Abstract
Salmonella enterica serovar Typhi can colonize the gallbladder and persist in an asymptomatic carrier state that is frequently associated with the presence of gallstones. We have shown that salmonellae form bile-mediated biofilms on human gallstones and cholesterol-coated surfaces in vitro. Here, we test the hypothesis that biofilms on cholesterol gallbladder stones facilitate typhoid carriage in mice and men. Naturally resistant (Nramp1+/+) mice fed a lithogenic diet developed cholesterol gallstones that supported biofilm formation during persistent serovar Typhimurium infection and, as a result, demonstrated enhanced fecal shedding and enhanced colonization of gallbladder tissue and bile. In typhoid endemic Mexico City, 5% of enrolled cholelithiasis patients carried serovar Typhi, and bacterial biofilms could be visualized on gallstones from these carriers whereas significant biofilms were not detected on gallstones from Escherichia coli infected gallbladders. These findings offer direct evidence that gallstone biofilms occur in humans and mice, which facilitate gallbladder colonization and shedding.
Keywords: biofilm, chronic infection, typhoid fever, cholesterol, bile
Global public health continues to be burdened with significant morbidity and mortality caused by salmonellae. Greater than 2,400 serovars of Salmonella enterica can be identified in nature that have evolved to infect a broad array of host organisms (1, 2). S. enterica serovar Typhi (S. Typhi) has adapted to induce disease exclusively in humans and is the etiologic agent of typhoid fever, a debilitating illness characterized by interstitial inflammation, a gradual onset of symptoms, and, ultimately, bacterial dissemination to systemic sites (3). The Centers for Disease Control and Prevention estimate that at least 22 million people worldwide are stricken with typhoid fever each year, yet many more go unreported due in part to poor diagnostic testing (4–6).
The hallmarks of salmonellosis caused by nontyphoidal S. enterica serovars such as Enteritidis or Typhimurium differ from those of enteric fever and include intestinal neutrophilia, localized inflammatory diarrhea, the loss of electrolytes, serum protein, and fluid, as well as sepsis-induced death in immunocompromised individuals (3, 7–9). Interestingly, much of what is known about the systemic pathogenesis of S. Typhi during typhoid fever has been extrapolated from infections of inbred mouse strains without a functional Nramp1 allele (e.g., BALB/c and C57BL/6) with nontyphoidal serovar Typhimurium. In this model, translocation across the intestinal epithelium at microfold (M) cells is followed by uptake by phagocytic cells of the underlying lymphoid tissue, a primary bacteraemia in the blood, and uncontrolled replication within macrophages of the liver, spleen, and bone marrow leading to death at 7–10 days postinfection (PI) (10–14).
In humans, serovar Typhi reaching the gallbladder can establish an acute, active infection accompanied by inflammation (cholecystitis) or persist in this organ long after symptoms subside, suggesting unique mechanisms used by the bacterium to mediate colonization in a bile-rich environment (15, 16). Bile is an important digestive secretion manufactured in the liver that serves as a potent emulsifying and antimicrobial agent in the gastrointestinal tract, yet salmonellae have adapted to resist its surface-acting, amphipathic, detergent-like properties (17). Additionally, bile acts as an environmental signal affecting virulence factor expression of many intestinal pathogens including Salmonella spp. (18).
Development of chronic typhoid carriage is frequently associated with the presence of gallbladder abnormalities, especially gallstones, yet the progression from infection to the carrier state remains undefined (19). Gallstone formation (cholelithogenesis) is attributed to a combination of environmental and genetic causes, typically linked to cholesterol supersaturation from bile, and can occur in patients for many years without symptoms (20, 21). The primary constituent of gallbladder stones is cholesterol, whereas calcium bilirubinate predominates in bile duct stones (22). In patients carrying both S. Typhi and cholesterol gallstones in the gallbladder, clinically administered antibiotics are typically ineffective against dissolution of the persistent bacterial infection (19) and the risk for developing hepatobiliary carcinomas is high (23, 24). To date, removal of the gallbladder (cholecystectomy) remains the most effective treatment option for chronic typhoid carriers with gallstones. Thus, there is a strong need for alternative treatment options in areas of the world where typhoid fever is endemic and surgical intervention is cost prohibitive.
Over the past two decades, bacterial biofilms associated with medical and industrial settings have been implicated as the cause of many antibiotic-resistant, persistent infections in humans (25, 26). Biofilm formation occurs in sequential, highly regulated stages that include adherence of free-swimming bacteria to a surface, development of a protective, self-initiated extracellular matrix (ECM), and continual release of planktonic cells from this sessile community into the environment (27).
We have demonstrated that S. enterica serovars Typhimurium, Enteritidis, and Typhi bile-dependent mature biofilm formation with characteristic ECM production (O-antigen capsule) occurs on human gallstones and on cholesterol-coated Eppendorf tubes in vitro (28, 29). The criteria for a disease-associated biofilm has been described as one in which the bacteria are (i) adherent to a surface, (ii) enclosed in a matrix and/or clustered in infected tissue during disease progression, (iii) confined to the bound location (although dissemination may occur secondarily), and (iv) difficult to eradicate with antibiotics (30). Given this description, the clinical data from typhoid carriers and our in vitro data, we hypothesize that asymptomatic S. Typhi carriage is facilitated by the formation of biofilms on cholesterol gallstones.
S. Typhi biofilm formation on cholesterol gallstones has not been investigated as a mechanism mediating the chronic carrier state of typhoid fever in vivo. To address this issue, we present clinical evidence that serovar Typhi forms biofilms on gallstones in asymptomatic, human carriers. In addition, we have developed a murine model of Salmonella persistence in mice with lithogenic diet-induced cholesterol gallstones. Interestingly, mice with gallstones harbor significantly more serovar Typhimurium in the gallbladder tissue, bile, and fecal matter than control mice, and salmonellae can be recovered from homogenized gallstones in increasing amounts over time post-infection. Furthermore, serovar Typhimurium biofilms were observed on cholesterol gallstones taken from mice 3 weeks postinfection. These findings offer direct evidence that gallstone biofilms occur in individuals carrying S. Typhi and points to a unique therapeutic target against the spread of typhoid fever.
Results
Salmonella-Resistant Mice Form Cholesterol Gallstones.
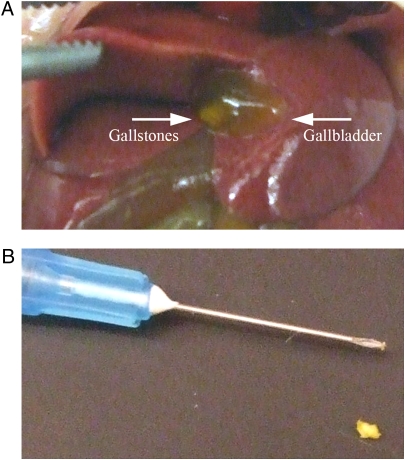
Serovar Typhimurium is a facultative intracellular pathogen that mediates a systemic, typhoid fever-like illness and rapid death in mouse strains lacking the macrophage-specific protein Nramp1 (for a review, see ref. 31), but persists without symptoms in naturally resistant mice (Nramp1+/+) (32). Recent experiments monitoring Salmonella infections in wild-type (Nramp1+/+) mice reported recovery of salmonellae from the gallbladders of asymptomatic animals at late time points PI (33, 34). The murine gallbladder has also been shown to carry diet-induced gallstones in certain inbred strains used to study cholesterol gallstone genetics and pathogenesis (35, 36). To determine whether strains resistant to serovar Typhimurium infection could harbor gallbladder stones, female 129 × 1/SvJ mice were fed a gallstone-inducing diet for 8 weeks. Consumption of the lithogenic diet (normal mouse chow supplemented with 1% cholesterol and 0.5% cholic acid) induced obvious, visible cholesterol gallstone formation in >80% of the gallbladders of 129 × 1/SvJ mice (Fig. 1A), and these materials could be surgically removed for further examination (Fig. 1B). Mouse gallstones were small, tended to clump together, and roughly of similar size. Some mice did have more gallstones than others and each gallbladder always harbored more than a single stone.
Fig. 1.
Effects of an 8-week lithogenic diet (mouse chow supplemented with 1% cholesterol and 0.5% cholic acid) in mice naturally resistant to serovar Typhimurium infections (n = 30, two independent experiments). (A) Cholesterol diet-induced gallstones in the gallbladder of Nramp1+/+ 129 × 1/SvJ mice. (B) Gallbladder stones were removed and photographed with a 25-gauge needle for scale.
Cholesterol Gallstones Enhance Salmonella Gallbladder Colonization and Fecal Shedding During Persistent Murine Infections.
We hypothesize that S. Typhi persistence in the gallbladders of asymptomatic human carriers is facilitated in large part by the formation of biofilms on cholesterol gallstones and have previously demonstrated that S. enterica serovars Typhimurium, Enteritidis, and Typhimurium formed bile-induced biofilms on human gallstones and cholesterol-coated surfaces in vitro (28, 29). To determine whether cholesterol gallbladder stones enhanced gallbladder colonization and carriage of salmonellae in vivo, 129 × 1/SvJ mice were fed a lithogenic diet or normal mouse chow for 8 weeks and inoculated intraperitoneally with 1.4 × 104 colony-forming units (CFU) serovar Typhimurium or left as uninfected controls.
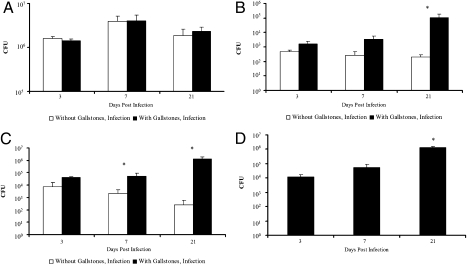
Similar splenic burdens between diet groups were observed at days 3, 7, and 21 PI (Fig. 2A), indicating that hypercholesterolemia did not impair general host immunity during infection. Over 106 CFU were recovered from the spleen 3 weeks PI (Fig. 2A) whereas no salmonellae were obtained in this organ from uninfected controls.
Fig. 2.
CFU enumerations (CFU/mL) from organs, bile, and gallstones of 129 × 1/SvJ mice fed normal chow or lithogenic diet at days 3, 7, and 21 post i.p.infections with 1.4 × 104 serovar Typhimurium 14028s (n = 42, 21 mice for each diet group, 7 per time point; two individual experiments; means + SD are given). (A) Intake of excessive dietary cholesterol did not enhance general infection or spleen burden at any time point. (B) The presence of gallstones significantly increased the amount of salmonellae recovered from gallbladder tissues at late time points compared with normal mouse chow controls during persistent murine infections. (C) Bacterial numbers from gallbladder bile increased significantly with time postinfection in mice fed a lithogenic diet for 8 weeks compared to infected mice fed normal chow. (D) Salmonellae were recovered from homogenized gallstones, and CFU values increased over time postinfection. No bacteria were recovered from the gallstones of uninfected mice. *, statistical significance (P < 0.01) based on a two-tailed Student t test.
Salmonella spp. initiate invasion into nonphagocytic cells by a type III secretion system-mediated event. However, their interaction with gallbladder epithelia has not been examined. To investigate the prevalence of serovar Typhimurium associated with the gallbladder epithelium during persistent infections, gallbladder tissues were separated from luminal contents, homogenized, and plated to enumerate CFU. The number of salmonellae harbored by the gallbladder epithelium of mice with diet-induced cholesterol gallstones increased over time PI and was significantly higher than observed in mice without gallbladder stones at time points 3 (4-fold), 7 (12-fold), and 21 (541-fold) days PI (Fig. 2B). Gallbladder tissues from uninfected mice in both diet groups yielded no salmonellae.
The presence of gallstones also correlated with higher levels of serovar Typhimurium recovered from gallbladder bile, and these amounts increased as the infection progressed (Fig. 2C). Conversely, the amounts of salmonellae detected in gallbladder bile of mice without cholesterol gallstones decreased over time PI. Those mice with gallstones showed dramatic increases (versus mice with no gallstones) in Salmonella CFU at time points of 3 (5-fold), 7 (24-fold), and 21 (5,300-fold) days (Fig. 2C). No bacteria were recovered from uninfected control mice. These results suggest that cholesterol gallstones enhance colonization and persistence of Salmonella in gallbladder tissues and bile during murine infection.
To determine whether gallstones themselves supported serovar Typhimurium growth, gallbladder stones were removed and homogenized with a microspatula followed by vigorous pipetting, dilution, and plating on LB agar. As shown in Fig. 2D, the association between salmonellae and cholesterol gallstones was robust, and the amount of salmonellae recovered from gallstone material increased by over two logs from days 3 to 21 PI. These results indicate that salmonellae colonize gallbladder stones, likely developing a biofilm as has been demonstrated in vitro (28, 29). We did not observe any correlation of gallstone number and carriage.
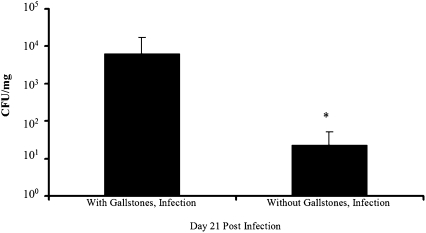
It was demonstrated previously that subsets of persistently infected 129 × 1/SvJ mice shed high levels of serovar Typhimurium in feces and that these groups rapidly transmitted the infection to naive mice (37). To determine whether cholesterol gallbladder stones enhanced shedding of serovar Typhimurium, ≈5–7 fresh fecal pellets were collected from isolated mice at day 21 PI, weighed, and plated on Salmonella shigella agar to detect salmonellae. Interestingly, mice harboring gallstones shed three logs more serovar Typhimurium than infected mice on the control diet, whereas stools from uninfected groups were Salmonella-free (Fig. 3). Therefore, the observed association of salmonellae with cholesterol gallstones and its elevated recovery from gallbladder tissue, bile, and feces at late time points postinfection suggest that the presence of diet-induced gallstones in 129 × 1/SvJ mice mediates enhanced gallbladder colonization and shedding following serovar Typhimurium infection.
Fig. 3.
Serovar Typhimurium is shed in fecal matter from infected 129 × 1/SvJ mice. Mice harboring cholesterol gallstones shed three logs more serovar Typhimurium than those on a normal chow diet. Means + SD are given for two independent experiments using seven infected animals per diet group. *, statistical significance (P < 0.01) based on a two-tailed Student t test.
Serovar Typhimurium Forms Biofilms on Cholesterol Gallbladder Stones During Chronic Murine Infection.
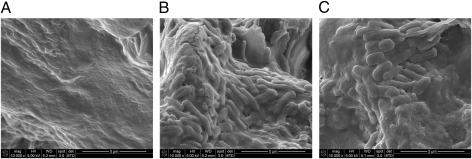
Cholesterol gallstones were removed from mouse gallbladders at 3, 7, and 21 days postinfection and examined by scanning electron microscopy (SEM) for biofilm formation. No significant groupings of bacteria were observed on stones collected from uninfected controls or from mice infected with serovar Typhimurium for 3 and 7 days. However, gallbladder stones taken from mice 21 days postinfection harbored bacterial biofilm communities covering an estimated 50% (by SEM) of the surface of each gallstone (Fig. 4). These results suggest that Salmonella biofilms on cholesterol gallstones mediate, at least in part, gallbladder colonization during persistent, asymptomatic infection.
Fig. 4.
Serovar Typhimurium biofilm formation on cholesterol gallstones at late time points during chronic murine infection. (A) SEM micrograph showing the surface of a cholesterol gallstone taken from an uninfected 129 × 1/SvJ control mouse fed a lithogenic diet. B and C show bacterial biofilms on gallstones taken from two different mice infected with serovar Typhimurium for 21 days.
Serovar Typhi Forms Biofilms on Gallstones of Human Asymptomatic Carriers.
In 1982, Levine et al. demonstrated that serovar Typhi could be recovered in stool samples for over 1 year from at least 2–5% of those suffering acute typhoid illnesses (38). It is now understood that gallbladder stones are frequently present in asymptomatic carriers of serovar Typhi (19). To better define the association between gallstones and typhoid carriage in an area endemic to typhoid fever, individuals seeking surgical gallbladder removal due to gallstones (cholecystectomy) at the Hospital General de México (age range 21–92 years, mean = 44.3 years, 12 unreported) were analyzed for the asymptomatic presence of serovar Typhi. Multiplex PCR using genomic DNA purified from gallbladder tissues, bile, gallstone sections, and stool samples, as well as traditional plating and bacterial enumeration, revealed that 5/103 (4.9%) patients with cholelithiasis tested positive for typhoid carriage, of which 3 were female (ages 52, 58, and 60) and 2 were male (ages 41 and 46). CFUs for the 5 typhoid positive biological samples are depicted in Table 1. All 5 carriers were positive for serovar Typhi in gallstone washings, 2 of which also harbored salmonellae in gallbladder epithelial tissues. Only 1 carrier tested positive for serovar Typhi in bile, and none were diagnosed by stool determinations. Additionally, the presence of Escherichia coli was detected in 13 typhoid negative patients and Shigella flexneri was recovered from three stool samples.
Table 1.
Gallstone biofilm formation and bacterial burden in patient samples
| No. | GB tissue | Bile | Gallstones | Biofilm formation |
| 1 | S. Typhi 1.2 x 103 CFU/mL | ND | S. Typhi 1.4 x 103 CFU/mL | ++ |
| 2 | ND | ND | S. Typhi 80 CFU/mL | ++ |
| 3 | S. Typhi 20 CFU/mL | S. Typhi 40 CFU/mL | S. Typhi 20 CFU/mL | ++ |
| 4 | ND | ND | S. Typhi 20 CFU/mL | — |
| 5 | ND | ND | S. Typhi 20 CFU/mL | ND* |
| 6 | E. coli 5 x 105 CFU/mL | E. coli 3 x 105 CFU/mL | E. coli 4.5 x 105 CFU/mL | + |
| 7 | E. coli 1 x 105 CFU/mL | E. coli 1 x 105 CFU/mL | E. coli 1 x 105 CFU/mL | + |
| 8 | E. coli 3 x 104 CFU/mL | E. coli 1 x 105 CFU/mL | E. coli 1 x 105 CFU/mL | — |
| 9 | E. coli 1 x 105 CFU/mL | E. coli 1 x 105 CFU/mL | E. coli 1 x 105 CFU/mL | — |
| 10 | E. coli 1 x 105 CFU/mL | E. coli 1 x 105 CFU/mL | E. coli 1 x 105 CFU/mL | — |
| 11 | E. coli 3.6 x 105 CFU/mL | E. coli 5 x 105 CFU/mL | E. coli 3 x 105 CFU/mL | — |
Gallstones from patients seeking cholecystectomy (those that were S. Typhi positive and a sampling of those E. coli positive) were visualized by SEM for the presence of bacterial biofilms. ++, robust biofilm formation covering an estimated 80–95% of gallstone surface; +, little biofilm present (covering ≈5–20% gallstone surface); —, absence of biofilm formation on gallstone surface. Bacteria recovered from the gallbladder tissues, bile, and gallstone sections in patients with gallstones were plated for CFU and further identified using multiplex PCR as described in Materials and Methods. ND, none detected.
*This gallstone was misplaced and thus was not examined further by electron microscopy.
Gallstones from four of the five asymptomatic serovar Typhi carriers were sent to the Ohio State University (OSU) and examined by SEM to determine whether typhoid biofilms on gallstones contributed to chronic carriage. Interestingly, biofilms were visualized with an estimated 80–95% surface coverage on three of four gallstones from typhoid carriers. A representative sample is highlighted in Fig. 5 at various magnifications (A–D), whereas specimens from the other typhoid positive patients are presented in Fig. 6 A and B. A single gallstone from this group lacked biofilm formation (Fig. 6C), an observation likely attributed to a variety of gallstone- and patient-specific factors including the uniqueness in surface structure, the limited sample size, and by the possibility that the primary constituent of this stone was not cholesterol, but rather calcium bilirubin as indicated by its black color. We have demonstrated previously that Eppendorf tubes coated with bilirubin supported significantly less biofilm than those coated with cholesterol in vitro, suggesting that serovar Typhi may not form biofilms on gallstones composed of calcium bilirubinate in vivo (29).
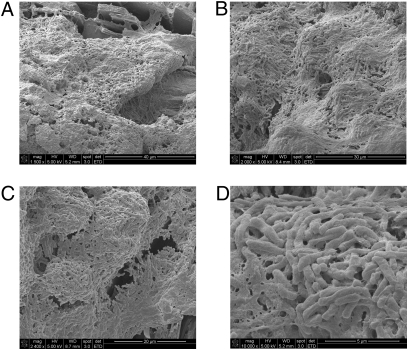
Fig. 5.
Gallbladder stones from an asymptomatic typhoid carrier in Mexico City support biofilm formation. SEM micrographs show serovar Typhi bacilli embedded in biofilms on the surfaces of gallstones at magnifications of 1,500× (A), 2,000× (B), 2,400× (C), and 16,000× (D).
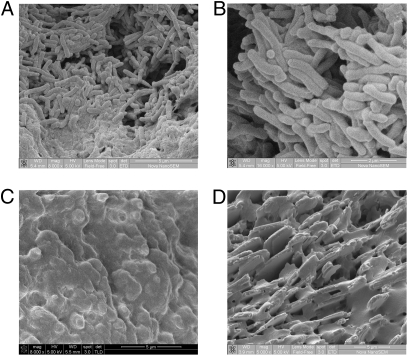
Fig. 6.
Biofilm formation on human gallstones was observed only in patients seeking cholecystectomy that were positive for typhoid carriage. SEM micrographs show bacterial biofilms on gallstones of two asymptomatic typhoid carriers in A and B. (C) Biofilm formation was not detected on the gallstone of one of the serovar Typhi carriers. The unique surface texture and pigment were suggestive of black, calcium bilirubinate gallstones. (D) Absence of biofilm formation on gallbladder stones from a patient positive for E. coli and S. flexneri.
To determine whether gallstone biofilm formation was mediated specifically by serovar Typhi, gallstones from 6/13 E. coli positive patients were also sent to OSU and examined by SEM. Of this group, 4 samples lacked any bound bacteria, whereas small patches of bacteria covering an estimated 5–20% of the surface were visualized on 2 others. Additionally, no biofilm formation was observed on gallstones from a patient positive for both E. coli and S. flexneri (Fig. 6D) or from patients free of culturable enterobacteraciae. Table 1 provides a summary of biofilm formation and respective tissue burden for patients in this study. These results provide unique clinical evidence that gallstone biofilms are associated with asymptomatic typhoid carriage.
Discussion
Bacterial biofilms develop on live and dead tissues and medical devices, are slow to produce overt symptoms, resist cellular and humoral immune host responses and antibiotic regimens, and persist for long periods of time with intermittent recurrence of symptoms until the sessile community is physically removed (for a review see ref. 26). Biofilm formation is thought to contribute to 65% of chronic infections treated by physicians (39) and has been observed directly in a variety of clinical manifestations. The asymptomatic, chronic carrier state of S. enterica serovar Typhi occurs in the bile-rich gallbladder, is frequently associated with the presence of cholesterol gallstones, and does not subside with antibiotic treatment (19, 38), yet the mechanism behind progression from infection to persistence remains unknown. We hypothesize that gallbladder typhoid carriage is often a result of biofilm formation on cholesterol gallstone surfaces. We have previously demonstrated that salmonellae form bile-induced biofilms on human gallstones and cholesterol-coated surfaces in vitro (28, 29). In this work, we develop a murine model of Salmonella chronic carriage in naturally resistant mice harboring Salmonella bound to cholesterol gallstones and present clinical evidence that biofilms occur on gallstones from asymptomatic, human typhoid carriers.
Our understanding of the molecular mechanisms of S. Typhi pathogenesis and associated pathology during typhoid fever has primarily been inferred from infections with nontyphoidal serovar Typhimurium of susceptible inbred mouse strains lacking a functional Nramp1 allele (11, 12). Relatively recently, it was demonstrated that naturally resistant Nramp+/+ mice inoculated with serovar Typhimurium carried salmonellae asymptomatically in spleens and gallbladders long after the initial infection (32, 33). Interestingly, gallbladder abnormalities are typically studied using a C57 variant that develops gallstones following an 8-week consumption of a cholesterol-rich diet (35, 36). Here, resistant 129 × 1/SvJ mice fed a lithogenic diet (normal chow supplemented with 1% cholesterol and 0.5% cholic acid) consistently formed gallstones that enhanced colonization and persistence of serovar Typhimurium in gallbladder tissue and bile following i.p. infection. The presence of this gallstone material was associated with significant numbers of salmonellae that increased over time, and these gallstone-containing mice had a 3-log increase in fecal shedding of serovar Typhimurium compared with infected controls lacking gallstones. Equivalent numbers of serovar Typhimurium were recovered from spleens between groups with or without gallstones at all time points, suggesting that, unlike observations in Mycobacterium tuberculosis (40), hypercholesterolemia did not impair general host immunity during murine infection with salmonellae. In addition, it was observed that serovar Typhimurium formed biofilms on cholesterol gallbladder stones at late time points during chronic murine infection. Taken together, these results indicate that persistently infected 129 × 1/SvJ mice harboring diet-induced cholesterol gallstones increased gallbladder colonization and thus have the potential to serve as a model for the study of typhoid carriage.
An estimated 25% of asymptomatic carriers have no history of typhoid fever and shedding is known to be sporadic (6), making the chronic carrier state difficult to confirm and an understudied aspect of global human health. It has been shown that typhoid carriage accelerates person-to-person transmission, is almost always associated with abnormalities of the gallbladder, including gallstones, and represents the largest risk factor for cancer of this organ (19, 23, 24). To better understand the association between gallstones and the chronic carrier state, individuals seeking cholecystectomy in an area endemic to typhoid fever were analyzed for the subclinical carriage of serovar Typhi. Approximately 5% of this population was shown to be positive for serovar Typhi in gallbladder tissue, bile, or gallstone sections analyzed by multiplex PCR and/or traditional plating on solid media. Interestingly, three of four gallstones from serovar Typhi positive patients visualized by SEM showed bacterial biofilms nearly completely covering the surface. The negative sample was black pigmented and had a unique surface composition, suggesting that the main constituent of this gallstone was calcium bilirubinate, a substrate shown in vitro to support significantly less Salmonella biofilm formation than cholesterol (29). The finding of S. Typhimurium associated with the gallbladder tissue (in mouse and man) suggests that invasion of gallbladder epithelial cells and subsequent sloughing of these cells may also augment carriage.
Gallstones from patients positive for E. coli were also analyzed by SEM and shown to be biofilm negative, although two of the six samples had small patches of bacteria on ≈5–20% of the surface. Additionally, no biofilm formation was detected on gallstones from an E. coli and S. flexneri double positive individual or gallbladders free of aerobic bacteria, suggesting that, of those culturable bacteria, biofilm formation on gallstones is mediated specifically by serovar Typhi.
We have developed a murine model of cholesterol gallstone-mediated typhoid carrier state, and provided unique clinical evidence that typhoid biofilms on gallstones may facilitate bacterial persistence in the hostile gallbladder during chronic carriage. These observations and this model system provide a better understanding of the typhoid carrier state and an important alternative, therapeutic target for alleviating asymptomatic gallbladder carriage of S. Typhi and human-to-human transmission.
Materials and Methods
Bacterial Growth Conditions.
Serovar Typhimurium cultures were grown in Luria-Bertani (LB) broth with or without 3% crude ox bile extract (Sigma) overnight on a rotating drum at 37 °C, diluted 1:100, and grown to mid-to-late exponential phase [optical density at 600 nm (OD600) of 0.6–0.8] for use in mouse colonization assays.
Serovar Typhimurium Infections in Mice.
Female 129 × 1/SvJ mice (Jackson Laboratories) weighing 16–18 grams (6–8 weeks) were fed a normal rodent diet (Harlan-Teklad, cat. no. 2014) or a lithogenic, gallstone-inducing diet consisting of mouse chow (Harlan-Teklad, cat. no. 7912) supplemented with 1% cholesterol (Sigma) and 0.5% cholic acid (Sigma) for 8 weeks. To make the lithogenic diet, equal parts (weight:volume) powdered ingredients and UltraPure distilled water (Invitrogen) were mixed until homogenous, and pellets weighing between 5 and 7 grams were shaped by hand and air dried overnight in a Laminar flow hood. Mice were inoculated (three independent experiments; total n = 69 where 39 mice were fed lithogenic diet and 30 mice were given the control diet) intraperitoneally with ≈1 × 104 CFU or left as uninfected controls (n = 43; 23 mice fed lithogenic diet and 20 mice fed control diet), and sacrificed at days 3, 7, and 21 postinfection. Dilutions of the culture inoculum were plated on LB agar to determine the actual number of delivered bacteria. For analysis of organ burdens (CFU), spleens and gallbladders were aseptically removed at the stated time points. To enumerate CFU, gallstones (present only in the lithogenic diet groups) and gallbladder tissues were separated and homogenized in a 50-mL conical tube containing 1 mL of 1× PBS. Gallbladder bile was removed before homogenizing, diluted, and plated. To monitor shedding of serovar Typhimurium, infected mice from both diet groups were isolated overnight in individual cages before killing at day 21. Approximately five to seven fresh pellets were collected from each mouse, weighed in 50-mL conical tubes, homogenized in 1× PBS, diluted, and plated on SS-agar (BD) for CFU determination.
Sample Collection from Human Donors.
Patients seeking surgical removal of their gallbladder (cholecystectomy) at the Hospital General de México (Mexico City) were tested for asymptomatic typhoid carriage at the Hospital Infantil de México “Federico Gómez” during a 2-year study. Patients selected for the study were not taking antibiotics and had no symptoms that would indicate that they were ill due to an infectious disease. This study was conducted using approved protocols by the institutional review boards of the Ohio State University and both Mexico City hospitals. To diagnose the presence of serovar Typhi, gallbladder tissues, gallstone sections, and stool samples were homogenized and plated, and bile was obtained and plated directly. If bacteria were present, serotyping was performed using standard clinical techniques. Direct tissue, gallstone and bile samples, as well as genomic DNA from culture positive samples were purified and selectively amplified by multiplex PCR for Vi antigen (viaB), flagellar antigens (fliC-d and fliC-a), and O-antigen synthesis (tyv and prt) as previously described (41) to confirm identity of serovar Typhi. Gallstones and gallbladder tissues from positive patients and negative controls were sent to OSU and analyzed for gallstone biofilms by scanning electron microscopy (SEM) or tissue inflammation and cellular infiltration by histopathology.
Scanning Electron Microscopy.
Gallstones recovered from human patients at the Hospital General de México and from mice at OSU were rinsed with sterile PBS, fixed in 2% glutaraldehyde, and air dried in a Laminar flow hood. All specimens were dehydrated postfixation using a Cressington critical point drier at the Campus Microscopy and Imaging Facility (OSU), sputter coated with a Pelco Model 3, and viewed using a FEI Nova NanoSEM at 5.00 kV under varying magnifications.
Acknowledgments
We thank Brian Kemmenoe of the Ohio State University (OSU) Campus Microscopy and Imaging Facility for technical support with SEM work and personnel at Hospital General de México and Universidad Nacional Autónoma de México, both in Mexico City, for their assistance with obtaining and processing gallbladder/gallstone samples.This study was supported by National Institutes of Health Grant AI066208 (to J.S.G.) and a graduate education fellowship from OSU Public Health Preparedness for Infectious Diseases (to R.W.C.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Boyle EC, Bishop JL, Grassl GA, Finlay BB. Salmonella: From pathogenesis to therapeutics. J Bacteriol. 2007;189:1489–1495. doi: 10.1128/JB.01730-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rabsch W, Tschäpe H, Bäumler AJ. Non-typhoidal salmonellosis: Emerging problems. Microbes Infect. 2001;3:237–247. doi: 10.1016/s1286-4579(01)01375-2. [DOI] [PubMed] [Google Scholar]
- 3.Tsolis RM, Young GM, Solnick JV, Bäumler AJ. From bench to bedside: Stealth of enteroinvasive pathogens. Nat Rev Microbiol. 2008;6:883–892. doi: 10.1038/nrmicro2012. [DOI] [PubMed] [Google Scholar]
- 4.Butler T, Bell WR, Levin J, Linh NN, Arnold K. Typhoid fever. Studies of blood coagulation, bacteremia, and endotoxemia. Arch Intern Med. 1978;138:407–410. doi: 10.1001/archinte.138.3.407. [DOI] [PubMed] [Google Scholar]
- 5.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 6.Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. Typhoid fever. N Engl J Med. 2002;347:1770–1782. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- 7.Dharmana E, Keuter M, Netea MG, Verschueren IC, Kullberg BJ. Divergent effects of tumor necrosis factor-alpha and lymphotoxin-alpha on lethal endotoxemia and infection with live Salmonella typhimurium in mice. Eur Cytokine Netw. 2002;13:104–109. [PubMed] [Google Scholar]
- 8.Fleisher GR. Management of children with occult bacteremia who are treated in the emergency department. Rev Infect Dis. 1991;13(Suppl 2):S156–S159. doi: 10.1093/clinids/13.supplement_2.s156. [DOI] [PubMed] [Google Scholar]
- 9.Santos RL, Zhang S, Tsolis RM, Bäumler AJ, Adams LG. Morphologic and molecular characterization of Salmonella typhimurium infection in neonatal calves. Vet Pathol. 2002;39:200–215. doi: 10.1354/vp.39-2-200. [DOI] [PubMed] [Google Scholar]
- 10.Jepson MA, Clark MA. The role of M cells in Salmonella infection. Microbes Infect. 2001;3:1183–1190. doi: 10.1016/s1286-4579(01)01478-2. [DOI] [PubMed] [Google Scholar]
- 11.Jones BD, Falkow S. Salmonellosis: Host immune responses and bacterial virulence determinants. Annu Rev Immunol. 1996;14:533–561. doi: 10.1146/annurev.immunol.14.1.533. [DOI] [PubMed] [Google Scholar]
- 12.Galán JE, Curtiss R., 3rd Distribution of the invA, -B, -C, and -D genes of Salmonella typhimurium among other Salmonella serovars: invA mutants of Salmonella typhi are deficient for entry into mammalian cells. Infect Immun. 1991;59:2901–2908. doi: 10.1128/iai.59.9.2901-2908.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richter-Dahlfors A, Buchan AM, Finlay BB. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J Exp Med. 1997;186:569–580. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salcedo SP, Noursadeghi M, Cohen J, Holden DW. Intracellular replication of Salmonella typhimurium strains in specific subsets of splenic macrophages in vivo. Cell Microbiol. 2001;3:587–597. doi: 10.1046/j.1462-5822.2001.00137.x. [DOI] [PubMed] [Google Scholar]
- 15.Vaishnavi C, et al. Prevalence of Salmonella enterica serovar typhi in bile and stool of patients with biliary diseases and those requiring biliary drainage for other purposes. Jpn J Infect Dis. 2005;58:363–365. [PubMed] [Google Scholar]
- 16.Sinnott CR, Teall AJ. Persistent gallbladder carriage of Salmonella typhi. Lancet. 1987;1:976. doi: 10.1016/s0140-6736(87)90319-9. [DOI] [PubMed] [Google Scholar]
- 17.van Velkinburgh JC, Gunn JS. PhoP-PhoQ-regulated loci are required for enhanced bile resistance in Salmonella spp. Infect Immun. 1999;67:1614–1622. doi: 10.1128/iai.67.4.1614-1622.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunn JS. Mechanisms of bacterial resistance and response to bile. Microbes Infect. 2000;2:907–913. doi: 10.1016/s1286-4579(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 19.Lai CW, Chan RC, Cheng AF, Sung JY, Leung JW. Common bile duct stones: A cause of chronic salmonellosis. Am J Gastroenterol. 1992;87:1198–1199. [PubMed] [Google Scholar]
- 20.Maurer KJ, Carey MC, Fox JG. Roles of infection, inflammation, and the immune system in cholesterol gallstone formation. Gastroenterology. 2009;136:425–440. doi: 10.1053/j.gastro.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shoheiber O, Biskupiak JE, Nash DB. Estimation of the cost savings resulting from the use of ursodiol for the prevention of gallstones in obese patients undergoing rapid weight reduction. Int J Obes Relat Metab Disord. 1997;21:1038–1045. doi: 10.1038/sj.ijo.0800513. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann AF. Helicobacter and cholesterol gallstones: Do findings in the mouse apply to man? Gastroenterology. 2005;128:1126–1129. doi: 10.1053/j.gastro.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 23.Dutta U, Garg PK, Kumar R, Tandon RK. Typhoid carriers among patients with gallstones are at increased risk for carcinoma of the gallbladder. Am J Gastroenterol. 2000;95:784–787. doi: 10.1111/j.1572-0241.2000.01860.x. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S, Kumar S, Kumar S. Infection as a risk factor for gallbladder cancer. J Surg Oncol. 2006;93:633–639. doi: 10.1002/jso.20530. [DOI] [PubMed] [Google Scholar]
- 25.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–138. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 26.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 27.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 28.Prouty AM, Schwesinger WH, Gunn JS. Biofilm formation and interaction with the surfaces of gallstones by Salmonella spp. Infect Immun. 2002;70:2640–2649. doi: 10.1128/IAI.70.5.2640-2649.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crawford RW, Gibson DL, Kay WW, Gunn JS. Identification of a bile-induced exopolysaccharide required for Salmonella biofilm formation on gallstone surfaces. Infect Immun. 2008;76:5341–5349. doi: 10.1128/IAI.00786-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parsek MR, Singh PK. Bacterial biofilms: An emerging link to disease pathogenesis. Annu Rev Microbiol. 2003;57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- 31.Skamene E, Schurr E, Gros P. Infection genomics: Nramp1 as a major determinant of natural resistance to intracellular infections. Annu Rev Med. 1998;49:275–287. doi: 10.1146/annurev.med.49.1.275. [DOI] [PubMed] [Google Scholar]
- 32.Nauciel C, Ronco E, Guenet JL, Pla M. Role of H-2 and non-H-2 genes in control of bacterial clearance from the spleen in Salmonella typhimurium-infected mice. Infect Immun. 1988;56:2407–2411. doi: 10.1128/iai.56.9.2407-2411.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monack DM, Bouley DM, Falkow S. Salmonella typhimurium persists within macrophages in the mesenteric lymph nodes of chronically infected Nramp1+/+ mice and can be reactivated by IFNgamma neutralization. J Exp Med. 2004;199:231–241. doi: 10.1084/jem.20031319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng SX, et al. Quantitative studies of the regular distribution pattern for Salmonella enteritidis in the internal organs of mice after oral challenge by a specific real-time polymerase chain reaction. World J Gastroenterol. 2008;14:782–789. doi: 10.3748/wjg.14.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reihnér E, Ståhlberg D. Lithogenic diet and gallstone formation in mice: integrated response of activities of regulatory enzymes in hepatic cholesterol metabolism. Br J Nutr. 1996;76:765–772. doi: 10.1079/bjn19960082. [DOI] [PubMed] [Google Scholar]
- 36.Wittenburg H, Lyons MA, Paigen B, Carey MC. Mapping cholesterol gallstone susceptibility (Lith) genes in inbred mice. Dig Liver Dis. 2003;35(Suppl 3):S2–S7. doi: 10.1016/s1590-8658(03)00085-9. [DOI] [PubMed] [Google Scholar]
- 37.Lawley TD, et al. Host transmission of Salmonella enterica serovar Typhimurium is controlled by virulence factors and indigenous intestinal microbiota. Infect Immun. 2008;76:403–416. doi: 10.1128/IAI.01189-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levine MM, Black RE, Lanata C. Precise estimation of the numbers of chronic carriers of Salmonella typhi in Santiago, Chile, an endemic area. J Infect Dis. 1982;146:724–726. doi: 10.1093/infdis/146.6.724. [DOI] [PubMed] [Google Scholar]
- 39.Schaechter M, Neidhardt FC. In: Escherichia coli and Salmonella Typhimurium Cellular and Molecular Biology. Neidhardt FC, editor. Washington, DC: American Society for Microbiology; 1987. pp. 1–2. [Google Scholar]
- 40.Martens GW, et al. Hypercholesterolemia impairs immunity to tuberculosis. Infect Immun. 2008;76:3464–3472. doi: 10.1128/IAI.00037-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirose K, et al. Selective amplification of tyv (rfbE), prt (rfbS), viaB, and fliC genes by multiplex PCR for identification of Salmonella enterica serovars Typhi and Paratyphi A. J Clin Microbiol. 2002;40:633–636. doi: 10.1128/JCM.40.2.633-636.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]