Abstract
Dopamine neurotransmission controls motor and perseverative behavior, is mediated by protein phosphorylation, and may be perturbed in disorders of attention and hyperactivity. To assess the role of casein kinase I (CK1) in the regulation of dopamine signaling, we generated a genetically modified mouse line that overexpresses CK1δ (CK1δ OE) specifically in the forebrain. Overexpression was confirmed both at the mRNA and at the protein levels. Under basal conditions, CK1δ OE mice exhibited horizontal and vertical hyperactivity, reduced anxiety, and nesting behavior deficiencies. The CK1δ OE mice also presented paradoxical responses to dopamine receptor stimulation, showing hypoactivity following injection of d-amphetamine or methylphenidate, indicating that CK1 activity has a profound effect on dopamine signaling in vivo. Interestingly, CK1δ overexpression led to significantly reduced D1R and D2R dopamine receptor levels. All together, under basal conditions and in response to drug stimulation, the behavioral phenotype of CK1δ OE mice is reminiscent of the symptoms and drug responses observed in attention-deficit/hyperactivity disorder and therefore the CK1δ OE mice appear to be a model for this disorder.
Keywords: CK1, D1R, methylphenidate, protein kinase amphetamine
Casein kinase 1 (CK1) represents an evolutionarily conserved eukaryotic protein kinase family consisting of several isoforms including CK1δ, which is one of the most enriched in brain. The CK1 Ser/Thr kinase family plays a crucial role in numerous biological functions ranging from cell cycle regulation (1–5) to more complex behavioral traits (6–8). In the central nervous system, CK1δ is involved in a variety of physiological (e.g., cell signaling, circadian rhythm, cellular trafficking) and pathological (e.g., amyloid-β formation and tauopathies) processes (9–14). In the basal ganglia, CK1 regulates the state of phosphorylation of DARPP-32 (dopamine- and cAMP-regulated phospho-protein Mw of 32 kDa), a key striatal protein, which integrates synaptic input signals from various origins including the dopaminergic and glutamatergic systems (15–17). Imbalance of dopamine neurotransmission in the nigrostriatal pathway has been linked to various neurodevelopmental disorders, such as attention-deficit/hyperactivity disorder (ADHD) (18–22). Indeed, the fronto-striatal network has been largely implicated in the occurrence of the cardinal features of ADHD, which are hyperactivity, inattention, and impulsivity (for a review see ref. 23).
In addition to regulation of DARPP-32, CK1 is likely to influence other aspects of neuronal function. The consensus sequence for CK1-dependent phosphorylation is well established (24, 25). However, the frequent requirement for a phospho-Ser or phospho-Thr residue close to the site actually phosphorylated by CK1 makes it challenging to predict if a given protein will be phosphorylated by CK1. Moreover, existing CK1 inhibitors are not brain permeable. To circumvent these caveats, we chose to investigate the physiological importance of CK1 in vivo, in the context of striatal signaling, by engineering a genetically modified mouse line overexpressing CK1δ, specifically in the forebrain. We used the tetracycline-inducible system to overexpress CK1 specifically in the striatum, cortex, and hippocampus. We report here the consequences of CK1δ overexpression (CK1δ OE) on the behavioral performance of mice under basal conditions and after exposure to drugs targeting the dopaminergic or glutamatergic systems. Interestingly, both D1R and D2R dopaminergic pathways are altered by CK1δ overexpression, whereas glutamatergic function appeared unchanged. Because some of the behavioral characteristics of the CK1δ OE mice resemble those of ADHD (22), we hypothesize that CK1 may represent a molecular target that plays an important role in perturbation of dopaminergic signaling mechanisms underlying ADHD.
Results
CK1δ Overexpression in the Forebrain.
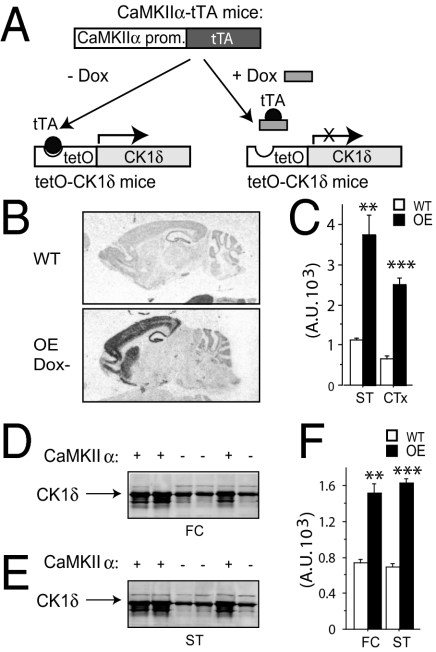
A transgenic mouse line with inducible CK1δ overexpression in the forebrain was generated by taking advantage of the tetracycline-inducible system. The exogenous CK1δ overexpression is controlled by a tetO promoter specifically activated by the transcriptional activator tTA expressed in a CaMKIIα specific manner (Fig. 1A). Mice were kept doxycycline-free from conception. As shown by in situ hybridization, the overexpression of CK1δ (CK1δ+/tTA+) was mainly detected in the striatum and cerebral cortex in the CK1δ OE mice (6–8 weeks of age) compared with control littermate mice (CK1δ+/tTA-) (WT)(Fig. 1B). Quantification of in situ hybridization autoradiographs revealed that the CK1δ mRNA level was increased both in the striatum (3.3-fold) and in the cortex (3.8-fold) of CK1δ OE mice compared to WT control littermates (Fig. 1 B and C). Western blot analysis indicated that protein levels of CK1δ were higher in the striatum (2.3-fold) and frontal cortex (2.0-fold) of CK1δ OE mice compared to WT littermate animals (Fig. 1 D–F).
Fig. 1.
Generation of CK1δ overexpressing mice (OE). (A) Mouse breeding strategy to generate CK1δ OE mice by crossing the tetO-CK1δ transgenic mice with CaMKIIα-tTA transgenic mice. (B) Autoradiographs of in situ hybridization with 33pUTP-labeled CK1δ probe to detect mRNA expression in sagittal slices. (Top) WT; (Bottom) CK1δ OE -dox. (C) Quantification of autoradiograms from B using National Institutes of Health image software. Data represent mean values ± SEM; n = 5 sections per brain area and three brains per genotype. ANOVA Fisher’s PLSD test, **, P < 0.005 and ***, P < 0.0001. (D and E) Western blot analysis showing the protein level of CK1δ in frontal cortex (FC). (D) and striatum (ST). (E) of CK1δ OE mice (+) and WT littermates (−). (F) Quantification of autoradiograms (major band indicated by an arrow) from D and E using National Institutes of Health image. Data represent mean values ± SEM, ANOVA n = 3 each genotype. **, P < 0.005 and ***, P < 0.0005. Error bar represents SEM.
CK1δ OE Mice Exhibit Increased Horizontal and Vertical Locomotion.
CK1δ overexpression did not affect overall health, appearance, feeding, or reproduction. In addition, the OE mice exhibited normal social interaction patterns, such as social avoidance, social contact, and social time, compared to WT mice as determined by social interaction tests (Fig. S1).
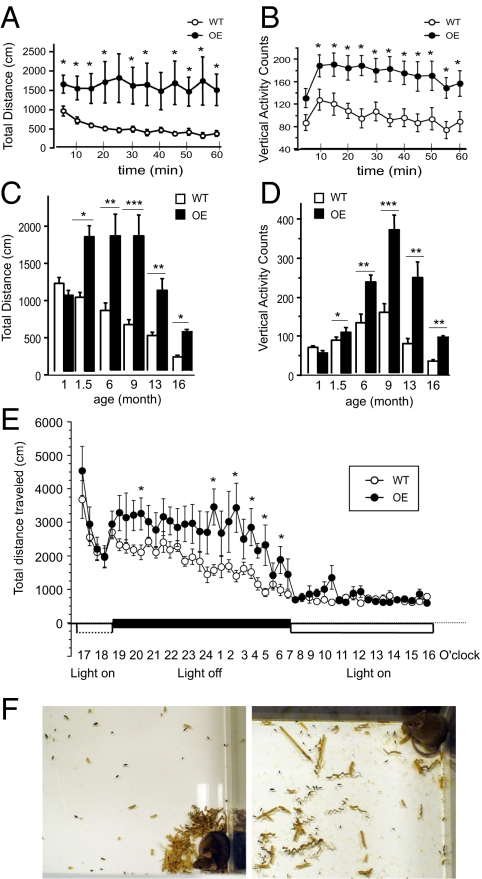
Locomotor activity of CK1δ OE mice was assessed in the open field (OF) paradigm. CK1δ OE mice exhibited significantly increased horizontal and vertical activity compared to WT littermates (3 months old) (Fig. 2 A and B). It was found that this phenotypic difference appeared when mice were 6 weeks old and persisted at least until they were 16 months old (Fig. 2 C and D).
Fig. 2.
Locomotor activities of CK1δ OE mice in the OF paradigm. (A–B) Locomotor activity in 3-month-old mice was recorded in the OF paradigm for 60 min (5 min bins per data point). (C–D) Bar graphs show the mean values ± SEM of total distance traveled (C) and vertical activities (D) in the OF paradigm for 60 min in mouse groups of various ages. (E) Total distance traveled by CK1δ OE and WT mice in an OF test for 24 h (30 min per point) under a light cycle of 12-h light and 12-h dark (horizontal black bar for dark period and horizontal open bar for light period). (F) Photographs of OF boxes following the 24-h OF experiment (Left: WT; Right CK1δ OE). ANOVA Fisher’s PLSD test; 1-month-old group: n = 5 WT, n = 6 OE; 1.5-month-old group: n = 7 WT, n = 7 OE; 3-month-old group: n = 10 WT, n = 11 OE; other groups: n = 8 for each genotype. *, P < 0.05; **, P < 0.005; and ***, P < 0.0001. Error bar represents SEM.
CK1δ and CK1ε are important regulators of circadian rhythm (6–8). Therefore, we investigated the possibility that CK1δ OE mice might have an altered day/night activity pattern. We scored locomotor activity over a period of 24 h under a standard 12-h light cycle in an OF paradigm. Although the CK1δ OE mice were confirmed to be overactive, under these conditions, the periods of activity and inactivity for both groups were fully overlapping (Fig. 2E), demonstrating that CK1δ OE mice do not have any obvious altered day/night activity pattern. Notably, after 24 h spent in the OF boxes, the WT mice organized the bedding material into a nest (three cornered nests and one centered nest). In contrast, none of the four CK1δ OE mice constructed a nest. The OF box was also more homogenously messy for the CK1δ OE mice compared to the WT mice (Fig. 2F).
CK1δ OE Mice Exhibit Lower Anxiety-Like Behaviors.
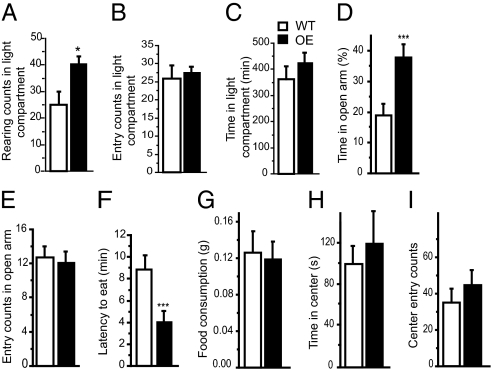
Four different behavioral paradigms were used to probe for anxiety-like behaviors in CK1δ OE mice. In the dark-light choice test (DLC), the CK1δ OE mice exhibited more rearing in the light compartment compared to their WT littermates (Fig. 3A). However, no difference was observed between the two groups of mice when scoring the entry and the time spent in the light compartment (Fig. 3 B and C). In the elevated place maze (EPM), CK1δ OE mice spent more time in the open arm than did their control littermates (Fig. 3 D and E). The novelty suppressed feeding paradigm (NSF), in which latency to begin feeding is measured (26, 27), has been employed to test anxiety-like behaviors. The CK1δ OE mice showed a shorter latency to start feeding in the novel environment than the WT mice (Fig. 3F)(n = 10; ANOVA; **P = 0.0035). No difference was observed in food consumption in the home cage (Fig. 3G). There was no difference between the CK1δ OE and WT mice in terms of the frequency of entering into, or the time spent in, the center of the OF, indicating no difference in the general anxiety-related behavior in CK1δ OE mice (Fig. 3 H and I). Forced swimming (FS) and tail suspension (TS) tests are paradigms often used to measure depression-like behaviors in rodents by scoring their immobility time when left in water (25 °C) or suspended by their tails. No difference in immobility time was observed in these tests for the CK1δ OE mice compared to control littermates (immobility in FS test: WT= 58 s ± 16 vs. CK1δ OE= 51 s ± 19; immobility in TS test: WT= 100 s ± 21 vs. CK1δ OE= 109 s ± 16).
Fig. 3.
Behavioral performance of CK1δ OE mice in anxiety-related paradigms. To evaluate the CK1δ OE mice emotional state, mice were tested using the DLC paradigm (A–C), the EPM test (D–E), the NSF test (F–G), the FS test (H), and the TS test (I). Rearing (A), entry (B), and time (C) spent in the light compartment were recorded for 30 min (n = 11 each genotype). Time spent (D) and entries (E) in the open arm were recorded for 6 min (n = 21 each genotype). (F) The latency time to start eating was recorded (n = 16 WT, n = 20 OE). (G) Food consumption in the home cage immediately after the NSF test was measured for 5 min (n = 16 WT, n = 20 OE). (H) The time spent in the center of the OF arena (n = 10 WT, n = 9 OE). (I) Entry counts in the center of the OF arena (n = 10 each genotype). ANOVA *, P < 0.05, **, P < 0.01, ***, P < 0.005. Error bar represents SEM.
CK1δ OE Mice Have Altered Sensitivity to Amphetamine and Methylphenidate.
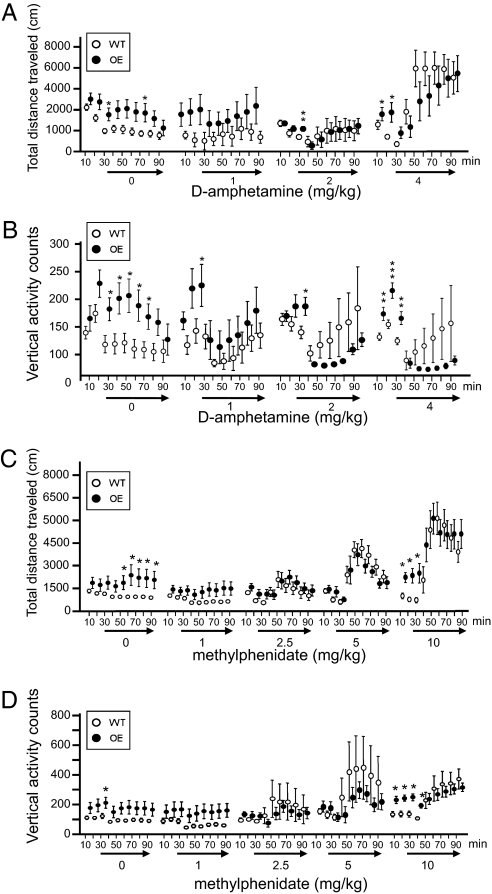
CK1δ OE mice differed from WT mice in their behavioral response to acute D-amphetamine injection (1, 2, and 4 mg/kg). At the highest dose tested, D-amphetamine induced hyperlocomotion of CK1δ OE mice but the full response was delayed by almost 40 min compared to control mice (Fig. 4A). Control littermates showed amphetamine-induced hyperactivity in a dose-dependent manner reaching a full response within 10 min following drug administration (4 mg/kg) (Fig. 4A). Moreover, the altered sensitivity to amphetamine between the two genotypes was even more apparent on the other phenotypical feature of the CK1δ OE mice, namely, vertical activity (e.g., frequent and repetitive jumping). The vertical activity was entirely suppressed in the CK1δ OE mice in a dose dependent manner (Fig. 4B). In contrast, vertical activity in the WT mice was enhanced at all three doses tested (Fig. 4B).
Fig. 4.
Effects of D-amphetamine and methylphenidate treatment on locomotion. Locomotor activities were measured in the OF paradigm for 30 min before injection and for another 60 min after injection of D-amphetamine (A and B) or methylphenidate (C and D) at the doses indicated. Graphs show the mean values ± SEM (10 min bins). ANOVA Fisher’s PLSD test, for D-amphetamine treatment: n = 12 WT, n = 9 OE in saline group; n = 8 WT, n = 7 OE in 1 mg/kg group; n = 6 WT, n = 7 OE in 2 mg/kg group; n = 10 each genotype in 4 mg/kg group; and for methylphenidate treatment: n = 18 WT, n = 13 OE in saline group; n = 8 WT, n = 7 OE in 1 mg group; n = 8 each genotype in 2.5 and 5 mg group; n = 7 WT, n = 10 OE in 10 mg group; *, P < 0.05, **, P < 0.01, ***, P < 0.005.
Interestingly, methylphenidate, the most efficient and widely used drug to treat ADHD, diminished the horizontal hyperactivity of the CK1δ OE mice to the level of the WT mice and diminished the vertical hyperactivity of the CK1δ OE mice to a lower level than that of the WT mice (Fig. 4 C and D).
Effect of D1R and D2R Agonists/Antagonists on Locomotor Activity of CK1δ OE Mice.
Our previous studies have indicated that CK1 is involved in modulating dopamine transmission through the phosphorylation of the major integrator for dopamine signaling in the striatum, DARPP-32 (28). Because the nigrostriatal dopaminergic pathway is implicated in regulation of motor function, we characterized the impact of D1R and D2R agonists or antagonists on the locomotor activity of CK1δ OE mice.
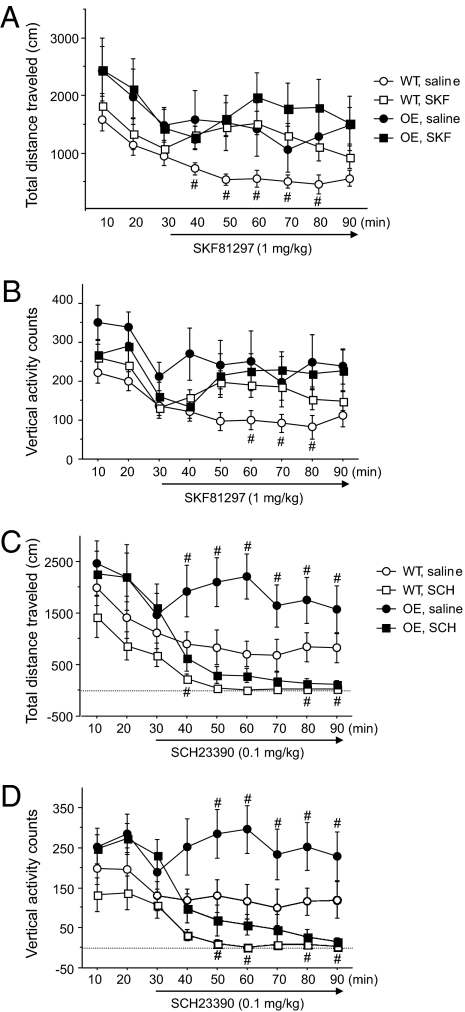
As expected, the D1R agonist, SKF 81297 (1 mg/kg), increased horizontal and vertical locomotor activity in WT mice (Fig. 5 A and B). SKF 81297 did not induce hyperactivity in CK1δ OE mice. The D1R antagonist, SCH23390, inhibited the locomotion of mice for both genotypes, but it was more effective at the three doses tested (0.1, 0.25, and 0.5 mg/kg) in suppressing hyperactivities in CK1δ OE mice considering that the basal activity was higher (Fig. 5 C and D).
Fig. 5.
Effects of D1R agonist and antagonist treatments on locomotor activity. Locomotor activities were measured in the OF paradigm for 30 min before injection and for another 60 min after injection of SKF 81297 (A and B) or SCH23390 (C and D). Graphs show the mean values ± SEM (10 min bins). ANOVA Fisher’s PLSD test, n = 10 each genotype in the SKF 81297 experiment, n = 7 each genotype in the SCH23390 experiment; #, P < 0.05 (treatment effect for a given genotype).
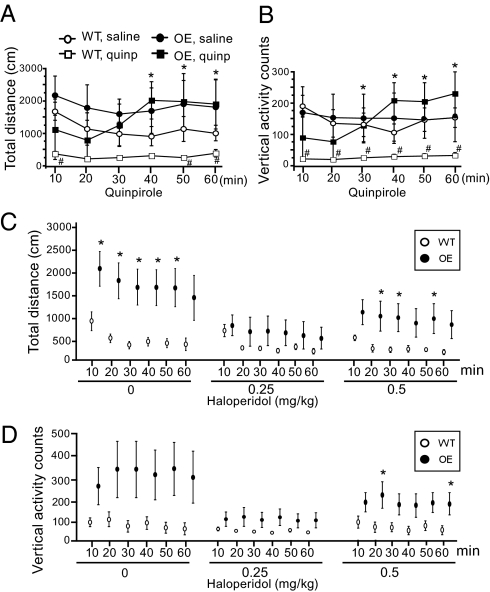
The administration of a D2R agonist, quinpirole (1 mg/kg), decreased horizontal and vertical activities for both the CK1δ OE and WT mice (Fig. 6 A and B). Interestingly, the inhibitory effect of quinpirole (1 mg/kg) on CK1δ OE mice was short-lasting: a clear trend of activity reappeared 40 min post-injection. In contrast, activity in WT mice was inhibited for up to 60 min post-injection (Fig. 6 A and B). The D2R antagonist, haloperidol, inhibited the hyperactivity of CK1δ OE mice as expected (Fig. 6 C and D). Noticeably, the effect on the CK1δ OE mice was greater at the lower dose tested. There was no obvious effect of haloperidol on WT mice, possibly due to the low basal activity of these mice. No dose-sensitive effect was observed for the WT mice.
Fig. 6.
Effects of D2R agonist and antagonist treatments on locomotor activity. Locomotor activities were measured in the OF paradigm for 60 min, specifically from 30 min to 90 min after injection of quinpirole (1mg/kg) (A and B) or haloperidol (C and D) at the doses indicated. Graphs show the mean values ± SEM (10 min bins). ANOVA Fisher's PLSD test, n = 7 each genotype in the quinpirole experiment, and n = 10 each genotype in the haloperidol experiment, *, P < 0.05 (genotype difference), #, P < 0.05 (treatment effect).
Effect of MK801, an NMDA Antagonist, on CK1δ OE Mice.
MK801, a noncompetitive antagonist of the NMDA receptor, had opposite effects on horizontal versus vertical activity of CK1δ OE mice. This compound rapidly increased horizontal activity, whereas it rapidly reduced vertical activity. The effects of MK801 on horizontal and vertical activities were also different in the WT mice (Fig. S2 A and B) (ANOVA; n = 10); the effect on CK1δ OE mice was very rapid, whereas the effect on wild type mice took 20–30 min to develop.
Effect of CK1δ OE on D1R, D2R, and NMDA Receptor Densities.
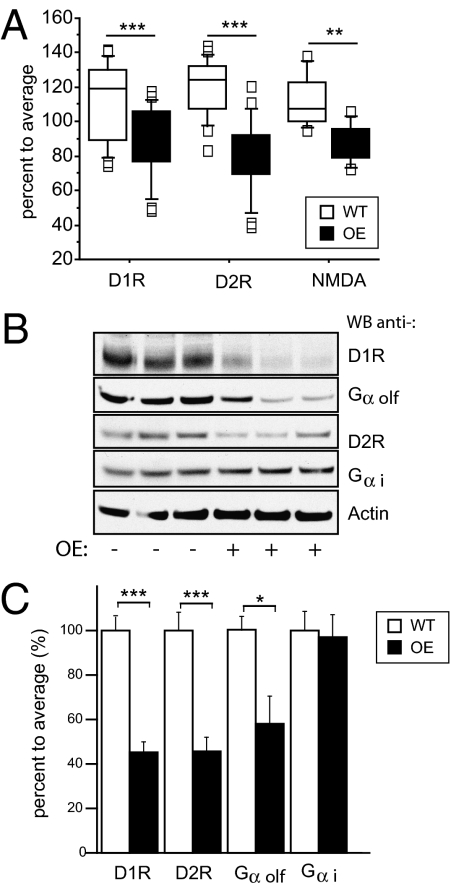
Three-month-old CK1δ OE animals were used for ligand binding experiments in situ to evaluate the expression levels of dopamine and NMDA receptors, taking advantage of radiolabeled ligands (3H-SCH23390, 3H-quinpirole, and 3H-MK801). D1R, D2R, and NMDA expression levels at the cell surface were decreased 15, 30, and 9%, respectively, in the CK1δ OE mice (Fig. 7A). Results from Western blot analysis indicated that total D1R, D2R, and Gαolf were down-regulated by 59%, 54%, and 50%, respectively, in the striatum of CK1δ OE mice (Fig. 7 B and C).
Fig. 7.
Expression levels of D1, D2, and NMDA receptors. (A) Box-and-Whisker plot illustrates quantitation of autoradiographs from ligand binding assays with 3H-SCH23390 (D1R specific antagonist), 3H-quinpirole (D2R specific antagonist), and 3H-MK801 (NMDA receptor antagonist) (n = 5 sections of 3 brains per genotype). Whiskers represent the 1.5 interquartile range. ANOVA, n = 5 or 7 each genotype, P < 0.005, and ***, P < 0.0005. Graph shows the mean values ± SEM. (B) Western blotting analysis of mouse striatal tissue using anti-D1R, anti-Gαolf, anti-D2R, anti-Gαi antibodies, and anti-actin. Three representative samples are shown for each genotype. (C) The Western blots were quantified and data were normalized to beta actin levels (n = 11 per genotype, *, P < 0.01, ***, P < 0005, error bars are SEM).
Discussion
For this study, we generated mice overexpressing CK1δ specifically in the forebrain. Phenotypically, the CK1δ OE mice display hyperlocomotion, frequent jumping, and lower behavioral inhibition. They exhibit altered behavioral responses to the dopamine agonists, amphetamine, SKF81297, and quinpirole. Moreover, their basal hyperactivity was suppressed by the dopamine antagonists, SCH23390 and haloperidol. Although CK1δ OE mice show enhanced locomotion and vertical activities, they do not have other motor impairments, such as motor neuron dyskinesia or Parkinsonian disorder-like symptoms up to 2 years of age.
One of the marked behavioral phenotypes of CK1δ OE mice is increased locomotor activity, especially vertical activity, attributable largely to repetitive jumping as observed in the OF chambers, but also in home cages. The CK1δ OE mice jumped repeatedly even after hitting the open field box lids or the metal grids of their home cages. Recent studies have focused on mechanisms of behavioral inhibition and pinpointed the integration of a network including the orbitofrontal cortex, the dorsomedial striatum, and the subthalamic nucleus, that normally inhibits many forms of behavior, including both impulsivity and compulsivity (for a review, see ref. 29). Our behavioral data suggest that overexpression of CK1δ in the forebrain might disturb this network balance and produce some form of impulsivity. Indeed, it is interesting to note that the CK1δ OE mice were not significantly less anxious in the OF test or in the DLC test. Moreover, there was no effect of CK1δ OE on fear conditioning (context or cue) (Fig. S3) or depression-related behaviors (TS and FS). Differences were, however, found in EPM and NSF tests. The difference observed in these tests might in fact reflect increased impulsivity rather than lessened anxiety, considering the results obtained in OF and DLC.
The administration of amphetamine rapidly and transiently reduced the horizontal activity and strongly suppressed the vertical activity in the CK1δ OE mice. Amphetamine increased both locomotor activities in WT mice as expected. The reduction of locomotor activities in CK1δ OE mice was less pronounced with methylphenidate than that observed with amphetamine, but a trend was clearly visible. Both amphetamine and methylphenidate increase synaptic dopamine concentration, resulting in locomotor hyperactivity in WT mice. However, they mediate their effects through different mechanisms. Amphetamine acts presynaptically, stimulating dopamine release, whereas methylphenidate increases synaptic dopamine concentration through inhibition of dopamine reuptake from synapses by blocking the activity of the dopamine transporter. The somewhat contrasting locomotor responses to amphetamine and methylphenidate in the CK1δ OE mice might indicate that the overexpression of CK1δ affects especially the presynaptic machinery to modulate neurotransmitter release. It is well established that protein phosphorylation can modulate neurotransmitter release (30, 31). It has been found that purified synaptic vesicles from rat brain were highly enriched in CK1. Thus, CK1δ may be involved either directly or indirectly in regulating the synaptic vesicle cycle. However, the clear reduction in the expression of D1R and D2R receptors in the striatum of these mice suggests that CK1 also has an impact on dopamine signaling at the postsynaptic level, possibly leading to long lasting phenomena such as altered transcriptional regulation. The mechanism(s) involved in the reduction in dopamine receptors is not known, but could involve altered expression or proteolytic turnover.
The impulsive phenotype of CK1δ OE mice bears a number of similarities to that of Coloboma mice, which have served as a murine model of ADHD (32, 33). ADHD is characterized by three major symptoms: inattention, hyperactivity, and impulsivity. Hyperactivity is predominantly seen in younger children affected by ADHD and is expected to decrease during adolescence (for a review, see ref. 34). ADHD is associated with dysfunction of dopaminergic cortico-subcortical networks related to executive functions and behavioral regulation, but whether ADHD is caused by hyper-or hypodopaminergic transmission is still a controversy (35). We measured dopamine levels in the CK1δ OE mice, but no difference was found compared to WT (Fig. S4), in contrast to what was found in the Coloboma mice. Mouse models of hyperdopaminergic transmission have been developed, such as mice with dopamine transporter knockout (DAT KO) or knock down, both of them displaying hyperactivity and responding to psychostimulants (36, 37). It has also been proposed that ADHD is caused by hypodopaminergic transmission because psychostimulants, including amphetamine and methylphenidate, enhance dopaminergic transmission and also improve ADHD symptoms (35). Interestingly, the densities of dopamine receptors, D1 and D2, are 59–54% lower in the striatum of the CK1δ OE mice than those of the control mice. These changes are very likely to affect the extent and balance in dopaminergic transmission mediated by the two dopamine receptor subtypes in striatonigral and striatopallidal neurons. Notably, in the Coloboma mouse model, the D2R has been proposed to mediate the effects linked to ADHD (38). Here we show that both D1R and D2R may be involved in the effects of CK1δ overexpression. This could suggest that the CK1δ OE mice do not exhibit ADHD symptoms for the same reasons as the Coloboma mouse model.
Because the CK1δ OE mice present clear signs of hyperactivity and impulsivity, and possibly some traits of inattention, and because they respond to amphetamine in a way comparable to ADHD patients and ADHD mouse models would, we raise the possibility that this mouse model that overexpresses CK1δ in forebrain could represent an additional ADHD model. Because the hyperactivity phenotype of the CK1δ OE mice is less pronounced than in other models—such as the Coloboma mutant mice, which result from a major deletion of SNAP-25, a component of the SNARE complex involved in the release of neurotransmitters (33, 39)—the characteristics of our mouse model might be closer to the human symptomatology compared with the Coloboma model and also more complete compared to nongenetic models, and therefore could present advantages over actual ADHD mouse models. Moreover, CK1δ OE mice do not present any health issues, are easy to breed, and the overexpression can easily be temporally controlled and even reversed if necessary. Altogether, the results presented here support the hypothesis that CK1 is an important player in the occurrence of ADHD symptoms. The results also raise the possibility that CK1δ might represent a viable drug target that would help alleviate the symptoms of ADHD.
Materials and Methods
Animals.
A transgenic mouse line, tetO-CK1δ, was engineered by inserting the tetO sequence with a mini-CMV promoter into the 5′ sequence of the CK1δ cDNA. The transgenic mouse line with inducible overexpression of CK1δ in the forebrain was generated by crossing the tetO-CK1δ positive mice with CaMKIIα-tTA positive mice (40) (Jackson Laboratory) (for details, see SI Materials and Methods). Animal use and procedures were in accordance with the National Institutes of Health guidelines and approved by the Rockefeller University Institutional Animal Care and Use Committees.
In Situ Hybridization.
The experiments were performed using standard procedures (see SI Materials and Methods for details).
In Situ Ligand Binding.
Fresh frozen sections (20 μm thick) were preincubated in assay buffer (50 mM Tris-HCl, 120 mM NaCl, 5 mM KCl, 2 mM CaCl2, and 1 mM MgCl2) for 30 min, and then incubated in assay buffer plus 2 nM 3H-SCH23390 (GE Healthcare; specific activity, 83 Ci/mmol) for the dopamine D1 receptor, and 100 nM 3H-quinpirole (GE Healthcare) for the D2 receptor, for 60 min. The slides were washed 2 × 10 min in ice-cold assay buffer, briefly rinsed with deionized water, dried, and exposed to Kodak MR film for 1–2 months at 4 °C.
OF.
Experiments were conducted in eight identical square boxes (40 × 40 × 30 cm) equipped with two rows of infrared photocells placed 20 and 50 mm above the floor, spaced 31 mm apart. The last photocell in a row was spaced 17.5 mm from the wall. Photocell beam interruptions were collected using the Superflex RBS program (AccuScan Instrument). Testing took place under ambient light conditions (500 lx).
DLC, EPM, NSF, Social Interaction (SI), and Fear Conditioning (FC).
The experiments were performed using standard procedures (see SI Materials and Methods for details).
Pharmacological Studies.
The drugs were injected in to mice i.p. (7–10 mice per test group) (see SI Materials and Methods for details). Locomotor activities were measured in the OF for 90 min including 30 min before drug injection except for haloperidol, quinpirole and MK801 which were measured for 60 min starting 30 min after drug injection.
Statistical Analysis.
StatView 5.0.1 was used to statistically analyze the data. When data were collected in multiple trials of a single session we used the “repeated measure ANOVA” test. When the results were significant with ANOVA, we used Fisher’s PLSD test to analyze the genotype or treatment effects as individual time point (data collected in a single trial of a single session).
Supplementary Material
Acknowledgments
We thank Drs. Jacob Bendor, Victor Bustos, Lars Brichta, and Hong Wang for helpful discussions. We thank Elisabeth Griggs for her help with graphic design. This work was supported in part by National Institutes of Health Grant DA10044 to A.C.N and P.G., and from The Army Medical Research and Materiel Command NETRP program (Award DAMD 17-02-1-0705 to P.G).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0915173107/DCSupplemental.
References
- 1.Hoekstra MF, et al. HRR25, a putative protein kinase from budding yeast: Association with repair of damaged DNA. Science. 1991;253:1031–1034. doi: 10.1126/science.1887218. [DOI] [PubMed] [Google Scholar]
- 2.Brockman JL, Gross SD, Sussman MR, Anderson RA. Cell cycle-dependent localization of casein kinase I to mitotic spindles. Proc Natl Acad Sci USA. 1992;89:9454–9458. doi: 10.1073/pnas.89.20.9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, et al. Prenylated isoforms of yeast casein kinase I, including the novel Yck3p, suppress the gcs1 blockage of cell proliferation from stationary phase. Mol Cell Biol. 1996;16:5375–5385. doi: 10.1128/mcb.16.10.5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behrend L, et al. IC261, a specific inhibitor of the protein kinases casein kinase 1-delta and -epsilon, triggers the mitotic checkpoint and induces p53-dependent postmitotic effects. Oncogene. 2000;19:5303–5313. doi: 10.1038/sj.onc.1203939. [DOI] [PubMed] [Google Scholar]
- 5.Li G, Yin H, Kuret J. Casein kinase 1 delta phosphorylates tau and disrupts its binding to microtubules. J Biol Chem. 2004;279:15938–15945. doi: 10.1074/jbc.M314116200. [DOI] [PubMed] [Google Scholar]
- 6.Kloss B, et al. The Drosophila clock gene double-time encodes a protein closely related to human casein kinase Iepsilon. Cell. 1998;94:97–107. doi: 10.1016/s0092-8674(00)81225-8. [DOI] [PubMed] [Google Scholar]
- 7.Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. 2001;107:855–867. doi: 10.1016/s0092-8674(01)00610-9. [DOI] [PubMed] [Google Scholar]
- 8.Xu Y, et al. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature. 2005;434:640–644. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- 9.Panek HR, et al. Suppressors of YCK-encoded yeast casein kinase 1 deficiency define the four subunits of a novel clathrin AP-like complex. EMBO J. 1997;16:4194–4204. doi: 10.1093/emboj/16.14.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murakami A, Kimura K, Nakano A. The inactive form of a yeast casein kinase I suppresses the secretory defect of the sec12 mutant. Implication of negative regulation by the Hrr25 kinase in the vesicle budding from the endoplasmic reticulum. J Biol Chem. 1999;274:3804–3810. doi: 10.1074/jbc.274.6.3804. [DOI] [PubMed] [Google Scholar]
- 11.Yasojima K, Kuret J, DeMaggio AJ, McGeer E, McGeer PL. Casein kinase 1 delta mRNA is upregulated in Alzheimer disease brain. Brain Res. 2000;865:116–120. doi: 10.1016/s0006-8993(00)02200-9. [DOI] [PubMed] [Google Scholar]
- 12.Lowrey PL, et al. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science. 2000;288:483–492. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flajolet M, et al. Regulation of Alzheimer’s disease amyloid-beta formation by casein kinase I. Proc Natl Acad Sci USA. 2007;104:4159–4164. doi: 10.1073/pnas.0611236104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oumata N, et al. Roscovitine-derived, dual-specificity inhibitors of cyclin-dependent kinases and casein kinases 1. Med Chem. 2008;51:5229–5242. doi: 10.1021/jm800109e. [DOI] [PubMed] [Google Scholar]
- 15.Desdouits F, Siciliano JC, Greengard P, Girault JA. Dopamine- and cAMP-regulated phosphoprotein DARPP-32: Phosphorylation of Ser-137 by casein kinase I inhibits dephosphorylation of Thr-34 by calcineurin. Proc Natl Acad Sci USA. 1995;92:2682–2685. doi: 10.1073/pnas.92.7.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greengard P, et al. The DARPP-32/protein phosphatase-1 cascade: A model for signal integration. Brain Res Brain Res Rev. 1998;26:274–284. doi: 10.1016/s0165-0173(97)00057-x. [DOI] [PubMed] [Google Scholar]
- 17.Liu F, et al. Regulation of cyclin-dependent kinase 5 and casein kinase 1 by metabotropic glutamate receptors. Proc Natl Acad Sci USA. 2001;98:11062–11068. doi: 10.1073/pnas.191353898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wahlstrom D, Collins P, White T, Luciana M. Developmental changes in dopamine neurotransmission in adolescence: Behavioral implications and issues in assessment. Brain Cogn. 2009 doi: 10.1016/j.bandc.2009.10.013. 10.1016/j.bandc.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris G, Schmidt R, Bergman H. Striatal action-learning based on dopamine concentration. Exp Brain Res. 2009 doi: 10.1007/s00221-009-2060-6. 10.1007/s00221-009-2060-6. [DOI] [PubMed] [Google Scholar]
- 20.Winsberg BG, Comings DE. Association of the dopamine transporter gene (DAT1) with poor methylphenidate response. J Am Acad Child Adolesc Psychiatry. 1999;38:1474–1477. doi: 10.1097/00004583-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Tripp G, Wickens JR. Research review: Dopamine transfer deficit: A neurobiological theory of altered reinforcement mechanisms in ADHD. J Child Psychol Psychiatry. 2008;49:691–704. doi: 10.1111/j.1469-7610.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- 22.Luman M, Tripp G, Scheres A. Identifying the neurobiology of altered reinforcement sensitivity in ADHD: A review and research agenda. Neurosci Biobehav Rev. 2009 doi: 10.1016/j.neubiorev.2009.11.021. in press. [DOI] [PubMed] [Google Scholar]
- 23.Bush G. Attention-deficit/hyperactivity disorder and attention networks. Neuropsychopharmacology. 2009;34:278–300. doi: 10.1038/npp.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rivers A, Gietzen KF, Vielhaber E, Virshup DM. Regulation of casein kinase I epsilon and casein kinase I delta by an in vivo futile phosphorylation cycle. J Biol Chem. 1998;273:15980–15984. doi: 10.1074/jbc.273.26.15980. [DOI] [PubMed] [Google Scholar]
- 25.Gietzen KF, Virshup DM. Identification of inhibitory autophosphorylation sites in casein kinase I epsilon. J Biol Chem. 1999;274:32063–32070. doi: 10.1074/jbc.274.45.32063. [DOI] [PubMed] [Google Scholar]
- 26.Lira A, et al. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry. 2003;54:960–971. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- 27.Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- 28.Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- 29.Eagle DM, et al. Serotonin depletion impairs waiting but not stop-signal reaction time in rats: Implications for theories of the role of 5-HT in behavioral inhibition. Neuropsychopharmacology. 2009;34:1311–1321. doi: 10.1038/npp.2008.202. [DOI] [PubMed] [Google Scholar]
- 30.Greengard P, Valtorta F, Czernik AJ, Benfenati F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science. 1993;259:780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- 31.Feng J, et al. Regulation of neurotransmitter release by synapsin III. J Neurosci. 2002;22:4372–4380. doi: 10.1523/JNEUROSCI.22-11-04372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson MC. Coloboma mouse mutant as an animal model of hyperkinesis and attention deficit hyperactivity disorder. Neurosci Biobehav Rev. 2000;24:51–57. doi: 10.1016/s0149-7634(99)00064-0. [DOI] [PubMed] [Google Scholar]
- 33.Hess EJ. The use of transgenes and mutations in the mouse to study the genetic basis of locomotor hyperactivity. Methods. 1996;10:374–383. doi: 10.1006/meth.1996.0115. [DOI] [PubMed] [Google Scholar]
- 34.Fox DJ, Tharp DF, Fox LC. Neurofeedback: An alternative and efficacious treatment for Attention Deficit Hyperactivity Disorder. Appl Psychophysiol Biofeedback. 2005;30:365–373. doi: 10.1007/s10484-005-8422-3. [DOI] [PubMed] [Google Scholar]
- 35.Swanson J, Castellanos FX, Murias M, LaHoste G, Kennedy J. Cognitive neuroscience of attention deficit hyperactivity disorder and hyperkinetic disorder. Curr Opin Neurobiol. 1998;8:263–271. doi: 10.1016/s0959-4388(98)80150-5. [DOI] [PubMed] [Google Scholar]
- 36.Gainetdinov RR, et al. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- 37.Zhuang X, et al. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci USA. 2001;98:1982–1987. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan X, Xu M, Hess EJ. D2 dopamine receptor subtype-mediated hyperactivity and amphetamine responses in a model of ADHD. Neurobiol Dis. 2010;37:228–236. doi: 10.1016/j.nbd.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hess EJ, Jinnah HA, Kozak CA, Wilson MC. Spontaneous locomotor hyperactivity in a mouse mutant with a deletion including the Snap gene on chromosome 2. J Neurosci. 1992;12:2865–2874. doi: 10.1523/JNEUROSCI.12-07-02865.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayford M, et al. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274(5293):1678–83. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.