Abstract
Neuronally expressed auxilin and ubiquitously expressed cyclin-G-dependent kinase (GAK) are homologous proteins that act as cochaperones to support the Hsc70-dependent clathrin uncoating of clathrin-coated vesicles. GAK was previously shown to be essential in mouse during embryonic development and in the adult. We have now engineered an auxilin knockout mouse. Mutant mice had a high rate of early postnatal mortality and surviving pups generally had a lower body weight than wild-type pups, although they had a normal life span. GAK was up-regulated as much as 3-fold in the brains of both surviving neonates and adult mutant mice. An increased number of clathrin-coated vesicles and empty cages were present at knockout synapses both in situ and in primary neuronal cultures. Additionally, clathrin-mediated endocytosis of synaptic vesicles in knockout hippocampal neurons was impaired, most likely due to sequestration of coat components in assembled coats and cages. Collectively, our results demonstrate the specialized role of auxilin in the recycling of synaptic vesicles at synapses, but also show that its function can be partially compensated for by up-regulation of GAK.
Keywords: Hsc70, gene targeting, synaptic transmission, vesicle recycling
Clathrin-mediated endocytosis is a major pathway for the internalization of selected protein cargo from the plasma membrane. Cargo is recruited to nascent clathrin-coated pits, which then mature, invaginate, and ultimately undergo fission to produce clathrin-coated vesicles (CCVs) (1, 2). The clathrin coat is then rapidly shed in an ATP-dependent reaction carried out by the molecular chaperone Hsc70 (3–5). This reaction also requires a cochaperone, either auxilin, which is expressed selectively in neurons, or the ubiquitously expressed cyclin-G-dependent kinase (GAK) (6).
Auxilin and GAK are highly homologous proteins that share a multidomain structure, except that GAK has an additional N-terminal kinase domain. GAK is ubiquitously expressed (7), whereas auxilin is neuron-specific and enriched in nerve terminals (8). Both auxilin and GAK are members of the J domain family of proteins, which are characterized by the canonical histidine-proline-asparatic acid motif that binds to Hsc70 (6). In addition, auxilin and GAK bind to clathrin and the clathrin adaptor AP2 (9), and via these interactions they recruit Hsc70 to CCVs (10, 11). Because Hsc70 has many different functions, several studies have targeted GAK and/or auxilin to define the role of Hsc70 in clathrin-mediated endocytosis. Levels of GAK and/or auxilin have been reduced in cells by using RNA interference (12–16). GAK has also been disrupted by expressing Cre recombinase in a mouse embryonic fibroblast cell line that was derived from the conditional GAK knockout mouse (12). These studies generally showed that reducing GAK and auxilin levels results in the impairment of clathrin-mediated endocytosis and of the clathrin-dependent traffic of cargo from the Golgi to the lysosome, although the extent of impairment observed was variable.
Studies of the conditional GAK knockout mouse showed that GAK is an essential protein both during development and in the adult (12). When GAK was knocked out selectively in the developing brain by expressing Cre recombinase using the nestin promoter, the brains of these animals showed anatomical defects as early as E15. In newborn mice, there was a dramatic loss of cells in the cerebral cortex and a reduction in thickness of the ventricular zones. Ultimately, these mice died within 4 days after birth. Although these results initially suggested that GAK might have a specialized role in neurons that auxilin could not perform (12), it is also possible that, by E15, levels of auxilin may not be sufficient to compensate for the absence of GAK. Therefore, it remains unclear whether GAK performs a unique function in neurons that cannot also be performed by auxilin.
The related question, of course, is whether the neuron-specific protein auxilin performs an essential unique function in neurons, in particular to support rapid recycling of synaptic vesicles during high-frequency firing. In nonneuronal cells, only GAK is present to cochaperone Hsc70-mediated uncoating activity on CCVs, but it is not known whether GAK alone could support synaptic activity in neurons. To answer this question, we engineered an auxilin knockout mouse. Our results suggest that auxilin has a specialized function in synaptic vesicle recycling that can compensated for, but only partially, by up-regulation of GAK.
Results
Viability Defects of Auxilin Knockout Mice.
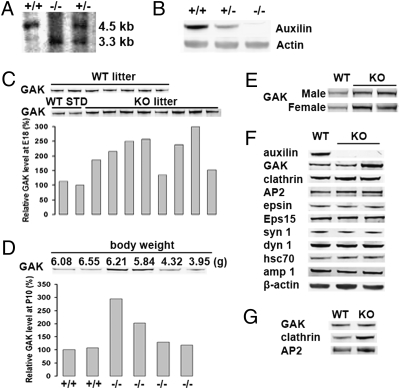
A conventional auxilin knockout mouse (−/−) was generated (Fig. S1), and offspring were backcrossed for eight generations before phenotypic analysis. The disruption of the auxilin gene was confirmed by Southern blotting (Fig. 1A) and by lack of auxilin expression in brain as revealed by western blotting (Fig. 1B). Heterozygous mice (+/−) showed a reduction in auxilin expression, indicating a gene-dosage effect. Lack of auxilin resulted in a marked reduction in viability. The offspring of heterozygous mice mating are predicted to be present at a 1:2:1 ratio for the +/+:+/−:−/− genotypes based on Mendelian genetics. Such a ratio was observed for E18–E19 embryos (Fig. S2A). However, χ2 analysis of the genotypes of the 3-week-old offspring generated by such mating revealed divergence from this ratio (P = 0.0038). Not only was the percentage of auxilin knockout mice lower than expected but the percentage of heterozygous mice was also significantly lower (Fig. S2B). Therefore, auxilin knockout mice show early postnatal mortality. In particular, the runts of the litter often died before weaning. Mice that survived for the first few weeks had apparently normal life span. Birth weights of knockout and heterozygous mice were also lower. At 1 week of age, wild-type pups were on average 40% heavier than auxilin knockout pups (Fig. S2C), and heterozygous mice had an intermediate weight. Female auxilin knockout mice also showed delayed sexual maturity (Fig. S2D), but after their first litter the adult knockout females generally had a normal reproductive cycle.
Fig. 1.
Analysis of endocytic proteins in auxilin knockout mice. (A) Southern blot analysis of knockout mice. The 5′ probe detects a 3.3-kb and a 4.5-kb band for the mutant and wild-type alleles, respectively. (B) Western blot analysis of auxilin from auxilin knockout mice. The brain cytosols from each genotype were analyzed to check the auxilin level. (C) GAK expression level in brain lysates of embryonic wild-type and auxilin knockout mice (E18). Brain lysates from embryos of a single wild-type (WT) litter and a single knockout (KO) litter, obtained from first-time mothers, were run on Western blots and then immunoblotted for GAK. In the western blot of GAK levels in the KO litter, the first two lanes are WT brain lysate standards (STD). The GAK intensity in KO brain lysates was plotted in the bar graph as relative GAK level by normalizing the intensity of the GAK bands to that of the WT lysate in lane 2. The same amount of protein from brain lysates was loaded in each lane. (D) Relationship between body weight and GAK expression level in auxilin knockout pups. Brain lysates from a single KO litter (P10) were analyzed for GAK level by Western blot. For comparison, brain lysates from a WT litter (P10) were run in lanes 1 and 2. The weights of the auxilin KO mice are shown above the blot, whereas the genotypes are shown below the graph. The relative GAK expression level is plotted directly beneath the immunoblot. (E) Western blots of GAK in neuronal tissue of wild-type and auxilin knockout mice at 5 weeks of age. (F) Western blots of the brain extract from wild-type and auxilin knockout mice at P7. Clathrin-mediated endocytosis-related proteins were detected from each brain extract using indicated antibodies. (G) Increased expression of clathrin and AP2 in neuronal tissue of auxilin knockout runts. Western blot for AP2 and clathrin of brain cytosol was obtained from a wild-type pup (P7) and an auxilin knockout runt (P7).
Up-Regulation of GAK.
The level of GAK was measured in the brain to determine whether up-regulation of GAK might compensate for the absence of auxilin in the knockout mice. GAK levels were first examined in embryos (E18). Analysis of five wild-type litters (total of 40 mice) showed less than a 25% variation in GAK levels among littermates (Fig. 1C Top). In contrast, GAK levels in the brain lysates from some of the knockout embryos were substantially greater than the GAK levels in the embryos of wild-type mice, as exemplified by the litter shown in Fig. 1C. Interestingly, there was a close relationship between brain GAK levels and body weight of the pups. As shown by the P10 litter depicted in Fig. 1D, there was no up-regulation of GAK in pups with the lowest body weights, whereas there was up-regulation of GAK in pups with body weights similar to wild-type mice. At 3–5 weeks of age, more than 80% of the auxilin knockout mice (analysis of 20 mice) showed approximately 3-fold up-regulation of GAK in their neuronal tissue compared to wild-type mice (Fig. 1E). As expected, given the selective expression of auxilin in the nervous system, GAK levels in nonneuronal tissues such as spleen, liver, kidney, and testes were not significantly different between auxilin knockout and wild-type mice (Fig. S1D).
The expression levels of various proteins associated with synaptic vesicle recycling were also examined by western blotting in brains from 1-week-old wild-type and auxilin knockout mice. Whereas levels of GAK were increased, no change was observed in auxilin knockout brains for several endocytic synaptic proteins such as dynamin 1, synaptojanin 1, epsin, eps15, amphiphysin 1, or Hsc70 (Fig. 1F). In general, AP2 and clathrin levels were also unchanged in the knockout mice, except for the runts, which showed an increase of 185 ± 35% (n = 6) and 165 ± 25% (n = 6) for clathrin and AP2 levels, respectively (Fig. 1G). This may be a consequence of an accumulation of abnormal CCVs and cages in nerve terminals, as described below.
Auxilin Is More Efficient than GAK in Inducing Clathrin Uncoating.
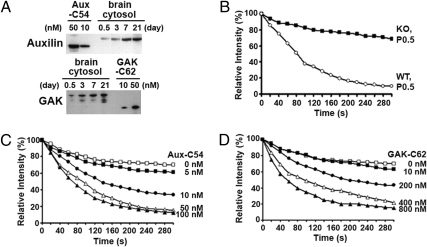
Because our data showed that a considerable up-regulation of GAK is required to rescue the growth and viability defects of auxilin knockout mice, we next examined the relative potency of auxilin and GAK in supporting the Hsc70-dependent uncoating of clathrin. To this aim, we used a clathrin-uncoating assay previously developed in our laboratory (17) and involving cells expressing GFP-clathrin. The assay monitors the loss of GFP fluorescence from digitonin-permeabilized cells upon incubation with uncoating factors. Based on semiquantitative western blot analysis (Fig. 2A), wild-type cytosol from newborn mice contains less auxilin than GAK, and at 7 days levels of auxilin and GAK are about equal. Yet the uncoating efficiency (the decrease in clathrin fluorescence associated with permeabilized cells as a function of time) of brain cytosol from newborn mice was dramatically impaired by the lack of auxilin (compare wild-type and knockout at P0.5 in Fig. 2B). This finding suggests that in combination with Hsc70 and ATP, auxilin uncoats clathrin more rapidly than GAK. This was confirmed using the C-terminal domains of auxilin and GAK, which we previously showed retain the uncoating activity of the full-length molecule (10). As shown in Fig. 2 C and D, to get the same time course of uncoating, 10 times more GAK-C62 fragment (GAK-C62) was required than Aux-C54 fragment.
Fig. 2.
Effect of knocking out auxilin on uncoating activity of brain cytosol. (A) Expression level of auxilin and GAK in wild-type brain cytosol from mice at P0.5, P3, P7, and P21. Western blots were used to determine the auxilin and GAK levels in brain cytosol by using protein standards (10 and 50 nM) of auxilin (Aux-C54) and GAK (GAK-C62). The auxilin and GAK were immunoblotted using anti-auxilin and anti-GAK antibodies, respectively. The same amount of protein from brain lysates was loaded in each lane. (B) Kinetics of clathrin uncoating by brain cytosol from wild-type and auxilin knockout mice. Clathrin-uncoating assays were conducted as described inMethods using permeabilized cells expressing GFP-clathrin. Brain cytosols (1 mg/200 μL) from newborn wild-type mice (P0.5) and auxilin knockout mice (P0.5) were used in the assay. (C) Time course of clathrin uncoating by Hsc70 as a function of Aux-C54 concentration. Varying concentrations of Aux-C54 (5–100 nM) were added to 2 μM Hsc70 and 1 mM ATP in the uncoating assay. (D) Time course of clathrin uncoating by Hsc70 as a function of GAK-C62 concentration. Varying concentrations of GAK-C62 (10–800 nM) were added to 2 μM Hsc70 and 1 mM ATP in the uncoating assay.
Increased Number of CCVs and Clathrin Cages in Nerve Terminals.
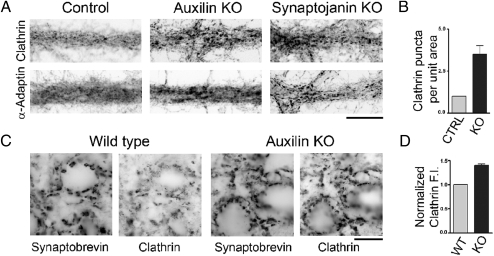
To determine the effect of knocking out auxilin on the structure of neuronal synapses, immunofluorescence and electron microscopic studies were performed on the brain of surviving adult knockout mice and in primary cortical neuronal cultures derived from auxilin knockout mice. As illustrated by Fig. 3A, large intensely fluorescent puncta containing clathrin and AP2 (labeled using antibodies to its α-adaptin subunit) were visible on the dendrites of cultured knockout neurons, whereas in control cultures, the immunoreactivity of these two proteins had a fine punctate pattern superimposed on diffuse fluorescence. Quantification of the results for neurons immunostained for clathrin is shown in Fig. 3B. Similar results, which had previously been observed in dynamin 1 (18, 19) and synaptojanin 1 (20) knockout neurons, were shown to be due to the accumulation of assembled coats in presynaptic nerve terminals: clathrin-coated pits in dynamin 1 knockout neurons and CCVs in synaptojanin 1 knockout neurons. Synaptojanin 1 knockout neurons immunostained for AP2 and clathrin are shown for comparison in Fig. 3A. A more intense punctate immunofluorescence pattern for clathrin in auxilin knockout synapses compared to wild-type synapses was also observed in sections of intact brain regions of adult mice, such as the deep cerebellar nuclei (Fig. 3 C and D). In contrast, the punctate immunofluorescence of synaptobrevin, a synaptic vesicle marker, was the same in both genotypes (Fig. 3C), indicating no change in the presynaptic content of synaptic vesicle membranes.
Fig. 3.
Immunoreactivity for endocytic clathrin coat components is clustered at auxilin knockout synapses, and clustering resembles the pattern observed in synaptojanin 1 knockout synapses. Immunofluorescence pictures are shown as negative images (fluorescence is shown in black). (A) High-magnification views of cultured neurons from an auxilin knockout mouse, a littermate pup, and a synaptojanin 1 knockout mouse immunostained for clathrin and AP2 (with α-adaptin antibodies). In the two knockout images, the clustering of clathrin and AP2 immunofluorescence, which reflects accumulations of clathrin-coated structures at synapses (16), is increased. (Scale bar, 20 μm.) (B) Number of clathrin puncta above threshold per unit area in auxilin knockout cultures. (C) Sections of deep cerebellar nuclei from auxilin knockout and wild-type mice immunostained for clathrin and for the synaptic vesicle marker synaptobrevin. Note that clathrin fluorescence, but not synaptobrevin fluorescence, is more intense at knockout synapses. (Scale bar, 20 μm.) (D) Clathrin immunofluorescence intensity (F.I.) normalized to the synaptobrevin immunofluorescence intensity. Error bars in B and C are SEM.
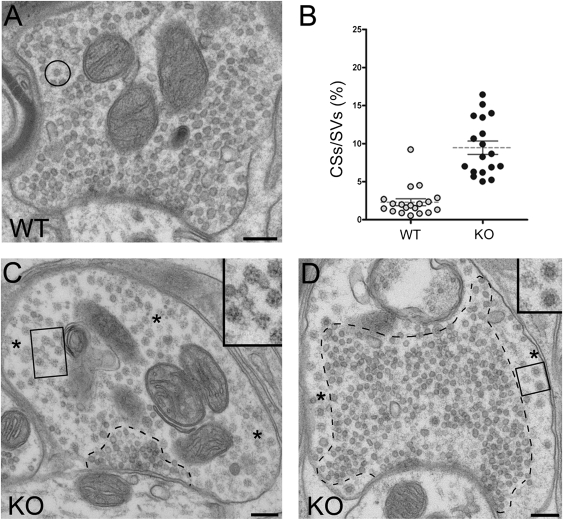
In agreement with the immunofluorescence studies, electron micrographs of wild-type synapses of the deep cerebellar nuclei from adult mice showed only occasional clathrin-coated profiles (Fig. 4A, circle), whereas CCVs and empty clathrin cages were very frequently observed in knockout synapses from the same region (Fig. 4 C and D and Fig. S3). However, there was great heterogeneity among the synapses: CCVs and cages were not visible in the sections of some synapses, whereas they were moderately abundant (Fig. 4D and Fig. S3) and very abundant in other synapses (Fig. 4C). A section of an axon nearly completely occupied by clathrin cages is shown in Fig. S4. Quantification of the differences between wild-type and knockout synapses in the percentage of assembled clathrin structures, including both CCVs and empty cages, relative to synaptic vesicles is shown in Fig. 4B. Despite the increase in CCV number, the overall number of synaptic vesicles was not statistically different between wild-type and knockout synapses, consistent with the observation that, although increased in number, CCVs represented only a small fraction of the total vesicles at the majority of synapses. Typically, both CCVs and cages were localized at the periphery of synaptic vesicle clusters and often lined the nerve terminal plasma membrane (Fig. 4D and Fig. S3). CCVs and empty cages also accumulated at the synapses of knockout cultured neurons (Fig. S5). Collectively, these data show that, in the absence of auxilin, there is a significant increase of assembled clathrin on vesicles and in cages, consistent with the well-characterized function of auxilin as a cochaperone in the Hsc70-dependent disassembly of clathrin on CCVs and clathrin cages in the cytosol.
Fig. 4.
Accumulation of CCVs and clathrin cages at auxilin knockout synapses from deep cerebellar nuclei of adult mice as revealed by electron microscopy. (A) Representative example of a wild-type synapse. A clathrin-coated structure is indicated by the circle. (B) Percentage of CCVs/cages (assembled clathrin structures, CSs) relative to synaptic vesicles (SVs) at wild-type and knockout synapses. (C and D) Examples of auxilin KO synapses where a variable number of CCVs or empty cages can be seen. Typically, CCVs/cages (asterisks indicate areas enriched in these structures) are located at the periphery of synaptic vesicle clusters (such clusters are delineated by dotted lines in the figures) and often along the plasma membrane. The insets in C and D are high-magnification views of the rectangular regions that show empty cages and CCVs, respectively. (Scale bars, 200 nm.)
Decreased Rate of Vesicle Endocytosis at Hippocampal Synapses.
To determine the effect of auxilin on the rate of synaptic vesicle endocytosis, hippocampal neurons from wild-type and auxilin knockout mice were transfected with synaptopHluorin, a fusion protein consisting of a pH-sensitive mutant of GFP fused to the luminal C-terminal end of the integral synaptic vesicle membrane protein VAMP-2 (21). Because the lumen of synaptic vesicles is acidic, the fluorescence of this fusion protein increases more than 20-fold upon exocytosis but decreases following endocytosis and reacidification of the internalized vesicles (22). Using this reporter, the time course of the decrease in fluorescence following excitation provides a measure of the rate of endocytosis.
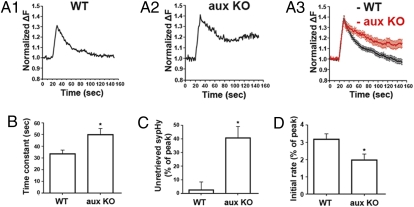
Exocytosis was induced by evoking action potentials via a bipolar platinum electrode (1 ms, 20 mA) positioned in the bath solution (22). A train of 20-Hz stimulation for 10 s increased the bouton fluorescence in wild-type and auxilin knockout neurons, indicating exocytosis of synaptic vesicles. Following stimulation, the fluorescence in boutons from wild-type mice returned monoexponentially to prestimulation levels with a time constant of 33.4 ± 3.4 s (n = 4 mice, 77 boutons; 12–28 boutons were imaged from each mouse). In contrast, only ∼60% of the fluorescence increase in auxilin knockout synapses decreased monoexponentially, and during this phase the time constant of decay was 49.5 ± 5.4 s (n = 4 mice, 48 boutons; 9–15 boutons were imaged from each mice; Fig. 5 A2 and A3), which is significantly slowed than for wild-type mice (Fig. 5B). At ∼120 s after stimulation, 37.7 ± 14.67% (n = 4 mice) of the fluorescence intensity increase induced by the stimulus was still observed, whereas in control neurons at the same time point only 2.63 ± 5.94% (n = 4 mice, P = 0.0045) of the fluorescence increase was still observed (Fig. 5C). The initial rate of the fluorescence decrease was also significantly decreased in auxilin knockout neurons, as compared to wild-type neurons (Fig. 5D). Taken together, these results suggest that the endocytic process is significantly impaired in the auxilin knockout mouse. After the stimulatory conditions used, 20-Hz stimulation for 10 s, clathrin-mediated endocytosis is expected to be the major form of endocytosis at cultured hippocampal synapses (23–26). Thus, the delay in endocytosis observed in our experiments is likely due to an impairment of clathrin-mediated endocytosis.
Fig. 5.
Auxilin knockout reduces the rate of endocytosis. (A) Representative examples of synaptopHluorin signal from a single hippocampal bouton (A1 and A2) and the averaged fluorescence trace (A3); 77 boutons from 4 wild-type mice and 48 boutons from 4 auxilin knockout mice after 200 AP at 20 Hz in WT (black) and auxilin KO (red) neurons. Traces are normalized to the basal fluorescence recorded before stimulation. The decay of the fluorescence signal indicates endocytosis. (B) The endocytic time constant of hippocampal boutons after 200 action potentials at 20 Hz in WT mice (n = 27) and auxilin KO mice (n = 14); *P = 0.01. (C) The percentage of fluorescence that remained unretrieved after 120 s in WT and auxilin KO mice; *P = 0.0045. The data were normalized to the peak of the fluorescence increase. (D) The initial endocytosis rate within 10 s after stimulation in WT and auxilin KO mice; *P = 0.0184. Error bars are SEM.
Discussion
The well-established role of auxilin in clathrin uncoating (6) and its high concentration at synapses had suggested a specialized function for this protein in the clathrin-dependent recycling of synaptic vesicles (27). Our study provides genetic support for this hypothesis and also shows that the function of GAK overlaps, but only partially, with the function of auxilin. Clearly, synaptic vesicle recycling was impaired but not blocked in the absence of auxilin. Furthermore, although auxilin knockout mice showed a marked increase in early postnatal mortality, some animals survived to adulthood though they had reduced body weight and delayed sexual maturity. Although it remains possible and likely that these animals may have impaired cognitive function given the impaired traffic of synaptic vesicles, clearly up-regulation of GAK can compensate for the lack of auxilin to allow for the basic functions of life. In these animals, a marked up-regulation of GAK levels in brain and a relationship between the extent of GAK up-regulation and body weight was observed.
As for why the absence of auxilin causes lethality, most likely this is due to neurological and cognitive impairment resulting from defective synaptic transmission. Similar early lethality was observed in mice harboring mutations in other genes implicated in the clathrin-dependent endocytosis of synaptic vesicles (18, 20).
Electron microscopy demonstrated that the absence of auxilin caused a marked increase in clathrin-coated structures, both CCVs and cages, in neuronal cultures from newborn mice, as well as in neurons from adult mouse brains, which show a compensatory increase in GAK expression. Therefore, even with the higher levels of GAK expression in adult brains, membrane-recycling defects were still present in the knockout mice, although these defects were not serious enough to cause obvious neurological problems. Apparently, even a several-fold increase in GAK above normal levels is not sufficient to completely compensate for the absence of auxilin.
The numerous empty cages observed at synapses suggest that auxilin and Hsc70 not only uncoat CCVs but more generally chaperone clathrin in the cytosol, thereby preventing the formation of clathrin baskets or triggering their rapid disassembly. These findings are consistent with photobleaching studies demonstrating that cytosolic clathrin no longer rapidly exchanges in the absence of auxilin/GAK (12, 16), as expected if clathrin were in an assembled/aggregated state. Furthermore, free cages were observed in the cytosol of HeLa cells depleted of auxilin/GAK (16) or when a dominant-negative Hsc70 was expressed in HeLa cells (28). The increase of clathrin levels in the brains of the most severely affected pups (Fig. 1G) may be explained by the accumulation of empty clathrin cages and CCVs. This accumulation may result from decreased clathrin degradation due to the enhanced pool of clathrin in an assembled state or to increased synthesis to compensate for the reduced availability of free clathrin.
As revealed by electrophysiological studies of cultured hippocampal neurons in primary culture, after stimulatory conditions that favor clathrin-mediated endocytosis, the compensatory endocytic response following a stimulus was strongly impaired by the lack of auxilin. More specifically, after an initial phase of slower (relative to control) but robust endocytosis, endocytosis nearly stopped. This effect was not due to a progressive depletion of synaptic vesicles due to repeated stimulation, because a defect of endocytosis also occurred after a single stimulation. We speculate that clathrin-mediated compensatory endocytosis fails due to sequestration of clathrin coat components and their accessory factors on CCVs and in empty cages so that new clathrin-coated pits cannot form.
An accumulation of CCVs was also observed at the synapses of synaptojanin knockout mice (18–20, 29, 30). Synaptojanin, a phosphoinositide phosphatase, plays a key role in the recycling of synaptic vesicles by hydrolyzing PtdIns(4,5)P2, a critical factor in the binding of the clathrin adaptors to the membrane. By catalyzing this reaction, synaptojanin 1 is thought to promote the dissociation of the adaptors during CCV uncoating (31). Interestingly, no empty cages were observed in the synaptojanin knockout synapses (19, 20, 29), consistent with the different functions of auxilin and synaptojanin 1 in uncoating: clathrin disassembly and chaperoning in the case of auxilin, adaptor shedding from the bilayer in the case of synaptojanin 1. Lack of adaptor dissociation may slow down clathrin removal by Hsc70/auxilin in synaptojanin 1 knockout neurons.
Although our results show that GAK overexpression can partially compensate for loss of auxilin, it is not known whether auxilin could compensate for the absence of GAK in neurons. Because GAK conventional knockout mice die embryonically, the role of GAK in mouse brain was examined in conditional knockout mice, where the GAK gene was disrupted in the brain by expressing Cre recombinase under the control of the nestin promoter. These mice died within 4 days after birth. However, because the nestin promoter is turned on very early in brain development whereas auxilin expression is turned on later (12), the possibility that auxilin compensates for the lack of GAK remains to be tested. GAK differs from auxilin not only in the presence of the kinase domain but also in the presence of two binding motifs for the clathrin adaptor AP1 that have been reported to be important for protein trafficking to the lysosome (15). Therefore, GAK may have unique housekeeping functions in neurons that cannot be compensated by the presence of auxilin. To determine whether this is the case, GAK needs to be knocked out in the mouse nervous system using a neuron-specific promoter that turns on late during mouse development, when auxilin is already present. These mice will allow us to determine the function of a nervous system in which only auxilin is expressed in mouse neurons.
Methods
Chemicals.
DNase type IV, poly-L-lysine (0.01%), and cytosine β-D-arabinofuranoside, L-cysteine, 6-cyano-7-nitroquinoxaline-2,3-dione, 1,2-amino-5-phosphonovaleric acid, and Mayer’s hematoxylin were obtained from Sigma. Neurobasal-A medium, Modified Eagle Medium, HBSS, Hepes, B-27, Na-pyruvate, GlutaMAX, penicillin-streptomycin, and FBS were from Invitrogen. Matrigel and bovine transferrin were obtained from BD Biosciences and Calbiochem, respectively. The list of antibodies is given in SI Text.
Generation of the Auxilin Knockout Mouse.
The targeting scheme to generate auxilin knockout embryonic stem cells is described in SI Methods. Positive embryonic stem cell clones were microinjected into blastocysts and then reimplanted into pseudopregnant foster females. The resulting chimeras were bred with C57BL/6 mice to obtain F1 offspring, and germline transmission was determined by Southern blot analysis and/or PCR. Auxilin heterozygote mice were backcrossed with C57BL/6 mice for more than eight generations before phenotypic analysis. All mice were handled in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Primary Cortical Neuronal Cultures for Microscopic Studies.
Primary cortical neurons were prepared from neonatal mouse brain (P0–P1). Cortical tissue was dissected out, placed in ice-cold HBSS, and cut into small pieces. Tissue was then digested for 30–40 min in HBSS containing papain (20 U/mL) and DNase (20 μg/mL) at 37 °C, followed by gentle trituration. The cells were plated on poly-D-lysine–coated coverslips at a high density (20,000–40,000 cells/cm2.) using FBS-containing MEM. After 8–12 h, the medium was changed to a neuronal medium (2 mM GlutaMAX, 2.5% B-27, 100 U/mL penicillin-streptomycin in neurobasal-A medium) and the cells were maintained at 37 °C in a 5% CO2 humidified incubator. Cultures were used for morphological studies after 3–4 weeks after plating.
Hippocampal Cultures and Electrophysiology.
CA1–CA3 regions were dissected from newborn mice, dissociated, and plated onto Matrigel-coated glass coverslips. Cells were maintained in culture medium (MEM, 0.5% glucose, 0.1 g/L bovine transferrin, 0.3 g/L glutamine, 10% FBS, 2% B-27, 3 μM cytosine-b-D-arabinofuranoside) at 37 °C in a 95% air/5% CO2 humidified incubator. Calcium-phosphate-mediated gene transfer was used to transfect 6- to 8-day-old cultures with synaptopHluorin (21). Coverslips were mounted in a rapid-switching, laminar-flow perfusion and stimulation chamber (RC-21BRFS chamber; Warner Instruments) 6−10 days posttransfection. The total volume of the chamber was ∼150 μL. Two hundred action potentials were delivered by passing 1-ms current pulses of 20 mA at 20 Hz through the chamber via platinum electrodes. Except if otherwise noted, cells were continuously perfused at room temperature (∼25 °C) in a saline solution containing 119 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 25 mM Hepes (buffered to pH 7.4), 30 mM glucose, plus 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione and 50 μM d,l-2-amino-5-phosphonovaleric acid (AP5).
Miscellaneous Procedures.
Western blotting, immunofluorescence of primary neuronal cultures and brain frozen sections, and electron microscopy were carried out by standard procedures (see SI Methods for details). Clustering of immunofluorescence in neuronal cultures was quantified as previously described (16).
Clathrin-Uncoating Assay.
CHO cells stably expressing GFP-clathrin were grown in eight-well chambers and permeabilized with digitonin as described previously (17). To measure the uncoating of clathrin, GFP fluorescence intensity was measured at 20-s intervals on a Zeiss confocal microscope on addition of either brain cytosols or purified proteins to the permeabilized cells. Brain cytosols used in the uncoating assay were prepared from newborn wild-type and knockout mice. Uncoating was also measured using purified Hsc70 and either recombinant Aux-C54 or GAK-C62. These latter protein fragments, which are the 54-kDa and 62-kDa C-terminal domains of auxilin and GAK, respectively, were prepared as described previously (10). All assays were conducted in the presence of 1 mM ATP and the following buffer: 20 mM imidazole, 2 mM magnesium acetate, 25 mM KCl, 10 mM (NH4)2SO4 (pH 7.0).
Supplementary Material
Acknowledgments
We thank Dr. Chengyu Liu for assistance in making the knockout mice, Valentina Cappello for help with electron microscopy, Dr. Mary Anne Conti for helpful discussions, and Dr. JingPing Lin for help with the statistical analysis. This work was supported in part by National Institutes of Health Grants NS36251 and DA018343 to P.D.C.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/1000738107/DCSupplemental.
References
- 1.Ehrlich M, et al. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Loerke D, et al. Cargo and dynamin regulate clathrin-coated pit maturation. PLoS Biol. 2009;7:e57. doi: 10.1371/journal.pbio.1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlossman DM, Schmid SL, Braell WA, Rothman JE. An enzyme that removes clathrin coats: Purification of an uncoating ATPase. J Cell Biol. 1984;99:723–733. doi: 10.1083/jcb.99.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chappell TG, et al. Uncoating ATPase is a member of the 70 kilodalton family of stress proteins. Cell. 1986;45:3–13. doi: 10.1016/0092-8674(86)90532-5. [DOI] [PubMed] [Google Scholar]
- 5.Sousa R, Lafer EM. Keep the traffic moving: Mechanism of the Hsp70 motor. Traffic. 2006;7:1596–1603. doi: 10.1111/j.1600-0854.2006.00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisenberg E, Greene LE. Multiple roles of auxilin and Hsc70 in clathrin-mediated endocytosis. Traffic. 2007;8:640–646. doi: 10.1111/j.1600-0854.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- 7.Kanaoka Y, Kimura SH, Okazaki I, Ikeda M, Nojima H. GAK: A cyclin G associated kinase contains a tensin/auxilin-like domain. FEBS Lett. 1997;402:73–80. doi: 10.1016/s0014-5793(96)01484-6. [DOI] [PubMed] [Google Scholar]
- 8.Ahle S, Ungewickell E. Auxilin, a newly identified clathrin-associated protein in coated vesicles from bovine brain. J Cell Biol. 1990;111:19–29. doi: 10.1083/jcb.111.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheele U, Kalthoff C, Ungewickell E. Multiple interactions of auxilin 1 with clathrin and the AP-2 adaptor complex. J Biol Chem. 2001;276:36131–36138. doi: 10.1074/jbc.M106511200. [DOI] [PubMed] [Google Scholar]
- 10.Ma Y, et al. Identification of domain required for catalytic activity of auxilin in supporting clathrin uncoating by Hsc70. J Biol Chem. 2002;277:49267–49274. doi: 10.1074/jbc.M203695200. [DOI] [PubMed] [Google Scholar]
- 11.Scheele U, et al. Molecular and functional characterization of clathrin- and AP-2-binding determinants within a disordered domain of auxilin. J Biol Chem. 2003;278:25357–25368. doi: 10.1074/jbc.M303738200. [DOI] [PubMed] [Google Scholar]
- 12.Lee DW, Zhao X, Yim YI, Eisenberg E, Greene LE. Essential role of cyclin-G-associated kinase (Auxilin-2) in developing and mature mice. Mol Biol Cell. 2008;19:2766–2776. doi: 10.1091/mbc.E07-11-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee DW, Zhao X, Zhang F, Eisenberg E, Greene LE. Depletion of GAK/auxilin 2 inhibits receptor-mediated endocytosis and recruitment of both clathrin and clathrin adaptors. J Cell Sci. 2005;118:4311–4321. doi: 10.1242/jcs.02548. [DOI] [PubMed] [Google Scholar]
- 14.Zhang CX, et al. Multiple roles for cyclin G-associated kinase in clathrin-mediated sorting events. Traffic. 2005;6:1103–1113. doi: 10.1111/j.1600-0854.2005.00346.x. [DOI] [PubMed] [Google Scholar]
- 15.Kametaka S, et al. Canonical interaction of cyclin G associated kinase with adaptor protein 1 regulates lysosomal enzyme sorting. Mol Biol Cell. 2007;18:2991–3001. doi: 10.1091/mbc.E06-12-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirst J, et al. Auxilin depletion causes self-assembly of clathrin into membraneless cages in vivo. Traffic. 2008;9:1354–1371. doi: 10.1111/j.1600-0854.2008.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yim YI, et al. Exchange of clathrin, AP2 and epsin on clathrin-coated pits in permeabilized tissue culture cells. J Cell Sci. 2005;118:2405–2413. doi: 10.1242/jcs.02356. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson SM, et al. A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science. 2007;316:570–574. doi: 10.1126/science.1140621. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi M, et al. Cell- and stimulus-dependent heterogeneity of synaptic vesicle endocytic recycling mechanisms revealed by studies of dynamin 1-null neurons. Proc Natl Acad Sci USA. 2008;105:2175–2180. doi: 10.1073/pnas.0712171105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cremona O, et al. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell. 1999;99:179–188. doi: 10.1016/s0092-8674(00)81649-9. [DOI] [PubMed] [Google Scholar]
- 21.Miesenböck G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 22.Sankaranarayanan S, Ryan TA. Real-time measurements of vesicle-SNARE recycling in synapses of the central nervous system. Nat Cell Biol. 2000;2:197–204. doi: 10.1038/35008615. [DOI] [PubMed] [Google Scholar]
- 23.Harata NC, Choi S, Pyle JL, Aravanis AM, Tsien RW. Frequency-dependent kinetics and prevalence of kiss-and-run and reuse at hippocampal synapses studied with novel quenching methods. Neuron. 2006;49:243–256. doi: 10.1016/j.neuron.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 24.Granseth B, Odermatt B, Royle SJ, Lagnado L. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron. 2006;51:773–786. doi: 10.1016/j.neuron.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 25.Balaji J, Ryan TA. Single-vesicle imaging reveals that synaptic vesicle exocytosis and endocytosis are coupled by a single stochastic mode. Proc Natl Acad Sci USA. 2007;104:20576–20581. doi: 10.1073/pnas.0707574105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Q, Li Y, Tsien RW. The dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles. Science. 2009;323:1448–1453. doi: 10.1126/science.1167373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cremona O, De Camilli P. Synaptic vesicle endocytosis. Curr Opin Neurobiol. 1997;7:323–330. doi: 10.1016/s0959-4388(97)80059-1. [DOI] [PubMed] [Google Scholar]
- 28.Newmyer SL, Christensen A, Sever S. Auxilin-dynamin interactions link the uncoating ATPase chaperone machinery with vesicle formation. Dev Cell. 2003;4:929–940. doi: 10.1016/s1534-5807(03)00157-6. [DOI] [PubMed] [Google Scholar]
- 29.Kim WT, et al. Delayed reentry of recycling vesicles into the fusion-competent synaptic vesicle pool in synaptojanin 1 knockout mice. Proc Natl Acad Sci USA. 2002;99:17143–17148. doi: 10.1073/pnas.222657399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mani M, et al. The dual phosphatase activity of synaptojanin1 is required for both efficient synaptic vesicle endocytosis and reavailability at nerve terminals. Neuron. 2007;56:1004–1018. doi: 10.1016/j.neuron.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wenk MR, De Camilli P. Protein-lipid interactions and phosphoinositide metabolism in membrane traffic: Insights from vesicle recycling in nerve terminals. Proc Natl Acad Sci USA. 2004;101:8262–8269. doi: 10.1073/pnas.0401874101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.