The study of the folding of membrane proteins has lagged far behind that of small soluble proteins—yet proteins that reside within biological membranes account for approximately a third of all proteomes. The article by Huysmans et al. in this issue of PNAS (1) represents a breakthrough by reporting a comprehensive φ-value analysis of the folding of a membrane protein (i.e., PagP) into a lipid bilayer. φ-value analysis is the most powerful tool for experimental analysis of protein folding pathways (2). It combines protein engineering with equilibrium and kinetic measurements to determine which regions of a protein are largely folded (high φ-values) or largely unfolded (low φ-values) at the rate-limiting transition state.
There are two major structural classes of integral membrane proteins: α-helical bundles and β-barrels. The latter are found in the outer membranes of Gram-negative bacteria and mitochondria, whereas helical structures are ubiquitous, occurring, for example, in plasma and inner membranes. PagP has a β-barrel structure and resides in the outer membrane of Escherichia coli. The analysis presented by Huysmans et al. (1) sheds light on the transition state structure for formation of this β-barrel in a bilayer. The work complements a previous φ-value study of membrane protein folding that was the first to map out a membrane protein transition state, but in this case, for folding of an α-helical protein, bacteriorhodopsin, into lipid-detergent mixtures (3). Thus detailed insight into the folding mechanisms of the two major membrane protein structural classes is now emerging.
A key feature of this current study (1) is the examination of folding into a lipid bilayer. To achieve this, Huysmans et al. (1) exploited a previously demonstrated trait of β-barrel proteins: they can be reversibly refolded from a urea-denatured state into lipid bilayer vesicles, and the kinetics and thermodynamics of the folding reaction can be determined (4, 5). The successful demonstration of this for PagP (6) paved the way for a φ-value study. Identification of conditions for reversible folding of PagP relied on manipulation of the lipid to protein ratio. At low ratios, PagP folding was protein concentration-dependent, but at the lipid-to-protein ratio used here (3,200:1), refolding was completely reversible and protein concentration–independent. The folding and unfolding kinetics were determined, and, perhaps surprisingly, conditions could be found (urea concentrations greater than approximately 8 M) in which folding could be characterized as a simple two-state reaction. It is also of note that the choice of a β-barrel protein means that folding can be studied from an apparently fully unfolded, denatured state. Most helical membrane proteins retain significant residual structure in common denaturants such as urea and SDS, but the denatured state of PagP in 10 M urea, although associated with the bilayer, is essentially unstructured (as judged by circular dichroism and fluorescence spectra). The use of urea gives added importance to the PagP work, as urea apparently does not partition into the bilayer, unlike SDS used for studies of the folding of several α-helical proteins.
Nineteen variants of PagP were investigated, at least one mutation in each β-strand, thus giving a snapshot across the entire protein. The protein folds via a polarized transition state with the barrel partly formed and the C-terminal part of the protein significantly more structured than the N-terminal regions. The authors propose that the results are consistent with a tilted folding-insertion mechanism (Fig. 1), as has been seen in simulations for insertion of folded OmpA into a bilayer (7). The results also agree with previously proposed models of concerted folding and insertion of β-barrels into membranes (8); as an unfolded barrel protein within a bilayer is unlikely, as it would expose potential hydrogen bonding groups (9).
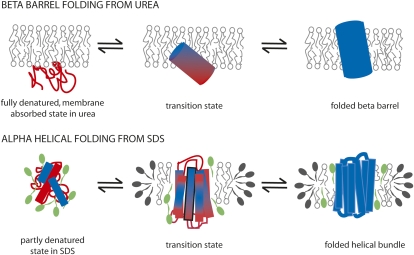
Fig. 1.
Schematic diagrams of proposed folding models for β-barrel and α-helical membrane proteins, highlighting potential transition state structures from φ-value studies. Folding of a β-barrel protein occurs from a fully denatured, membrane-absorbed state in urea with a tilted, partly inserted transition state as proposed by Huysmans et al. (1). In contrast, folding of an α-helical protein such as bacteriorhodopsin occurs from a partly denatured state in SDS, which contains some helical content. The transition state is proposed to have significant native helix content. Only one transmembrane helix has been analyzed by φ-values (3), and this suggests a largely formed helix with partial helix formation at the cytoplasmic side (shown in the bottom of this diagram, with this helix outlined in black). The degree of structure in other helices is estimated from previous studies (e.g., ref 15). Unfolded structure is shown in red and folded structure in blue. SDS is shown in green. PagP was folded into lipid bilayers vesicles, whereas bacteriorhodopsin was folded into mixed DMPC/CHAPS micelles (the detergent CHAPS is shown here capping the edges of a DMPC disc).
Helical membrane proteins, by contrast, seem to fold by a fundamentally different mechanism than barrels (9, 10). Individual transmembrane helices can be stable entities in bilayers, as backbone hydrogen bonding is satisfied locally within the helix. Thus partially folded states occur within a membrane, as conceptualized by the classic two-stage folding model, whereby helices first form within a membrane (10). Final helix packing and tertiary structure formation then occurs as a second stage (Fig. 1). Models emerging for in vivo folding also propose this type of folding mechanism, as transmembrane helices form within the translocon complex during cotranslational folding, and this is followed by final helix packing and folding within the membrane (11, 12). Investigations of folding mechanisms by studies in vitro are also consistent with the overall two-stage idea, although the situation is more complex than two simple steps (9, 13).
The increased hydrophobicity of helical membrane proteins compared with β-barrels makes the former more challenging to experimental manipulation. Consequently, the folding system used for the bacteriorhodopsin φ-value study (3) is more complex than the urea/bilayer one used by Huysmans et al. for PagP (1). A partially denatured state of bacteriorhodopsin in SDS was used; this contains just more than half the native helix content and seems to represent a critical helical core that is a prerequisite for correct folding. More extensive denaturation of bacteriorhodopsin requires acidic organic acids that are incompatible with refolding in detergent or lipid systems. Two-state reversible folding conditions were established for bacteriorhodopsin using lipid/detergent mixtures of 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) and CHAPS that seem to form elongated micelles or DMPC bilayer discs capped by CHAPS detergent, referred to as bicelles. Overall, these bilayer discs are not much larger than the embedded protein, but seem to provide a good bilayer mimic for helical proteins. The SDS denaturant will also partition; thus folding is studied in DMPC/CHAPS/SDS mixtures. The folding process studied for bacteriorhodopsin represents final helix formation and packing, which is a key folding event for helical proteins. φ-values were determined throughout one helix of the seven-helical bacteriorhodopsin. These revealed that most of the helix is formed in the transition state, but intermediate and low φ-values toward the cytoplasmic end of the helix suggest only partial helix formation and tertiary contacts. Intermediate φ-values can also arise from multiple transition states or folding paths, which could be envisaged for packing of stable helical entities. Many intermediate φ-values are also seen in the PagP study (1). The possible contributions of the bilayer environment and intermolecular forces to such intermediate values await further examination for both protein classes.
Membrane proteins may reside within a heterogeneous and largely hydrophobic environment, which complicates experimental study, but given careful consideration of experimental conditions, membrane protein folding can be investigated in detail, and many of the approaches that have worked well for soluble proteins can be suitably transferred. These successful applications of φ-value analysis are especially propitious, as they will reveal structures of key membrane protein intermediates and transition states. The membrane environment itself directly affects protein folding (13), with the membrane elastic properties influencing the folding rates, yields, and pathways of both helical and β-barrel proteins. Matching of the hydrophobic thickness of the bilayer to the protein is also vital, with a mismatch affecting the yield, cooperativity, thermodynamics, and tilt of the folding protein structure. This requires comprehensive investigation of the lipid properties. An excellent start has previously been made for β-barrel proteins using another E. coli outer membrane protein, OmpA (4), in which lipids with a hydrophobic thickness of approximately 30 Å were found to give the best match to the protein. The lipids used for PagP are shorter, fully saturated lipids (1, 6), which have different elastic properties and thicknesses than those used for OmpA. It is interesting that different lipids give rise to two-state reversible folding for two different β-barrel proteins from the same outer membrane. This further emphasizes how diverse conditions may be needed to probe the folding of apparently similar structures. A full understanding of the influence of the lipid bilayer will require further investigation of several proteins under contrasting lipid conditions, together with a quantitative understanding of the relevant lipid properties.
In some respects, the structures of membrane proteins, with only two main structural classes, are generally less complex than those of soluble proteins. Huysmans et al. (1) suggest that there might be a generic tilted folding-insertion mechanism for β-barrel membrane proteins. Conservation of folding mechanisms in protein families has been observed before—particularly in all β-proteins in which the topology is complex (14). This lends further support to the notion that there may be general folding mechanisms for the two folding classes: insertion and packing of preformed helical elements or concerted folding and insertion. Intriguingly, the early events in the folding of the β-barrel proteins might also be more complex—in the study by Huysmans et al., the folding kinetics of PagP became much more difficult to interpret below 7.8 M urea—suggesting that early stable intermediates might be populated.
The importance of this study and that of bacteriorhodopsin is that they show it is possible to investigate the folding mechanisms of membrane proteins in detail and to explicitly test simulations of their folding. Neither system is trivial to work on, but these studies (1, 3) pose a challenge to other experimentalists in the field. In studies of the folding of soluble proteins, the assumption is that the folding pathway in vitro is essentially the same as pathway in vivo. Is the same true of membrane proteins? It is clear that the physiochemical properties of membranes have an effect on the physical/chemical properties of membrane proteins. These two studies, which elucidate the mechanism of the folding of membrane proteins, open the door to experimental investigations of these complex questions.
Acknowledgments
J.C. is a Wellcome Trust Senior Research Fellow. P.J.B. thanks the Royal Society for a Wolfson Research Merit Award.
Footnotes
The authors declare no conflict of interest.
See companion article on page 4099.
References
- 1.Huysmans GHM, Baldwin SA, Brockwell DJ, Radford SE. The transition state for folding of an outer membrane. Proc Natl Acad Sci USA. 2010;107:4099–4104. doi: 10.1073/pnas.0911904107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fersht AR, Matouschek A, Serrano L. The folding of an enzyme. I. Theory of protein engineering analysis of the stability and pathway of protein folding. J Mol Biol. 1992;224:771–782. doi: 10.1016/0022-2836(92)90561-w. [DOI] [PubMed] [Google Scholar]
- 3.Curnow P, Booth PJ. The transition state for integral membrane protein folding. Proc Natl Acad Sci USA. 2009;106:773–778. doi: 10.1073/pnas.0806953106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong H, Tamm LK. Elastic coupling of integral membrane protein stability to lipid bilayer forces. Proc Natl Acad Sci USA. 2004;101:4065–4070. doi: 10.1073/pnas.0400358101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Surrey T, Jähnig F. Refolding and oriented insertion of a membrane protein into a lipid bilayer. Proc Natl Acad Sci USA. 1992;89:7457–7461. doi: 10.1073/pnas.89.16.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huysmans GHM, Radford SE, Brockwell DJ, Baldwin SA. The N-terminal helix is a post-assembly clamp in the bacterial outer membrane protein PagP. J Mol Biol. 2007;373:529–540. doi: 10.1016/j.jmb.2007.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bond PJ, Sansom MS. Insertion and assembly of membrane proteins via simulation. J Am Chem Soc. 2006;128:2697–2704. doi: 10.1021/ja0569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamm LK, Arora A, Kleinschmidt JH. Structure and assembly of beta-barrel membrane proteins. J Biol Chem. 2001;276:32399–32402. doi: 10.1074/jbc.R100021200. [DOI] [PubMed] [Google Scholar]
- 9.Bowie JU. Solving the membrane protein folding problem. Nature. 2005;438:581–589. doi: 10.1038/nature04395. [DOI] [PubMed] [Google Scholar]
- 10.Popot J-L, Engelman DM. Membrane protein folding and oligomerization: the two-stage model. Biochemistry. 1990;29:4031–4037. doi: 10.1021/bi00469a001. [DOI] [PubMed] [Google Scholar]
- 11.Skach WR. The expanding role of the ER translocon in membrane protein folding. J Cell Biol. 2007;179:1333–1335. doi: 10.1083/jcb.200711107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rapoport TA, Goder V, Heinrich SU, Matlack KE. Membrane-protein integration and the role of the translocation channel. Trends Cell Biol. 2004;14:568–575. doi: 10.1016/j.tcb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Booth PJ. Sane in the membrane: designing systems to modulate membrane proteins. Curr Opin Struct Biol. 2005;15:435–440. doi: 10.1016/j.sbi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Zarrine-Afsar A, Larson SM, Davidson AR. The family feud: do proteins with similar structures fold via the same pathway? Curr Opin Struct Biol. 2005;15:42–49. doi: 10.1016/j.sbi.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Compton EL, et al. Kinetics of an individual transmembrane helix during bacteriorhodopsin folding. J Mol Biol. 2006;357:325–338. doi: 10.1016/j.jmb.2005.12.042. [DOI] [PubMed] [Google Scholar]