Bacteriophage can convert their bacterial host from a nonpathogenic form to a pathogenic form by providing the bacterium with virulence genes, in a process called lysogenic phage conversion. Vibrio cholerae is a bacterium prevalent in marine environments that can infect humans to cause the devastating diarrheal disease cholera, which is endemic in much of Asia and Africa. Although the health and economic burdens of cholera are enormous, the disease is sometimes overshadowed by other diseases, but cholera's predilection for epidemic spread commands attention.
Cholera toxin, an A-B type exotoxin encoded by the ctxAB genes, is the main cause of the voluminous watery diarrhea that is characteristic of cholera (1–3). V. cholerae isolates that cause cholera encode the ctxAB genes in the genome of a filamentous bacteriophage CTXϕ (Fig. 1A) (4). CTXϕ is a small, positive, single-stranded DNA [(+) ssDNA)] virus that can be found either in a replicative form or, more commonly, integrated site-specifically in the host genome to form stable lysogens. Whether the ssDNA or dsDNA form of the virus is the substrate for recombination and integration between the dsDNA host genome and CTXϕ is open to debate (5, 6). Many different CTXϕ types and arrangements have been observed in the host V. cholerae genome (7). The basis of specificity and efficiency of integration of CTXϕ is not known, nor is the mechanism of emergence of strains with novel CTXϕ chromosomal arrangements. In PNAS, a report by Das et al. resolves many of the questions surrounding the mechanism of CTXϕ integration and the capacity of variant CTXϕ genomes to integrate at each of the known attachment sites (att) (8).
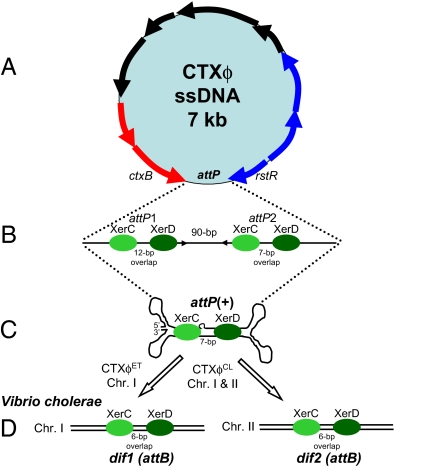
Fig. 1.
(A) Schematic representation of the single-stranded CTXϕ genome. Arrows indicate ORFs identified and characterized in CTXϕ. Phage attachment site is indicated by attP. (B) Linear representation of ssDNA attP site and regions identified by Huber and Waldor (9) and by McLeod and Waldor (5) as essential for efficient integration of CTXϕ into chromosome I (ChrI) of V. cholerae. Green oval shapes represent XerC and XerD attached at their respective binding sites on attP sites 1 (attP1) and 2 (attP2), identified by McLeod and Waldor (5). Also shown are the spacer or overlap regions between XerC and XerD binding sites, 12 bp for attP1 and 7 bp for attP2. (C) Secondary fork and stem structure formed when attP1 and attP2 base pair, creating the new attP+ site, identified by Val et al. (6). (D) The bacterial attachment sites (attB), dif1 and dif2 on chromosome I and chromosome II, the primary function of which is to resolve chromosomal dimers during cell division.
Like many bacteriophage, CTXϕ integrates its genome into the chromosome of a host V. cholerae, thereby ensuring stable vertical transmission within the bacterial host. Subsequent to CTXϕ particle adsorption to the V. cholerae cell wall, viral ssDNA is injected into the cell cytoplasm and forms a circular pCTX, which then integrates into the V. cholerae genome at a site-specific attachment site (4, 9). CTXϕ integration requires a number of phage-encoded and host-encoded factors (5, 9). A recombinase (integrase), which ordinarily catalyzes this integration in other phages, is not present in the CTXϕ genome; instead, it commandeers two host-encoded tyrosine recombinases, XerC and XerD (9). The XerCD proteins are conserved among eubacteria, as they serve to resolve chromosome dimers during cell division (10, 11). In Escherichia coli, XerCD proteins bind and catalyze recombination at homologous 28-bp dif sites, composed of two 12-bp binding sites for XerC and XerD separated by a 6-bp spacer or overlap region, which allows for XerC-XerD interactions that ensure stable synapsis (10, 11). Because V. cholerae harbors two distinct, nonhomologous circular chromosomes (chromosome I and II) (12, 13), two dif sites are present, dif1 in chromosome I and dif2 in chromosome II (9); similar to E. coli, the same FstK-dependent mechanism coordinates dimer resolution on each chromosome with cell division (14). The dif1 site differs from dif2 at four polymorphic sites, one of which is located in the XerC binding site and the other three sites are located in the 6-bp spacer region (9, 14).
The arrangement of CTXϕ in the V. cholerae genome depends on whether it is integrated at one or two of the chromosome dimer resolution sites, dif1 and dif2, and the number of copies present at each site. For example, in many El Tor strains, the cause of the seventh and ongoing cholera pandemic, the El Tor phage CTXϕET is arranged in tandem and interspersed with a related element, RS1, to give an RS1-CTXET-RS1-CTXET-RS1 arrangement on chromosome I. The El Tor Strain C6709, isolated in Peru in 1991, encodes a CTXET-RS1 arrangement on chromosome I (7, 15). In V. cholerae classical biotype isolates, which were the cause of earlier pandemics and are now extinct, the classical type phage CTXϕCL is integrated on both chromosomes and never contains an RS1 element (16, 17). Interestingly, recent El Tor strains isolated in Mozambique and India have been found to contain a single CTXϕCL integrated on chromosome I (18); but how these novel arrangements and strains emerge remains an open question.
Huber and Waldor (9) proposed that the integration of CTXϕ resulted from recombination between a 200-bp intergenic region (attP) of the replicative double-stranded form of CTXϕ and dif1 (attB) on chromosome I of V. cholerae (Fig. 1A). These authors demonstrated that the recombination reaction was catalyzed by XerC and XerD; but here, unlike in E. coli chromosomal dimer resolution, the cell division protein FtsK was not required. Unlike other phage site-specific integration mechanisms, CTXϕ integration was irreversible (9). CTXϕ virons are generated from a chromosomally integrated phage by a process analogous to rolling circle replication (19). Further detailed molecular characterization of the CTXϕ attP and chromosome I dif1 (attB) interaction was carried out using purified recombinases to show XerC and XerD binding to attP and subsequent single-strand exchange (5), but surprisingly, the mechanism did not follow that executed by other known tyrosine recombinases.
McLeod and Waldor found that XerC and XerD also bound to a second site ≈90-bp downstream of the first binding site (Fig. 1B) (5). The precise role of second site was undetermined, although it was required for integration (5). In addition, the XerC cleavage on attP was separated from XerD cleavage by 12-bp, which is wholly unexpected given the 6–8 bp spacing of XerC and XerD binding at two homologous dif sites, which is essential for interaction between the two recombinases that control synapse formation and catalysis (10, 11). McLeod and Waldor's data suggested that synapsis of the two duplexes performed by XerCD at the attP and dif1 sites are potentially less stable than recombination between homologous dif sites. The reasons why CTX integration was irreversible were also unresolved.
Following from these data, an alternative model for CTXϕ integration suggested that the (+) ssDNA genome is the form exploited for integration at dif1 by XerC and XerD, which is further supported by Das et al. (Fig. 1C) (6, 8). Val et al. (6) uncovered a double-forked hairpin structure within the region encompassing attP in the (+) ssDNA of form of CTXϕ that creates an alternative attP+ site in the stem of the secondary structure (Fig. 1C). Val et al. further demonstrated that XerC can catalyze a single pair of strand exchanges between this target, attP+, and dif1 in the presence of XerD, resultant in CTXϕ integration upon conversion of the ensuing Holliday junction by repair and/or replication, similar to the earlier findings by McLeod and Waldor (5, 6). However, at the time, neither the ssDNA nor the dsDNA integration models explained how CTXϕ integrates at dif2 on chromosome II, given the lack of complementary base pair interactions that would result, which are required to stabilize the exchange of strands catalyzed by XerC. Das et al. have clarified this issue, showing how and why there is specific integration between CTXϕET at dif1 and none at dif2, in contrast to the integration of CTXϕCL at both dif1 and dif2 (8). The authors demonstrate that this altered integration behavior of CTXϕCL is due to two base changes in the overlap region of attP2 in this phage, which allows XerCD recombination between CTXϕCL attP+ with dif1 and dif2. Indeed, they show that alteration of these two bases in the El Tor CTXϕ attP+ site results in efficient integration into both dif1 and dif2. Their data determine that the specificity of integration of the different CTXϕ variants is governed by the potential of the ssDNA CTXϕ to form base pair interactions that stabilize strand exchanges. Furthermore, the authors show that complementary base pairing may account for recent El Tor isolates that contained a dif-like site (difG) with an overlap region different from dif1 and dif2. The authors further observe that neither the El Tor nor the classical CTXϕ attP+ could recombine with difG, but they identified a variant attPG+ site that allowed recombination to take place with difG.
The wide distribution of the XerCD recombinase system among bacteria and the prevalence of dif sites among filamentous phages suggest that this system may be the paradigm for filamentous phage integration into the bacterial genome. One question that is still puzzling is how CTXϕCL are transferred between natural isolates. Although the data explain the specificity of the integration mechanism, they do not account for the recent emergence of V. cholerae El Tor strains containing CTXϕCL. Recent studies have shown that CTXϕCL isolated in a range of strains cannot produce CTXϕ virons; the infectious form of CTXϕCL is only ever present integrated in the genome (17, 18, 20). It is possible that these novel strains acquired CTXϕCL via a mechanism alternative to lysogenic conversion, such as lytic phage and/or transformation mechanisms, although the in vitro efficiencies of these events have been shown to be quite low (20, 21).
Acknowledgments
Research in the Boyd laboratory is funded by National Science Foundation CAREER Grant DEB-0844409, National Science Foundation Grant IOS-0918429, and US Department of Agriculture NRI CSREES Grant 2008-01198.
Footnotes
The author declares no conflict of interest.
See companion article on page 4377.
References
- 1.De SN. Enterotoxicity of bacteria-free culture-filtrate of Vibrio cholerae. Nature. 1959;183:1533–1534. doi: 10.1038/1831533a0. [DOI] [PubMed] [Google Scholar]
- 2.Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981;292:413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- 3.Mekalanos JJ, et al. Cholera toxin genes: Nucleotide sequence, deletion analysis and vaccine development. Nature. 1983;306:551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- 4.Waldor MK, Mekalanos JJ. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 5.McLeod SM, Waldor MK. Characterization of XerC- and XerD-dependent CTX phage integration in Vibrio cholerae. Mol Microbiol. 2004;54:935–947. doi: 10.1111/j.1365-2958.2004.04309.x. [DOI] [PubMed] [Google Scholar]
- 6.Val ME, et al. The single-stranded genome of phage CTX is the form used for integration into the genome of Vibrio cholerae. Mol Cell. 2005;19:559–566. doi: 10.1016/j.molcel.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Mekalanos JJ. Duplication and amplification of toxin genes in Vibrio cholerae. Cell. 1983;35:253–263. doi: 10.1016/0092-8674(83)90228-3. [DOI] [PubMed] [Google Scholar]
- 8.Das B, Bischerour J, Val M, Barre F-X. Molecular keys of the tropism of integration of the Cholera toxin phage. Proc Natl Acad Sci USA. 2010;107:4377–4382. doi: 10.1073/pnas.0910212107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huber KE, Waldor MK. Filamentous phage integration requires the host recombinases XerC and XerD. Nature. 2002;417:656–659. doi: 10.1038/nature00782. [DOI] [PubMed] [Google Scholar]
- 10.Sherratt DJ, et al. Recombination and chromosome segregation. Philos Trans R Soc Lond B Biol Sci. 2004;359:61–69. doi: 10.1098/rstb.2003.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barre FX, et al. Circles: The replication-recombination-chromosome segregation connection. Proc Natl Acad Sci USA. 2001;98:8189–8195. doi: 10.1073/pnas.111008998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trucksis M, Michalski J, Deng YK, Kaper JB. The Vibrio cholerae genome contains two unique circular chromosomes. Proc Natl Acad Sci USA. 1998;95:14464–14469. doi: 10.1073/pnas.95.24.14464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heidelberg JF, et al. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature. 2000;406:477–483. doi: 10.1038/35020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Val ME, et al. FtsK-dependent dimer resolution on multiple chromosomes in the pathogen Vibrio cholerae. PLoS Genet. 2008;4:e1000201. doi: 10.1371/journal.pgen.1000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waldor MK, Mekalanos JJ. Emergence of a new cholera pandemic: Molecular analysis of virulence determinants in Vibrio cholerae O139 and development of a live vaccine prototype. J Infect Dis. 1994;170:278–283. doi: 10.1093/infdis/170.2.278. [DOI] [PubMed] [Google Scholar]
- 16.Sharma C, et al. Molecular characterization of Vibrio cholerae O1 biotype El Tor strains isolated between 1992 and 1995 in Calcutta, India: Evidence for the emergence of a new clone of the El Tor biotype. J Infect Dis. 1997;175:1134–1141. doi: 10.1086/516453. [DOI] [PubMed] [Google Scholar]
- 17.Davis BM, Moyer KE, Boyd EF, Waldor MK. CTX prophages in classical biotype Vibrio cholerae: Functional phage genes but dysfunctional phage genomes. J Bacteriol. 2000;182:6992–6998. doi: 10.1128/jb.182.24.6992-6998.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faruque SM, et al. Genomic analysis of the Mozambique strain of Vibrio cholerae O1 reveals the origin of El Tor strains carrying classical CTX prophage. Proc Natl Acad Sci USA. 2007;104:5151–5156. doi: 10.1073/pnas.0700365104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moyer KE, Kimsey HH, Waldor MK. Evidence for a rolling-circle mechanism of phage DNA synthesis from both replicative and integrated forms of CTXphi. Mol Microbiol. 2001;41:311–323. doi: 10.1046/j.1365-2958.2001.02517.x. [DOI] [PubMed] [Google Scholar]
- 20.Udden SM, et al. Acquisition of classical CTX prophage from Vibrio cholerae O141 by El Tor strains aided by lytic phages and chitin-induced competence. Proc Natl Acad Sci USA. 2008;105:11951–11956. doi: 10.1073/pnas.0805560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyd EF, Waldor MK. Alternative mechanism of cholera toxin acquisition by Vibrio cholerae: Generalized transduction of CTXPhi by bacteriophage CP-T1. Infect Immun. 1999;67:5898–5905. doi: 10.1128/iai.67.11.5898-5905.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]