Abstract
Measuring the binding curve and stoichiometry of protein complexes in living cells is a prerequisite for quantitative modeling of cellular processes. Dual-color fluorescence fluctuation spectroscopy provides a general framework for detecting protein interactions, but lacks suitable methods for quantifying protein heterointeractions in the cell. We address this challenge by introducing heterospecies partition (HSP) analysis for protein heterointeractions of the type D + nA↔DAn. HSP directly identifies the heterointeracting species from the sample mixture and determines the binding curve and stoichiometry of the protein complex. The HSP method is applied to provide the first direct characterization of the ligand-dependent binding of the retinoic X receptor to the coactivator transcription intermediate factor 2. A previous study based on protein fragments observed a higher binding stoichiometry than biologically expected. We address this difference in stoichiometry by measuring the binding curves of the full-length proteins in living cells. This study provides proof-of-principle experiments that illustrate the potential of HSP as a general and robust analysis tool for the quantitative characterization of protein heterointeractions by dual-color fluorescence fluctuation spectroscopy in living cells.
Keywords: coactivator, fluorescence correlation spectroscopy, fluorescence fluctuation spectroscopy, nuclear receptor, time-integrated fluorescence cumulant analysis
Protein–protein interactions are central to most biological processes in cells. The ready availability of fluorescent proteins as markers for cellular proteins has opened up the possibility to observe protein interactions in real time by fluorescence methods. Both Förster resonance energy transfer (FRET) and fluorescence correlation spectroscopy (FCS) are widely used for detecting the presence of interactions between proteins in cells (1–4), yet quantitative characterization of protein interactions and their stoichiometry remains challenging. Fluorescence fluctuation spectroscopy (FFS), a technique closely related to FCS, provides another measure of protein interactions via brightness analysis. Brightness characterizes the average fluorescence intensity of a single particle and encodes the stoichiometry of a protein complex (5–7). If two fluorescently labeled monomers form a homodimer, the fluorescence intensity of the particle and therefore its brightness doubles. Single-color brightness analysis utilizes this effect to characterize the binding curve and the stoichiometry of protein homocomplexes directly from cellular data (5). An extension of single-color brightness analysis for the identification of protein heterocomplexes has been described (8, 9), but the applicability of the technique is very limited.
The desire to study heterointeractions motivated the introduction of dual-color FFS (10, 11). Unlike homointeractions, heterointeractions between two proteins D and A require differently colored labels to distinguish the proteins. Each of the two detection channels primarily receives the signal from one of the two colored labels. Dual-color FFS retains color-information of a species by measuring its brightness in each of the two detection channels. Thus, dual-color brightness analysis could in principle reveal the binding curve and stoichiometry of heteroprotein interactions, because each protein species (such as D, A, DA, DA2) has its own distinct dual-color brightness. Unfortunately, a general analysis method for extracting this information from dual-color brightness data is not available. Because of this limitation, dual-color brightness analysis is currently restricted to the detection of protein heterointeractions without the ability to quantify the reaction.
We overcome this shortcoming of the dual-color brightness technique by introducing heterospecies partition (HSP) analysis. HSP isolates the heterointeracting proteins into a single species with a brightness that directly reflects the degree of binding between the proteins D and A. We specifically consider the reaction D + nA↔DAn and prove that its binding curve is described by the brightness of the heterospecies as a function of the total protein concentration of A as long as the protein expression ratio between A and D exceeds a certain threshold. In addition, HSP analysis identifies the stoichiometry of the protein complex. Thus, this analysis method provides an important tool for the quantitative characterization of heteroprotein interactions in living cells.
We apply HSP-analysis to study the interaction of a nuclear receptor (NR) with a coactivator. NRs are transcription factors that regulate gene expression in a ligand-specific manner (12). Binding of an agonist ligand triggers a conformational change in the NR that leads to the recruitment of a coactivator and ultimately results in transcriptional activation. Because most NRs interact with their hormone response element as dimers, it is generally thought that two NRs bind a single coactivator molecule (13), but this model has never been directly confirmed. Because the NR/coactivator interaction is crucial for transcriptional activation, we investigate the interaction between the NR retinoic X receptor (RXR) and the coactivator transcription intermediate factor 2 (TIF-2) in the nucleoplasm with HSP analysis and determine the binding curve and binding stoichiometry. In addition to the full-length RXR, we also work with its ligand binding domain (RXRLBD) and characterize its binding with TIF-2. The coactivator TIF-2 belongs to the p160 family and interacts with NRs through the LXXLL motif (14, 15), which is referred to as an NR box (Fig. 1A). Although it is assumed that two NRs bind to the coactivator (Fig. 1B), the nuclear interacting domain (NID) of all p160 coactivators contains three NR boxes (Fig. 1A). The reason for the existence of three NR boxes, when only two are needed, is currently unknown. We previously demonstrated that all three NR boxes of the NID fragment of the coactivator can be occupied by RXRLBD (Fig. 1C) (9). Because the binding of three NRs to a coactivator is unexpected, it raises the question whether this result is a consequence of using protein fragments. HSP-analysis provides the tool to address this question using full-length proteins. The binding studies show that full-length RXR associates in the expected stoichiometry of two NRs per coactivator. This result provides direct experimental verification of the binding model between NR and its coactivator. The experimental study serves as a proof-of-principle that illustrates the promising potential of HSP-analysis for quantifying heteroprotein interactions in living cells from dual-color brightness data.
Fig. 1.
(A) Schematic representation of the TIF-2 coactivator gene, whose NID domain contains three NR boxes. (B) Binding stoichiometry of TIF-2 interacting with the nuclear receptors RXR. (C) Binding stoichiometry of RXRLBD interacting with TIF-2 and the NID domain.
Results
Heterospecies Partition Model.
Two proteins D and A are labeled with spectrally distinct fluorophores with A emitting further in the red than D. In this paper D refers to proteins labeled with EGFP, whereas A refers to proteins labeled with mCherry. The fluorescence signal is split into a “red” and a “green” detection channel for dual-color FFS experiments. The brightness of species S in detection channel j is described by λj,S, where j = R for the red and j = G for the green detection channel. For convenience, the brightness values of both channels are sometimes reported in vector notation, 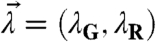 . Despite spectral overlap between the two fluorescent proteins, it is generally possible to select filters so that the fluorescence of the protein emitting at longer wavelengths is only detected by the red channel (16), which leads to a vanishing brightness of A in the green channel, λG,A = 0. The experiments described in this paper are conducted under conditions whereλG,A = 0.
. Despite spectral overlap between the two fluorescent proteins, it is generally possible to select filters so that the fluorescence of the protein emitting at longer wavelengths is only detected by the red channel (16), which leads to a vanishing brightness of A in the green channel, λG,A = 0. The experiments described in this paper are conducted under conditions whereλG,A = 0.
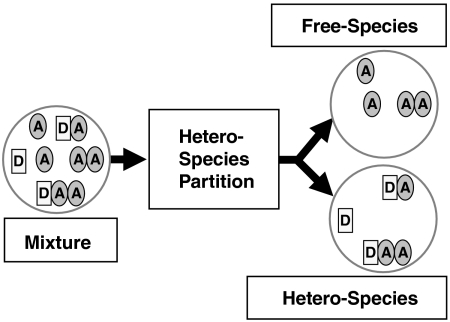
The following interactions between proteins are allowed: Protein D has s binding sites for A, which leads to species DA,DA2,…,DAs. Protein A may also form homocomplexes such as A2. However, we impose the following restriction: Protein D cannot form homocomplexes. This constraint leads to simple analytical equations and is sufficient for the experiments described in this manuscript. In general, such a system is described by a mixture of a large number of molecular species, {D,A,A2,DA,DA2,…}. Resolving this complex mixture into its individual components by FFS is not feasible. As an alternative, we propose to resolve the mixture into two different classes of species, which we will refer to as heterospecies H and its complement, the free species F (Fig. 2). The free species F contains A and its oligomers (F = {A,A2,A3,…}), whereas the heterospecies H includes D and its complexes with A (H = {D,DA,DA2,…}). This two species model is described by five parameters: the number of molecules of each species, NH and NF, the brightness of the heterospecies (λG,H,λR,H), and the brightness of the free species in the red channel, λR,F. The green-channel brightness of the free species is by definition zero, λG,F = 0. We refer to this separation into two species as HSP.
Fig. 2.
Conceptual picture illustrating the projection of a mixture of brightness species into two different classes. One class contains the molecules of A that are not interacting with D, which is referred to as free species (A,A2,…,Ar). The other class includes all species that contain the molecule D (D,DA,DA2,…,DAs) and is called heterospecies. The analysis method that accomplishes the separation is described in the text. The FFS parameters describing the heterospecies characterize the binding between D and A.
The separation into the two classes of species is achieved by a fit of the experimental data to the HSP model described above. Data analysis by photon counting histogram (17, 18) or moment-related techniques (19–21) are feasible. Here we perform cumulant analysis of the photon counts, because it provides analytical results that are crucial for interpreting the fit parameters of the heterospecies model. The factorial cumulants of the photon counts of the HSP model are given by (22),
| 1 |
where δi,0 is the Kronecker delta and γr is the rth shape factor of the point spread function (20). We now derive formulas describing the model parameter by comparing the factorial cumulants 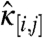 of the HSP model with the exact cumulants of the mixture. We first describe the heterodimer reaction, which is the simplest case.
of the HSP model with the exact cumulants of the mixture. We first describe the heterodimer reaction, which is the simplest case.
Case 1: Heterodimer Reaction: D + A↔DA.
Even the simple reaction, 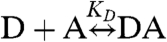 , involves more than two species and direct resolution into its components remains a challenge. The degree of heterodimer formation depends on the dissociation coefficient KD and the total concentration of both proteins, [Dt] and [At]. The cumulants describing this reaction depend on the brightness values and number of molecules of each participating species. For a mixture of three species D, A, and DA, the cumulants are calculated by
, involves more than two species and direct resolution into its components remains a challenge. The degree of heterodimer formation depends on the dissociation coefficient KD and the total concentration of both proteins, [Dt] and [At]. The cumulants describing this reaction depend on the brightness values and number of molecules of each participating species. For a mixture of three species D, A, and DA, the cumulants are calculated by
| 2 |
where NDA is the number of molecules of the heterodimer and λi,DAis the brightness of the heterodimer in channel i. We have assumed throughout the paper that the sampling time T is shorter than the diffusion time for simplicity. Generalization to arbitrary sampling time is straightforward (22). In the absence of FRET, the brightness vector of the heterodimer is the sum of the brightness vectors 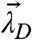 of D and
of D and 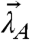 for A,
for A,
| 3 |
Setting the cumulants of first and second order {κ[0,1],κ[1,0],κ[1,1],κ[0,2],κ[2,0]} of Eq. 2 equal to the corresponding cumulants of Eq. 1 and solving for the FFS parameters of the HSP model results in
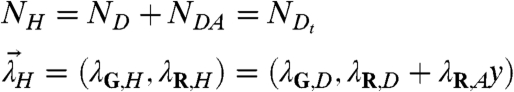 |
4 |
with
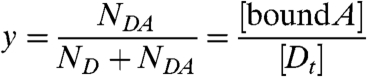 |
5 |
Note, Eqs. 4 and 5 are exact and independent of the detailed reaction mechanism. Even if the reaction is not at equilibrium, the equations still holds. The parameter y describes the average number of molecules of A bound to D and represents the degree of binding. The parameters describing the free species F are NF = NA/(1 + y × ND/NA) and λR,F = λR,A(1 + y × ND/NA). Because the paper focuses on heterointeractions, the free species will not be discussed further.
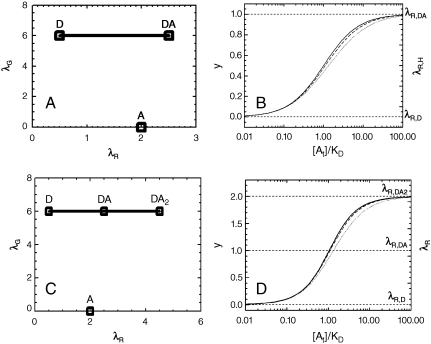
We take a closer look at the FFS parameters of the heterospecies H described by Eq. 4. The number of molecules NH equals the total number of molecules of D. The possible brightness values of the heterospecies are restricted to the line shown in Fig. 3A. The green-channel brightness λG,H is constant and equal to that of protein D in the green channel. The red-channel brightness λR,H varies linearly from λR,D to λR,D + λR,A with the degree of binding. The brightness starts at the brightness vector  of D for y = 0 (no binding) and moves along a straight segment to that of DA for y = 1 (complete binding).
of D for y = 0 (no binding) and moves along a straight segment to that of DA for y = 1 (complete binding).
Fig. 3.
Modeling of HSP analysis. (A) Brightness plot for D + A↔DA. The brightness of the heterodimer DA is the sum of the brightness values of D and A. The brightness of the heterospecies H is confined to the straight line connecting D and DA. The value of the red-channel brightness characterizes the degree of binding y. (B) Binding curve of the reaction D + A↔DA versus [At]/KD for protein expression ratios rAD = [At]/[Dt] of 10 (solid line), 5 (dashed line), and 2 (dotted line). The right-hand axis displays the brightness titration plot with λR,H = λR,D + λR,Ay. (C) Brightness plot for D + 2A↔DA2. The brightness of the species D, A, DA, and DA2 is indicated. The possible brightness values of the heterospecies Hare marked by the solid line and moves from D in the absence of binding to DA2 for complete binding. (D) Binding curve of the reaction D + 2A↔DA2 versus [At]/KD for protein expression ratios rAD of 20 (solid line), 10 (dashed line), and 4 (dotted line). The binding curve is calculated for identical binding sites with dissociation coefficient KD. The left-hand axis shows the degree of binding, while the right-hand axis displays the red-channel brightness of the heterospecies. The horizontal lines mark the red-channel brightness values of D, DA, and DA2.
The brightness plot by itself is insufficient to characterize the binding between both proteins, because the degree of binding y depends not only on the dissociation coefficientKD, but also on the total concentration of both species. A binding curve of the reaction is typically constructed from measurements where the concentration of one protein is kept constant, while the other is systematically varied. This approach is not feasible for cell measurements, because the protein expression levels vary from cell to cell. Therefore we explore an alternative approach. Because the protein expression ratio rAD = [At]/[Dt] of both proteins is easily determined from the intensity ratio rF of the green and red channel (SI Text), we examine the influence of rAD on y for the reaction 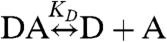 in equilibrium with dissociation coefficient KD,
in equilibrium with dissociation coefficient KD,
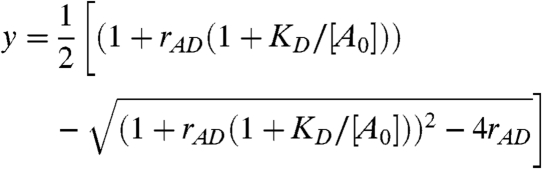 |
6 |
The degree of binding y is graphed as a function of [A0]/KD for several values of the protein expression ratio rAD in Fig. 3B. Dividing the total concentration [A0] by KD provides a rescaling into a unitless concentration. In the limit of large rAD, Eq. 6 reduces to [A0]/([A0] + Kd), which is identical to the standard form of the sigmoidal binding curve for y. We notice that y is very insensitive to the expression ratio once rAD≥2. The relative deviation from the standard curve for rAD = 2 is less than 10%, which is to first order negligible, because the uncertainty of brightness measurements in cells typically exceeds 10%. Thus, as long as we choose cells with an expression ratio larger than two, the degree of binding y follows the standard binding curve, independent of the actual protein expression ratio. For the presentation of experimental data, we prefer to plot the red-channel brightness λR,H = λR,D + λR,Ay of the heterospecies instead of y against the total concentration[A0], which we will refer to as the brightness titration plot. The right-hand axis in Fig. 3B illustrates the concept of the brightness titration plot.
Case 2: Hetero-N-mer Reaction: D + nA↔DAn.
Consider a protein D with n binding sites for A. The heterospecies contains the following molecular species: {D,DA,DA2,…,DAn}. The brightness of DAi in the absence of FRET is 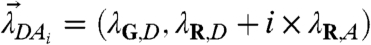 . Performing the same analysis as for the heterodimer reaction determines the FFS parameters of the heterospecies H:
. Performing the same analysis as for the heterodimer reaction determines the FFS parameters of the heterospecies H:
| [7] |
with 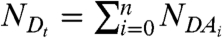 representing the total number of molecules of D. The parameters are identical to the ones for the heterodimer case (Eq. 4). The degree of binding
representing the total number of molecules of D. The parameters are identical to the ones for the heterodimer case (Eq. 4). The degree of binding
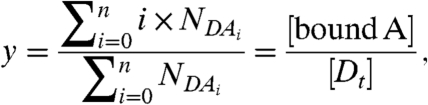 |
8 |
which describes the average number of molecules of A bound to a molecule of D, ranges from zero for no binding to n for complete binding. Thus, the red-channel brightness λR,H provides a faithful measure of the degree of binding y for the reaction: D + nA↔DAn. The possible brightness values of the heterospecies are indicated by the line shown in Fig. 3C for the case n = 2. The brightness vector starts at 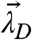 for y = 0 and ends at
for y = 0 and ends at 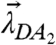 for y = 2.
for y = 2.
We calculated brightness titration plots for various values of n and found that the degree of binding y is very insensitive to the protein expression ratio provided that rAD≥2n. The brightness titration plot for a heterotrimer reaction (D + 2A↔DA2) with equivalent binding sites is shown as an example in Fig. 3D for expression ratios rAD of 10n, 5n, and 2n. Thus, brightness titration plots characterize the degree of binding y of higher-order oligomers as long as the total concentration of species A is at least a factor of 2n larger than the total concentration of species D.
HSP for mCherry.
Previous experiments reveal that mCherry, unlike EGFP, cannot be described by a single brightness state (16). It was shown that modeling mCherry with two brightness states is sufficient to describe FFS experiments. The two-state mixture of mCherry has been incorporated into the HSP analysis (SI Text). We specifically examined the influence of the two-state model on the brightness titration curve for the heterodimer and heterotrimer reaction. As shown in Figs. S1 and S2, the titration curves for both the two-state model and the one-state model of A result in identical binding curves. Thus, the determination of binding curves with HSP analysis remains valid for mCherry.
HSP in the Presence of FRET.
The text describes HSP analysis for conditions where FRET is absent. This description is sufficient for the data presented in the paper. The presence of FRET reduces the brightness of the donor and increases the brightness of the acceptor. General modeling of FRET in FFS experiments is feasible (8), but complicated if several acceptors are present. In order to gauge the influence of FRET on HSP analysis, the reaction D + A↔DA was modeled for different FRET efficiencies (SI Text). Fig. S3 illustrates that the brightness titration curve shifts to higher concentrations with increasing FRET efficiency. However, the shape of the titration curve is to very good approximation not affected by FRET. The experimental uncertainty of brightness measurements inside cells is sufficiently large that the change in the brightness titration curve is negligible as long as the FRET efficiency is less than 30%.
Nuclear Receptor and Coactivator Interactions.
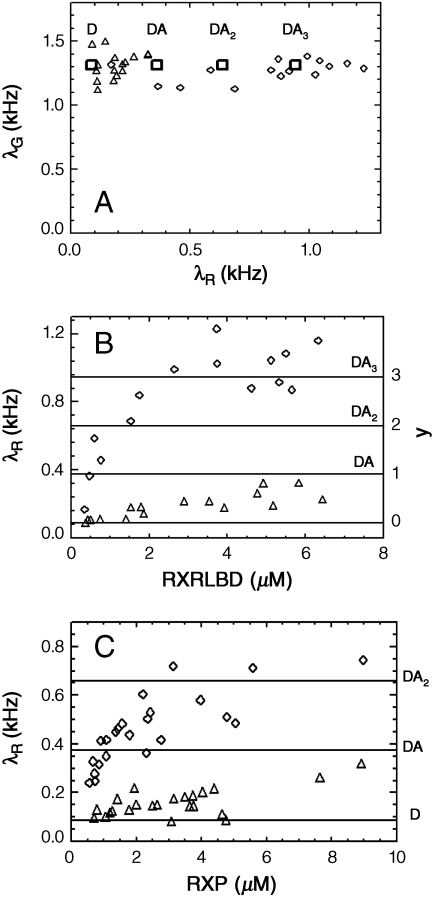
The previous section introduced the HSP model. This section applies HSP analysis to analyze experimental binding data. The following nomenclature is used to specify fusion proteins: Protein X labeled with EGFP is written as G-X, whereas protein Y labeled with mCherry is referred to as Ch-Y. The first study probes the interaction between the NR fragment RXRLBD and the full-length coactivator TIF-2. Cells are cotransfected with G-TIF-2 and Ch-RXRLBD. Because the coactivator contains three potential NR binding sites, we select cells with an expression ratio exceeding six. The expression level of the full-length coactivator is in general very low, which makes it very easy to identify cells with sufficient excess of Ch-RXRLBD over G-TIF2. We measure cells with different concentrations of Ch-RXRLBD, and perform dual-color time-integrated fluorescence cumulant analysis of the data by fitting the experimental cumulants to the HSP model. Fig. 4A shows the two-channel brightness vectors of the heterospecies in the presence (diamond) and absence (triangle) of the agonist 9-cis retinoic acid. Each symbol in the figure represents the brightness measured from a single cell. We notice that the brightness vectors are located on a straight line with constant brightness in the green channel. This result signals the absence of FRET in the interaction between both proteins. Thus, based on the results of the proceeding section, the red-channel brightness is a direct measure of the degree of binding y.
Fig. 4.
Interaction between G-TIF-2 and Ch-RXRLBD. (A) Brightness plot of the heterospecies in the absence (Δ) and in the presence (◊) of the ligand 9-cis retinoic acid. (B) Brightness titration plot of the heterospecies in the absence (Δ) and presence (◊) of the ligand. The left-hand axis indicates the red-channel brightness and the right-hand axis displays the degree of binding y. The horizontal lines mark the brightness values corresponding to protein complexes of D, DA, DA2, and DA3. (C) Brightness titration plot for interaction between G-TIF-2 and Ch-RXR in the absence (Δ) and presence (◊) of the ligand 9-cis retinoic acid.
For a clearer depiction of the binding, Fig. 4B shows the red-channel brightness of the heterospecies as a function of the total concentration of Ch-RXRLBD. Each symbol in the figure represents the measurement of a single cell. In the absence of the ligand 9-cis retinoic acid, the brightness of the heterospecies remains close to that of EGFP, indicating that the interaction between TIF-2 and RXRLBD is very weak. The horizontal lines of the figure indicate the brightness values for the stoichiometric complexes DAi (with D = G-TIF-2 and A = Ch-RXRLBD) to aid in the interpretation of the brightness titration curve. In addition, the right-hand axis displays the degree of binding y of the titration curve. Without ligand, less than one molecule of Ch-RXRLBD is bound per coactivator on average. However, upon adding ligand to the cells, the red-channel brightness of the heterospecies increases dramatically as a function of RXRLBD concentration. At low concentration, no binding between G-TIF-2 and Ch-RXRLBD occurs (y ≈ 0). The degree of binding y grows with concentration and finally saturates at a brightness value that corresponds to the heterotetramer TIF-2 - RXRLBD3.
This binding stoichiometry suggests that all three NR boxes of TIF-2 are occupied by RXRLBD (Fig. 1C). This result agrees with an earlier study where it was shown that the NID fragment of steroid receptor coactivator-1, which contains the three NR boxes, is able to bind three RXRLBD (9). Whereas the earlier study was limited to a coactivator fragment, the present data show the same surprising result for the full-length coactivator. This stoichiometry of the complex is surprising, because there is ample evidence that only two NRs interact with their respective DNA hormone response element (23, 24).
The ligand binding domain (LBD) of the NR is often used as a model system to study interactions between NR proteins. However, examining the behavior of full-length proteins is important, because these represent the biologically relevant molecule. Thus, the next set of experiments measures full-length RXR and TIF-2 in the presence and absence of the agonist ligand 9-cis retinoic acid. Cells expressing Ch-RXR and G-TIF-2 are measured if the expression ratio exceeds the threshold. The brightness of the heterospecies is determined by a fit of the cumulants to the HSP model. Examination of the brightness vectors reveals a constant value for the green-channel brightness, which demonstrates the absence of FRET. The brightness titration curve of the data is shown in Fig. 4C as a function of Ch-RXR concentration. Without ligand, the interaction between RXR and coactivator is weak with less than one NR bound per coactivator molecule. The strength of interaction increases if the ligand is present. At the lowest concentration, the brightness of the heterospecies is close to that of EGFP, indicating that G-TIF-2 and Ch-RXR do not interact with each other. The brightness increases with growing RXR concentration and saturates at a brightness, which corresponds to the heterotrimer TIF-2 - RXR2. Thus, the experiment demonstrates that RXR and TIF-2 interact in the presence of ligand, but in contrast to RXRLBD only two receptors are recruited by the coactivator. This result suggests that the access to the NR binding sites of the coactivator is changed by the additional domain present in the full-length RXR. It also restores the stoichiometry expected from biological considerations.
Discussion
Conventional dual-color brightness analysis fails to establish a direct link with the heterointeracting protein species of the sample. Yet such information is a prerequisite for the quantitative characterization of heteroprotein interactions. HSP analysis establishes the necessary relationship between dual-color FFS data and the composition of the sample in two steps. First, HSP analysis of dual-color FFS data separates the signal of all heterointeracting species of the mixture into a single brightness species (heterospecies) by exploiting the spectral characteristics of the fluorescent labels. Second, quantitative interpretation of the interaction is provided by the heterospecies brightness for reactions of the type D + nA↔DAn and yields the degree of binding. We demonstrated that the brightness titration plot, which graphs the red-channel brightness of the heterospecies versus the total protein concentration [At], represents the binding curve as long as the expression ratio rAD≥2n. It is usually easy to choose conditions where cells express one species in excess over the other. This condition also simplifies the selection of cells for measurement. Instead of finding cells with a specific expression ratio, all cells exceeding a threshold value of rAD are suitable. Note that the condition rAD≥2n simplifies the experiment, but is not required. Although other values of the coexpression ratio shift the binding curve, its influence can be accounted for in the analysis.
A single-color brightness technique for determining the stoichiometry of heteroprotein complexes under stoichiometric binding conditions has been described (9). The protein expression ratio that leads to the highest single-color brightness identifies the stoichiometry of the heteroprotein complex. However, because this technique ignores the color of the labels, it cannot distinguish heterointeracting proteins from the rest of the sample. Thus a direct measurement of the protein binding curve is not feasible. Another constraint of the technique is the need to express proteins at specific concentration ratios, which in many cases is not experimentally feasible. For example, the expression level of the full-length coactivator is very low compared to that of NR. The interaction between these proteins cannot be characterized by the single-color brightness approach.
HSP analysis of dual-color brightness data overcomes these limitations. It neither requires the high-expression concentrations associated with stoichiometric binding nor are specific expression ratios mandated. It not only provides the stoichiometry, but also the binding curve of heteroprotein interactions. Thus, characterization of the binding between full-length TIF-2 and RXR is now feasible, despite the low expression of the full-length coactivator. Our experiments identify a nontrivial 3∶1 stoichiometry between RXRLBD and TIF-2 in the presence of agonist (Fig. 1C), which agrees with a previous study limited to the NID fragment of a related coactivator (9). If both proteins are full-length, a change in the stoichiometry occurs with two RXR bound to the coactivator TIF-2 (Fig. 1B). Thus, full-length NR binds to the coactivator in the stoichiometry expected from biology (12). Although only the RXRLBD domain is known to interact directly with the NR box of the coactivator, the remaining domains of RXR exert an influence on the binding of the coactivator by a currently unknown mechanism.
The binding curve for the NR/coactivator reaction is potentially influenced by endogenous protein that competes with labeled protein in the formation of protein complexes. In addition, partially expressed exogenous protein would also influence the brightness binding curve. We performed control experiments described in the SI Text to rule out these potentially complicating factors. Furthermore, HSP analysis assumes a reaction of the type D + nA↔DAn. In other words, G-TIF-2 is not allowed to form homocomplexes. The brightness vector of Fig. 4A indicates the absence of G-TIF-2 homointeractions, because the green-channel brightness remains monomeric. A direct measurement of the brightness of G-TIF-2 confirms the absence of homointeractions (see SI Text and Fig. S4).
It is worth pointing out that FRET approaches, which are commonly used to detect protein heterointeractions, are not applicable to studying NR/coactivator binding. The brightness plots clearly show the absence of FRET for this experimental system. Thus, a dual-color brightness measurement by FFS is currently the only approach for studying the interactions of this important protein system.
Conclusions
The HSP-analysis technique described in this paper presents a simple and fairly general method for quantifying protein heterointeractions in cells from dual-color brightness data. Analysis is especially straightforward if mCherry-labeled protein exists in sufficient excess over the EGFP-labeled protein. The analysis technique is very robust because it always employs a two-species fit of the data, independent of the detailed interaction scheme. HSP analysis of the heterospecies provides an easy-to-understand graphical result. The brightness of the heterospecies is plotted as a function of total concentration of the mCherry-labeled species. If there is no interaction, the brightness stays close to that of EGFP. If there is an interaction, the brightness increases with concentration and saturates when all binding sites are occupied. The saturated brightness provides information about the stoichiometry of the protein complex. The shape of the brightness titration curve can be used to estimate the dissociation coefficient KD of the interaction.
The introduction of HSP analysis was crucial for achieving an experimental characterization of the binding model between NR and coactivator. Previous in vitro and in vivo studies have been based on fragments of the proteins and were unable to elucidate the binding model (9, 25, 26). We experimentally demonstrated that the use of fragments can alter the binding stoichiometry. For the full-length proteins, a 2∶1 stoichiometry of NR to coactivator TIF-2 was observed, which confirms the binding model suggested from the interaction of NR dimers with the hormone response element. This study demonstrates the significant potential of HSP-analysis for the quantitative characterization of cellular protein interactions.
Materials and Methods
FFS Instrumentation.
The instrumentation for two-color fluorescence fluctuation experiments is based on a modified Axiovert 200 microscope (Zeiss) as described previously (22). A 63X Plan-Apochromat oil immersion objective (NA = 1.4) was used to focus the laser and collect the fluorescence. An excitation wavelength of 1,000 nm was used for all experiments. The fluorescence emission was separated into two different detection channels with a 580 nm dichroic mirror (585DCXR, Chroma Technology). The green channel was equipped with an 84-nm-wide band pass filter centered at 510 nm (FF01-510/84-25 Semrock) to eliminate the reflected fluorescence of mCherry.
Sample Preparation and Data Analysis.
The construction of the plasmids and transfection protocol are described in the SI Text. The recorded photon counts of each measurement are converted into factorial cumulants as described previously (22, 27). A fit of these cumulants to an n-species model determines the FFS parameters for each fluorescent species: the number N of molecules in the observation volume and its brightness in the green and red channel (λG, λR). HSP analysis requires a two-species fit as described in the SI Text. The protein expression ratio and the total concentration of mCherry-labeled protein are determined from a dual-color intensity measurement (see SI Text).
Supplementary Material
Acknowledgments.
This work was supported by grants from the National Institutes of Health (GM64589), the National Science Foundation (PHY-0346782), and the American Heart Association (0655627Z).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905670107/DCSupplemental.
References
- 1.Selvin PR. The renaissance of fluorescence resonance energy transfer. Nat Struct Biol. 2000;7:730–734. doi: 10.1038/78948. [DOI] [PubMed] [Google Scholar]
- 2.Llopis J, et al. Ligand-dependent interactions of coactivators steroid receptor coactivator-1 and peroxisome proliferator-activated receptor binding protein with nuclear hormone receptors can be imaged in live cells and are required for transcription. Proc Natl Acad Sci USA. 2000;97:4363–4368. doi: 10.1073/pnas.97.8.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SA, Heinze KG, Waxham MN, Schwille P. Intracellular calmodulin availability accessed with two-photon cross-correlation. Proc Natl Acad Sci USA. 2004;101:105–110. doi: 10.1073/pnas.2436461100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosales T, et al. Quantitative detection of the ligand-dependent interaction between the androgen receptor and the co-activator, Tif2, in live cells using two color, two photon fluorescence cross-correlation spectroscopy. Eur Biophys J. 2007;36:153–161. doi: 10.1007/s00249-006-0095-1. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Wei LN, Muller JD. Probing protein oligomerization in living cells with fluorescence fluctuation spectroscopy. Proc Natl Acad Sci USA. 2003;100:15492–15497. doi: 10.1073/pnas.2533045100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saffarian S, Li Y, Elson EL, Pike LJ. Oligomerization of the EGF receptor investigated by live cell fluorescence intensity distribution analysis. Biophys J. 2007;93:1021–1031. doi: 10.1529/biophysj.107.105494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Digman MA, Wiseman PW, Choi C, Horwitz AR, Gratton E. Stoichiometry of molecular complexes at adhesions in living cells. Proc Natl Acad Sci USA. 2009;106:2170–2175. doi: 10.1073/pnas.0806036106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Wei LN, Muller JD. Unraveling protein-protein interactions in living cells with fluorescence fluctuation brightness analysis. Biophys J. 2005;88:4366–4377. doi: 10.1529/biophysj.105.059170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Muller JD. Determining the stoichiometry of protein heterocomplexes in living cells with fluorescence fluctuation spectroscopy. Proc Natl Acad Sci USA. 2007;104:3147–3152. doi: 10.1073/pnas.0606557104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bacia K, Schwille P. Practical guidelines for dual-color fluorescence cross-correlation spectroscopy. Nat Protoc. 2007;2:2842–2856. doi: 10.1038/nprot.2007.410. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, et al. Dual-color photon-counting histogram. Biophys J. 2005;88:2177–2192. doi: 10.1529/biophysj.104.048413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu L, Glass CK, Rosenfeld MG. Coactivator and corepressor complexes in nuclear receptor function. Curr Opin Genet Dev. 1999;9:140–147. doi: 10.1016/S0959-437X(99)80021-5. [DOI] [PubMed] [Google Scholar]
- 13.Mangelsdorf DJ, et al. The nuclear receptor superfamily: The second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leo C, Chen JD. The SRC family of nuclear receptor coactivators. Gene. 2000;245:1–11. doi: 10.1016/s0378-1119(00)00024-x. [DOI] [PubMed] [Google Scholar]
- 15.Heery DM, Kalkhoven E, Hoare S, Parker MG. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733–736. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- 16.Wu B, Chen Y, Müller JD. Fluorescence fluctuation spectroscopy of mCherry in living cells. 2009;96:2391–2404. doi: 10.1016/j.bpj.2008.12.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Müller JD, So PT, Gratton E. The photon counting histogram in fluorescence fluctuation spectroscopy. Biophys J. 1999;77:553–567. doi: 10.1016/S0006-3495(99)76912-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kask P, Palo K, Ullmann D, Gall K. Fluorescence-intensity distribution analysis and its application in biomolecular detection technology. Proc Natl Acad Sci USA. 1999;96:13756–13761. doi: 10.1073/pnas.96.24.13756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer AG, Thompson NL. High-order fluorescence fluctuation analysis of model protein clusters. Proc Natl Acad Sci USA. 1989;86:6148–6152. doi: 10.1073/pnas.86.16.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müller JD. Cumulant analysis in fluorescence fluctuation spectroscopy. Biophys J. 2004;86:3981–3992. doi: 10.1529/biophysj.103.037887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sergeev M, Costantino S, Wiseman PW. Measurement of monomer-oligomer distributions via fluorescence moment image analysis. Biophys J. 2006;91:3884–3896. doi: 10.1529/biophysj.106.091181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu B, Chen Y, Müller JD. Dual-color time-integrated fluorescence cumulant analysis. Biophys J. 2006;91:2687–2698. doi: 10.1529/biophysj.106.086181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 24.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 25.Teichert A, et al. Quantification of the vitamin D Receptor-Coregulator interaction. Biochemistry-US. 2009;48:1454–1461. doi: 10.1021/bi801874n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Margeat E, et al. The human estrogen receptor alpha dimer binds a single SRC-1 coactivator molecule with an affinity dictated by agonist structure. J Mol Biol. 2001;306:433–442. doi: 10.1006/jmbi.2000.4418. [DOI] [PubMed] [Google Scholar]
- 27.Wu B, Müller JD. Time-integrated fluorescence cumulant analysis in fluorescence fluctuation spectroscopy. Biophys J. 2005;89:2721–2735. doi: 10.1529/biophysj.105.063685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.