Abstract
The idea that sensitivity to self-produced motion could lie at the foundations of the clear-cut divide that the brain operates between the two basic domains of inanimate and animate objects dates back to Aristotle. Sensitivity to self-propelled objects is apparent in human infants from around the fifth month of age, which leaves undetermined whether it is acquired by experience with animate objects or whether it is innately predisposed in the brain. Here, we report that newly hatched, visually naïve domestic chicks presented with objects exhibiting motion either self-produced or caused by physical contact prefer to associate with self-propelled objects. This finding supports the idea of an evolutionarily ancient, predisposed neural mechanism in the vertebrate brain for the detection of animacy.
Keywords: animated object, self-propelled motion, filial imprinting, predisposition
“Of the proper subjects of motion some are moved by themselves and others by something not themselves, and some have a movement natural to themselves and others have a movement forced upon them which is not natural to them. Thus the self-moved has a natural motion. Take, for instance, any animal: the animal moves itself, and we call every movement natural, the principle of which is internal to the body in motion.”
Aristotle, Physics (vol. V, p. 307)
Self-produced motion provides one of the most powerful cues about what makes an object “animate”—i.e., a type of object distinct from one that can be put into motion only as a result of physical contact (1–6). This idea dates back to at least Aristotle (Physics) (7), and it has been incorporated, with some important specification, into developmental psychology doctrine (8, 9). Developmental studies have shown that at a young age infants know that stationary objects start to move if, and only if, they are contacted by another moving object unless provided with an inner mechanism that permits self-produced motion (10). Current research, however, distinguishes between representations of animacy (entities that are capable of self-propelled motion and of taking on the role of mechanical/causal agent) and representations of intentional agency (entities with goals, attentional states, capable of perception and mental states like beliefs and desires). It is now recognized that self-propulsion is not a sufficient cue for intentional agency detection (11–13).
Here, we shall be concerned only with self-produced motion as a pure animacy cue [i.e., with causal/mechanical agency, or the presence of an internal force of action (14)].
We address two issues: First, does the basic distinction between inert and self-propelled objects also hold true in nonhuman animal species? Second, does such a distinction emerge as a result of learning from experience of the world or is it rather part of an animal’s innate representational repertoire?
From previous research in nonhuman primates it remains unclear as to what role self-propelled motion and animate/inanimate objects play in forming an expectation about an object’s potential capacity to change its spatial location (15). Such research employed adult animals and was not aimed at testing the role of past experiences. This issue could be definitively addressed with controlled-rearing experiments on newborn subjects. To this purpose we used a methodology that takes advantage of the phenomenon of filial imprinting, a learning process by which the young of some animal species learn the characteristics of an object—usually a social partner—when exposed to it for a short time soon after hatching (16–18). The newly hatched domestic chicken (Gallus gallus) was used as our animal model (19–21) because it is a precocial species, which allows for the rigorous control of sensory experiences. Chicks were exposed to computer-presented animation sequences picturing two oval-shaped objects of different color to which motion could be attributed either a causal-agentive role (i.e., the object appeared as self-propelled) or a receptive role (i.e., the object appeared as moved by an external force). After exposure, chicks were tested for their spontaneous preference between the two objects.
Results
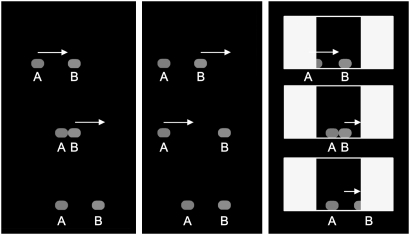
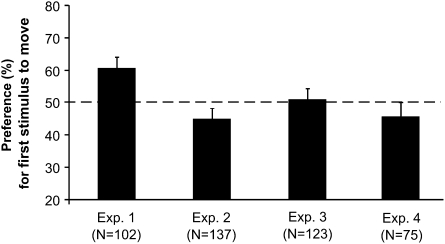
In Experiment 1, chicks saw the two objects performing the classical example of Michotte's perceived physical causality (22)—i.e., the launching effect. The stimuli employed were similar to those used to test for perception of causality in 7-month-old infants (14). In the animation sequence, one object (A) moved toward a second, stationary object (B). Immediately after contact, object B started to move along the same direction as A, while object A became stationary. Object B moved with identical speed and covered the same distance that had been traveled by object A (Fig. 1). In this sort of display, human (22) and nonhuman animals (23) perceive object A as being a “self-propelled causal agent” launching object B and causing its movement. When tested for their preferences for objects A and B (see Materials and Methods for details of the stimuli used at test), we found that chicks showed a clear preference for object A (Fig. 2), the self-propelled object playing the causal-agentive role during the exposure phase (mean ± SEM = 60.700 ± 3.294, one-sample two-tailed t test, t101 = 3.248, P = 0.002).
Fig. 1.
Schematic representation of the animation sequences used during exposure. In Experiment 1 (left-most sequence), the classical launching effect (22) was reproduced: To humans the sequence appears as object A being a “self-propelled causal agent” hitting object B and causing its movement. In Experiment 2 (central sequence), the order of movement was swapped: The sequence appears as the independent motion of two objects, both self-propelled. In Experiment 3, chicks were imprinted onto a sequence identical to that used in Experiment 1 except for the presence of a 3-sec delay between the objects contacting one another and the start of motion of object B. In Experiment 4 (right-most sequence), no cues were available about the nature of motion of object A because of the presence of occluding screens, although the causality of the launching effect was preserved. Note that for reasons of comprehension (and differently from the left-most and central sequences) the top and bottom images of the right-most sequence do not represent the first frame and the last frame of the video.
Fig. 2.
Overall results. Average percentage of time (mean ± SEM) spent by the chicks near the first stimulus to move. The dotted line represents chance level (50%).
To check whether the perceived causality was crucial for the results obtained in Experiment 1, the order of the displacements was swapped temporally in Experiment 2: thus, object B moved first and object A started its movement only after object B had stopped. In this new animation sequence, any physical causality between the movements of the two objects was disrupted (no contact between A and B), whereas distances traveled and perceptual features of the two objects were identical to those of the launching effect. Both objects would thus appear to be self-propelled. At test, no significant preference was shown by chicks for either object [mean ± SEM = 44.999 ± 3.103, one-sample two-tailed t test, t136 = −1.611, P = 0.109 (Fig. 2)].
Results of Experiment 1 could not, therefore, be due to a preference for the stimulus that moved first in the animation because no preference for stimulus B (which moved first) was apparent in Experiment 2. However, given that in Experiment 2 any physical contact was removed, we wondered whether chicks’ preferences in Experiment 1 could be accounted for in terms of which object applied physical contact over the other object, which perhaps may have acted as a cue of “causal agency.” To determine this, chicks were imprinted onto a noncausal physical animation in Experiment 3. The stimulus sequence was identical to the launching effect used in Experiment 1 except for the presence of a 3-sec delay between the time of contact and the motion of B. In human subjects, the presence of a delay is known to abolish any impression of physical causality (22): object B appeared in this case as being self-propelled, as was object A. Moreover, it has been recently demonstrated that human infants as young as 6 months of life expect an object to be self-propelled when it resists moving after having been hit (24). Using those kind of stimuli at test, chicks did not exhibit any preference for either stimulus [mean ± SEM = 51.002 ± 3.298, one-sample two-tailed t test, t122 = 0.304, P = 0.762 (Fig. 2)].
It remained unclear, however, whether the results of Experiment 1 were due to a preference for the self-propelled stimulus or to a preference for the object that was the “cause” of the motion sequence. To determine this, chicks were exposed in Experiment 4 to a video animation identical to the one used in Experiment 1 except for the presence of two opaque screens, one of which occluded the object at the beginning of the motion sequence and the other at the end of the motion sequence. In this way, no cues were available about the self-/not-self-propelled nature of object A, although it continued to be perceivable as the cause of motion of object B. At test, chicks did not show any significant preference for either object [mean ± SEM = 45.796 ± 4.330, one-sample two-tailed t test, t74 = −0.971, P = 0.335 (Fig. 2)].
The analysis of variance (ANOVA) revealed a significant heterogeneity between chicks’ preferences in the four experiments (F3,423 = 5.218, P = 0.002). No other significant main effects (test stimuli: F1,423 = 3.170, P = 0.076; color: F1,423 = 0.427, P = 0.514) or interactions (Experiment × Type of stimuli: F2,423 = 0.225, P = 0.798; Experiment × Color: F3,423 = 1.558, P = 0.199; Type of stimuli × Color: F1,423 = 0.001, P = 0.973; Experiment × Type of stimuli × Color: F2,423 = 2.829, P = 0.060) were observed. The heterogeneity was due to preferences expressed by the chicks in Experiment 1: when excluding this experiment, the ANOVA did not show any significant heterogeneity between the remaining three experiments (F2,325 = 1.994, P = 0.138). Preferences in Experiment 1 were significantly different from those observed in Experiment 2 (t237 = 3.425, P = 0.001), Experiment 3 (t223 = 2.062, P = 0.040), and Experiment 4 (t175 = 2.790, P = 0.006).
Discussion
The results of the experiments showed that the color of the two stimuli and the first vs. second stimulus to move during the exposure phase were immaterial with respect to the chicks’ subsequent preference at test. Only when one of the two objects appeared as being self-propelled and the other not self-propelled did a preference emerge, as a choice for the self-propelled stimulus. Physical contact, which was not accompanied by physical causation (Experiment 3), or physical causation without any cues about the nature of the motion of “the causal object” (Experiment 4), sufficed to abolish any preference, showing that physical contact in itself, when both objects appeared as being self-propelled, or causation in itself, when no cue was given about the nature of “the causal object” (self-propelled/not self-propelled), were not capable of producing any preference.
Subjects came from a dark incubator and hatchery; hence, they had not had any chance to be exposed visually to the motion of animate objects, except for the controlled imprinting–exposure phase. During this phase, the total amount of exposure to both objects was the same; thus, the preference for the self-propelled object cannot be accounted for in terms of any specific learning.
The results thus demonstrate that newly hatched chicks show an innate sensitivity to differentiate and prefer a self-propelled causal agent as a target for imprinting. The minimal interpretation of the finding is that chicks are innately sensitive to self-propulsion as a crucial cue to animacy [or mechanical agency in the sense of having internal cause—“force”—of action (14)]. These data may even suggest that chicks are sensitive to contact causality (because they consistently preferred the object acting as causal self-propelled agent). Thus, there may be at least two aspects—i.e., self-propulsion and contact causality—innately represented by chicks as a cue to animacy. Those capabilities may be sufficient to adaptively constrain the early commitment of a highly precocial animal as to what to imprint on. It remains of course to be determined whether chicks are also innately endowed with a sensitivity to other movement properties, such as efficiency of goal approach [as humans and adult primates seem to be (25)], that are cues to intentional agency.
Our findings are compatible with the idea that many vertebrate species, including humans, have primitive neural pathways that ensure a bias to attend toward, or preferentially process, sensory information about other living entities. For example, newly hatched chicks and newborn humans attend to patterns that correspond to the head region of their likely caregivers (26, 27) and to the general biomechanical characteristics of vertebrate motion irrespective of the species (28, 29). The possibility of performing controlled-rearing studies with animals (30) and the generality of the underlying basic mechanisms in different species (31–33) opens the door to direct investigation of their neural and genetic bases.
Materials and Methods
Subjects were domestic chicks (Gallus gallus) hatched in the laboratory. Fertilized eggs were obtained from a local commercial hatchery (Agricola Berica, Montegalda, Vicenza, Italy). On arrival, eggs were placed in an MG 70/100 incubator (45 × 58 × 43 cm, 100-egg capacity) until day 19 of incubation. Temperature was maintained at 37.5 °C and humidity was maintained at 55–60%, providing standard conditions for optimal incubation. From day 19, the eggs were placed in a hatchery (60 × 65 × 66 cm) with the same temperature as the incubator, but at a lower humidity, ideal conditions for hatching. The incubator, the hatchery, and the hatching room were maintained in complete darkness.
Imprinting stimuli consisted of computer-presented animation sequences featuring two oval objects of the same size (width = 3.5 cm, height = 2.5 cm) but of different color [red: RGB = r255, g0, b0, with alpha = 100%; purple: RGB = r255, g0, b255, with alpha = 100% (Fig. 1)]. Some chicks (n = 222; Experiment 1, n = 56; Experiment 2, n = 64; Experiment 3, n = 60; Experiment 4, n = 42) were exposed to A-red and B-purple; the remaining chicks (n = 215; Experiment 1, n = 46; Experiment 2, n = 73; Experiment 3, n = 63; Experiment 4, n = 33) were exposed to A-purple and B-red. Animation sequences were generated by “Flash® 8” software. Animations of Experiments 1, 2, and 4 lasted 8 sec (480 frames; 60 frames/sec) whereas animations of Experiment 3 lasted 11 sec (660 frames; 60 frames/sec); each object in the animation covered a distance of 6.5 cm at 4.3 cm/sec and maintained both its starting and final position for 2 sec. At the end of the sequence, a black screen appeared for 1 sec before the animation restarted. Videos were produced by looping a 60-frames/sec animation and saved with 75 quality AVI. Stimuli were presented on a Samsung SyncMaster 931BF (LCD, 19 inches) screen.
The imprinting apparatus consisted of a set of eight clear-fronted plywood boxes (10 × 10 cm), each housing one chick. The imprinting animation was shown on a computer screen placed 40 cm away, with the center of the stimulus coaxial to the center of the imprinting apparatus to guarantee an appropriate view to all of the chicks. The computer screen was placed at the end of a black-plastic tunnel to prevent chicks from seeing any other visual stimulus. Apart from the light arising from the monitor, the room was maintained in complete darkness.
The test apparatus consisted of a white plywood runway (75 × 20 × 30 cm), with the testing stimuli presented at its opposite ends.
Two different pairs of test stimuli were employed. The first pair consisted of two animations, each reproducing either object A or object B continuously moving back and forth (moving 1.5 cm in either direction) on a black background. Although chicks saw the stimuli when they were already set in motion (thus preventing a possible cue to self-propelling), the moving back should be seen as self-propelled (24) rather than as physical bouncing (although the latter was the impression reported by adult human observers). If both objects were perceived as being self-propelled at test, one could wonder which of the two effects underlies the chicks’ preference in Experiment 1: imprinting on the object whose motion is self-propelled or aversion to the object that behaves in a novel manner [i.e., whose motion changed from passive (during imprinting) to self-propelled (during the test); it could be that chicks are equally drawn to the two objects during the imprinting session but subsequently avoid an object that changes its motion at test]. In either case, there would be evidence for sensitivity to self-propelled motion. However, because it is only the first interpretation that shows that chicks preferentially imprint onto self-propelled objects, another pair of test stimuli was used, in which the objects appeared and disappeared from behind two side partitions (spaced 6.5 cm apart). In this stimulus pair, object A (or B) moved along one direction (i.e., the same one experienced by the chick during exposure). By using these screens, the nature of the motion (self-propelled vs. not self-propelled) of the two objects remained undetermined and any preference for either object would be due to only the role they played in the imprinting animation sequence (note that, in the case of a stimulus moving unidirectionally, human infants attribute to it a self-propelled nature only if its trajectory is completely visible).
Both pairs of test stimuli were used in Experiments 1, 2, and 3 (with separate groups of chicks), whereas only stimuli with occluded trajectory were used in Experiment 4 (this was done because the first three experiments did not reveal any statistically significant effect of the type of test stimuli used).
The animation sequences were produced by looping a 60-frames/sec animation and saved with 75 quality AVI, presented on a Samsung SyncMaster 931BF (LCD, 19 inches) screen in a completely dark room.
On day 1 of life, in the early morning, chicks were taken from the dark hatchery in a closed cardboard box and placed individually in each imprinting box, where they were exposed to the imprinting stimulus. After ∼90 min of continuous exposure, each chick was taken from its box and placed in the central area of the test apparatus, which was subdivided virtually into a middle (15 cm long) and two side areas (each one 30 cm long), with the two monitors placed at its opposite ends. The chicks’ position at the starting point, as well as the position of the two stimuli, was balanced across animals. Chicks’ behavior was observed for a total of 6 consecutive minutes. Permanence of the chick in the mid-compartment was assumed to indicate no choice, whereas movement of the chick to one of the end-side compartments was regarded as a preference for the object presented at that end. Time (in seconds) spent by the chick in each of the two side areas was scored on-line by an experimenter blind to the purposes of the research.
Acknowledgments
The experiments comply with the current Italian (Ministero della Sanità, Dipartimento Alimenti, Nutrizione e Sanità Pubblica Veterinaria-Ufficio X°) and European Community laws for the ethical treatment of animals. We thank Susan Carey, Lesley J. Rogers, and Elizabeth Spelke for reading the manuscript and providing helpful comments, and Elena Lo Sterzo for invaluable help provided with chick testing and care. This work is part of the project Evolution and Development of Cognitive, Behavioural, and Neural Lateralisation—2006–2009, supported by the Commission of the European Communities within the framework of the specific research and technological development program “Integrating and strengthening the European Research Area” (initiative “What it means to be Human”), through a financial grant to L.R. and G.V.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Caramazza A, Shelton J-R. Domain-specific knowledge systems in the brain: the animate-inanimate distinction. J Cogn Neurosci. 1998;10:1–34. doi: 10.1162/089892998563752. [DOI] [PubMed] [Google Scholar]
- 2.New J, Cosmides L, Tooby J. Category-specific attention for animals reflects ancestral priorities, not expertise. Proc Natl Acad Sci USA. 2007;104:16598–16603. doi: 10.1073/pnas.0703913104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woodward A-L, Phillips A, Spelke E-S. Proceedings of the Fifteenth Annual Meeting of the Cognitive Science Society. Hillsdale, NJ: Erlbaum; 1993. pp. 1087–1091. [Google Scholar]
- 4.Ellsworth C-P, Muir D-W, Hains S-M. Social competence and person–object differentiation: an analysis of the still-face effect. Dev Psychol. 1993;29:63–73. [Google Scholar]
- 5.Mandler J-M. Perceptual and conceptual processes in infancy. J Cogn Dev. 2000;1:3–36. [Google Scholar]
- 6.Molina M, Van de Walle G-A, Condry K, Spelke E-S. The animate-inanimate distinction in infancy: developing sensitivity to constraints on human actions. J Cogn Dev. 2004;5:399–426. [Google Scholar]
- 7.Aristotle . The Physics. Cambridge, MA: Harvard Univ Press; 1980. [Google Scholar]
- 8.Premack D. The infant's theory of self-propelled objects. Cognition. 1990;36:1–16. doi: 10.1016/0010-0277(90)90051-k. [DOI] [PubMed] [Google Scholar]
- 9.Leslie A-M. In: Mapping the Mind: Domain Specificity in Cognition and Culture. Hirschfeld L, Gelman S, editors. New York: Cambridge Univ Press; 1994. pp. 119–148. [Google Scholar]
- 10.Luo Y, Baillargeon R. Can a self-propelled box have a goal? Psychological reasoning in 5-month-old infants. Psychol Sci. 2005;16:601–608. doi: 10.1111/j.1467-9280.2005.01582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Csibra G. Goal attribution to inanimate agents by 6.5-month-old infants. Cognition. 2008;107:705–717. doi: 10.1016/j.cognition.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Johnson S-C, Shimizu Y-A, Ok S-J. Actors and actions: the role of agent behavior in infants’ attribution of goals. Cogn Dev. 2007;22:310–322. doi: 10.1016/j.cogdev.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimizu Y-A, Johnson S-C. Infants’ attribution of a goal to a morphologically novel agent. Dev Sci. 2004;7:425–430. doi: 10.1111/j.1467-7687.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- 14.Leslie A-M, Keeble S. Do six-month-old infants perceive causality? Cognition. 1987;25:265–288. doi: 10.1016/s0010-0277(87)80006-9. [DOI] [PubMed] [Google Scholar]
- 15.Hauser M-D. A nonhuman primate’s expectations about object motion and destination: the importance of self-propelled movement and animacy. Dev Sci. 1998;1:31–37. [Google Scholar]
- 16.Bateson PPG. In: The Evolution Of Cognition. Heyes C, Huber L, editors. Cambridge, MA: MIT Press; 2000. pp. 85–102. [Google Scholar]
- 17.Horn G. Pathways of the past: the imprint of memory. Nat Rev Neurosci. 2004;5:108–120. doi: 10.1038/nrn1324. [DOI] [PubMed] [Google Scholar]
- 18.Suge R, McCabe B-J. Early stages of memory formation in filial imprinting: Fos-like immunoreactivity and behavior in the domestic chick. Neuroscience. 2004;123:847–856. doi: 10.1016/j.neuroscience.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Andrew R-J. Neural and Behavioural Plasticity. The Use of the Chick as a Model. Oxford: Oxford Univ Press; 1991. [Google Scholar]
- 20.Rogers L-J. The molecular neurobiology of early learning, development and sensitive periods, with emphasis on the avian brain. Mol Neurobiol. 1993;7:161–187. doi: 10.1007/BF02769174. [DOI] [PubMed] [Google Scholar]
- 21.Vallortigara G. In: Comparative Cognition: Experimental Explorations of Animal Intelligence. Wasserman E-A, Zentall T-R, editors. Oxford: Oxford Univ Press; 2006. pp. 41–58. [Google Scholar]
- 22.Michotte A. The Perception of Causality. New York: Basic Books; 1963. [Google Scholar]
- 23.O’Connell S, Dunbar R-I-M. The perception of causality in chimpanzees (Pan spp.) Anim Cogn. 2005;8:60–66. doi: 10.1007/s10071-004-0231-1. [DOI] [PubMed] [Google Scholar]
- 24.Luo Y, Kaufman L, Baillargeon R. Young infants’ reasoning about physical events involving inert and self-propelled objects. Cognit Psychol. 2009;58:441–486. doi: 10.1016/j.cogpsych.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rochat M-J, Serra E, Fadiga L, Gallese V. The evolution of social cognition: goal familiarity shapes monkeys’ action understanding. Curr Biol. 2008;18:227–232. doi: 10.1016/j.cub.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 26.Rosa Salva O, Regolin L, Vallortigara G. Faces are special for chicks: evidence for inborn domain-specific mechanisms underlying spontaneous preferences for face-like stimuli. Dev Sci. 2009 doi: 10.1111/j.1467-7687.2009.00914.x. in press. [DOI] [PubMed] [Google Scholar]
- 27.Vallortigara G, Regolin L, Marconato F. Visually inexperienced chicks exhibit a spontaneous preference for biological motion patterns. PLoS Biol. 2005;3:1312–1316. doi: 10.1371/journal.pbio.0030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson M-H, Morton J. Biology and Cognitive Development: The Case of Face Recognition. Oxford: Blackwell; 1991. [Google Scholar]
- 29.Simion F, Regolin L, Bulf H. A. predisposition for biological motion in the newborn baby. Proc Natl Acad Sci USA. 2008;105:809–813. doi: 10.1073/pnas.0707021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vallortigara G, Sovrano V-A, Chiandetti C. Doing Socrates experiment right: controlled-rearing studies of geometrical knowledge in animals. Curr Opin Neurobiol. 2009;19:20–26. doi: 10.1016/j.conb.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Landau B, Lakusta L. Spatial representation across species: Geometry, language, and maps. Curr Opin Neurobiol. 2009;19:1–8. doi: 10.1016/j.conb.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carey S. The Origin of Concepts. New York: Oxford Univ Press; 2009. [Google Scholar]
- 33.Spelke E-S. In: Language in Mind: Advances in the Investigation of Language and Thought. Gentner D, Goldin-Meadow S, editors. Cambridge, MA: MIT Press; 2003. pp. 277–311. [Google Scholar]