Abstract
The long-term response to chronic stress is variable, with some individuals developing maladaptive functioning, although other “resilient” individuals do not. Stress reduces neurogenesis in the dentate gyrus subgranular zone (SGZ), but it is unknown if stress-induced changes in neurogenesis contribute to individual vulnerability. Using a chronic social defeat stress model, we explored whether the susceptibility to stress-induced social avoidance was related to changes in SGZ proliferation and neurogenesis. Immediately after social defeat, stress-exposed mice (irrespective of whether they displayed social avoidance) had fewer proliferating SGZ cells labeled with the S-phase marker BrdU. The decrease was transient, because BrdU cell numbers were normalized 24 h later. The survival of BrdU cells labeled before defeat stress was also not altered. However, 4 weeks later, mice that displayed social avoidance had more surviving dentate gyrus neurons. Thus, dentate gyrus neurogenesis is increased after social defeat stress selectively in mice that display persistent social avoidance. Supporting a functional role for adult-generated dentate gyrus neurons, ablation of neurogenesis via cranial ray irradiation robustly inhibited social avoidance. These data show that the time window after cessation of stress is a critical period for the establishment of persistent cellular and behavioral responses to stress and that a compensatory enhancement in neurogenesis is related to the long-term individual differences in maladaptive responses to stress.
Keywords: dentate gyrus, nestin-GFP, posttraumatic stress disorder, social defeat, subgranular zone
Acentral component of optimal functioning and survival is allostasis, or an individual’s dynamic response to internal and external sources of stress required to maintain physiological and behavioral homeostasis (1). For some individuals, exposure to chronic stress leads to a pathologically enhanced allostatic response (“allostatic overload”) (2), which ultimately results in disease states like major depressive disorder or posttraumatic stress disorder. Interestingly, the vast majority of stress-exposed individuals do not develop stress-related psychopathology (3, 4). Recently, neuroadaptations in the mesolimbic reward system were identified that contribute to the susceptibility to stress (5), raising the question as to whether neuroadaptations occur in other brain regions that also influence individual responses to stress. Because stress potently modulates learning and memory, and the hippocampus is intimately linked to these processes, it is critical to evaluate how hippocampal structure and function are altered by stress.
One notable stress-induced hippocampal neuroadaptation is inhibition of cell proliferation in the subgranular zone (SGZ), a region that gives rise to adult-generated dentate gyrus granule neurons (1, 6). This inhibition generalizes across species and stress paradigms and may represent a long-lasting maladaptive response to stress (1, 6). Previous attempts to link SGZ proliferation to a stress-induced behavioral response have failed (7, 8). However, neurogenesis is a complex multistage and multiweek process involving proliferation; neuronal differentiation; and, ultimately, survival and integration into circuitry (9–11). Because previous studies have focused on short-term effects of stress on proliferation, it is unclear if there are longer lasting stress-induced changes in neurogenesis that may influence behavior.
To examine the behavioral significance of stress-induced changes in hippocampal neurogenesis, we employed a social defeat stress paradigm that produces cohorts of mice that can be classified as “susceptible” or “unsusceptible” to stress (5). Susceptible mice show long-lasting social avoidance behavior and a depressive-like phenotype (anhedonia, social anxiety, heightened sensitization to psychostimulants, and circadian changes) that suggests a maladaptive phenotype (5). In contrast, unsusceptible mice are indistinguishable from controls in these measures and are interpreted as being resilient in the face of social adversity. Because both susceptible and unsusceptible mice experience the same degree of stress, it is not surprising that they both exhibit some similar stress-related physiological responses like elevated corticosterone (CORT).
Given the persistent changes in social behavior 1 month after social defeat stress (5, 12), we wondered whether the proliferation and survival of adult-born hippocampal neurons contributed to this phenotype. Here, we show that independent of susceptibility to stress, there is a robust and transient reduction in SGZ proliferation immediately after stress with no change in long-term survival of cells that were born before stress. However, examination of neuronal survival after cessation of stress reveals that susceptible mice have enhanced dentate gyrus neurogenesis. Moreover, ablation of neurogenesis shows that adult hippocampal neurogenesis is required for stress-induced social avoidance, highlighting a unique functional role for newly born neurons.
Results
Chronic Social Defeat Stress Leads to Specific Avoidance of a Potential Aggressor.
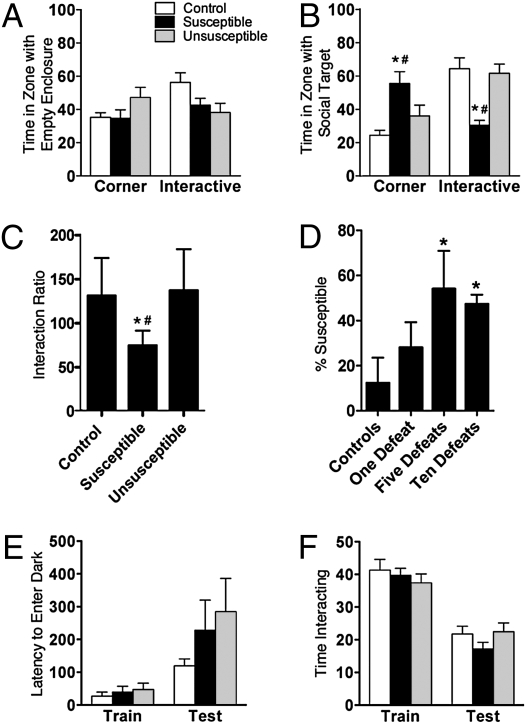
Mice were categorized as susceptible or unsusceptible to defeat stress based on the interaction ratio obtained from the social interaction test, as previously described (5, 12). Twenty-four hours after 10 days of social defeat, stressed (susceptible and unsusceptible) and control mice spent comparable time exploring the corners or interaction zone in the absence of a social target (Fig. 1A). However, in the presence of a social target, susceptible mice spent significantly more time in the corners and less time in the interaction zone (Fig. 1B). Thus, susceptible mice had a significantly reduced interaction ratio (ratio of time spent in interaction zone in the absence vs. presence of social target; Fig. 1C and Fig. S1). One day of defeat stress did not reduce the interaction ratio, but 5 or 10 days did (Fig. 1D). Because 10 days of defeat stress reliably produced ∼50% susceptible mice, this protocol was used for remaining studies. Moreover, because the susceptible phenotype occurs in 50% of the stressed populations, there was no significant difference in interaction ratio for the control vs. stressed (susceptible and unsusceptible combined) mice [t(41) = 1.43, P = not significant (ns); n = 10–33 mice per group].
Fig. 1.
Chronic social defeat stress produces specific social avoidance of an aggressor. (A) In the presence of an empty enclosure, time (sec) spent in corners opposite the interaction zone (F(2,37) = 1.8, P = ns) and interaction zone (F(2,37) = 2.6, P = ns). (B) In the presence of a social target, time spent in corners (F(2,37) = 4.8, P < 0.05) and interaction zone (F(2,37) = 7.5, P < 0.005). (C) Interaction ratio determined by proportion of time in presence vs. absence of social target (F(2,39) = 6.6, P < 0.005) (n = 18–31 mice per group in A–C). (D) Proportion of mice with a susceptible phenotype with an interaction ratio <100 after 1, 5, or 10 days of defeat stress (F(3,9) = 8.0, P < 0.05; n = 5–36 mice per group). (E) Passive avoidance performance expressed as latency (sec) to enter the dark punished compartment at training and testing (group: F(2,36) = 1.4, P = ns; time: F(1,36) = 11.4, P < 0.005; n = 6–8 mice per group). (F) Social behavior expressed as time (sec) interacting with a juvenile during the first exposure (training) and exposure to the same juvenile 3 days later (test) (group: F(2,84) = 0.7, P = ns; time: F(1,84) = 77.4, P < 0.001; n = 6–8 mice per group). *P < 0.05; #P < 0.05 compared with control and unsusceptible mice in a Bonferroni post hoc test, respectively (mean ± SEM).
We previously demonstrated that avoidance behavior is associated with depression- and anxiety-like behavioral abnormalities, and thus can be interpreted as maladaptive (5). The avoidance behavior also generalizes to nonaggressor mice and is reversed by antidepressants (5, 12). However, another interpretation is that avoidance is adaptive and may be associated with enhanced learning or memory to avoid a potential threat. We tested whether susceptible mice were different from controls in avoidance of a nonsocial adverse stimulus, social interaction, or memory. The lack of difference in passive avoidance behavior (Fig. 1E) suggested that susceptible mice do not have specific differences in avoidance of nonsocial stimuli or alterations in short-term memory. There was also no difference in social interaction between the groups as assessed in the juvenile interaction test (Fig. 1F). Thus, avoidance of the aggressor mouse is a specific behavior that occurs in susceptible mice in the presence of other maladaptive behaviors and does not generalize to deficits in avoidance, social interaction, or short-term memory.
Stress Transiently Reduces the Number of S-Phase SGZ Cells in Susceptible and Unsusceptible Mice.
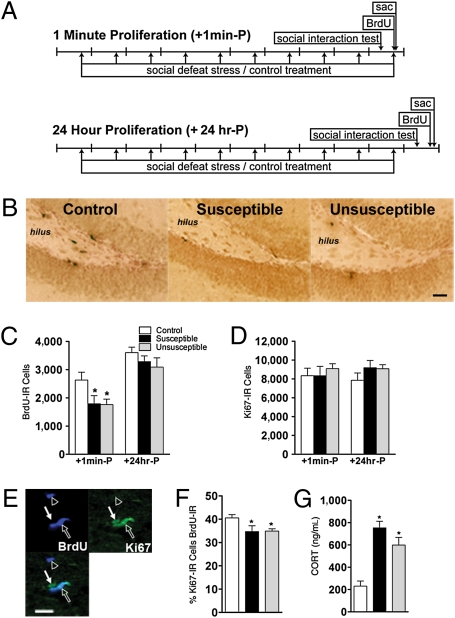
The number of cells in S-phase of the cell cycle in dentate gyrus subregions (Fig. S2) was examined by quantifying BrdU-immunoreactive (IR) cells immediately [+1 min-proliferation (P)] and 24 hr (+24 hr-P) after stress (Fig. 2A and SI Text). The frequency of susceptible mice was 52% and 44% for the +1 min-P and +24 hr-P groups, respectively (Fig. S3). Both susceptible and unsusceptible mice had fewer BrdU-IR SGZ cells immediately after stress, which normalized 24 hr later (Fig. 2 B and C). The reduction occurred across the septotemporal axis of the SGZ but not in other dentate gyrus regions (Figs. S2 and S4 A–D), emphasizing the restriction of this significant decrease to this neurogenic region. There was also no correlation between BrdU-IR cell number and interaction ratio in +1 min-P (r = 0.28, P = ns) or +24 hr-P (r = 0.07, P = ns) mice.
Fig. 2.
Defeat stress results in a transient decrease in the number of BrdU-IR cells regardless of susceptibility. (A) Schematic of study groups. The “+1 min-P” group received BrdU immediately after the last defeat episode (n = 9–18 mice per group). The “+24 hr-P” group received BrdU 24 hr after the last defeat episode (n = 7–12 mice per group). sac, sacrifice. (B) BrdU-IR cells in stressed mice (susceptible and unsusceptible) compared with control mice (+1 min-P). (Scale bar: 100 μm.) (C) BrdU-IR cell number in SGZ (F(2,54) = 4.3, P < 0.05). (D) Ki67-IR cell number in SGZ (F(2,51) = 0.9, P = ns). (E) IHC reveals BrdU-IR cells (open arrowhead), Ki67-IR cells (closed arrow), and cells that were both BrdU-IR and Ki67-IR (open arrow). (Scale bar: 25 μm.) (F) Percentage of Ki67-IR cells that are BrdU-IR in +1 min-P group (F(2,29) = 5.1, P < 0.05; n = 7–13 mice per group). (G) CORT levels in mice 30 min (+1 min-P) after last defeat stress (F(2,26) = 23.2, P < 0.001; n = 6–13 mice per group). *P < 0.05 compared with control mice in a Bonferroni post hoc test (mean ± SEM).
Although stress resulted in an immediate decrease in S-phase SGZ cells (Fig. 2C), there was surprisingly no difference in the total number of proliferating cells between groups as measured by Ki67-IR SGZ cell number (13, 14) (Fig. 2D). To assess if stress altered cell cycle dynamics, the colocalization of Ki67-IR and BrdU-IR cells was used to determine the percentage of total cycling cells that were in S-phase (13) (Fig. 2E). Susceptible and unsusceptible mice had a significantly lower proportion of cells in S-phase immediately after the last stress (Fig. 2F), an effect that was normalized 24 hr later (Fig. S4E). This suggests that the transient stress-induced decrease in BrdU-IR cell number reflects a decreased S-phase cohort but not a general decrease in the number of proliferating cells.
When CORT was assessed immediately after stress, there was a significant increase in CORT in both susceptible and unsusceptible mice (Fig. 2G). In agreement with the findings of other investigators (15), CORT was negatively correlated with BrdU-IR SGZ cell number (r = −0.43, P < 0.05 for control and stressed mice in +1 min-P). However, 24 hr after stress, CORT levels normalized in both susceptible and unsusceptible mice relative to controls (Fig. S4F). Thus, an increase in CORT was concurrent with fewer cells in S-phase immediately after stress irrespective of behavioral phenotype, and these changes did not distinguish the mice based on their susceptibly to stress.
Mice with Long-Term Susceptibility to Stress Have Enhanced Dentate Gyrus Neurogenesis.
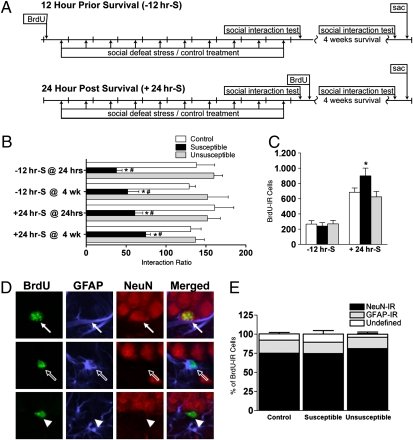
To determine if susceptibility to stress was related to neurogenesis, cell survival was measured for cells “born” before stress [BrdU given 12 hr before stress initiation (−12 hr-survival [S])] and cells born after 10 days of stress [BrdU given 24 hr after cessation of stress (+24 hr-S)] (Fig. 3A). Fifty-eight percent and 46% of the −12 hr-S and +24 hr-S mice were susceptible to defeat stress, respectively (Fig. 3B and Fig. S5). As with previous results (5), avoidance behavior in susceptible mice was stable and persistent, with a positive correlation between interaction ratios 24 hr and 4 weeks following stress (r = 0.5, P < 0.05; Fig. S5).
Fig. 3.
Increased neurogenesis in susceptible mice after cessation of stress. (A) Schematic of experiments in which “−12 hr-S” (Top) mice were given BrdU 12 hr before the first defeat episode (n = 7–14 mice per group). The “+24hr-S” mice (Bottom) were given BrdU 24 hr after the last defeat episode (n = 8–15 mice per group). Both groups were perfused 4 weeks after the last defeat stress. sac, sacrifice. (B) Interaction ratios 24 hr and 4 weeks after stress (group: F(2,97) = 24.8, P < 0.001; time: F(3,97) = 0.3, P = ns). (C) Number of surviving BrdU-IR cells in SGZ (F(1,39) = 61.7, P < 0.001). (D) BrdU-IR cells were classified as neurons [BrdU-IR (green) and NeuN-IR (red), closed arrow], glial [BrdU-IR (green) and GFAP-IR (blue), open arrow], or undefined (BrdU-IR only, arrowhead). (E) Phenotypic analysis of surviving BrdU-IR cells (F(2,81) = 228.3, P < 0.001; n = 7–15 mice per group). *P < 0.05; #P < 0.05 compared with control and unsusceptible mice in a Bonferroni post hoc test, respectively (mean ± SEM).
When S-phase cells were labeled with BrdU before initiation of stress (a time when BrdU labels equivalent number of cells in all groups) and examined 4 weeks after completion of the 10 days of defeat stress, there was no difference in surviving BrdU-IR cells (−12 hr-S; Fig. 3 A and C). In sharp contrast, when S-phase cells were labeled with BrdU 1 day after cessation of stress (at which time the stress-induced decrease in proliferation is normalized; Fig. 2), susceptible mice had significantly more surviving BrdU-IR cells 4 weeks later compared with both control and unsusceptible mice (+24 hr-S; Fig. 3 A and C). BrdU-IR cell number was also increased in the dentate gyrus molecular layer and trended toward an increase in the outer granule cell layer (Fig. S6). Differentiation was not grossly influenced, because over 75% of surviving BrdU-IR cells in stressed and control mice expressed the neuronal marker NeuN (Fig. 3 D and E).
Enhanced Dentate Gyrus Neurogenesis in Susceptible Mice Is Associated with Altered Number of Transient Amplifying Progenitors but Not with Altered BDNF Signaling or Cell Death.
One potential mechanism for the increased survival of adult-generated dentate gyrus neurons in susceptible mice is increased hippocampal levels of BDNF (16). Regional hippocampal dissections and subsequent immunoblotting revealed no change in protein levels between controls and either group of stressed mice for BDNF, its receptor TrkB (full length or truncated), or downstream signaling molecule ERK (Fig. S7). Thus, the increased neurogenesis in susceptible mice is not caused by grossly altered hippocampal BDNF signaling.
To determine if the enhanced survival of BrdU-IR cells was related to either cell death or the number of cells at the different stages of maturation at the time of BrdU labeling (+24 hr-P), both the number of activated caspase-3 (AC3-IR) SGZ cells and the phenotype of BrdU-IR cells were determined. There was no significant difference in AC3-IR cell number in +24 hr-P mice (susceptible: 227 ± 33, unsusceptible: 230 ± 31, control: 242 ± 28; F(2,17) = 0.07, P = ns; n = 5–7 mice per group). To assess the proportion of SGZ cells in distinct stages of neurogenesis (9, 17), BrdU-IR cells were phenotyped based on colocalization of BrdU with an immature neuron marker doublecortin (DCX) and GFP in nestin-GFP mice. Using morphology of DCX- and GFP-IR cells, four distinct cell types were identified that represent stages and a potential lineage of hippocampal neurogenesis (Fig. S8A, S9, and SI Text). Briefly, stem-like cells (Type-1: GFP+/DCX−, radial glial-like morphology) and early progenitors (Type-2a: GFP+/DCX−, compact morphology) give rise to late progenitors (Type-2b: GFP+/DCX+, compact morphology) and then to maturing progenitors/neuroblasts (Type-3: GFP−/DCX+). Susceptible and unsusceptible mice did not differ in the proportion of BrdU-IR cell that were Type-1 (Fig. S8B), in the number of Type-1 cells (Fig. S8C), or in the percentage of BrdU-IR cells that were Type-3 (mature progenitor; Fig. S8B). Notably, susceptible mice had a higher proportion of BrdU-IR cells that were Type-2a and a lower proportion that were Type-2b (Fig. S8B). These data suggest that the enhanced neurogenesis in susceptible mice is, in part, attributable to greater numbers of transient-amplifying progenitors immediately after stress.
X-Ray Irradiation Before Social Defeat Attenuates the Percentage of Mice that Have a Susceptible Phenotype.
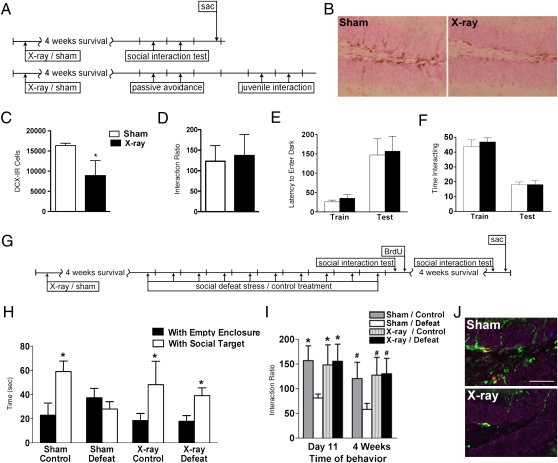
To explore the hypothesis that enhanced neurogenesis might contribute to behavioral susceptibility to stress, we ablated neurogenesis via cranial x-ray irradiation 4 weeks before social defeat and assessed effects on behaviors relevant to social interaction and short-term memory (Fig. 4A). Irradiated mice displaying severe deficits in BrdU-IR, Ki67-IR, and DCX-IR cells were compared with controls (Fig. 4 B and C), as previously shown (18). X-ray irradiated mice displayed no significant differences in social avoidance, passive avoidance, or juvenile interaction behavior (Fig. 4 A and D–F). Thus, cranial irradiation reduced SGZ proliferation and neurogenesis without overt changes in key behavioral measures.
Fig. 4.
Ablation of neurogenesis before stress reduces susceptibility to social avoidance behavior. (A) Mice received 5-Gy x-ray or sham treatment 4 weeks before assessment of social interaction and number of BrdU-IR cells present 2 hr following injection (n = 6 mice per group) (Top) or passive avoidance and juvenile interaction testing (n = 12 mice per group) (Bottom). sac, sacrifice. (B) DCX-IR cells in the dentate gyrus of sham and x-ray-irradiated mice 4 weeks postirradiation. (C) DCX-IR cell number in SGZ (t(8) = 4.4, P < 0.01). (D) Social interaction analysis expressed as interaction ratio (t(9) = 0.5, P = ns). (E) Passive avoidance performance expressed as latency (sec) to enter the dark punished compartment at training and testing (group: F(1,44) = 0.1, P = ns; time: F(1,44) = 17.8, P < 0.001). (F) Social behavior expressed as time (sec) interacting with a juvenile during the first exposure (training) and exposure to the same juvenile 3 days later (test) (group: F(1,44) = 0.3, P = ns; time: F(1,44) = 78.4, P < 0.001). (G) Mice received 5-Gy x-ray or sham treatment 28 days before 10 days of social defeat (n = 8–11 mice per group). (H) Time in interaction zone during social interaction testing completed 24 hr (day 11) following 10 days of social defeat stress in the presence of either the empty enclosure or a social target (main effect group: F(1,61) = 8.3, P < 0.01; main effect with/without: F(3, 61) = 0.59, P = ns; group with/without: F(3,61) = 2.2, P = ns). (I) Interaction ratio from social interaction testing completed 24 hr (day 11) and 4 weeks following 10 days of defeat stress (F(3,61) = 3.2, P < 0.05). (J) DCX-IR (green), Ki67-IR (red), and BrdU-IR (blue) cells 4 weeks after sham or x-ray exposure. (Scale bar: 50 μm.) *P < 0.05 compared with sham/defeat day 11 mice; #P < 0.05 compared with control 4-week mice in a Bonferroni post hoc test (mean ± SEM).
Sham- and x-irradiated mice underwent 10 days of social defeat stress and were tested for social interaction 1 day or 4 weeks later (Fig. 4G). Surprisingly, irradiated mice were unsusceptible to defeat stress and did not display social avoidance, resulting in a significantly increased interaction ratio when compared with sham-irradiated defeated mice (Fig. 4 H and I). The percentage of irradiated mice that displayed social avoidance following defeat stress was 25% and 37% at day 11 and 4 weeks, respectively. Importantly, control nondefeated mice that were sham- or x-ray irradiated had no significant difference in interaction ratios, supporting our previous finding that x-ray irradiation did not alter social interaction behavior (Fig. 4 D, H, and I). Four weeks after defeat, irradiated mice had very few SGZ cells labeled with BrdU, Ki67, or DCX (Fig. 4J) and had significantly fewer BrdU-IR cells surviving 4 weeks later compared with sham-irradiated mice (sham/defeat: 249 ± 42, x-ray irradiated/defeat: 23 ± 9; t(10) = 4.4, P < 0.005; n = 5–7 mice per group). Thus, ablation of adult neurogenesis via x-ray irradiation decreased the proportion of mice susceptible to stress-induced avoidance.
Discussion
The social defeat paradigm is a robust model of stress-induced social avoidance that produces multiple features of stress susceptibility in approximately one-half of subjects (5). As such, it is an ideal model in which to probe the neural mechanisms contributing to an organism’s response to chronic severe stress. Here, we explore the potential links between adult hippocampal neurogenesis and the “memory” of severe stress. It is well known that stress decreases SGZ proliferation, and we show that social defeat stress significantly but transiently reduces SGZ proliferation immediately after the last stress independent of a mouse's susceptibility. Surprisingly, we show that mice susceptible to defeat stress have significantly enhanced survival of dentate gyrus neurons that were generated after but not before defeat stress compared with either unsusceptible mice or controls. Thus, increased hippocampal neurogenesis is related to the long-term maintenance of social avoidance. When neurogenesis was ablated by x-ray irradiation, significantly fewer mice displayed social avoidance. Thus, hippocampal neurogenesis appears to be involved in the persistent social avoidance behavior. These data suggest that a compensatory enhancement in hippocampal neurogenesis is related to the long-term individual differences in maladaptive responses to stress.
Stress is generally associated with decreased SGZ proliferation, leading to decreased survival and neurogenesis (1, 6). Our current results concur with the preponderance of literature demonstrating decreased cell proliferation during or immediately after stress (e.g., 10, 19, 20) and with reports of no change in survival when cells are labeled with BrdU before stress (21). In contrast, when we label cells with BrdU 1 day after stress, we find enhanced cell survival, mirroring the enhanced survival seen postischemia (e.g., 22). Importantly, we find that enhanced neuronal survival is associated with sustained behavioral alterations poststress: The number of surviving BrdU-IR cells is increased only in mice with persistent social avoidance. When we analyze all defeated mice in this study, regardless of susceptibility to stress, there is no difference in BrdU-IR cell survival postdefeat. The lack of decrease in neurogenesis poststress between stressed and control mice is consistent with no change in cell survival 1 day after chronic unpredictable stress in rats (21) and the normal granule cell layer volume reported in many stress studies (23–25). However, by analyzing the susceptibility to stress through behavioral outcomes, we have uncovered a striking significant increase in surviving BrdU-IR cells in mice specifically susceptible to stress. These findings urge the use of a similar behavioral analysis in other stress models to clarify the neuroadaptations associated with the diverse behavioral coping mechanisms used after stress.
Mechanistically, we hypothesized that the increased cell survival in susceptible mice might be mediated by BDNF because this neurotrophic factor is linked to enhanced dentate gyrus neurogenesis (16) and plays a critical role in the nucleus accumbens of susceptible mice (5). However, hippocampal levels of key components of BDNF signaling were not different from those in controls at any time point postdefeat. These data highlight the regional specificity of BDNF actions within the nucleus accumbens following defeat. Because few factors are known that alter the survival of adult-generated hippocampal neurons independent of changes in proliferation, ongoing studies with gene profiling will likely provide previously undescribed mechanisms that could account for increased survival in mice susceptible to defeat stress.
Unlike the specific increase in neurogenesis observed solely in mice susceptible to stress, there was a transient decrease in BrdU-IR cell number immediately postdefeat in all mice (either susceptible or unsusceptible to stress). This decrease was restricted to the neurogenic SGZ, suggesting a region-specific mechanism for this regulation. This finding agrees with most of the literature showing fewer BrdU-IR cells immediately after chronic stress paradigms (e.g., 23, 24, 26, 27). The reduction in BrdU-IR cell number immediately after stress does not generalize to an overall decrease in the total number of proliferating cells, as assessed by immunoreactivity of the endogenous cell cycle protein Ki67. Although BrdU-IR and Ki67-IR cell numbers are often used as interchangeable indices of proliferation (14, 28), our current data and other work (29) caution against this, because cells in discrete phases of the cell cycle can be differentially influenced by stimuli (13, 30) and cells in S-phase may be particularly susceptible to certain stimuli (e.g., 31). Future work is warranted to explore the hypothesis that the decrease in BrdU-IR cell number without a corresponding decrease in Ki67 cell number could result from selective cell death of S-phase cells and/or a lack of movement of cells into S-phase as a result of local signals (e.g., 31). Our further analysis of BrdU-IR cells in nestin-GFP mice revealed that susceptible mice had a specific increase in the proportion of BrdU-IR cells that were early progenitors (Type-2a) compared with unsusceptible mice 1 day poststress. A reasonable hypothesis to test in the future is that the increased proportion in the putatively “younger” Type-2a cells with a decrease in “older” Type-2b cells may reflect a stalling of some Type-2a cells in this early stage of development and may result in a long-term and near-exponential decrease in neurogenesis. Although nestin-GFP mice have been used to determine the neurogenic stage affected by stimuli like running or drugs of abuse (e.g., 9, 31), the current data reveal how stress affects different stages of dentate gyrus neurogenesis. Because the populations of stem-like Type-1 and mature progenitor Type-3 cells appear to be largely unaffected by chronic stress, our data are consistent with previous work that manipulation of the rapidly dividing Type-2 population is a prime and robust way to modulate neurogenesis (9, 31).
Ablation of neurogenesis before social defeat inhibited social avoidance, which could be interpreted as an antidepressant-like effect (12). It seems counterintuitive that both antidepressants, which enhance neurogenesis, and ablation of neurogenesis inhibit stress-induced social avoidance (5). Perhaps the effect of antidepressants on social avoidance is mediated via other neural circuits (12). Alternatively, regulation of neurogenesis may be required for hippocampal-based plasticity, whether adaptive or maladaptive in nature. Social avoidance behavior appears to be mediated by situational encoding of information, because avoidance occurs in the presence of an awake but not anesthetized aggressor mouse (5, 12). The avoidance behavior in susceptible mice is also specific to potentially threatening stimuli, because avoidance did not generalize to passive avoidance, social interaction, or short-term memory. A role for hippocampal neurogenesis and learning and memory in stress-induced social avoidance is consistent with the naturally important role of strong memory formation in the presence of a threatening stimulus (32). However, stress does not consistently enhance learning and memory, which is likely attributable to the various types and durations of stress as well as its predictability (33).
We show that ablating neurogenesis via x-ray irradiation decreased social avoidance in defeated mice but did not change basal levels of social interaction in nondefeated controls. Although our data agree with work showing that ablation of neurogenesis does not alter behavioral measures associated with memory (34–37), they uniquely suggest that alterations in hippocampal neurogenesis provide the synaptic plasticity required for social avoidance. Our work also highlights the fact that the period after cessation of stress is a critical period for the establishment of persistent cellular and behavioral responses to stress, and thus is likely amenable to therapeutical intervention.
Methods
Mice, Social Defeat, and Social Interaction Protocol.
Adult (5–8 weeks of age) male mice expressing GFP under the control of the nestin promoter (17) were the subjects, and CD1 retired breeders (Charles River) were the aggressors. Social defeat (SI Text) was performed daily for 10 days similar to protocols used in previously published reports (5, 12, 38). Social interaction testing (SI Text) comprised two trials for 150 sec each (5, 12, 38). Briefly, for interaction testing, in the first trial, a mouse is placed into an open-field box and allowed to explore a plastic enclosure placed within the predefined interaction zone. In the second trial, the mouse is returned to the open-field arena containing a plastic enclosure now holding an awake CD1 aggressor mouse. An interaction score is calculated for each mouse (ratio of time spent in the interaction zone with the aggressor mouse present to time spent in interaction zone without an aggressor present). Control mice tend to interact with a social target and generally have an interaction ratio >100; susceptible mice are defined as mice having undergone social defeat with an interaction ratio <100.
Ablation of Neurogenesis Using X-Ray Irradiation.
Ionizing radiation (SI Text) was carried out using the X-RAD 320 self-contained irradiation system (Precision X-Ray, Inc.).
BrdU Injection, Tissue Collection, and Processing.
Mice received an i.p. injection of BrdU (150 mg/kg; Boehringer Mannheim) before being euthanized to label proliferating cells in the S-phase of the cell cycle. The time between BrdU injection and death varied across experimental groups (+1 min-P, +24 hr-P, −12 hr-S, +24 hr-S; Fig. 3A, Fig. 4A, and SI Text). Using stereological assessment (39, 40), BrdU-, Ki67-, DCX-, and GFP-IR cells were counted in every ninth section of the hippocampus at 400× magnification (SI Text). Colocalization of fluorescence was determined via confocal microscopy (630× magnification) using multitrack scanning. Colocalization was verified by 3D reconstruction (SI Text).
Supplementary Material
Acknowledgments
We thank Anita Autry, Kerstin Ure, Keren Leviel Kumar, and Mojgan Siahbazi for excellent technical assistance. This work was supported by National Institutes of Health Grants R01 DA016765, R21 DA023701, and K02 DA023555 (to A.J.E.), P50 MH66172 (to E.J.N.), F31 MH754572 (to M.H.D.), T32 DA007290 (to A.J.E. and N.A.D.) and F31 NS064632 (to N.A.D.); and by the National Aeronautics and Space Administration (to A.J.E.), by National Alliance for Research on Schizophrenia and Depression Young Investigator Awards (to O.B. and D.C.L.), and by a postdoctoral research fellowship from the Canadian Institute of Health Research (to D.C.L.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910072107/DCSupplemental.
References
- 1.Joëls M, Karst H, Krugers HJ, Lucassen PJ. Chronic stress: Implications for neuronal morphology, function and neurogenesis. Front Neuroendocrinol. 2007;28:72–96. doi: 10.1016/j.yfrne.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 3.Yehuda R, LeDoux J. Response variation following trauma: A translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Charney DS, Manji HK. Life stress, genes, and depression: Multiple pathways lead to increased risk and new opportunities for intervention. Sci STKE. 2004;2004:re5. doi: 10.1126/stke.2252004re5. [DOI] [PubMed] [Google Scholar]
- 5.Krishnan V, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: A convergence of mechanisms. Neuropsychopharmacology. 2007;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 7.Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: Reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- 8.Vollmayr B, Simonis C, Weber S, Gass P, Henn F. Reduced cell proliferation in the dentate gyrus is not correlated with the development of learned helplessness. Biol Psychiatry. 2003;54:1035–1040. doi: 10.1016/s0006-3223(03)00527-4. [DOI] [PubMed] [Google Scholar]
- 9.Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 11.Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 12.Berton O, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 13.Eisch AJ, Mandyam CD. Adult neurogenesis: Can analysis of cell cycle proteins move us “Beyond BrdU”? Curr Pharm Biotechnol. 2007;8:147–165. doi: 10.2174/138920107780906540. [DOI] [PubMed] [Google Scholar]
- 14.Kee N, Sivalingam S, Boonstra R, Wojtowicz JM. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods. 2002;115:97–105. doi: 10.1016/s0165-0270(02)00007-9. [DOI] [PubMed] [Google Scholar]
- 15.Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 16.Sairanen M, Lucas G, Ernfors P, Castrén M, Castrén E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamaguchi M, Saito H, Suzuki M, Mori K. Visualization of neurogenesis in the central nervous system using nestin promoter-GFP transgenic mice. NeuroReport. 2000;11:1991–1996. doi: 10.1097/00001756-200006260-00037. [DOI] [PubMed] [Google Scholar]
- 18.Wojtowicz JM. Irradiation as an experimental tool in studies of adult neurogenesis. Hippocampus. 2006;16:261–266. doi: 10.1002/hipo.20158. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- 20.Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- 21.Heine VM, Maslam S, Zareno J, Joëls M, Lucassen PJ. Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. Eur J Neurosci. 2004;19:131–144. doi: 10.1046/j.1460-9568.2003.03100.x. [DOI] [PubMed] [Google Scholar]
- 22.Jin K, et al. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Czéh B, et al. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci USA. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yap JJ, et al. Repeated brief social defeat episodes in mice: Effects on cell proliferation in the dentate gyrus. Behav Brain Res. 2006;172:344–350. doi: 10.1016/j.bbr.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 25.Jayatissa MN, Bisgaard C, Tingström A, Papp M, Wiborg O. Hippocampal cytogenesis correlates to escitalopram-mediated recovery in a chronic mild stress rat model of depression. Neuropsychopharmacology. 2006;31:2395–2404. doi: 10.1038/sj.npp.1301041. [DOI] [PubMed] [Google Scholar]
- 26.Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon M, Czéh B, Fuchs E. Age-dependent susceptibility of adult hippocampal cell proliferation to chronic psychosocial stress. Brain Res. 2005;1049:244–248. doi: 10.1016/j.brainres.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Wojtowicz JM, Kee N. BrdU assay for neurogenesis in rodents. Nat Protoc. 2006;1:1399–1405. doi: 10.1038/nprot.2006.224. [DOI] [PubMed] [Google Scholar]
- 29.Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: Paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Arguello AA, et al. Time course of morphine's effects on adult hippocampal subgranular zone reveals preferential inhibition of cells in S phase of the cell cycle and a subpopulation of immature neurons. Neuroscience. 2008;157:70–79. doi: 10.1016/j.neuroscience.2008.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shors TJ. Stressful experience and learning across the lifespan. Annu Rev Psychol. 2006;57:55–85. doi: 10.1146/annurev.psych.57.102904.190205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandi C, Pinelo-Nava MT. Stress and memory: Behavioral effects and neurobiological mechanisms. Neural Plast. 2007;2007:1–20. doi: 10.1155/2007/78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Airan RD, et al. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- 35.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 36.Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Surget A, et al. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry. 2008;64:291–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 38.Tsankova NM, et al. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 39.Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci USA. 2000;97:7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.