Abstract
Most carcinomas present some form of chromosome instability in combination with spindle defects. Numerical instability is likely caused by spindle aberrations, but the origin of breaks and translocations remains elusive. To determine whether one mechanism can bring about both types of instability, we studied the relationship between DNA damage and spindle defects. Although lacking apparent repair defects, primary Dido mutant cells formed micronuclei containing damaged DNA. The presence of centromeres showed that micronuclei were caused by spindle defects, and cell cycle markers showed that DNA damage was generated during mitosis. Although the micronuclei themselves persisted, the DNA damage within was repaired during S and G2 phases. DNA breaks in Dido mutant cells regularly colocalized with centromeres, which were occasionally distorted. Comparable defects were found in APC mutant cell lines, an independent system for spindle defects. On the basis of these results, we propose a model for break formation in which spindle defects lead to centromere shearing.
Keywords: centrosome, chromosome instability, double-strand break, H2A.X
Most solid tumors show changes in chromosome number, a phenomenon termed aneuploidy, which is caused by chromosome segregation errors and rarely observed in normal cells (1). Many tumors not only gain or lose whole chromosomes, but also accumulate intragenic mutations, thought to suppress negative control on proliferation. Mathematical models predict that chromosomal instability initiates tumorigenesis before the mutation of tumor suppressor genes, underlining the importance of aneuploidy (2). Other studies indicate that aneuploidy is required for sporadic carcinogenesis and collaborates with intragenic mutations by generating multiple copies of mutated chromosomes (3).
Because of their capacity to initiate and propagate intragenic mutations, DNA breaks are considered important in carcinogenesis (3). To examine the origin of breaks, several studies have analyzed the link between cancer and the repair of double-strand DNA breaks (DSBs) (4). But even though mutation of DSB repair genes results in DNA damage after irradiation, little effect was found in untreated cells (5, 6). In addition, sporadic carcinomas bear few if any mutations in DSB repair genes, and many tumor samples show augmented instead of reduced repair activity (7, 8). The role of DSB repair in de novo generation of structural defects thus remains uncertain.
Another potential route for DSB generation involves the mitotic spindle. Spindle defects, an important cause of aneuploidy (1), are frequent in solid tumors, and background γH2A.X levels correlate well with aneuploidy (9). γH2A.X has also been observed after the induction of mitotic arrest by spindle disruption (10, 11). When combined with chromosome end-to-end fusions, the mitotic spindle can damage chromosomes by a mechanism termed the breakage–fusion–bridge (BFB) cycle (12, 13). Still, it remains unclear whether chromosome breakage in the BFB cycle is caused by the spindle itself or by contraction of the midbody actin ring. Inactivation of genes that control the metaphase checkpoint causes severe spindle defects and generally is lethal due to extensive aneuploidy (14). In this study, we used primary cells bearing a mutated death inducer obliterator (Dido) gene. The main product of the Dido gene, Dido3, is a structural protein that localizes to the spindle pole in mitosis (15) and to the synaptonemal complex in meiosis (16). The Dido mutation compromises the spindle metaphase checkpoint, leading to an increased frequency of aberrant anaphases (17). Although they suffer from myelodysplastic/myeloproliferative diseases (18), Dido mutant mice are viable and can thus provide a cellular model in which to study downstream effects. For a second model, we used colon cancer cell lines lacking a functional adenomatous polyposis coli (APC) gene. APC is a microtubule-associated protein that regulates spindle dynamics and microtubule–chromosome attachments (19).
We encountered DSBs in micronuclei in Dido mutant mouse primary embryonic fibroblasts (MEFs) without signs of DNA damage in the main nucleus. DSBs were found in centromere-containing micronuclei, probably formed by uncorrected merotelic attachments. This shows an aneugenic origin combined with clastogenic effects. Most DSBs in Dido mutant MEFs were found adjacent to centromeres, which were occasionally distorted. APC mutant cell lines showed similar defects. Thus, two independent models of spindle defects show comparable centromere-localized DSBs. Although mitosis was thought to contribute to chromosomal instability only by chromosome missegregation, our results show that spindle defects can directly generate DSBs. We propose a model in which spindle defects promote tumor formation through several pathways.
Results
Localized DNA Damage in Dido Mutant Cells.
Primary MEFs from Dido mutants show a high frequency of spindle defects and lagging chromosomes (15, 17). When lagging chromosomes persist until mitotic exit, they form micronuclei in the subsequent interphase (20). Aside from spindle defects, faulty repair can generate micronuclei through the loss of centromere sequences (21). To compare micronucleus formation under different conditions, primary MEFs from Dido, Ku80, and ATM mutants were labeled with an antibody to the DSB marker γH2A.X (22), and the frequency of micronuclei was determined (Fig. 1). Ku80 has a critical role in nonhomologous end joining (NHEJ) and ATM is a kinase that phosphorylates several proteins involved in NHEJ and homologous recombination repair (HRR) (23, 24).
Fig. 1.
Localized DNA damage in mutant cells. MEFs were seeded on coverslips, labeled with anti-γH2A.X antibody (red), and studied by fluorescence microscopy. DNA was DAPI stained (blue). Quantitation (A) showed an increased proportion of γH2A.X-positive micronuclei in Dido mutant MEFs. Error bars indicate standard deviation. (B–E) Representative images of WT (B) cells and Dido (C), Ku80 (D), and ATM (E) mutants are shown. Micronuclei are indicated with arrowheads. Strong γH2A.X labeling was found in micronuclei (C), although Dido mutant MEFs show no further DNA damage. The weaker signals correspond to constitutive foci not related to DSBs. (Scale bar, 5 μm.)
A small proportion of wild-type (WT) MEFs contained micronuclei, and this proportion increased in Dido, Ku80, and ATM mutants (Fig. 1A). γH2A.X labeling revealed small constitutive γH2A.X foci (6, 25), not related to DSB, in all cell lines. The number and localization of constitutive γH2A.X foci is specific for each cell line. Although Ku80 mutant MEFs show an exceptionally high number of constitutive foci and undue H2A.X phosphorylation in heterochromatic regions, these phenomena are not associated with widespread DSBs. However, untreated WT, Ku80 mutant, or ATM mutant MEFs showed hardly any of the high-intensity γH2A.X label characteristic for DSBs (Fig. 1 B, D, and E). The Dido mutation caused a marked (fourfold) increase in micronuclei, many of which with high-intensity γH2A.X labeling (Fig. 1C). Ku80 or ATM inactivation, too, increased the frequency of micronuclei, but few of these were γH2A.X positive (Fig. 1 D and E). γH2A.X-positive micronuclei were also found in Dido mutant embryos (Fig. S1), showing in vivo DSB formation. In addition, a subset of Dido mutant MEFs showed DNA damage in the main nucleus (Fig S1). In contrast to radiation-induced damage, which gave rise to more or fewer globular regions of γH2A.X (Fig. 2), nuclear γH2A.X regions in Dido mutant MEFs were jagged. In conclusion, the Dido mutation gives rise to localized DSBs, but repair mutants do not show this kind of “spontaneous” DNA damage.
Fig. 2.
Normal DSB repair in Dido mutant MEFs. (A) MEFs from WT and mutant mice were seeded on coverslips, irradiated, and left to recover for the times indicated. Subsequently, cells were fixed, labeled with anti-γH2A.X antibodies, and the number of γH2A.X foci per cell was counted by fluorescence microscopy. Whereas mock-treated controls showed little γH2A.X, a single 2-Gray radiation dose induced ≈50 foci in WT and mutant cells. The number of foci gradually decreased during the 8-h recovery. Wild-type and mutant MEFs showed equal numbers of foci at each time point. Error bars indicate standard deviation. (B) Wild-type MEFs were seeded on glass coverslips, irradiated, and labeled with antibodies to γH2A.X and Dido. No significant colocalization of the two signals was found. (C and D) Dido mutant MEFs were seeded on glass coverslips and analyzed by phospho-ATM (C), or TUNEL and centromere (D) labeling, without treatment. DNA was DAPI stained. Colocalization of phospho-ATM or TUNEL with γH2A.X shows that H2A.X phosphorylation characterizes true DSBs. Representative images are shown. (Scale bar, 5 μm.)
Normal DSB Repair and H2A.X Phosphorylation in Dido Mutant Cells.
Even though repair gene mutations cause retention of γH2A.X after DSB induction, no effect is found in untreated cells (5, 6). Untreated Dido mutant MEFs, however, formed micronuclei containing damaged DNA. We therefore analyzed the DSB repair efficiency of the Dido mutant. Because the Dido mutation had no significant effect on overall radiation sensitivity (Fig. S2), DSB kinetics were assayed. MEFs were treated with 2 Gray γ-radiation and labeled with anti-γH2A.X antibodies at different time points (Fig. S3). High-intensity γH2A.X foci were counted to quantify DSBs (Fig. 2A). Both WT and Dido mutant MEFs showed the rapid appearance of γH2A.X foci after irradiation and gradually lost these as DSB repair took place. After 8 h, few γH2A.X foci remained in WT and Dido mutant MEFs. The number of γH2A.X foci did not differ significantly between WT and Dido mutant MEFs at any of the time points. Thus, although Dido mutants generated more γH2A.X-positive micronuclei than repair-deficient controls, their DSB repair capacity was comparable to that of WT animals.
Repair proteins are recruited to the actual break sites and thus colocalize with γH2A.X foci after induction of DSBs. To test whether Dido proteins are recruited to DSBs, MEFs were irradiated and labeled with antibodies to Dido and γH2A.X (Fig. 2B). Dido distributed heterogeneously in the nucleus but did not colocalize with γH2A.X foci, indicating that Dido has no overt role in DSB repair.
Although γH2A.X is used as a marker of DSB formation (22), γH2A.X can be formed without DSBs, such as a normal cell cycle (6, 25) or XY body formation in meiosis (26). To determine whether the γH2A.X in micronuclei is associated with DNA damage, we detected phosphorylation of the HRR protein ATM (27) and terminal transferase dUTP nick end labeling (TUNEL) (28) as supplementary markers. Whenever γH2A.X-positive micronuclei were found in untreated Dido mutant MEFs, these always yielded corresponding phospho-ATM and TUNEL signals (Fig. 2 C and D), confirming γH2A.X as a valid DSB marker. In addition, individual micronuclei were found positive for TUNEL, γH2A.X, and distorted centromeres (Fig. 2D). Micronuclei, centromere distortion, and DNA damage may thus share a common origin. Taken together, these data indicate that only de novo DSB generation, but not diminished repair or aberrant H2A.X phosphorylation, can explain the γH2A.X-positive micronuclei.
Micronuclei in Dido Mutants Are Caused by Spindle Defects.
Micronuclei can have two origins: loss of centromeres due to DNA damage, generating chromosomes no longer recognized by the mitotic spindle and defective kinetochore capture due to anomalies of the spindle itself. Because previous studies showed spindle defects in Dido mutants (15), we labeled Dido mutant MEFs with antibodies to centromeres and α-tubulin and studied these by confocal fluorescence microscopy (Fig. 3). We detected individual centromeres captured by microtubules from opposite poles (Fig. 3 A and B), which shows that merotelic attachments cause lagging chromosomes in the Dido mutant (29).
Fig. 3.
Merotelic attachments in Dido mutant cells. Dido mutant MEFs were seeded on coverslips and double labeled with antibodies against centromeres (green) and α-tubulin (red). DNA was stained with DAPI (blue), and cells were studied by confocal fluorescence microscopy. (A) Detection of merotelic centromere attachments in Dido mutant MEFs. An individual anaphase centromere attached to both spindle poles is shown. The image of a whole cell is from maximum projection (A), and the threefold amplification (B) is from a single confocal layer. (C–E) Appearance of centromeres in Dido mutant MEFs. Centromeres in micronuclei (C) and in a nuclear protrusion (D) are indicated (arrowheads). (E) In some cases, an individual centromere is distorted (arrow), whereas neighboring centromeres appear normal (arrowhead). Representative images are shown. (Scale bar, 5 μm.)
Uncorrected merotelic attachments lead to the exclusion of lagging chromosomes from the main nucleus during mitotic exit (21). In such an event, a centromere-positive micronucleus is generated. Centromeres in micronuclei thus are reliable indicators of spindle defects, but more easily discernible than wrongly attached kinetochores (21, 30). To confirm the origin of micronuclei in the Dido mutant, we labeled MEFs with antibodies to centromeres and α-tubulin, and studied these by fluorescence microscopy. Micronuclei in mutant cells were physically separated from the main nucleus, and nearly all (∼85%, n = 120) contained well-defined centromeres (Fig. 3C). Thus, even though the γH2A.X in the micronuclei suggests a clastogenic outcome (breakage), the presence of centromeres demonstrates an aneugenic origin (spindle defects). Uncorrected merotelic attachments can generate enough force to distort kinetochores (29). Accordingly, a small proportion (∼15%) of micronuclei in the Dido mutant showed a diffuse distorted centromere signal (Fig. 2D). In addition, we found distorted individual centromeres adjacent to centromeres that appeared normal (Fig. 3 D and E). These data confirm merotelic attachments as the probable cause of micronuclei and centromere distortion.
DNA Damage Is Generated During Mitosis.
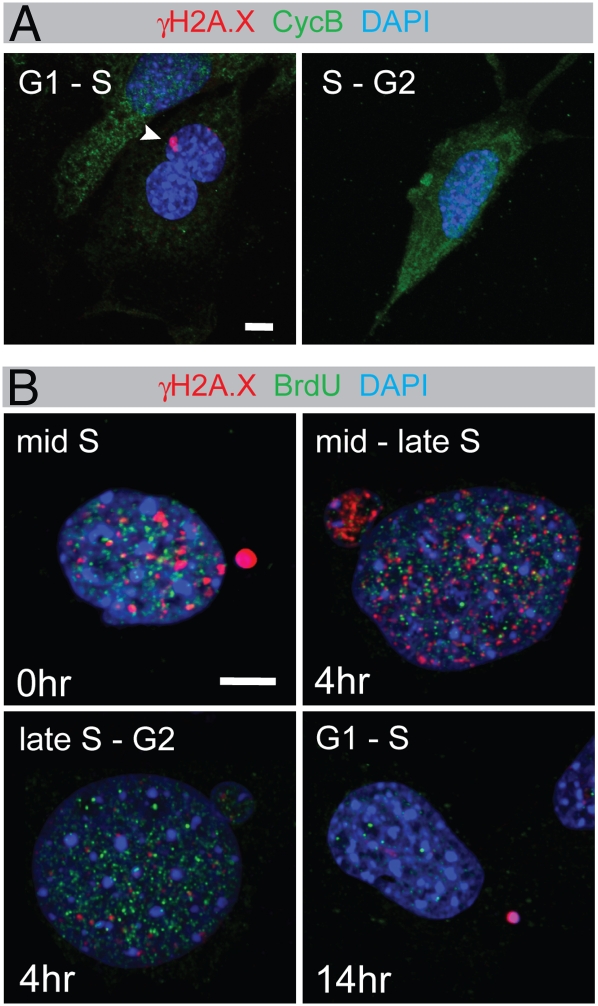
The merotelic attachments and γH2A.X-positive micronuclei indicate a mitotic origin for DSBs. Because standard methods to synchronize cells by itself induce DNA damage (10, 11), we established the time point of DSB generation in nonsynchronized cells. Dido mutant MEFs were labeled with antibodies to cyclin B1 and γH2A.X (Fig. 4A). Cyclin B1 accumulates in the cytoplasm from S phase to late G2, enters the nucleus at the beginning of mitosis, and is destroyed at anaphase onset (31). γH2A.X-positive micronuclei were regularly found in cells with low cyclin B1 levels characteristic of G1 and early S phase. However, γH2A.X was never found in cells with high cyclin B1, showing absence of DSBs in G2 phase. These data indicate that DNA damage is generated during mitosis, in agreement with break formation after spindle disruption (10, 11) and the mitotic origin of micronuclei (21). The lack of γH2A.X in G2 cells suggests that damage is eliminated during S phase.
Fig. 4.
Intramitotic DNA damage in Dido mutant cells. (A) MEFs were seeded on coverslips, labeled with anti-γH2A.X and anti-cyclin B1 antibodies, and studied by confocal microscopy. Whereas γH2A.X-positive micronuclei (arrowhead) were found in cyclin B1-negative cells, no γH2A.X was found in cells with a high cyclin B1 content. (B) MEFs were pulse labeled with BrdU for 1 h and chased in medium without BrdU for the time indicated. Subsequently, cells were labeled with anti-BrdU (green) and anti-γH2A.X antibodies (red) and studied by confocal microscopy. Micronuclei containing bright γH2A.X labeling were found in early-to-mid S phase cells. Late S phase cells showed micronuclei with diminishing or no γH2A.X. No G2 phase cells were found γH2A.X-positive, but cells that were chased until the next G1 phase again showed γH2A.X in micronuclei. Representative images are shown. (Scale bars, 5 μm.)
To study S phase in more detail, Dido mutant MEFs were pulse labeled with bromodeoxyuridine (BrdU) and chased various times with label-free medium (Fig. 4B). The combination of BrdU and γH2A.X labeling, showing spatial distribution of constitutive γH2A.X foci (6, 25), allows the discrimination of S phase stages (32). Middle S phase cells, showing a distributed BrdU pattern directly after labeling, were positive for γH2A.X. Micronuclei with low intensity γH2A.X signals were found in middle to late S phase cells, characterized by a distributed BrdU-labeling pattern after a 4-h chase. Out of over 100 examined G2 phase cells, BrdU labeled but without constitutive γH2A.X foci after a 4-h chase, none showed remaining DSBs. Thus, even though the micronuclei themselves persist, the DNA damage within is repaired in S phase. This means that new γH2A.X-positive micronuclei are formed during mitosis. Accordingly, we again found γH2A.X-positive micronuclei after a 14-h chase, when the BrdU-labeled cells had passed through mitosis and were in G1 or early S phase.
Centromere Localization of DNA Damage.
Spindle disruption not only generates micronuclei (21), but also causes kinetochore distortion and DNA damage (10, 11, 29), suggesting a common mechanism. The individual micronuclei in Dido mutant MEFs, positive for γH2A.X and distorted centromeres, corroborated this hypothesis. To further analyze the hypothesis of spindle-generated DNA damage, we evaluated colocalization of DSBs and spindle attachment sites. Dido mutant MEFs were labeled with antibodies to γH2A.X and centromeres and studied by confocal fluorescence microscopy. Whereas one subpopulation of cells showed extensive DSBs in micronuclei (Figs. 1–3), the DNA damage in another subset was restricted to smaller areas (Fig. 5A). In nearly all cases, however, γH2A.X surrounded or was adjacent to centromeres. DSBs thus typically localized to the spindle attachment sites on the chromosomes, in agreement with centromere distortion. Mitotic figures showed comparable centromeric γH2A.X labeling (Fig. 5B and Fig S4), consistent with a mitotic origin of DNA damage. DSB detection by TUNEL confirmed centromere association (Fig. 5C). In contrast to these spontaneous DSBs, few radiation-induced breaks localized to centromeres in Dido mutant MEFs (Fig. 5D). To quantify the frequency of centromere-associated DSBs under different conditions, we scored the number of γH2A.X foci that colocalized with centromeres. Whereas over 75% of DSBs localized to centromeres in untreated cells, less than 10% of radiation-induced DSBs showed this localization (Fig. 5E). Taken together, these results show clear differences between spindle- and radiation-induced γH2A.X and corroborate the role of the mitotic spindle in DSB formation.
Fig. 5.
DSBs in untreated Dido mutant MEFs are centromere associated. MEFs were seeded on coverslips, labeled with anti-γH2A.X antibodies (red, A, B, and D) or by TUNEL (C), and counter labeled with centromere antibodies (green). Cells were studied by confocal scanning laser microscopy. DNA was DAPI stained (blue). Insets show threefold magnification of the centromeric areas (arrowheads). An interphase (A) and anaphase (B) are shown. (C) TUNEL confirmed centromere association of DSBs. Representative images are shown. (D) In contrast to spindle-induced breaks, radiation-induced γH2A.X foci do not colocalize with centromeres. (E) Quantitation of DSB localization in untreated and irradiated MEFs. Error bars indicate standard deviation. (Scale bar, 5 μm.)
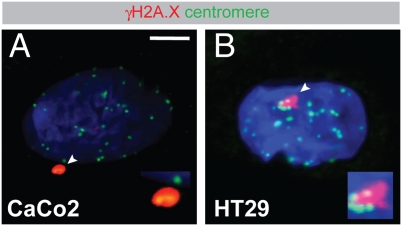
To determine whether a deregulated spindle causes DNA damage in an independent model, we confirmed these data in cell lines with a chromosomal instability (CIN) phenotype (Fig. 6 and Fig. S5). These cell lines, bearing a mutated APC gene, suffer from aberrant regulation of the cytoskeleton and chromosome segregation defects (19). The CIN cell lines, too, showed γH2A.X adjacent to centromeres, showing that shearing of centromeric chromatin is a common effect of spindle anomalies.
Fig. 6.
Centromere-associated DSBs in CIN cell lines. Caco2 (A) and HT29 (B) cells were seeded on glass coverslips, labeled with antibodies to γH2A.X (red) and centromeres (green), and then analyzed by confocal scanning microscopy. DNA was counterstained with DAPI (blue). Areas indicated with arrowheads are shown magnified twofold in Insets. Representative images are shown. (Scale bar, 5 μm.)
Discussion
Several lines of evidence suggest that aneuploidy is required for sporadic carcinogenesis in mice and collaborates with intragenic mutations during tumorigenesis (2, 3). Aneuploidy is caused by a dysfunctional mitotic spindle (33), but the origins of DNA breaks, which are thought to initiate intragenic mutations, remain poorly understood. Although repair mutants show persistent lesions after DSB-inducing treatments, the same mutations seem to have little or no effect in untreated cells, and mutations in repair genes are rarely found in sporadic tumors (5, 7, 8). In contrast, several lines of evidence implicate the mitotic spindle in DSB generation; genetic or drug-induced spindle abnormalities cause γH2A.X accumulation (10, 11), γH2A.X levels in untreated cells correlate with numerical chromosome instability (9), and cancer cell lines with a CIN phenotype show numerous translocations close to centromeric regions (34).
Here we show that cells with a Dido mutation, which causes spindle defects and an increased frequency of lagging chromosomes (15, 17), show localized DSBs in micronuclei, subnuclear regions, and nuclear protrusions. Because these DSBs were found in cells without apparent repair defects, they can only be explained by de novo generation. The micronuclei in Dido mutant primary MEFs contained centromeres, indicating an aneugenic (spindle) origin. In addition, we found DSBs adjacent to the centromeres, which form the spindle attachment points in mitosis. In agreement with a role of the spindle, breaks were generated in mitosis and again repaired later in the cell cycle. The APC mutants provide an alternative model in which chromosome segregation defects and spindle defects cause aneuploidy (19), confirming DSBs adjacent to centromeres.
The force generated by a single microtubule, ≈50 pN, is not considered enough to overcome the integrity of a DNA helix (35), which breaks at forces around 250 pN in vitro (36). During mitosis, however, microtubules form bundles termed K-fibers to amplify their strength (37). The combination of K-fiber formation and loss of spindle control brings about a potentially dangerous situation, in which the chromosomes are exposed to excessive force. Whereas dicentric chromosomes, the source of the breakage–fusion–bridge cycle, potentially experience the combined force of two K-fibers, lagging chromosomes typically result from a merotelic attachment. Even though merotelic attachments generally do not combine more microtubules than a single K-fiber, uncorrected merotelic attachments are able to overcome the structural integrity of the kinetochore (29). Approximately 15 microtubules connect to a single kinetochore in a merotelic attachment, which can generate a combined force three times the tensile strength of chromosomes (750 pN). Two K-fibers stretch the chromatin between centromeres in the BFB cycle, resulting in anaphase bridges (38). In a merotelic attachment, however, the microtubules act on a single centromere, which lacks the flexibility of intermediate chromatin. Thus, even though merotelic attachments do not generate as much force as two K-fibers, the small dimensions of individual kinetochores favor DNA rupture. Although the merotelic attachments themselves are relatively difficult to detect, distorted centromeres (Fig. 3F) point at merotelic attachments as the origin of micronuclei and nuclear protrusions in the Dido mutant. Also the isolated centromeric chromosome fragments (Fig. S6), the nuclear protrusions (Fig. S1), and the jagged form of damaged regions (Fig. S1) are consistent with a persistent physical force that shears the chromatin.
In agreement with the appearance of DSBs in arrested cells (11), we found no DNA damage in G2 phase, but detected localized DNA damage as early as prometaphase in mitosis (Fig. S4). These DSBs apparently persisted into the next interphase. Although intramitotic DSBs apparently cause H2A.X phosphorylation (11), they allow for completion of mitosis despite extensive lesions (39). The metaphase–anaphase transition thus seems to be a critical step, as neither spindle symmetry nor chromatin integrity is monitored at this moment (39, 40). This could explain why intramitotic DNA damage does not cause metaphase arrest but allows the cell cycle to continue.
The apparent randomness of structural changes may not reflect random breakpoint positioning, but could result from random transposition of related chromosome fragments (3); small pieces of chromatin consisting almost exclusively of centromeric material could be randomly transposed into noncentromeric sites. Another recurrent genetic defect in carcinomas is the loss or gain of complete chromosome arms (41). Colon cancer cell lines with a CIN phenotype, but not those with other mutations, frequently show this defect (33). The loss of whole chromosome arms is easily explained by centromeric DSBs, as this positions the break exactly at the point that separates the two chromosome arms. After chromosome segregation, free DNA ends with or without centromere identity can react with different partner substrates (Fig. S7). A single event, spindle-induced centromere shearing, may thus underlie a variety of genetic defects found in carcinomas.
Materials and Methods
Cell Culture and Immunofluorescence.
Dido, ATM, and Ku80 mutant mice have been described (18, 42, 43). All experiments were performed in compliance with the European Union and National Bioethics Committee directives. MEFs were seeded on poly-L-lysine-coated glass coverslips for immunofluorescence. For γH2A.X detection, cells were rinsed briefly in PBS, fixed in PBS containing 4% formaldehyde, and permeabilized in PBS with 0.5% Triton X-100. For tubulin detection, cells were rinsed briefly in PBS and fixed in methanol. After blocking in PBS containing 5% goat serum (1 h, room temperature), cells were incubated with primary antibodies (1 h, room temperature). Cells were then washed, incubated with secondary antibodies (Jackson Immunoresearch), washed again, and mounted in Prolong Gold antifade (Invitrogen). Images were captured on an IX81 laser scanning confocal microscope (Olympus), with sequential fluorophore excitation. Final images were obtained through maximum projection by ImageJ software.
Antibodies.
The CREST antiserum, recognizing CENP-A, and antibodies to Dido have been described (16, 44). We used commercial antibodies to γH2A.X (Millipore and Abcam), phospho-ATM (Rockland), cyclin B1 (BD Biosciences), or α-tubulin (Upstate). DNA strand breaks were determined by TUNEL assay, using a fluorescein-dUTP-based in situ cell death detection kit (Roche Diagnostics).
Cell Irradiation.
To assay DSB repair, MEFs were seeded on glass coverslips and irradiated with a 2-Gray dose from a calibrated cesium source 24 h after seeding. At the times indicated, coverslips were fixed and pooled for further processing. Cells were labeled with anti-γH2A.X antibodies, counterstained with DAPI, and studied by fluorescence microscopy. The number of γH2A.X foci in 50 cells was counted for each time point.
Bromodeoxyuridine Labeling.
To determine cell cycle phase, MEFs were pulse labeled for 1 h with 10 μM BrdU, washed three times in medium without BrdU, and then chased for the times indicated. Cells were fixed, DNA was partially denatured in 50 mM NaOH for 5 min, and BrdU was detected with fluorescein-coupled antibodies (BD Biosciences). DSBs and small constitutive γH2A.X foci were visualized with anti-γH2A.X antibodies, allowing precise S phase determination (32).
Supplementary Material
Acknowledgments
The authors thank Dr. Maria Blasco and Dr. Oscar Fernandez-Capetillo for mutant mice, Catherine Mark for editorial assistance, and Almudena Hernando for help during submission. This work was financed by Grants PI05/1965, PS09/0572 (Fondo de Investigación en Salud), and S-BIO-0189-2006 (Comunidad Autónoma de Madrid). K.H.M.v.W. is supported by Grant RyC2004-1886 (Ministerio de Educación y Ciencia). The Department of Immunology and Oncology was founded and is supported by the Consejo Superior de Investigaciones Científicas.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912143106/DCSupplemental.
References
- 1.Rajagopalan H, Lengauer C. Aneuploidy and cancer. Nature. 2004;432:338–341. doi: 10.1038/nature03099. [DOI] [PubMed] [Google Scholar]
- 2.Nowak MA, et al. The role of chromosomal instability in tumor initiation. Proc Natl Acad Sci USA. 2002;99:16226–16231. doi: 10.1073/pnas.202617399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pihan G, Doxsey SJ. Mutations and aneuploidy: Co-conspirators in cancer? Cancer Cell. 2003;4:89–94. doi: 10.1016/s1535-6108(03)00195-8. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal S, Tafel AA, Kanaar R. DNA double-strand break repair and chromosome translocations. DNA Repair (Amst) 2006;5:1075–1081. doi: 10.1016/j.dnarep.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 5.Stiff T, et al. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 2004;64:2390–2396. doi: 10.1158/0008-5472.can-03-3207. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki K, et al. Qualitative and quantitative analysis of phosphorylated ATM foci induced by low-dose ionizing radiation. Radiat Res. 2006;165:499–504. doi: 10.1667/RR3542.1. [DOI] [PubMed] [Google Scholar]
- 7.Hosoi Y, et al. Up-regulation of DNA-dependent protein kinase activity and Sp1 in colorectal cancer. Int J Oncol. 2004;25:461–468. [PubMed] [Google Scholar]
- 8.Pucci S, et al. Tumor specific modulation of KU70/80 DNA binding activity in breast and bladder human tumor biopsies. Oncogene. 2001;20:739–747. doi: 10.1038/sj.onc.1204148. [DOI] [PubMed] [Google Scholar]
- 9.Yu T, MacPhail SH, Banáth JP, Klokov D, Olive PL. Endogenous expression of phosphorylated histone H2AX in tumors in relation to DNA double-strand breaks and genomic instability. DNA Repair (Amst) 2006;5:935–946. doi: 10.1016/j.dnarep.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 10.Quignon F, et al. Sustained mitotic block elicits DNA breaks: One-step alteration of ploidy and chromosome integrity in mammalian cells. Oncogene. 2007;26:165–172. doi: 10.1038/sj.onc.1209787. [DOI] [PubMed] [Google Scholar]
- 11.Dalton WB, Nandan MO, Moore RT, Yang VW. Human cancer cells commonly acquire DNA damage during mitotic arrest. Cancer Res. 2007;67:11487–11492. doi: 10.1158/0008-5472.CAN-07-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung AL, Deng W. Telomere dysfunction, genome instability and cancer. Front Biosci. 2008;13:2075–2090. doi: 10.2741/2825. [DOI] [PubMed] [Google Scholar]
- 13.Lundblad V. Genome instability: McClintock revisited. Curr Biol. 2001;11:R957–R960. doi: 10.1016/s0960-9822(01)00573-5. [DOI] [PubMed] [Google Scholar]
- 14.Dobles M, Liberal V, Scott ML, Benezra R, Sorger PK. Chromosome missegregation and apoptosis in mice lacking the mitotic checkpoint protein Mad2. Cell. 2000;101:635–645. doi: 10.1016/s0092-8674(00)80875-2. [DOI] [PubMed] [Google Scholar]
- 15.Trachana V, van Wely KH, Guerrero AA, Fütterer A, Martínez-A C. Dido disruption leads to centrosome amplification and mitotic checkpoint defects compromising chromosome stability. Proc Natl Acad Sci USA. 2007;104:2691–2696. doi: 10.1073/pnas.0611132104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prieto I, et al. Synaptonemal complex assembly and H3K4Me3 demethylation determine DIDO3 localization in meiosis. Chromosoma. 2009;118:617–632. doi: 10.1007/s00412-009-0223-7. [DOI] [PubMed] [Google Scholar]
- 17.Rojas AM, et al. Death inducer obliterator protein 1 in the context of DNA regulation. Sequence analyses of distant homologues point to a novel functional role. FEBS J. 2005;272:3505–3511. doi: 10.1111/j.1742-4658.2005.04759.x. [DOI] [PubMed] [Google Scholar]
- 18.Fütterer A, et al. Dido gene expression alterations are implicated in the induction of hematological myeloid neoplasms. J Clin Invest. 2005;115:2351–2362. doi: 10.1172/JCI24177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan KB, et al. A role for the adenomatous polyposis coli protein in chromosome segregation. Nat Cell Biol. 2001;3:429–432. doi: 10.1038/35070123. [DOI] [PubMed] [Google Scholar]
- 20.Norppa H, Falck GC. What do human micronuclei contain? Mutagenesis. 2003;18:221–233. doi: 10.1093/mutage/18.3.221. [DOI] [PubMed] [Google Scholar]
- 21.Mateuca R, Lombaert N, Aka PV, Decordier I, Kirsch-Volders M. Chromosomal changes: Induction, detection methods and applicability in human biomonitoring. Biochimie. 2006;88:1515–1531. doi: 10.1016/j.biochi.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 23.Hartlerode AJ, Scully R. Mechanisms of double-strand break repair in somatic mammalian cells. Biochem J. 2009;423:157–168. doi: 10.1042/BJ20090942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shrivastav M, De Haro LP, Nickoloff JA. Regulation of DNA double-strand break repair pathway choice. Cell Res. 2008;18:134–147. doi: 10.1038/cr.2007.111. [DOI] [PubMed] [Google Scholar]
- 25.McManus KJ, Hendzel MJ. ATM-dependent DNA damage-independent mitotic phosphorylation of H2AX in normally growing mammalian cells. Mol Biol Cell. 2005;16:5013–5025. doi: 10.1091/mbc.E05-01-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandez-Capetillo O, et al. H2AX is required for chromatin remodeling and inactivation of sex chromosomes in male mouse meiosis. Dev Cell. 2003;4:497–508. doi: 10.1016/s1534-5807(03)00093-5. [DOI] [PubMed] [Google Scholar]
- 27.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 28.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cimini D, et al. Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J Cell Biol. 2001;153:517–527. doi: 10.1083/jcb.153.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirsch-Volders M, Vanhauwaert A, De Boeck M, Decordier I. Importance of detecting numerical versus structural chromosome aberrations. Mutat Res. 2002;504:137–148. doi: 10.1016/s0027-5107(02)00087-8. [DOI] [PubMed] [Google Scholar]
- 31.Pines J, Hunter T. Human cyclins A and B1 are differentially located in the cell and undergo cell cycle-dependent nuclear transport. J Cell Biol. 1991;115:1–17. doi: 10.1083/jcb.115.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kennedy BK, Barbie DA, Classon M, Dyson N, Harlow E. Nuclear organization of DNA replication in primary mammalian cells. Genes Dev. 2000;14:2855–2868. doi: 10.1101/gad.842600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kops GJ, Weaver BA, Cleveland DW. On the road to cancer: Aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5:773–785. doi: 10.1038/nrc1714. [DOI] [PubMed] [Google Scholar]
- 34.Abdel-Rahman WM, et al. Spectral karyotyping suggests additional subsets of colorectal cancers characterized by pattern of chromosome rearrangement. Proc Natl Acad Sci USA. 2001;98:2538–2543. doi: 10.1073/pnas.041603298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexander SP, Rieder CL. Chromosome motion during attachment to the vertebrate spindle: Initial saltatory-like behavior of chromosomes and quantitative analysis of force production by nascent kinetochore fibers. J Cell Biol. 1991;113:805–815. doi: 10.1083/jcb.113.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacKerell AD, Jr, Lee GU. Structure, force, and energy of a double-stranded DNA oligonucleotide under tensile loads. Eur Biophys J. 1999;28:415–426. doi: 10.1007/s002490050224. [DOI] [PubMed] [Google Scholar]
- 37.Maiato H, Sunkel CE. Kinetochore-microtubule interactions during cell division. Chromosome Res. 2004;12:585–597. doi: 10.1023/B:CHRO.0000036587.26566.81. [DOI] [PubMed] [Google Scholar]
- 38.Gisselsson D, et al. Chromosomal breakage-fusion-bridge events cause genetic intratumor heterogeneity. Proc Natl Acad Sci USA. 2000;97:5357–5362. doi: 10.1073/pnas.090013497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skoufias DA, Lacroix FB, Andreassen PR, Wilson L, Margolis RL. Inhibition of DNA decatenation, but not DNA damage, arrests cells at metaphase. Mol Cell. 2004;15:977–990. doi: 10.1016/j.molcel.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 40.Sluder G, Thompson EA, Miller FJ, Hayes J, Rieder CL. The checkpoint control for anaphase onset does not monitor excess numbers of spindle poles or bipolar spindle symmetry. J Cell Sci. 1997;110:421–429. doi: 10.1242/jcs.110.4.421. [DOI] [PubMed] [Google Scholar]
- 41.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 42.Zhu C, Bogue MA, Lim DS, Hasty P, Roth DB. Ku86-deficient mice exhibit severe combined immunodeficiency and defective processing of V(D)J recombination intermediates. Cell. 1996;86:379–389. doi: 10.1016/s0092-8674(00)80111-7. [DOI] [PubMed] [Google Scholar]
- 43.Barlow C, et al. Atm-deficient mice: A paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 44.Kremer L, del Mazo J, Avila J. Identification of centromere proteins in different mammalian cells. Eur J Cell Biol. 1988;46:196–199. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.