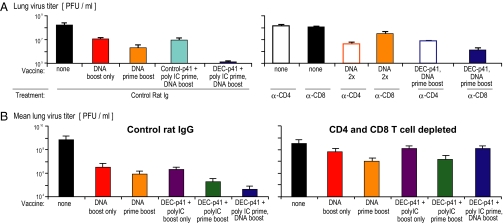
Fig. 2.
CD4+ and CD8+ T cells protect mice after protein-prime DNA-boost vaccine. (A) Mice vaccinated as indicated on the x axis and Fig. 1 were treated with control rat Ig or depleting antibodies to CD4 or CD8 at days −3, −2, and −1 before airway challenge with recombinant vaccinia gag virus (mean of two experiments). (B) As in (A), mice were treated with control rat IgG (Left) or depleted of both CD4+ and CD8+ T cells (Right) before challenge with vaccinia gag and measurement of vaccinia virus titers (PFU/lung).
Protein Priming Improves CD8+ T Cell Immunity to a DNA Vaccine.
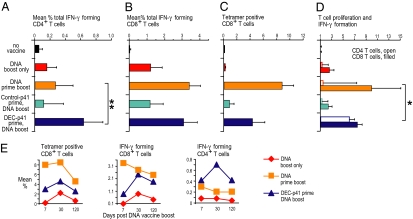
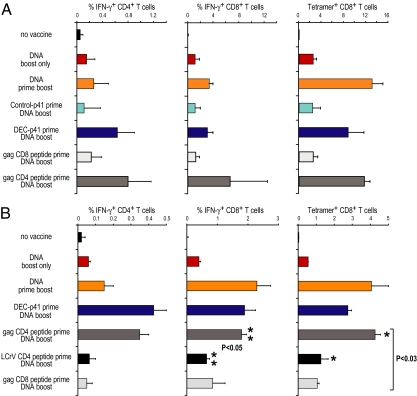
To assess T cell immunity after vaccination with DEC-targeted gag protein prime followed by gag DNA boost, we measured CD4+ and CD8+, gag-specific T cells at the single-cell level. One dose of DNA elicited weak CD4+ and CD8+ immunity (Fig. 3 A–C, red second row of data) whereas two doses induced a stronger response (orange, third row of data). Prior data had shown that one dose of DEC-targeted gag protein vaccine, together with polyIC, induced weak CD4+ T cell immunity, whereas two doses led to strong but primarily CD4+ T cell immunity (5) (and see below). In three experiments, priming with one dose of a DC targeted protein vaccine, but not nontargeted control Ig-gag vaccine, resulted in strong combined CD4+ and CD8+ T cell immunity to a single dose of DNA vaccine (Fig. 3 A–C, compare fourth and fifth rows, turquoise and dark blue). IFN-γ production by CD4+ T cells following complementary prime boost also was significantly higher than with two doses of DNA [Fig. 3, ** (P < 0.01)]. For mice primed with either two doses of DNA or complementary prime boost, the gag-specific CD8+ T cells were also capable of proliferating to HIV gag (Fig. 3D, *). However, CD4+ T cells immunized with the complementary prime boost approach expanded significantly better than CD4+ T cells immunized with two doses of DNA [Fig. 3D, *, open bars (P < 0.05)]. The gag-specific CD4+ and CD8+ T cells persisted at least 4 months after boosting in two long-term experiments (Fig. 3E, dark blue). Thus, complementary prime boosting provides improved and long-lived CD4+ and CD8+ T cell immunity to one dose of DNA vaccine.
Fig. 3.
Complementary protein prime DNA boost vaccination induces combined and durable CD4+ and CD8+ T cell immunity. (A) Female CxB6 F1 mice were vaccinated as in Fig. 1. Thirty days after the DNA boost, bulk splenocytes were assessed for T cell immunity. (A and B) Splenocytes were restimulated either with unreactive peptides or with an HIV gag peptide mix, and IFN-γ production in response to peptide was evaluated by intracellular cytokine staining 6 h later in CD4+ (A) or CD8+ (B) CD3+ T cells. (C) HIV gag-specific CD8+ T cells were enumerated by binding of gag tetramers. (D) HIV gag-dependent T cell proliferation and IFN-γ production was measured in CFSE-labeled splenocytes restimulated for 4 days with gag-specific peptides (or nonspecific control peptides), followed by intracellular staining for IFN-γ in CFSE-low CD4+ and CD8+ T cells. All data are mean ± SD of three experiments, each involving five F1 mice per group. (E) Means of two experiments to follow the longevity of the immune response in vaccinated mice for 120 days.
Complementary Protein Prime-DNA Boost Allows for More Rapid Accumulation of CD8+ T Cells to an Infection Challenge Site.
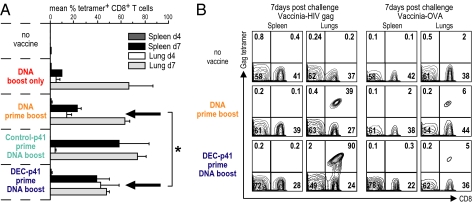
Because the above studies did not reveal a major quantitative difference in CD8+ T cells induced by two doses of DNA (“homologous” prime boost) vs. complementary prime boost, we asked whether the priming with DEC gag vaccine improved the quality of the CD8+ T cell response by examining the rapidity with which gag-specific CD8+ T cells were mobilized to a site of infection. In three experiments, 120 days after vaccination, we challenged the mice with recombinant vaccinia gag i.n. and then examined the site of challenge infection, the lungs, as well as the spleen 4 and 7 days later. Priming with DEC-gag p41 plus polyIC followed by boosting with one dose of DNA led to a better accumulation of CD8+ T cells to the lung (P = 0.05) than a single dose or two doses of DNA vaccine (Fig. 4A, compare lung day 4 data at arrows). To establish that the accumulation of gag-reactive CD8+ T cells in the lungs was specific to vaccine antigen, we showed that gag-reactive cells accumulated when mice were challenged with vaccinia gag but not vaccinia OVA (Fig. 4B). Therefore, complementary prime boost vaccination leads to more rapid accumulation of CD8+ T cells upon vaccinia gag challenge, and this is gag antigen-dependent not vaccinia "inflammation"- or infection-dependent.
Fig. 4.
Complementary protein prime DNA boost vaccine allows for more rapid accumulation of CD8+ T cells to an infection challenge site. As in Fig. 3, mice were vaccinated (y axis) and 90 days after the boost challenged with a lethal dose of vaccinia gag i.n. At day 4 and day 7, lungs were dissociated to enumerate HIV gag-specific tetramer binding cells. (A) A rapid accumulation of specific CD8+ T cells in the lungs after DC-targeted protein prime-DNA boost vaccine. Illustrative FACS data are in Fig. S3. Arrows point to rapidly accumulating gag-specific CD8+ T cells early (day 4) in mice challenged after two doses of DNA vaccine or complementary prime-boost vaccine. Shown are means of two experiments. (B) In HIV gag-vaccinated mice, gag-specific CD8+ T cells accumulate when mice are challenged with vaccinia gag (Left) but not vaccinia-OVA (Right).
CD4 Restricted HIV gag Peptides Provide Help for HIV gag DNA Vaccine.
To test whether priming with DEC-gag p41 protein could be replaced with previously defined gag peptides that are recognized by primed CD4+ T cells (4), we synthesized three of these peptides (C57BL/6 pool 1, peptide #6, amino acids 145–159, QAISPRTLNAWVKVV; B6 pool 4, peptide #8, amino acids 297–311, VDRFYKTLRAEQASQ; Balb/C pool 3, peptide #10, amino acids 257–271, PVGEIYKRWIILGLN) and used them to prime and boost CxB6 F1 mice. In parallel, we primed mice with a single peptide (GHQAAMQMLKETINE, amino acids 193–207) that includes a nanomer recognized by gag-specific CD8+ T cells (AMQMLKETI) presented on H-2d, as well as CD4-restricted peptides from a different protein, the LcrV protein from Yersinia pestis as described (13). As expected, the peptides primed the mice in a specific way; i.e., CD4-negative T cells were primed to CD8-restricted gag peptides (Fig. S2, arrow second row) whereas CD4+ T cells were primed selectively to either LcrV or gag CD4-restricted peptides (Fig. S2, arrows, third and fourth rows).
In three experiments, we again observed strong CD8+ responses to a DNA boost in mice primed with DEC-gag p41 protein vaccine plus polyIC but not control Ig gag p41 plus polyIC (Fig. 5A; compare turquoise and dark blue). Importantly, when we replaced the protein vaccine with peptides, only helper peptides recognized by CD4+ T cells, and not the peptide recognized by gag-specific CD8+ T cells, were able to prime for a strong CD8+ T cell response to DNA gag vaccine (Fig. 5A, lower two rows). Thus, priming CD4+ T cells with peptides allows for better CD8+ T cell responses to a single dose of DNA vaccine. Next, to evaluate the specificity of the help provided by CD4+ T cells, we immunized CxB6 F1 mice with either HIV gag- or LCRV CD4-restricted peptides and boosted with HIV gag DNA as above. Only gag CD4-restricted peptides enhanced CD8+ T cell responses to gag DNA vaccine (P < 0.03) (Fig. 5B; compare fifth and sixth rows, ** and *). This indicated that help provided to CD8+ T cells was cognate antigen-dependent.
Fig. 5.
Helper HIV gag peptides improve CD8 T cell responses to DNA vaccine. Two experiments in which CxB6 F1 mice were vaccinated as in Figs. 2 and 3, but we added groups of mice primed with HIV gag peptides recognized by CD4+ and CD8+ T cells, or LcrV CD4-restricted peptides, to compare with DEC gag p41 protein vaccine (y axis). Thirty days after the DNA vaccine boost we measured HIV gag-specific IFN-γ-producing CD4+ and CD8+ IFN-γ-secreting T cells, and tetramer binding CD8+ T cells. Shown are means of two experiments.
Complementary Protein Prime-DNA Boost Vaccine Requires CD40.
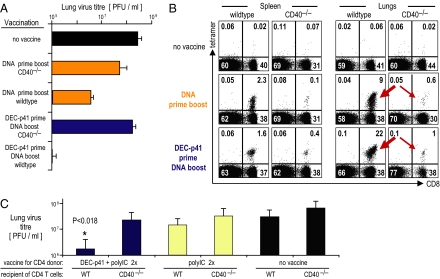
To begin to understand mechanisms required for the helper effect of a DC-targeted protein vaccine, we pursued the fact that helper T cells express CD40L, which acts to mature DCs presenting antigens to CD8+ T cells (10, 14–16). Indeed, CD40−/− mice developed reduced CD4+ and CD8+ T cell immunity to vaccination with either two doses of DNA or complementary protein prime DNA boost, including T cells that could proliferate and produce IFN-γ in response to HIV gag antigen (Fig. S3; compare Left and Right). Complementary prime boost did not allow for reduced virus titers in lungs (Fig. 6A; compare fourth and fifth rows). When we also followed CD8+ T cell accumulation to a challenge site, the CD40−/− mice lacked gag-specific CD8+ T cells in the lungs, with either two DNA vaccines or the complementary prime boost strategy (Fig. 6B; compare thick and thin arrows for wild-type and CD40−/− mice). Thus, our prime boost vaccination increased lung CD8+ T cells in a CD40-dependent manner.
Fig. 6.
Help from DC-targeted protein vaccine requires CD40. (A) Female wild-type and CD40−/− CxB6 F1 mice were vaccinated as indicated on the y axis. Thirty days after the last DNA boost mice were challenged with vaccinia gag i.n. to assess protection in terms of body weight and lung virus titers as in Fig. 1. (B) Likewise, during the challenge with vaccinia gag the numbers of CD8+ gag-specific tetramer binding cells were measured at day 4 and day 7 in spleen and lungs. (C) Female CxB6 F1 mice were vaccinated with DEC-p41 and polyIC twice. A spleen equivalent of CD4+ T cells was then adoptively transferred into wild-type or CD40−/− CxB6 F1 mice and boosted with a single dose of HIV gag DNA. Four weeks later the mice were challenged with vaccinia gag. Data represent a mean ± SD of 10 mice per group.
To determine whether CD40 was operating at the level of antigen-presenting cells, we adoptively transferred gag-primed CD4+ T cells into wild-type or CD40−/− mice and then boosted with DNA vaccine. Before T cell transfer, we first confirmed priming of gag-specific CD4+ T cells in the donor mice (Fig. S4). One day later the mice were boosted with a single dose of gag DNA, and 4 weeks later the mice were challenged i.n. with vaccinia gag. Upon challenge, wild-type, but not CD40−/−, mice showed a 10 log-fold reduction in lung virus titer when provided with immune CD4+ T cells [Fig. 6C; compare two left blue bars (P < 0.018)]. Thus, adoptively transferred wild-type CD4+ T cells do not enhance the protection afforded by a DNA vaccine in CD40−/− mice.
Discussion
Protein and DNA vaccines, though safe, do not induce a high frequency of CD4+ and CD8+ T cells as detected by cytokine production and cell proliferation. Our results show that antigen-specific CD4+ helper T cells can be elicited by a priming dose of a DEC-targeted protein vaccine, and this improves the induction of T cell immunity with a DNA vaccine. In addition to strong, combined, durable T cell immunity, this approach improved protection against a challenge with recombinant vaccinia gag virus in the airway. We are also finding that the DNA vaccine can either precede or follow the DEC-gag protein vaccine.
Multiple strategies have been exploited to augment the induction of effective immunity following DNA vaccination (reviewed in refs. 17–19), including the coadministration of toll-like receptor ligands (20). Importantly, in prime boosted mice challenged with vaccinia gag, the CD8+ gag-specific T cells induced by our complementary prime boost approach were more rapidly mobilized in the lung. A similar finding has been reported in an elegant report carried out during our study; i.e., that helper cells formed during infection improved the influx of CD8+ T cells to the infection site (21).
CD4+ helper cells are known to improve CD8+ T cell immunity (reviewed in ref. 22). CD4+ T cells can provide IL-2 required to sustain CD8+ T cell memory, although these CD4+ T cells do not have to be primed to a specific antigen (23). Alternatively, Heath et al. (24), in studies of the strong CD8+ T cell response to influenza and HSV, showed that CD4+ and CD8+ T cells each had to respond to the same antigenic component of the virus. We also found, consistent with previous data (24, 25), that the CD4+ and CD8+ T cells needed to recognize peptides from the same protein.
With respect to mechanism, CD40 was required for the protective efficacy of the complementary prime boost vaccination. CD40L is expressed by CD4+ helper T cells, and one consequence of CD40L function is to ligate CD40 on DCs, driving their maturation and enhancing immunity to antigens presented by DCs (10, 14–16). In addition, intravital microscopy of intact lymph nodes indicates that CD4+ helper cells recruit CD8+ T cells to the antigen presenting DCs (26) by producing CCL3 and CCL4 chemokines (27), the same chemokines that block HIV infection via CCR5 coreceptors (28). We verified that the helper effect from priming CD4+ T cells operated through CD40 on non-T cells, presumably DCs. In addition, we found that CD40-mediated help led to the more rapid accumulation of CD8+ T cells at a site of challenge infection, the lung.
There is concern that HIV-specific CD4+ T cells could provide a permissive environment for HIV replication (29), but the reciprocal is also possible, that CD4+ helper cells produce chemokines that block the CCR5 coreceptor (30) and, as we emphasize here, valuably help protective CD8+ T cell (and antibody) responses (31). In particular, antigen-specific CD4+ T cells allow for the more rapid accumulation of antigen-specific CD8+ T cells after challenge with a pathogenic virus. In macaques showing protection after vaccination with attenuated delta nef SIV vaccine (32) or recombinant adenovirus-SIV gag (33), broad gag-specific CD4+ T cell immunity was evident, raising the possibility that gag-specific CD4+ T cells can be helpful, not harmful, in resisting immunodeficiency virus. A key distinction is that the CD4+ T cells should be virus-specific to provide help for strong protective CD8+ resistance as opposed to more abundant activated T cells specific for disparate antigens, which can serve as a permissive site for SIV and HIV but fail to offer protective value.
The complementary prime boost approach, in which DC-targeted protein vaccine favors helper cell formation and DNA vaccine favors killer cell formation, does not require microbial vectors, thus simplifying vaccine manufacture; it also reduces antivector immunity as well as competition between peptides presented from vector and vaccine antigens. By directing the helper response to defined proteins, it may be feasible to improve the quality of the CD8+ T cell response to DNA and other vaccines, and potentially resistance to infectious and malignant diseases.
Materials and Methods
Mice and DNA Vaccination.
CxB6 F1 mice from Harlan were maintained under specific-pathogen-free conditions and used at 6–10 weeks, according to Rockefeller University guidelines. DNA vaccine (prepared with Qiagen endotoxin free GIGA kit) was injected i.m. in saline with electroporation (Ichor Medical Systems) and 1.25 mg per mouse nembutal anesthesia i.p. The skin above the anterior tibialis muscles was shaved and sterilized with ethanol.
Antibodies.
Antibodies to CD3, CD4, CD8α, and cytokines (IFN-γ, IL-2, and TNF-α) were purchased from BD Biosciences-Pharmingen.
Fusion HIV gag mAbs.
These were generated as described (4, 5). Western blotting with HRP-anti-p24 (ImmunoDiagnostics) was used to determine the specificity of the gag fusion constructs. mAb binding was verified on CHO cells stably transfected with mouse DEC-205 by FACS, using either PE-conjugated goat anti-mouse IgG (Jackson ImmunoResearch) or FITC-conjugated anti-p24 (Coulter KC57-FITC). All mAbs were endotoxin-free in limulus amebocyte lysate assay (QCL-1000; Cambrex).
Immunizations.
Female CXB6 F1 mice were injected once i.p. with fusion mAb together with polyIC (50 μg; Invivogen) as adjuvant. Eight weeks later, mice were boosted with 10 μg of HIV gag p41 DNA. One hundred micrograms of peptides was injected i.m. with or without polyIC. Four weeks after the last peptide injection, mice were boosted with 10 μg of HIV gag p41 DNA.
Assays for HIV-Specific Immune T Cells.
To detect HIV gag–specific T cell responses, bulk splenocytes were restimulated with peptides spanning the entire gag p41 sequence (4, 5, 12) or a negative unreactive control peptide mix consisting of HIV gag p17 pool 1 in the presence of 2 μg/mL anti-CD28 (clone 37.51) for 6 h, adding 10 μg/mL brefeldin A (Sigma-Aldrich) for the last 4 h to accumulate intracellular cytokines. Overlapping (staggered by 4 aa) 15-mer peptides spanning LcrV (13) and HIV gag p41—i.e., HIV gag p17 and HIV gag p24 (4)—were synthesized by the Proteomic Resource Center (The Rockefeller University). The 90-member gag p41 library was resuspended at 1 mg/mL each peptide in 100% DMSO. For FACS, dead cells were excluded using LIVE/DEAD fixable dead stain kit (Aqua LIVE/DEAD; Invitrogen). After blocking Fcγ receptors, the cells were stained with antibodies to CD3-pacific blue, CD4-percp, CD8-alexa-750, and Aqua LIVE/DEAD stain for 20 min at 37 °C. Cells were washed, fixed (Cytofix/Cytoperm; BD Biosciences), permeabilized with Permwash and stained with antibodies to IFN-γ (IFN-γ-alexa-700), IL-2 (IL-2-FITC), and TNF-α (TNF-α-PE-CY7) for 15 min at room temperature. All antibodies were from eBioscience, and HIV gag CD8 tetramers (AMQMLKETI) were H-2Kd PE from Beckman Coulter. We use BD Biosciences LSRII with data analysis in FlowJo (Tree Star).
Vaccinia gag Protection Assays.
Nembutal-anesthetized mice were challenged i.n. with 105 PFU per mouse recombinant vaccinia gag virus in 35 μL of PBS with Mg/Ca. A negative control was vaccinia-OVA virus. Animal weights (groups of five) were determined daily for 7 days following challenge. Then, mice were euthanized, and their lungs were harvested, homogenized in transport medium (0.1% gelatin in PBS), and stored in duplicate at –80 °C before virus titration. Lung virus titers of individual mice in each group were determined by plaque assay on monolayers of CV-1 cells as described (4, 5, 12).
Statistics.
Postchallenge mean vaccinia lung virus titers and mean percentage in weight loss were compared between vaccination groups using one-tailed Student's t test. Differences were considered significant at P < 0.05 after analysis using Prism 3 (GraphPad).
Supplementary Material
Acknowledgments
We thank J. Kuroiwa for the depleting antibodies to CD4 and CD8, H. Zebroski (Rockefeller University proteomics facility) for synthesizing peptides, B. Moltedo and C. B. Lopez (Depatment of Microbiology, Mount Sinai School of Medicine) for vaccinia viruses, R. Bernard and D. Hannaman (Ichor Medical Systems) for electroporation equipment and advice, and J. Adams for help with graphics. This work was supported by National Institutes of Health Grants AI40045 and AI40874 and the Bill and Melinda Gates Foundation Center for AIDS Vaccine Discovery.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/1000621107/DCSupplemental.
References
- 1.McConkey SJ, et al. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat Med. 2003;9:729–735. doi: 10.1038/nm881. [DOI] [PubMed] [Google Scholar]
- 2.Harari A, et al. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J Exp Med. 2008;205:63–77. doi: 10.1084/jem.20071331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banchereau J, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 4.Trumpfheller C, et al. Intensified and protective CD4+ T cell immunity in mice with anti-dendritic cell HIV gag fusion antibody vaccine. J Exp Med. 2006;203:607–617. doi: 10.1084/jem.20052005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trumpfheller C, et al. The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc Natl Acad Sci USA. 2008;105:2574–2579. doi: 10.1073/pnas.0711976105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 7.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janssen EM, et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 9.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith CM, et al. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat Immunol. 2004;5:1143–1148. doi: 10.1038/ni1129. [DOI] [PubMed] [Google Scholar]
- 11.Ryu SJ, et al. Cognate CD4 help is essential for the reactivation and expansion of CD8 memory T cells directed against the hematopoietic cell-specific dominant minor histocompatibility antigen, H60. Blood. 2009;113:4273–4280. doi: 10.1182/blood-2008-09-181263. [DOI] [PubMed] [Google Scholar]
- 12.Nchinda G, et al. The efficacy of DNA vaccination is enhanced in mice by targeting the encoded protein to dendritic cells. J Clin Invest. 2008;118:1427–1436. doi: 10.1172/JCI34224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Do Y, et al. Broad T cell immunity to the LcrV virulence protein is induced by targeted delivery to DEC-205/CD205-positive mouse dendritic cells. Eur J Immunol. 2008;38:20–29. doi: 10.1002/eji.200737799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoenberger SP, Toes REM, van der Voort EIH, Offringa R, Melief CJM. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 15.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 16.Bennett SRM, et al. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 17.Scheerlinck JY. Genetic adjuvants for DNA vaccines. Vaccine. 2001;19:2647–2656. doi: 10.1016/s0264-410x(00)00495-3. [DOI] [PubMed] [Google Scholar]
- 18.Barouch DH, Letvin NL, Seder RA. The role of cytokine DNAs as vaccine adjuvants for optimizing cellular immune responses. Immunol Rev. 2004;202:266–274. doi: 10.1111/j.0105-2896.2004.00200.x. [DOI] [PubMed] [Google Scholar]
- 19.Kutzler MA, Weiner DB. Developing DNA vaccines that call to dendritic cells. J Clin Invest. 2004;114:1241–1244. doi: 10.1172/JCI23467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwissa M, et al. Adjuvanting a DNA vaccine with a TLR9 ligand plus Flt3 ligand results in enhanced cellular immunity against the simian immunodeficiency virus. J Exp Med. 2007;204:2733–2746. doi: 10.1084/jem.20071211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8+ T lymphocyte mobilization to virus-infected tissue requires CD4+ T-cell help. Nature. 2009;462:510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu Rev Immunol. 2006;24:519–540. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- 23.Wilson EB, Livingstone AM. Cutting edge: CD4+ T cell-derived IL-2 is essential for help-dependent primary CD8+ T cell responses. J Immunol. 2008;181:7445–7448. doi: 10.4049/jimmunol.181.11.7445. [DOI] [PubMed] [Google Scholar]
- 24.Heath WR, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 25.Castellino F, Germain RN. Chemokine-guided CD4+ T cell help enhances generation of IL-6RαhighIL-7Rαhigh prememory CD8+ T cells. J Immunol. 2007;178:778–787. doi: 10.4049/jimmunol.178.2.778. [DOI] [PubMed] [Google Scholar]
- 26.Beuneu H, Garcia Z, Bousso P. Cutting edge: cognate CD4 help promotes recruitment of antigen-specific CD8 T cells around dendritic cells. J Immunol. 2006;177:1406–1410. doi: 10.4049/jimmunol.177.3.1406. [DOI] [PubMed] [Google Scholar]
- 27.Castellino F, et al. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 28.Cocchi F, et al. Identification of RANTES, MIP-1 α, and MIP-1 β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 29.Douek DC, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 30.Guan Y, Abdelwahab S, Kamin-Lewis R, DeVico AL, Lewis GK. Self-protection of individual CD4+ T cells against R5 HIV-1 infection by the synthesis of anti-viral CCR5 ligands. PLoS One. 2008;3:e3481. doi: 10.1371/journal.pone.0003481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kannanganat S, et al. Human immunodeficiency virus type 1 controllers but not noncontrollers maintain CD4 T cells coexpressing three cytokines. J Virol. 2007;81:12071–12076. doi: 10.1128/JVI.01261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynolds MR, et al. Macaques vaccinated with live-attenuated SIV control replication of heterologous virus. J Exp Med. 2008;205:2537–2550. doi: 10.1084/jem.20081524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.