Abstract
Islet transplantation is a promising treatment for diabetes but long-term success is limited by progressive graft loss. Aggregates of the beta cell peptide islet amyloid polypeptide (IAPP) promote beta cell apoptosis and rapid amyloid formation occurs in transplanted islets. Porcine islets are an attractive alternative islet source as they demonstrate long-term graft survival. We compared the capacity of transplanted human and porcine islets to form amyloid as an explanation for differences in graft survival. Human islets were transplanted into streptozotocin-diabetic immune-deficient mice. Amyloid deposition was detectable at 4 weeks posttransplantation and was associated with islet graft failure. More extensive amyloid deposition was observed after 8 weeks. By contrast, no amyloid was detected in transplanted neonatal or adult porcine islets that had maintained normoglycemia for up to 195 days. To determine whether differences in IAPP sequence between humans and pigs could explain differences in amyloid formation and transplant viability, we sequenced porcine IAPP. Porcine IAPP differs from the human sequence at 10 positions and includes substitutions predicted to reduce its amyloidogenicity. Synthetic porcine IAPP was considerably less amyloidogenic than human IAPP as determined by transmission electron microscopy, circular dichroism, and thioflavin T binding. Viability assays indicated that porcine IAPP is significantly less toxic to INS-1 beta cells than human IAPP. Our findings demonstrate that species differences in IAPP sequence can explain the lack of amyloid formation and improved survival of transplanted porcine islets. These data highlight the potential of porcine islet transplantation as a therapeutic approach for human diabetes.
Keywords: amylin, diabetes, xenotransplantation, pig, islet transplantation
Islet transplantation holds great promise as a treatment for patients with type 1 diabetes. The prospect of better glucose control and fewer complications has considerable appeal compared to traditional glucose monitoring and insulin injection regimens. The long-term success of human islet transplants has been limited, however, with 75% of patients who achieved insulin independence requiring insulin within 2 years posttransplant (1). Although immune rejection of islet allografts certainly plays a role in graft failure, nonimmune-mediated beta cell loss is also likely to play an important role (2–6).
Islet morphology in type 2 diabetes is characterized by progressive apoptotic beta cell loss (7) and the deposition of islet amyloid (8–10). The presence of amyloid in type 2 diabetic humans and in transgenic animal models is associated with beta cell loss and hyperglycemia (9, 11). Human islets transplanted into diabetic, immune-deficient murine recipients rapidly develop amyloid (12). Amyloid is detectable within a few weeks in transplanted human islets, compared to the many months or years thought to be required in type 2 diabetic humans and nonhuman primates. Interestingly, a recent histological study of human islets engrafted into the liver of a diabetic patient found extensive amyloid deposition within islets, only 5 years following transplantation (13). Transplanted murine islets transgenic for human islet amyloid polypeptide (IAPP) also display progressive deposition of amyloid and gradually lose their capacity to maintain normoglycemia (14). These studies raise the possibility that rapid amyloid formation in transplanted islets may be detrimental to graft function and mass, and may therefore be an unappreciated contributor to islet graft failure.
Islet amyloid forms by aggregation of IAPP (or amylin), a peptide that is produced and secreted by pancreatic beta cells (10). IAPP is released from beta cells in response to glucose and other stimuli that also trigger insulin secretion (15). IAPP is found in the beta cells of all mammals; however, not all species develop islet amyloid. The ability of IAPP to aggregate into amyloid fibrils is dependent upon the primary sequence of the peptide, which has now been determined in a number of species. These fibrils and smaller prefibrillar aggregates are cytotoxic and induce beta cell apoptosis, contributing to beta cell loss in type 2 diabetes. Analysis of the primary sequence of IAPP from mammalian species led to the initial suggestion that the region corresponding to residues 20–29 is a key determinant of its ability to form amyloid (16). In particular, rodent IAPP contains three proline residues in this region. Proline is well known to disrupt β-structure and is not compatible with the cross β-structure of amyloid. Rodent IAPP is soluble, does not form fibrils or prefibrillar aggregates, and unlike human IAPP, is not cytotoxic. More recent investigations have shown that additional regions of the peptide, in particular amino acids 8–20 and 30–37, are also likely to contribute to amyloid formation (17–20).
Xenotransplantation of pancreatic islets, using pigs or other animals as islet donors, has received increasing interest in recent years, given the limited number of human islets available for clinical transplantation. Many factors support the use of pigs as donors in islet xenotransplantation. Neonatal porcine islets show particular promise, as they are easily isolated, are highly resistant to hypoxia and hyperglycemia (21, 22), and have shown remarkable ability to expand their beta cell mass following transplantation (23). Notably, porcine islets have been shown to maintain long-term function following intraportal transplantation into nonhuman primates with production of detectable porcine C-peptide and restoration of normoglycemia (24–28). In a limited number of case reports, xenotransplantation of porcine islets into diabetic humans has improved glycemic control (29).
We hypothesized that one explanation for the promising reports of sustained glycemic control in porcine islet transplants may be the inability of porcine IAPP to form toxic aggregates and amyloid and therefore that transplanted pig islets will not be subject to rapid amyloid formation and IAPP-induced toxicity. To critically evaluate this hypothesis, we sequenced and synthesized pig IAPP and assessed its fibrillogenicity and toxicity compared to synthetic human IAPP. We also determined whether amyloid formation in human islets transplanted into immune-deficient mice is associated with graft failure and whether amyloid formation occurs in transplanted porcine islets.
Results
Rapid Amyloid Formation Is Associated with Human but Not Porcine Islet Graft Failure.
Islet amyloid forms rapidly in human islets transplanted into immune-deficient, diabetic murine recipients (14). To determine whether such aggregation correlates with graft dysfunction, we transplanted human islets into nonobese diabetic/severe combined immune deficiency (NOD/SCID) recipients. Before transplantation, amyloid was not detectable by thioflavin S staining in donor islets. Over a period of 4–8 weeks, some grafts consistently maintained normoglycemia whereas other grafts failed to maintain normoglycemia as demonstrated by glucose levels >15 mM at 4 or 8 weeks posttransplantation (Table S1).
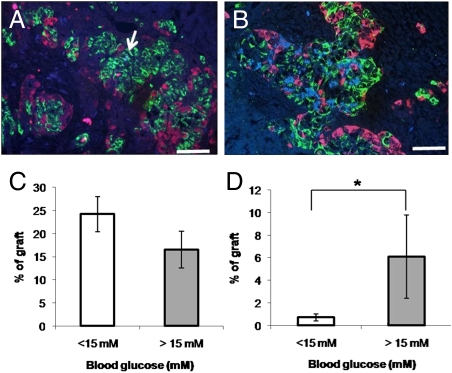
Variable amounts of amyloid were detectable in most grafts by 4 weeks (Fig. 1A). Amyloid deposition was usually greater by 8 weeks (Fig. 1B) posttransplantation. Upon graft harvest, mice whose grafts had consistently maintained normoglycemia tended to have a greater proportion of the graft area occupied by beta cells than those mice whose grafts had failed to maintain normoglycemia (Fig. 1C). The proportion of the graft occupied by amyloid was 0.7 ± 0.3% in normoglycemic mice and 6.1 ± 3.7% in mice in hyperglycemic recipients (P < 0.05; Fig. 1D). These experiments demonstrate that amyloid deposition is increased in failed human islet grafts in this mouse model and is associated with loss of beta cells.
Fig. 1.
Rapid amyloid formation is associated with human islet graft failure. Human islets were grafted in streptozotocin-diabetic NOD/SCID recipients as described in Materials and Methods (n = 43). Small amounts of amyloid (arrow) were detected by thioflavin S stain (blue) in grafts in normoglycemic recipients at 4 weeks posttransplant (A) but were more marked at 8 weeks posttransplant and in hyperglycemic recipients (B). Amyloid appeared adjacent to insulin-positive cells (green) and areas of apparent islet cell loss, but not glucagon-positive cells (red). (Scale bar, 50 μm.) Beta cell area (C) tended to be reduced and amyloid area was increased (D) in recipients of grafts with blood glucose values >15 mM at the time of graft harvest. The number of recipients in the normoglycemic and hyperglycemic recipients were 31 and 12, respectively. *, denotes statistically significant difference from normoglycemic (<15 mM) group (P < 0.05).
Adult porcine grafts maintained normoglycemia for up to 195 days posttransplantation, with the exception of one recipient out of six studied (Table S2). This recipient was borderline diabetic at 34 days posttransplantation and never achieved normoglycemia. Neonatal porcine islet grafts were unable to correct diabetes immediately after transplantation but eventually established and maintained euglycemia as transplanted beta cells were supplemented by the generation of new beta cells within the graft (23) (Table S3). Upon restoration of normoglycemia, neonatal porcine islet grafts consistently maintained glycemic control until time of sacrifice between 4 weeks and 3 months posttransplantation. Transplanted neonatal (n = 10) and adult (n = 6) porcine islets failed to stain positively for the presence of amyloid by thioflavin S even after 12 weeks posttransplantation (Fig. 2 D and E). In contrast, transplanted islets demonstrated strong insulin staining. Islet amyloid was also not detected in adult porcine pancreas (Fig. 2C).
Fig. 2.
Lack of amyloid in native or transplanted porcine islets. (A) Human islet. (B) Human islet graft. (C) Adult porcine islet. (D) Neonatal porcine islet graft. (E) Adult porcine islet graft. Amyloid is detectable by thioflavin S (blue) stain in human but not adult porcine islets. Amyloid develops rapidly in transplanted human islets but is not found in transplanted neonatal or adult porcine islets. Red immunostaining, insulin. (Scale bar, 50 μm.)
Porcine IAPP Sequence Explains Differences in Amyloid Formation and Toxicity.
To determine whether differences in the primary structure of the porcine polypeptide might explain the lack of amyloid formation in transplanted pig islets, we cloned and sequenced a porcine IAPP cDNA reverse transcribed using primers based on an EST sequence (BF712755) for porcine hypothalamus and RNA extracted from neonatal porcine islets. The sequence of mature porcine IAPP predicts a 37-amino-acid peptide (Fig. 3) that is well conserved in the N- and C-terminal regions of the molecule, but has unique amino acid differences in the midportion. The predicted sequence (GenBank accession GU396090) matches partial sequences (amino acids 3–34) that are deposited in SwissProt (Q29119) and GenBank (AAB05919.1). Comparison of the porcine and human sequences reveal amino acid differences in 10 positions, 5 of which reside within the 20–29 region thought to be critical for amyloid formation. Noteworthy differences include the substitution of a positively charged arginine at position 20 and a proline at position 29. Overall, porcine IAPP contains six residues that are potentially charged at neutral pH whereas human IAPP contains only three. These sequence differences are predicted to reduce the ability of the peptide to form amyloid as they increase the net charge and reduce the net hydrophobicity (30). This raises the question as to whether porcine IAPP is capable of forming amyloid.
Fig. 3.
Primary sequences of human and porcine islet amyloid polypeptide. (A) Human IAPP (hIAPP) sequence. (B) Porcine IAPP (pIAPP) sequence. All peptides have an amidated C terminus and free N terminus with a Cys-2 to Cys-7 disulfide bridge. Residues are numbered according to their position in mature IAPP. Residues differing from the human sequence are indicated in bold.
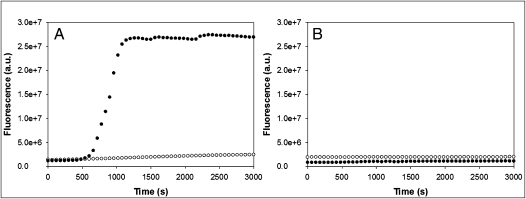
Amyloid formation is a complex process and the kinetics exhibit several distinctive features. A lag phase is observed in which no significant production of amyloid occurs followed by a rapid growth phase leading to a steady state in which soluble peptide is in equilibrium with amyloid fibrils. The ability of porcine IAPP to aggregate and form amyloid fibrils was investigated and compared to its human counterpart at a pH value mimicking that of the extracellular space (pH 7.4). Thioflavin T binds amyloid fibrils formed by aggregation of IAPP but does not bind to preamyloidogenic species and is a useful probe to assess the time course of IAPP aggregation. The experimental curve observed for human IAPP aggregation displayed the characteristic features expected for kinetics of amyloid formation (Fig. 4). At a concentration of 32 μM human IAPP, a short lag phase was observed in which no significant change in thioflavin-T fluorescence was detected, followed by a rapid rise in fluorescence leading to a plateau phase. These experiments were performed multiple times and similar results were obtained each time. The time for the reaction to reach 50% completion, the t50 value, was 862 ± 53 s for the human peptide, where the uncertainty is the apparent standard deviation based on three measurements. The behavior of the porcine peptide was dramatically different. At the same concentration of porcine IAPP, no significant increase in thioflavin-T fluorescence was observed even when the measurements were extended to times much longer than that required for human IAPP to fully convert to amyloid (Fig. 4). Identical results were obtained with independently prepared samples of porcine IAPP on different days.
Fig. 4.
Kinetics of human and porcine IAPP aggregation. (A) Thioflavin-T monitored aggregation kinetics of human (black circles) and porcine (white circles) IAPP indicate that porcine IAPP is less amyloidogenic than its human counterpart. (B) Thioflavin-T kinetics of porcine IAPP seeded by human IAPP aggregates. Seeding experiments were conducted by adding porcine IAPP to a solution containing either 3.2 μM (black circles) or 6.4 μM (white circles) preformed human IAPP amyloid fibrils. All reactions were monitored at pH 7.4 in 2% HFIP and 25 °C. Peptide concentration was 32 μM. Experiments were conducted with constant stirring to maintain solution homogeneity. Experiments were performed a minimum of three separate times and similar results were obtained.
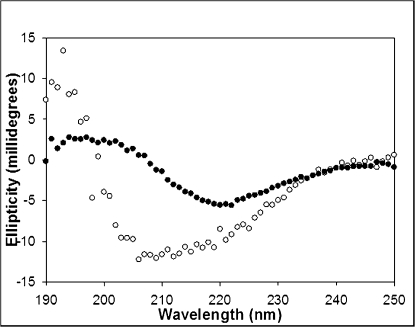
Amyloid fibrils are rich in β-sheet structure and therefore UV circular dichroism (CD) offers another convenient probe of amyloid formation. CD spectra taken of aliquots removed at the end of the respective thioflavin-T kinetic studies confirm that porcine and human IAPP have very different tendencies to form β-sheet (Fig. 5). The CD spectrum of human IAPP exhibits a strong minimum at 218 nm, a band associated with β-structure that is typical of the CD spectrum of IAPP-derived amyloid. In contrast, the CD spectrum of porcine IAPP displays a wide band ranging from 208 to 222 nm, indicative of a mixture of helical, random, and β-sheet structures. CD spectra were recorded on independently prepared samples of porcine IAPP on different days and similar results obtained.
Fig. 5.
Far UV CD spectra of human and porcine IAPP. Conformational analysis of human (black circles) and porcine (white circles) IAPP after 12-h incubation indicates that porcine IAPP has significantly less β-sheet propensity than the human peptide. Human IAPP adopts a classic β-sheet conformation signified by a signal minima at 218 nm and positive ellipticity below 200 nm. In contrast, porcine IAPP shows a wide band ranging from 208 to 222 nm, indicative of a mixture of helical and β-sheet structures. All reactions were monitored at pH 7.4 in 2% HFIP and 25 °C . Peptide concentration was 32 μM. Spectra are the average of three repeats. All samples contained 16 mM Tris HCl.
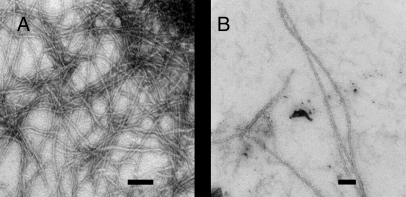
Neither CD nor thioflavin-T fluorescence provides direct information about the morphology of any aggregates formed. Consequently, transmission electron microscopy (TEM) was used to image the reaction products. TEM micrographs of human IAPP revealed dense clusters of rod-like fibrils with classic amyloid morphology (Fig. 6). These images are typical of those previously reported for human IAPP under similar conditions (19). A few long fibril-like structures were observed after incubation of 32 μM porcine IAPP for 24 h, but these fibrils were much fewer in number and appeared dispersed among a mat of smaller amorphous aggregates. Three independently prepared samples of porcine IAPP were imaged and similar TEMs obtained each time. The images displayed in Fig. 6 confirm that porcine IAPP is significantly less amyloidogenic than its human counterpart.
Fig. 6.
TEM micrographs of human and porcine IAPP at physiological pH. (A) After 24 h of incubation, human IAPP samples appear as dense mats of intertwined amyloid fibrils with the classic linear and unbranched morphology. (B) Porcine IAPP samples incubated for the same length of time show the presence of a few fibrillar strands among a bed of amorphous aggregates. TEM samples were incubated at pH 7.4 in 2% HFIP and 25 °C. Peptide concentration was 32 μM. (Scale bar, 100 nm.)
The inability of porcine IAPP to form amyloid may reflect the fact that it cannot form the critical nucleus required to initiate amyloid formation and/or that the sequence is not capable of binding to a nucleus. These two possibilities can be partially distinguished by seeding experiments in which a small amount of preformed human IAPP amyloid fibrils are added to a solution of porcine IAPP. We added porcine IAPP to a small amount of preformed human IAPP amyloid to test the ability of the human peptide to seed porcine IAPP aggregation. Thioflavin-T fluorescence measurements showed that porcine IAPP is incapable of fibril formation even when seeded by human IAPP (Fig. 4B). Although this result may not be particularly surprising given that seeding often occurs with high specificity and even seemingly minor changes in sequence can affect the ability to seed (31, 32), it is entirely consistent with our findings that porcine IAPP is not amyloidogenic.
Differences in Primary Sequence of Porcine and Human IAPP Explain Species Differences in IAPP Cytotoxicity.
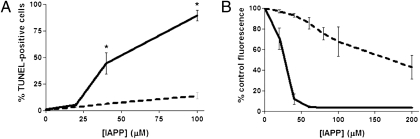
Given that synthetic porcine IAPP is much less amyloidogenic than human IAPP, we predicted that it would also be less toxic. The effect of porcine IAPP on beta cell toxicity was next assessed in transformed (INS-1) beta cells by Alamar blue reduction (Fig. 7B). As expected, addition of human IAPP (40 μM) substantially reduced INS-1 cell viability after 24 h (12 ± 5% viability) and further increases in IAPP concentration virtually eliminated any viable cells. In contrast, at the same concentration of porcine IAPP, INS-1 cell viability was significantly greater (93 ± 2%, P < 0.001). To confirm the reduced toxicity of porcine compared to human IAPP, we assessed the induction of apoptosis in INS-1 cells by transferase-mediated dUTP nick-end labeling (TUNEL) staining after 16 h of incubation of cells with either peptide (Fig. 7A). In the presence of 40 μM human IAPP, the proportion of apoptotic beta cells was 44 ± 10% compared to only 6 ± 1% (P < 0.001) following incubation in the presence of porcine IAPP. Even in the presence of a higher concentration of IAPP (100 μM), the proportion of TUNEL-positive beta cells was still much higher when cells were exposed to human (90 ± 5%) compared to porcine (14 ± 3%) IAPP (P < 0.001). We confirmed these results with porcine IAPP synthesized by two independent sources. Thus, porcine IAPP is much less fibrillogenic and cytotoxic than its human counterpart.
Fig. 7.
Porcine IAPP is less cytotoxic than human IAPP. (A) TUNEL staining following 16-h incubation of INS-1 cells in the presence of human or porcine IAPP. *, denotes statistically significant difference from porcine IAPP-treated cells (P < 0.05). (B) Alamar blue viability assay following 24-h incubation of INS-1 beta cells in the presence of human or porcine IAPP. The EC50 for human IAPP was 27 μM compared to 172 μM for porcine IAPP (P < 0.05). Solid line, human IAPP; dashed line, porcine IAPP.
Discussion
IAPP-derived amyloid deposits are hallmarks of islet pathology in type 2 diabetes. Whereas in type 2 diabetes islet amyloid formation takes months or years to develop, islet amyloid has been observed to form rapidly in cultured human islets (33), in human islets transplanted into diabetic murine recipients (34), and in islets from transgenic mice with beta cell expression of human IAPP (14, 35). In addition, a recent case report described the presence of extensive amyloid formation in transplanted islets, engrafted into the liver obtained at autopsy of a diabetic subject (13). We demonstrate here that rapid amyloid deposition in human islets transplanted into diabetic, immune-deficient mice is associated with loss of beta cells and graft failure. Taken together, these data support the hypothesis that amyloid deposition can play a significant role in reducing the viability of human islet grafts. Transplanted porcine islets, in contrast, formed no detectable amyloid following transplantation. Moreover, the sequence of porcine IAPP predicts a 37-amino-acid peptide with substantial differences from human IAPP. Indeed, we found synthetic porcine IAPP to be much less fibrillogenic and less toxic to beta cells than its human counterpart. We propose that the lower amyloidogenic potential and toxicity of porcine IAPP is likely to be a significant factor contributing to the better long-term survival and function of transplanted porcine islets in animal models, compared to human islets.
IAPP is found in the islet beta cells of most mammals; however, only those species in which an amyloidogenic sequence in the midportion of the IAPP molecule is conserved have a propensity to form toxic IAPP aggregates (16). Sequencing of the mature form of pig IAPP revealed that human and pig IAPP differ by 10 residues. Notably, porcine IAPP has a serine-for-proline substitution at residue 29, although feline IAPP, which is also amyloidogenic, also has a proline residue at position 29. Therefore, other amino acid substitutions within porcine IAPP, including V17D, S20R, and N31K, all of which involve the substitution of a neutral residue by a charged residue, are likely to be important. Interestingly, an S20G IAPP mutation in a subset of Japanese type 2 diabetic patients increases the amyloidogenicity of human IAPP and may accelerate the onset of diabetes in these patients (36).
We have found that amyloid also forms rapidly in cultured human islets and is associated with islet cell death (33, 37). Importantly, both amyloid formation and beta cell death can be prevented in cultured human islets by addition of short peptide inhibitors of IAPP aggregation (37) or by siRNA-mediated suppression of IAPP synthesis (33), indicating that IAPP aggregates are toxic to islet cells in situ. Our finding that amyloid formation is associated with graft failure supports the idea that inhibition of IAPP aggregation or synthesis may have therapeutic value in preserving human islet transplant viability. It seems likely that similar mechanisms may underlie the rapid amyloid formation that occurs in both transplanted and cultured islets. One plausible hypothesis is that IAPP or its precursors are unable to diffuse rapidly enough from the islet following secretion from beta cells, allowing it to aggregate within the islet. A second possibility is that beta cell dysfunction is characteristic of both cultured and transplanted islets and may manifest as impaired beta cell prohormone processing. Indeed, we have recently found that islet transplant recipients have impaired proinsulin processing (38) and that impaired processing of the IAPP precursor proIAPP leads to amyloid formation and beta cell death (39).
Given that the number of islet donors is far exceeded by the number of type 1 diabetic patients who could benefit from islet transplantation (40), new sources of transplantable beta cells are sorely needed. Pig islets show great potential in xenotransplantation. Neonatal porcine islets have been shown to maintain normoglycemia in immune-deficient murine recipients for more than 100 days, with no spontaneous failure (41), and similarly adult pig islet autografts were found to function for up to two years (42). Our findings are in keeping with two previous reports that also did not observe evidence of fibrils or islet amyloid in adult porcine pancreas or transplanted pig islets (42, 43).
In summary, our findings point to the importance of the amyloid-forming potential of IAPP and the toxicity of IAPP aggregates as a limiting factor to islet transplant survival. Should rapid formation of amyloid and associated beta cell loss be, as we now suspect, a significant limitation to human islet transplant success, we propose that use of pig islets will circumvent this limitation.
Materials and Methods
Porcine IAPP Sequencing.
Primers (Invitrogen) were designed according to EST sequence (Q29119) from porcine hypothalamus homologous to the predicted sequence for mature (amino acids 1–37) porcine IAPP (for details, see SI Materials and Methods). Reverse transcription was performed on RNA isolated from neonatal porcine islets using SuperScript First-Strand (Invitrogen). PCR was performed using Accuprime TaqDNA Polymerase High Fidelity (Invitrogen).
Peptide Synthesis and Preparation.
Porcine IAPP and porcine IAPP fragments were purchased from Bachem or synthesized in the Raleigh laboratory using methods previously optimized for the synthesis of human IAPP (44, 45). For details, see SI Materials and Methods.
Thioflavin-T Fluorescence Assay.
Thioflavin-T fluorescence was used to measure the development of structurally ordered fibrils over time. Fluorescence was measured on a Jobin Yvon Horiba Fluorescence Spectrophotometer. The excitation wavelength used was 445 nm and the emission was 485 nm; excitation and emission slits were set at 5 nm. A 1.0-cm cuvettete was used. All kinetic experiments were performed by diluting 68 μL of filtered peptide stock into Tris buffer containing thioflavin T. Final solution conditions were 16 mM Tris HCl and 65 μM thioflavin T at pH 7.4 (±0.3). The peptide concentration for kinetic assays was 32 μM in 2% HFIP at 25 °C. All solutions were stirred during fluorescence experiments to maintain solution homogeneity and increase the rate of the reaction to allow data collection in real time. Experiments were repeated multiple times on independently prepared samples of human and porcine IAPP. Similar results were obtained. The uncertainly in the t50 value for the human IAPP reaction was estimated as the apparent standard deviation based upon three repeats.
Circular Dichroism.
All CD experiments were performed at 25 °C on an Aviv 62A DS CD spectrophotometer. A few minutes before data collection, 300 μL of peptide solution from the kinetic assay was directly pipetted into a 0.1-cm quartz cuvette. Far-UV CD spectra are the average of five repeats over a range of 190–250 nm. Spectra were recorded at 1-nm intervals with an averaging time of 3 s. Background spectra were subtracted from collected data. The final peptide concentration for all CD experiments was 32 μM in 2% HFIP and 16 mM Tris HCl. The spectra are the average of three repeats. Spectra were recorded of multiple, independently prepared, samples of porcine IAPP and similar results were obtained each time.
Transmission Electron Microscopy.
Transmission electron microscopy (TEM) was performed at the University Life Science Microscopy Center at the State University of New York at Stony Brook. Aliquots (4 μL) were removed from the end of the thioflavin-T monitored kinetic experiments, placed on a carbon-coated 200-mesh copper grid, and negatively stained with saturated uranyl acetate. Three independently prepared samples of the porcine peptide were studied and the TEM images collected at the end of each independent kinetic run were indistinguishable. Multiple images were collected for each grid and the micrographs displayed in Fig. 6 are representative of the complete grid.
IAPP Cytoxicity Assays.
To assess toxicity of human and porcine IAPP, transformed rat insulinoma (INS-1) beta cells were used (for details, see SI Materials and Methods). Porcine and human IAPP were solubilized in RPMI culture media and added directly to cells. After 24 h, Alamar blue (Biosource International) was diluted 10-fold in culture media and cells were incubated for 3 h at 37 °C. Fluorescence (excitation 530; emission 590 nm) was measured by a Fluoroskan Ascent plate reader. After 14 h, cells were fixed in 4% paraformaldehyde (20 min), permeabilized with 0.5% Triton X-100 in PBS, and incubated with TUNEL reaction mixture (Roche Diagnostics) for 1 h at 37 °C. Cells were costained with Hoechst-33342 for 10 min. Images were taken using an Olympus BX-61 fluorescent microscope. Image quantification was performed using Image Pro-6.2. IAPP was tested at concentrations between 20 and 200 μM. Experiments were repeated in triplicate.
Immunohistochemistry and Thioflavin S Staining.
For double insulin and thioflavin S staining, sections were blocked in PBS containing 2.0% normal goat serum (Vector Laboratories) and incubated with guinea pig anti-insulin antibody (Dako) at a 1:100 dilution in PBS/1% BSA for one hour, followed by incubation with Texas Red–conjugated goat anti-guinea pig antibody (Jackson ImmunoResearch) for 1 h. All steps were performed at room temperature. Slides were then incubated in 0.5% thioflavin S solution for 2 min and rinsed with 70% ethanol.
Human Islet Transplantation.
All human islet transplant experiments were performed at the University of British Columbia (UBC) in compliance with institutional guidelines and approved by the UBC Animal Care Committee. Recipient NOD.scid mice were previously rendered hyperglycemic by a single i.p. injection of 180 mg/kg streptozotocin (STZ) (Sigma) in citrate buffer. Recipient mice were transplanted 3–5 days after STZ injection, when blood glucose levels were above 20 mmol/L. Human islets for these studies were isolated from pancreas obtained from 12 cadaveric organ donors by previously described isolation procedures (46). Recipient mice received transplants of 300–500 hand-picked human islets (or ≈1,000 IE) beneath the kidney capsule. Blood glucose was monitored twice weekly. After 4 or 8 weeks, the graft-containing kidney was removed from normoglycemic mice by nephrectomy and the animals allowed to recover, to ensure that normoglycemia was being maintained by the islet graft. Graft failure (return to hyperglycemia) was determined by two consecutive blood glucose measurements above 15 mM.
Neonatal Porcine Islet Isolation and Transplantation.
Neonatal porcine islets (NPI) were isolated from 1 to 3 day old Landrace-Yorkshire neonatal pigs (1.5–2.0 kg) using the method developed by Korbutt et al. (23) and cultured for 7–10 days at 37 °C, 20% CO2 in supplemented Ham’s F-10 medium (for details, see SI Materials and Methods). A single i.p. injection of STZ (200 mg/kg; Sigma) was administered to 8- to 10-week-old male SCID Biege mice to induce diabetes, and animals were considered to be diabetic after two consecutive blood glucose measurements ≥20 mM. Grafts containing a mass of 2,000 NPI were transplanted under the left kidney capsule in confirmed diabetic mice. Animals were monitored weekly for nonfasting blood glucose levels.
Adult Porcine Islet Isolation and Transplantation.
Pig pancreata were harvested and islets isolated and transplanted as detailed in SI Materials and Methods.
Statistical Analysis.
Data are expressed as mean ± SEM. Statistical analyses were performed using one-way ANOVA followed by a Newman-Keuls post hoc test. P < 0.05 was taken as significant. Data are representative of a minimum of three independent experiments performed in triplicate.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (GM078114 to D.P.R. and U42RR023245 to J.O.), the Canadian Institutes of Health Research (MOP-84516 and PPP-93068 to P.E.F.), the Juvenile Diabetes Research Foundation (#21-2006-882 to G.S.K.), and the Canadian Diabetes Association (C.B.V.). Support for human islet studies was provided by the Chicago Diabetes Project and NIH grant U42RR023245 (J.O.), the Michael Smith Foundation for Health Research (MSFHR) Centre for Human Islet Transplantation and Beta Cell Regeneration (G.W., C.B.V.), and the I.K. Barber Human Islet Laboratory. C.B.V. Is a Senior Scholar of the MSFHR. We thank D. Dai and G. Soukhatcheva for technical assistance and Dr. T. Kieffer for assistance in sequencing porcine IAPP.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909024107/DCSupplemental.
References
- 1.Shapiro AM, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 2.Menger MD, Yamauchi J, Vollmar B. Revascularization and microcirculation of freely grafted islets of Langerhans. World J Surg. 2001;25:509–515. doi: 10.1007/s002680020345. [DOI] [PubMed] [Google Scholar]
- 3.Avila JG, et al. Improved outcomes in islet isolation and transplantation by the use of a novel hemoglobin-based O2 carrier. Am J Transplant. 2006;6:2861–2870. doi: 10.1111/j.1600-6143.2006.01551.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee Y, et al. Metabolic mechanisms of failure of intraportally transplanted pancreatic beta-cells in rats: Role of lipotoxicity and prevention by leptin. Diabetes. 2007;56:2295–2301. doi: 10.2337/db07-0460. [DOI] [PubMed] [Google Scholar]
- 5.Noguchi H, et al. Activation of c-Jun NH2-terminal kinase (JNK) pathway during islet transplantation and prevention of islet graft loss by intraportal injection of JNK inhibitor. Diabetologia. 2007;50:612–619. doi: 10.1007/s00125-006-0563-2. [DOI] [PubMed] [Google Scholar]
- 6.Yin D, et al. Liver ischemia contributes to early islet failure following intraportal transplantation: Benefits of liver ischemic-preconditioning. Am J Transplant. 2006;6:60–68. doi: 10.1111/j.1600-6143.2005.01157.x. [DOI] [PubMed] [Google Scholar]
- 7.Butler AE, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 8.Clark A, et al. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: Quantitative changes in the pancreas in type 2 diabetes. Diabetes Res. 1988;9:151–159. [PubMed] [Google Scholar]
- 9.Kahn SE, Andrikopoulos S, Verchere CB. Islet amyloid: A long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes. 1999;48:241–253. doi: 10.2337/diabetes.48.2.241. [DOI] [PubMed] [Google Scholar]
- 10.Westermark P, et al. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci USA. 1987;84:3881–3885. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verchere CB, et al. Islet amyloid formation associated with hyperglycemia in transgenic mice with pancreatic beta cell expression of human islet amyloid polypeptide. Proc Natl Acad Sci USA. 1996;93:3492–3496. doi: 10.1073/pnas.93.8.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westermark GT, Westermark P, Nordin A, Törnelius E, Andersson A. Formation of amyloid in human pancreatic islets transplanted to the liver and spleen of nude mice. Ups J Med Sci. 2003;108:193–203. doi: 10.3109/2000-1967-113. [DOI] [PubMed] [Google Scholar]
- 13.Westermark GT, Westermark P, Berne C, Korsgren O, Nordic Network for Clinical Islet Transplantation Widespread amyloid deposition in transplanted human pancreatic islets. N Engl J Med. 2008;359:977–979. doi: 10.1056/NEJMc0802893. [DOI] [PubMed] [Google Scholar]
- 14.Udayasankar J, et al. Amyloid formation results in recurrence of hyperglycaemia following transplantation of human IAPP transgenic mouse islets. Diabetologia. 2009;52:145–153. doi: 10.1007/s00125-008-1185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahn SE, et al. Evidence of cosecretion of islet amyloid polypeptide and insulin by beta-cells. Diabetes. 1990;39:634–638. doi: 10.2337/diab.39.5.634. [DOI] [PubMed] [Google Scholar]
- 16.Westermark P, Engström U, Johnson KH, Westermark GT, Betsholtz C. Islet amyloid polypeptide: Pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci USA. 1990;87:5036–5040. doi: 10.1073/pnas.87.13.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scrocchi LA, et al. Identification of minimal peptide sequences in the (8-20) domain of human islet amyloid polypeptide involved in fibrillogenesis. J Struct Biol. 2003;141:218–227. doi: 10.1016/s1047-8477(02)00630-5. [DOI] [PubMed] [Google Scholar]
- 18.Abedini A, Raleigh DP. The role of His-18 in amyloid formation by human islet amyloid polypeptide. Biochemistry. 2005;44:16284–16291. doi: 10.1021/bi051432v. [DOI] [PubMed] [Google Scholar]
- 19.Abedini A, Raleigh DP. Destabilization of human IAPP amyloid fibrils by proline mutations outside of the putative amyloidogenic domain: Is there a critical amyloidogenic domain in human IAPP? J Mol Biol. 2006;355:274–281. doi: 10.1016/j.jmb.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 20.Green J, et al. Full-length rat amylin forms fibrils following substitution of single residues from human amylin. J Mol Biol. 2003;326:1147–1156. doi: 10.1016/s0022-2836(02)01377-3. [DOI] [PubMed] [Google Scholar]
- 21.Harb G, Korbutt GS. Effect of prolonged in vitro exposure to high glucose on neonatal porcine pancreatic islets. J Endocrinol. 2006;191:37–44. doi: 10.1677/joe.1.06812. [DOI] [PubMed] [Google Scholar]
- 22.Emamaullee JA, Shapiro AM, Rajotte RV, Korbutt G, Elliott JF. Neonatal porcine islets exhibit natural resistance to hypoxia-induced apoptosis. Transplantation. 2006;82:945–952. doi: 10.1097/01.tp.0000238677.00750.32. [DOI] [PubMed] [Google Scholar]
- 23.Korbutt GS, et al. Large scale isolation, growth, and function of porcine neonatal islet cells. J Clin Invest. 1996;97:2119–2129. doi: 10.1172/JCI118649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cardona K, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12:304–306. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 25.Dufrane D, Gianello P. Pig islet xenotransplantation into non-human primate model. Transplantation. 2008;86:753–760. doi: 10.1097/TP.0b013e3181840f55. [DOI] [PubMed] [Google Scholar]
- 26.van der Windt DJ, et al. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant. 2009;9:2716–2726. doi: 10.1111/j.1600-6143.2009.02850.x. [DOI] [PubMed] [Google Scholar]
- 27.Hering BJ, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12:301–303. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 28.Casu A, et al. Metabolic aspects of pig-to-monkey (Macaca fascicularis) islet transplantation: Implications for translation into clinical practice. Diabetologia. 2008;51:120–129. doi: 10.1007/s00125-007-0844-4. [DOI] [PubMed] [Google Scholar]
- 29.Elliott RB, et al. Live encapsulated porcine islets from a type 1 diabetic patient 9.5 yr after xenotransplantation. Xenotransplantation. 2007;14:157–161. doi: 10.1111/j.1399-3089.2007.00384.x. [DOI] [PubMed] [Google Scholar]
- 30.Chiti F, Stefani M, Taddei N, Ramponi G, Dobson CM. Rationalization of the effects of mutations on peptide and protein aggregation rates. Nature. 2003;424:805–808. doi: 10.1038/nature01891. [DOI] [PubMed] [Google Scholar]
- 31.O’Nuallain B, Williams AD, Westermark P, Wetzel R. Seeding specificity in amyloid growth induced by heterologous fibrils. J Biol Chem. 2004;279:17490–17499. doi: 10.1074/jbc.M311300200. [DOI] [PubMed] [Google Scholar]
- 32.Krebs MR, Morozova-Roche LA, Daniel K, Robinson CV, Dobson CM. Observation of sequence specificity in the seeding of protein amyloid fibrils. Protein Sci. 2004;13:1933–1938. doi: 10.1110/ps.04707004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marzban L, et al. Small interfering RNA-mediated suppression of proislet amyloid polypeptide expression inhibits islet amyloid formation and enhances survival of human islets in culture. Diabetes. 2008;57:3045–3055. doi: 10.2337/db08-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westermark P, Eizirik DL, Pipeleers DG, Hellerström C, Andersson A. Rapid deposition of amyloid in human islets transplanted into nude mice. Diabetologia. 1995;38:543–549. doi: 10.1007/BF00400722. [DOI] [PubMed] [Google Scholar]
- 35.de Koning EJ, et al. Intra- and extracellular amyloid fibrils are formed in cultured pancreatic islets of transgenic mice expressing human islet amyloid polypeptide. Proc Natl Acad Sci USA. 1994;91:8467–8471. doi: 10.1073/pnas.91.18.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma Z, et al. Enhanced in vitro production of amyloid-like fibrils from mutant (S20G) islet amyloid polypeptide. Amyloid. 2001;8:242–249. doi: 10.3109/13506120108993820. [DOI] [PubMed] [Google Scholar]
- 37.Potter KJ, et al. Amyloid inhibitors enhance survival of cultured human islets. Biochim Biophys Acta. 2009;1790:566–574. doi: 10.1016/j.bbagen.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Klimek AM, et al. Impaired proinsulin processing is a characteristic of transplanted islets. Am J Transplant. 2009;9:2119–2125. doi: 10.1111/j.1600-6143.2009.02740.x. [DOI] [PubMed] [Google Scholar]
- 39.Marzban L, et al. Impaired NH2-terminal processing of human proislet amyloid polypeptide by the prohormone convertase PC2 leads to amyloid formation and cell death. Diabetes. 2006;55:2192–2201. doi: 10.2337/db05-1566. [DOI] [PubMed] [Google Scholar]
- 40.Naftanel MA, Harlan DM. Pancreatic islet transplantation. PLoS Med. 2004;1(3):e58. doi: 10.1371/journal.pmed.0010058. quiz e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rayat GR, Gill RG. Indefinite survival of neonatal porcine islet xenografts by simultaneous targeting of LFA-1 and CD154 or CD45RB. Diabetes. 2005;54:443–451. doi: 10.2337/diabetes.54.2.443. [DOI] [PubMed] [Google Scholar]
- 42.Emamaullee JA, et al. Porcine marginal mass islet autografts resist metabolic failure over time and are enhanced by early treatment with liraglutide. Endocrinology. 2009;150:2145–2152. doi: 10.1210/en.2008-1116. [DOI] [PubMed] [Google Scholar]
- 43.Lukinius A, Korsgren O, Grimelius L, Wilander E. Expression of islet amyloid polypeptide in fetal and adult porcine and human pancreatic islet cells. Endocrinology. 1996;137:5319–5325. doi: 10.1210/endo.137.12.8940352. [DOI] [PubMed] [Google Scholar]
- 44.Abedini A, Raleigh DP. Incorporation of pseudoproline derivatives allows the facile synthesis of human IAPP, a highly amyloidogenic and aggregation-prone polypeptide. Org Lett. 2005;7:693–696. doi: 10.1021/ol047480+. [DOI] [PubMed] [Google Scholar]
- 45.Abedini A, Singh G, Raleigh DP. Recovery and purification of highly aggregation-prone disulfide-containing peptides: Application to islet amyloid polypeptide. Anal Biochem. 2006;351:181–186. doi: 10.1016/j.ab.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 46.Warnock GL, Kneteman NM, Evans MG, Rajotte RV. Isolation of purified large mammal and human islets of Langerhans. Horm Metab Res Suppl. 1990;25:37–44. [PubMed] [Google Scholar]
- 47.Bottino R, et al. Isolation outcome and functional characteristics of young and adult pig pancreatic islets for transplantation studies. Xenotransplantation. 2007;14:74–82. doi: 10.1111/j.1399-3089.2006.00374.x. [DOI] [PubMed] [Google Scholar]
- 48.Balamurugan AN, et al. Harmful delayed effects of exogenous isolation enzymes on isolated human islets: Relevance to clinical transplantation. Am J Transplant. 2005;5:2671–2681. doi: 10.1111/j.1600-6143.2005.01078.x. [DOI] [PubMed] [Google Scholar]
- 49.Bertera S, et al. Gene transfer of manganese superoxide dismutase extends islet graft function in a mouse model of autoimmune diabetes. Diabetes. 2003;52:387–393. doi: 10.2337/diabetes.52.2.387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.