Abstract
Natural fluctuations in circulating estradiol are associated with behavioral changes, including severe disturbances in mood and cognition in some women. Common genetic variation in some of the molecular mediators of estradiol effects on these behaviors, in brain regions such as the hippocampus, may explain individual variation in estradiol effects on behavior. We tested whether the common human variant BDNF Val66Met interacts with estradiol in the control of hippocampal function in cycling female mice homozygous for the wild-type Val or BDNF Met variant. BDNF Met increased anxiety behavior, impaired memory, and increased expression of BDNF and its receptor TrkB in the hippocampal formation. BDNF Met also dramatically altered the fluctuation of spatial memory, hippocampal Akt phosphorylation, and PSD-95 protein expression across the estrous cycle. The variant BDNF Val66Met should therefore be considered as a strong candidate for mediating genetic differences in ovarian steroid-related behavioral changes and disorders.
Keywords: estrogen, TrkB, synaptic plasticity, PSD-95, Akt
Natural fluctuations in circulating estrogens across the human menstrual cycle and in menopause are associated with changes in hippocampal function and hippocampal-dependent behaviors such as mood and cognition (1–5). Most women report mood and cognitive changes associated with the menstrual cycle, and 5–10% meet strict diagnostic criteria for premenstrual dysphoric disorder (PMDD) (6). The nature and extent of these behavioral changes associated with the menstrual cycle in individual women may stem from as-yet-undefined genetic risk factors.
In rodent models, the neurotrophin BDNF is one mediator of estradiol effects in the hippocampus. Increases in circulating estradiol induce hippocampal BDNF mRNA and protein, and increase activation of the BDNF receptor TrkB, in female mice and rats (7–13). BDNF signaling is important for estradiol to enhance hippocampal synaptic plasticity, because TrkB inhibitors block the estradiol-mediated increase in hippocampal excitability, synaptic protein expression, and dendritic spine formation in rat hippocampal slice cultures (7, 14). Because of the importance of BDNF in estradiol-mediated plasticity, we hypothesized that a common variant of the BDNF gene, Val66Met, interacts with estradiol in the control of hippocampal function.
The BDNF variant Val66Met is carried by 20–30% of Caucasians (15, 16). This single nucleotide polymorphism in the pro region of the BDNF gene measurably affects human behavior and susceptibility to neuropsychiatric disorders (17). Neurons expressing BDNF Met show impaired trafficking of pro-BDNF and ≈30% less activity-dependent BDNF secretion relative to neurons expressing BDNF Val (18–20).
We tested whether BDNF genotype and ovarian steroids interact to control hippocampal function in cycling female mice homozygous for the wild-type BDNF Val or BDNF Met variant. Two behavioral tests of hippocampal-dependent memory were used to assess mnemonic and nonmnemonic behavior. After behavioral testing, the mice were killed in high-estradiol (proestrus) or low-estradiol (diestrus) estrous cycle stages. Quantitative in situ hybridization and immunocytochemistry were used to relate the behavioral findings to molecular changes in hippocampal BDNF and estradiol signaling.
Results
BDNF Met Mice Are Reproductively Normal.
Any effect of the variant BDNF Val66Met on the hypothalamic-pituitary-gonadal (HPG) axis that controls the rhythm of circulating reproductive hormones could confound the results of this study in cycling mice. By monitoring estrous cycles, reproductive behavior, and progestin receptor (PR) expression in the medial preoptic area of the hypothalamus (MPO) in the Met females, we ensured that here were no significant changes in HPG axis function in the Met females (SI Text and Fig. S1).
Variant BDNF Met Affects Memory in Females.
Hippocampal-dependent memory was assessed by using the object recognition (OR) and object placement (OP) tests. Both OP and OR tests rely on hippocampal function, although the parahippocampal cortices and other brain regions also are important for OR performance (21–24).
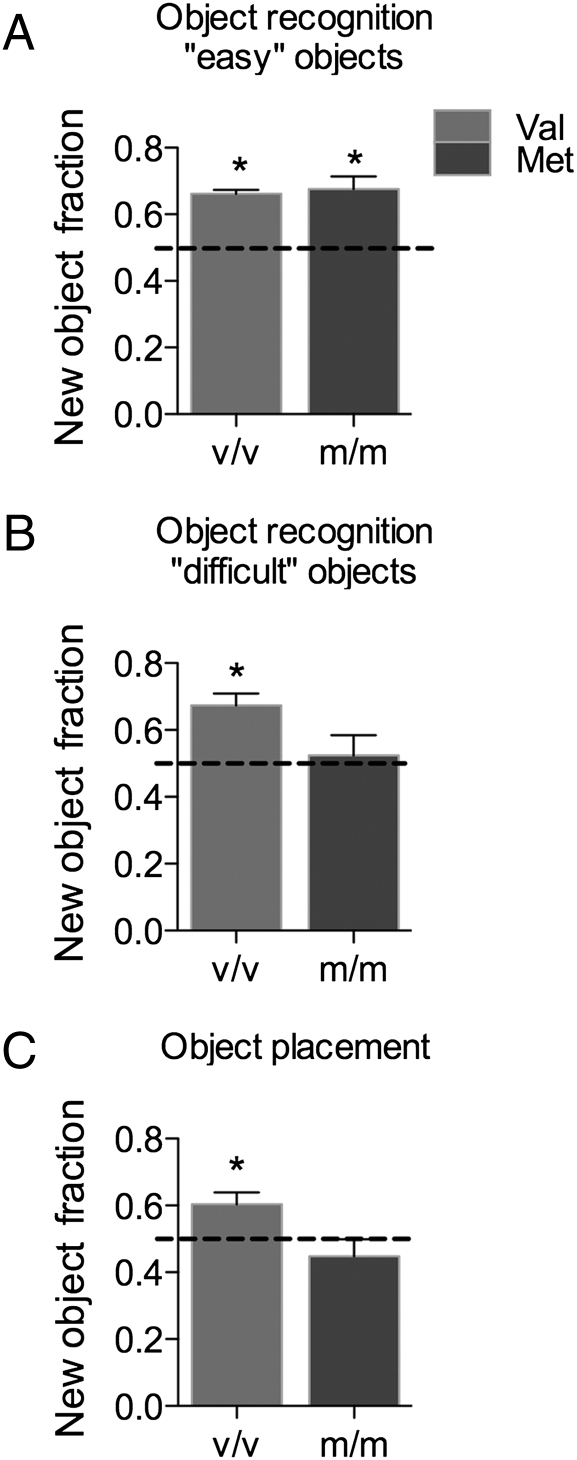
Successful OR and OP performance depends on two main factors: the mouse’s preference for novelty (neophilia) and recognition memory. To demonstrate these traits in Val and Met mice, each mouse was tested on the OR test by using an easily differentiated pair of objects chosen from experience (Fig. 1A). The mice were in mixed estrous cycle stages during this test. Novel object recognition was measured by using the novel object fraction, where a fraction of 0.5 represents chance performance. Both Val and Met mice had a novel object fraction significantly greater than 0.5 [Val, t(4) = 17.9, P < 0.0001; Met, t(4) = 4.567, P = 0.0103], indicating that both genotypes recognized the novel object. Thus Val and Met mice exhibited neophilia and recognition memory.
Fig. 1.
BDNF Val66Met impairs memory. OP and OR performance is represented by the novel object fraction, where chance performance (0.5) is indicated by a dotted line. (A) OR performance using an easy object pair. (B) OR performance using a more difficulty object pair. (C) OP performance on a test with 5-minute intertrial delay. *, P < 0.05 relative to chance. n = 8 for OP; 5 for OR. Error bars represent SEM.
To investigate differences in recognition memory between Val and Met mice, each mouse was tested on the OR task by using a new, less easily differentiated, object pair (Fig. 1B). This time, Val mice recognized the novel object [t(4) = 4.752, P = 0.0090], whereas Met mice did not. The mice were also tested on the OP task, again in mixed estrous cycle stages (Fig. 1C). Val mice recognized the novel object placement [t(9) = 2.884, P = 0.0181], whereas Met mice did not. Thus, compared to wild-type Val females, Met females exhibited impaired object and place recognition.
Variant BDNF Met Affects Nonmnemonic Behavior in Females.
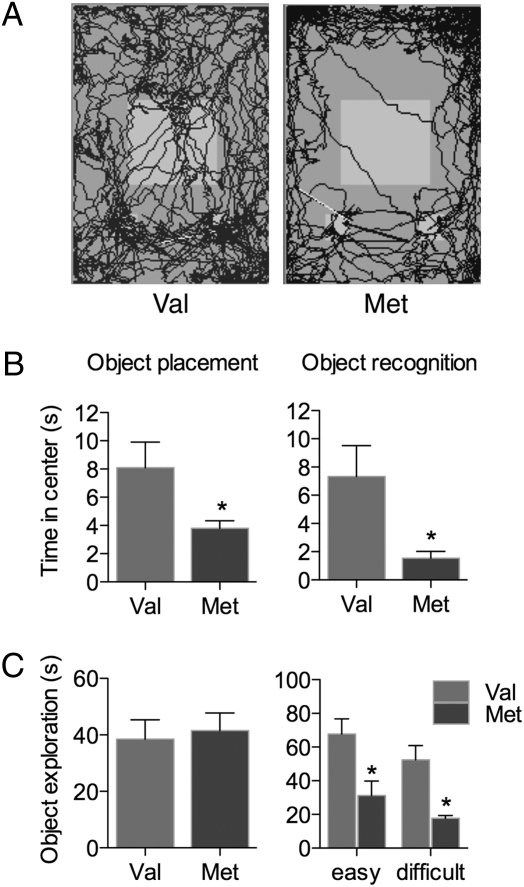
In addition to memory, anxiety-type and exploratory behaviors were also assessed during the OR and OP tests. Anxiety-type behavior was measured during OP and OR tests by recording the amount of time each mouse spent in the center of the open field, where less time signifies increased anxiety-type behavior (Fig. 2 A and B). Met mice spent significantly less time than Val mice in the center of the open field during the sample trial of the OP test [t(17) = 2.179, P = 0.0437] and the OR test by using easy objects [t(7) = 2.871, P = 0.0240]. Thus, Met female mice showed increased anxiety-type behavior.
Fig. 2.
BDNF Val66Met affects nonmnemonic behaviors in female mice. (A) Diagram of the path of one representative mouse of each genotype during a sample trial of the OR test. The two small white squares at the bottom of the field represent objects, and the larger white square represents the defined center of the open field. (B) Anxiety-type behavior measured as time in the center during the sample trials of both OP and OR tests. (C) Exploratory behavior measured as total object exploration during the sample trials of both OP and OR tests. *, P < 0.05 relative to Val. n = 8 for OP; 5 for OR. Error bars represent SEM.
Exploratory behavior was measured by recording object exploration time (Fig. 2 A and C). There was no difference between Val and Met mice in time spent exploring the two identical, familiar objects during the sample trial of the OP test. In contrast, Met mice spent significantly less time than Val mice exploring the unfamiliar objects during the sample trial of both OR tests [t(8) = 2.904, P = 0.0198 for easy objects and t(8) = 4.055, P = 0.0037 for difficult objects]. This effect was independent of performance, as it occurred during the sample trials of both easy and difficult object tests. Moreover, this effect was independent of object novelty, because Met mice also spent significantly less time than Val mice exploring the familiar objects during the recognition trial of the OR test (for easy objects, 27.4 ± 2.1 seconds for Val, 15.6 ± 2.4 seconds for Met; t(8) = 2.453, P = 0.0398]. Thus, Met mice showed decreased exploration of both novel and familiar objects during the OR test.
Estrous Cycle Interacts with BDNF Genotype To Affect Memory in Females.
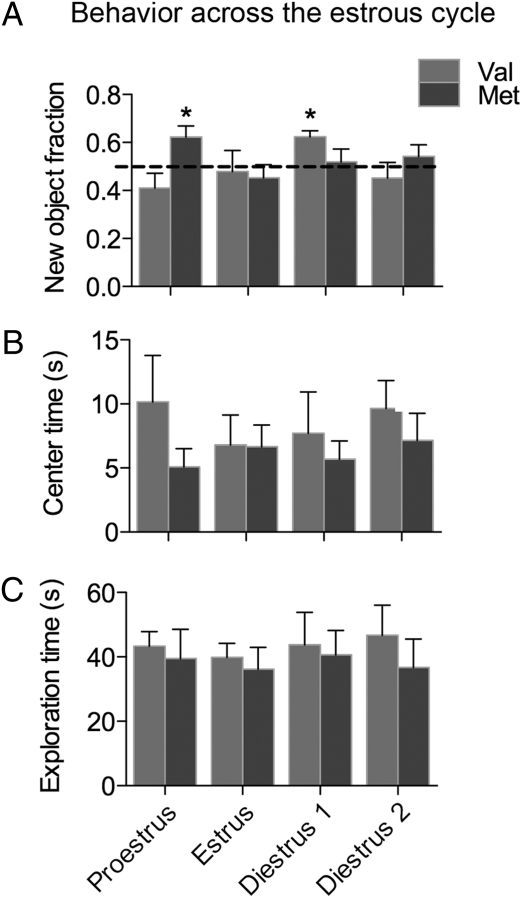
To investigate whether the estrous cycle interacts with BDNF in the control of hippocampal function, each mouse was tested on the OP test in four estrous cycle stages—proestrus, estrus, diestrus 1, and diestrus 2—Because all Val females recognized the new object placement on their first OP test (Fig. 1C), the task was made more difficult by increasing the intertrial delay from 5 to 30 min to increase the likelihood of detecting differences in performance across the cycle.
OP performance fluctuated across the estrous cycle for both Val and Met mice (Fig. 3A). The new object fraction was significantly greater than chance in diestrus for Val [t(5) = 5.097, P = 0.0038], and proestrus for Met [t(7) = 2.674, P = 0.0318]. Spatial memory was therefore best in diestrus in Val mice and in proestrus in Met mice. When the novel object fractions were compared by using a two-way ANOVA, there was a significant interaction between genotype and cycle stage on object placement test performance (F(3, 36) = 2.962, P = 0.0450), indicating that the effect of the Met variant on OP performance depended on estrous cycle stage. In contrast, there was no effect of estrous cycle or interaction between genotype and estrous cycle on anxiety or exploratory behaviors during the sample trial (Fig. 3 B and C). Thus, independent of nonmnemonic factors that could influence performance, spatial memory improved during different estrous cycle stages in wild-type Val and variant Met mice.
Fig. 3.
Estrous cycle and BDNF genotype interact to control OP performance. (A) Performance of Val and Met mice on the OP task in different estrous cycle stages. Anxiety-type (B) and exploratory (C) behavior from the sample trial in different estrous cycle stages. *, P < 0.05 relative to chance. n = 8. Error bars represent SEM.
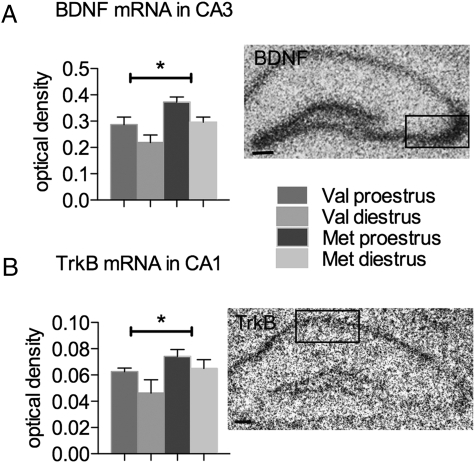
Variant BDNF Met Alters the Hippocampal Expression of BDNF and TrkB.
Changes in the expression of BDNF or its receptor TrkB could account for the different fluctuation of OP performance across the estrous cycle in Met and Val mice. To explore this possibility, expression of BDNF and TrkB was assayed by using in situ hybridization (Fig. 4). BDNF mRNA was measured in the CA3 pyramidal cell layer, where expression fluctuates across the estrous cycle in female rats (7). Similar to rats, BDNF mRNA was higher in proestrus than diestrus for both Val and Met mice; one-way ANOVA showed a significant overall effect of cycle stage (F(1, 15) = 7.994, P = 0.0127). Furthermore, compared to the wild-type Val mice, Met mice had increased BDNF mRNA (Fig. 4A); one-way ANOVA showed a significant overall effect of genotype (F(1, 15) = 10.17, P = 0.0061). TrkB mRNA was measured in the CA1 pyramidal cell layer, where expression was strongest and the anatomy most well defined. There was no significant fluctuation of TrkB mRNA across the estrous cycle. As with BDNF, TrkB mRNA was increased in Met compared to Val mice (Fig. 4B); one-way ANOVA showed a significant overall effect of genotype (F(1, 13) = 6.576, P = 0.0235). Thus, the overall expression of both BDNF and TrkB was significantly increased in Met female mice, and BDNF mRNA increased significantly during proestrus in mice of both genotypes.
Fig. 4.
BDNF Met mice have increased hippocampal BDNF and TrkB expression. (A) Optical density of BDNF mRNA in the CA3 pyramidal cell layer (box) from in situ hybridization films. (B) Optical density of TrkB mRNA in the CA1 pyramidal cell layer (box) from in situ hybridization films. *, P < 0.05 for genotype. n = 6 for Val proestrus, 4 for Val diestrus, 5 for Met proestrus, and 4 for Met diestrus. Error bars represent SEM. (Scale bars: 100 μm.)
Variant BDNF Met Alters Estrous Cycle Fluctuation of pAkt and PSD-95.
Several molecular changes have been associated with estrogen effects on hippocampal synaptic plasticity. For example, phosphorylation of the serine/threonine kinase Akt increases during proestrus and after estradiol administration in the rat and mouse hippocampus (12, 13, 25). In addition, the expression of several synaptic proteins, including the postsynaptic scaffolding protein PSD-95, increases during proestrus and after estradiol administration (13, 26). Akt phosphorylation and PSD-95 expression were therefore measured in the Val and Met mice as molecular measures of hippocampal sensitivity to ovarian hormones.
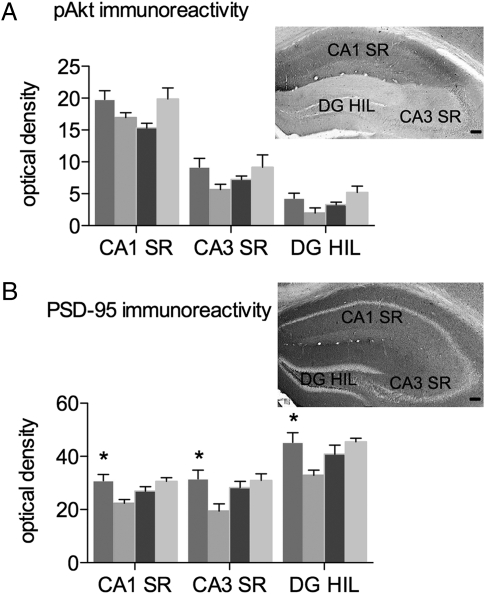
Akt phosphorylation was measured as pAkt-immunoreactivity (pAkt-ir). The pAkt antibody labels hippocampal neuropil, with the densest labeling seen in the CA1 stratum radiatum (Fig. 5A). pAkt-ir was measured in the CA1 and CA3 strata radiatum and dentate hilus. pAkt-ir in all three subregions increased during proestrus in Val mice and decreased during proestrus in Met mice. Estrous cycle stage affected pAkt-ir differently in Val and Met females; two-way ANOVA showed a significant interaction between cycle stage and genotype in CA1 stratum radiatum and dentate hilus, and a barely statistically significant interaction in the CA3 stratum radiatum (CA1 stratum radiatum, F(1, 15) = 8.615, P = 0.0102); CA3 stratum radiatum, F(1, 15) = 4.372, P = 0.054); and dentate hilus, F(1, 15) = 7.493, P = 0.0153).
Fig. 5.
Estrous cycle fluctuation of pAkt-ir and PSD-95-ir is altered in BDNF Met/Met mice. pAkt-ir (A) and PSD-95-ir (B) in the CA1 stratum radiatum, CA3 stratum radiatum, and dentate hilus. *, P < 0.05 compared to diestrus. SR, stratum radiatum; DG HIL, hilus of the dentate gyrus. Error bars represent SEM. (Scale bars: 100 μm.)
PSD-95-ir was found in the hippocampal neuropil and absent from pyramidal and dentate granule cell bodies (Fig. 5B). Similar to pAkt-ir, PSD-95-ir increased during proestrus in Val mice and decreased during proestrus in Met mice. Two-way ANOVA confirmed that estradiol affected PSD-95 differently in Val and Met mice, with a significant interaction between cycle stage and genotype in all hippocampal subregions (CA1 stratum radiatum, F(1, 15) = 9.088, P = 0.0087); CA3 stratum radiatum, F(1, 15) = 6.457, P = 0.0226; and dentate hilus, F(1, 15) = 7.161, P = 0.0173). Post hoc tests showed significantly increased PSD-95-ir in proestrus Val mice compared to diestrus Val mice (P < 0.05 for all three subregions). Thus, the fluctuation of hippocampal pAkt-ir and PSD-95-ir across the estrous cycle was altered in Met mice compared to wild-type Vals.
Discussion
In this study of female transgenic BDNF Met mice, we demonstrate unique effects of this allele on memory, exploratory behavior, and the expression of BDNF and its receptor TrkB. Furthermore, the impact of estrous cycle on spatial memory and molecular measures associated with dendritic spine formation differed in the Met mice compared with wild-type controls.
Variant BDNF Met Affects Behavior and BDNF/TrkB Expression.
The behavioral phenotype of increased anxiety and impaired memory in Met female mice is consistent with the behavior of male Met mice (19), which show increased anxiety-related behavior and impaired hippocampal-dependent fear conditioning. In humans, Met carriers have an increased prevalence of anxiety traits and disorders and poorer episodic memory compared to noncarriers (18, 44–46). Thus, these aspects of the Met phenotype are conserved between mice and humans, and not sexually dimorphic. Increased anxiety may exacerbate poor memory in Met mice by interfering with willingness to explore objects (as in the OR test) or with memory formation. Decreased total object exploration, as exhibited by Met mice in the OR test, may also contribute to memory impairment. The reason for this phenotype in the OR test but not the OP test is unclear and may stem from differences in the types of objects that were used.
This study finds increased hippocampal BDNF and TrkB mRNA expression in Met mice. Previous work may have missed a specific increase in hippocampal BDNF by comparing levels of BDNF immunoreactivity in whole brain lysate (19). Interestingly, a recent human study found increased serum BDNF in Met carriers (27). The hippocampus of Met mice may attempt to compensate for the decrease in activity-dependent BDNF release by increasing BDNF and TrkB expression. This putative compensation may either mitigate or contribute to the behavioral phenotype of the Met mice.
Estradiol Affects Hippocampal Signaling and Spatial Memory in Wild-Type Mice.
The significant increase in PSD-95, and nonsignificant increase in pAkt-ir in Val mice during proestrus, is consistent with our previous demonstration that PSD-95-ir and pAkt-ir increase during proestrus in wild-type mice and rats (13, 28). Furthermore, the increase in BDNF mRNA during proestrus is consistent with previous studies in female rats, which showed that BDNF mRNA and protein expression increase during proestrus (7, 10, 11).
The finding that wild-type Val mice performed best on the OP test during diestrus, when estradiol levels are low, was surprising in light of previous studies indicating that estradiol enhances spatial memory. These studies showed that in proestrus, OP performance improves in rats (29), as does performance on a 1-day water maze test in mice (30). OP performance also improves after acute estradiol administration in rats (31) and after 6 days of estradiol administration in mice (26). The differences between our findings and these previous studies underscore the complexity of estradiol effects on behavior (32) and may be explained by several factors. Differences in the strategy used to perform the OP test between mice and rats may alter the effects of estradiol on this behavior (32–34). In addition, different hormone replacement regimens and studies of natural fluctuations in hormones can lead to different behavioral outcomes (35), due to variations in the levels of circulating hormones and the fluctuation of many different hormones during the estrous cycle. Finally, difficulty of the OP test varies with the extramaze cues, objects, box size or shape, and intertrial delay. When the test is too easy, such as in our previous study (13), no differences between groups will be detected.
Hippocampal Function Fluctuates Differently Across the Estrous Cycle in Val and Met Mice.
OP performance was best in diestrus for Val mice and proestrus for Met mice. As a result, proestrus Met mice actually performed better than proestrus Val mice on the 30-min OP test. This finding was surprising in light of the overall impairment exhibited by the Met mice on the 5-min OP test. Thus, in some cases, the effects of circulating ovarian steroids may be robust enough to overcome the global behavioral phenotype of this BDNF variant.
For PSD-95, post hoc analyses showed that PSD-95-ir increased during proestrus in Val mice but not Met mice. For these wild-type Val mice, lower levels of PSD-95 protein during diestrus favored better OP performance. This inverse correlation between PSD-95 and spatial memory is contrary to what one might expect, given that PSD-95 is thought to promote hippocampal synaptic plasticity and memory (36–40). It is possible that a widespread increase in PSD-95 could obscure the specific synaptic events required for short-term memory formation during the sample trial. In support of this idea, overexpression of PSD-95 in neurons of the rat hippocampus occludes the synaptic potentiation seen in control neurons by inducing a widespread increase in postexcitatory synaptic potentials (41). In Met mice, the absence of any change in PSD-95, when combined with other estradiol-mediated changes, may contribute to the observed enhancement of spatial memory during proestrus.
Several studies have supported a role for BDNF in modulating PSD-95 expression and Akt activity (7, 14, 42, 43). Consistent with this work, our findings indicate that activity-dependent BDNF secretion is important for the fluctuation of pAkt-ir and PSD-95-ir across the estrous cycle in female mice. The different estrous cycling of pAkt and PSD-95 in Val and Met mice is not explained by changes in BDNF mRNA expression, because all mice showed increased hippocampal BDNF mRNA during proestrus. Instead, it may be explained by downstream changes in BDNF signaling in Met mice, such as the sites of BDNF release or the ratio of activity-dependent to constitutive BDNF secretion.
Implications for Human Behavior.
Based on the current study, we hypothesize that BDNF Met may affect vulnerability to cognitive and affective disorders in women. Specifically, Met carrier and noncarrier women may react differently to fluctuations in circulating ovarian steroids. BDNF genotype may therefore alter risk for the development of PMDD and other behavioral disorders associated with the menstrual cycle and could assist in the diagnosis or treatment of these disorders. BDNF genotype may also predict changes in mood and cognitive function associated with menopause and hormone replacement therapy. Such predictions could influence therapeutic decisions at the menopausal transition.
In summary, we show here a reproducible behavioral phenotype of increased anxiety and impaired memory that parallels the phenotype of human Met carriers. We identify unique interactions between BDNF genotype and the ovarian cycle for spatial memory and molecular markers of dendritic spine formation in the hippocampal formation. Efficient translation of this information from the mouse model to clinical studies could result in useful application of BDNF genotype to women’s health in the foreseeable future.
Methods
Animals.
All animal procedures were approved by the Institutional Animal Care and Use Committees of The Rockefeller University and Weill Cornell Medical College and were in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Mice aged 8–13 weeks were housed on a 12-h light/dark cycle (lights on: 7 a.m.) with food and water available ad libitum. BDNF Val66Met mice were generated by using a knock-in allele with the point mutation G to A in the BDNF coding region as described (19). This mutation changes the Valine at position 66 to a Methionine. The mice were crossed onto a C57BL/6J background for at least 11 generations before these experiments and were bred and genotyped at Weill Cornell Medical College.
Estrous Cycling.
Vaginal swabs were taken daily between 9:30 and 10:30 a.m. and vaginal cytology was observed under a microscope after staining with the Hema 3 Stain Set from Fisher Scientific. Cycle stage was determined as proestrus, estrus, diestrus 1, or diestrus 2 according to published criteria (47). Mice without progression through these distinct cytological stages were excluded from the studies. Vaginal cytology was monitored for at least one complete cycle before behavioral testing. Upon the completion of testing, mice were killed in the late morning between the hours of 11 a.m. and noon.
Behavioral Testing.
BDNF Val66Met mice underwent behavioral testing on the OR and OP tests as described (ref. 26, adapted from ref. 31) in an open field measuring 38.1 × 53.3 cm. Objects used for the OR test were novel, whereas for the OP test, mice were acclimated to the objects before testing. The objects for these tests were chosen based on previous experience with object tests in mice. For the object recognition test, two different object pairs of varying levels of difficultly were used. The “easy” objects were a sake cup and a cup of similar size constructed from Legos. The “difficult” objects were a small pill bottle and skinny nail polish bottle of similar size and height. For the object placement test, the objects were Lego constructions with contrasting shades and surface textures.
Before testing, mice were acclimated to the apparatus in addition to the objects for OP testing. Once testing began, the mice were tested no more than once each day and at least once every 5 days. Each mouse was tested on the OP test once with a 5-min intertrial delay, and four times with a 30-min intertrial delay in each of the following estrous cycle stages: proestrus, estrous, diestrus 1, and diestrus 2. The same two objects were used throughout testing. Each mouse was tested on the OR task with a 30-min intertrial delay twice with different object pairs. Starting objects or object start locations and new object locations were counterbalanced across mice and trials. In the initial OP test and the two OR tests, mice were in mixed estrous cycle stages. Trials were recorded and analyzed by using the Noldus Ethovision XT software (Noldus Information Technology). An area of 1 cm surrounding the objects was delineated by using the software, and object exploration was defined as when the nose of the mouse was within this object surround area. To compare nonmnemonic behaviors that may influence task performance, the following parameters were also measured from the sample trial: time spent in the center of the open field and time spent exploring objects.
Tissue Preparation.
Mice were anesthetized with an overdose of sodium pentobarbital (150 mg/kg intraperitoneal) and perfused through the aorta with saline/heparin followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4; PB). Brains were removed from the skull and postfixed in 4% paraformaldehyde for 1 h, sunk in 30% sucrose in PB for 48 h at 4 °C, and frozen at −80 °C. Sections (40 μm thick) through the hippocampal formation were cut on a freezing microtome (Microm HM440E). Sections were stored in a cryoprotectant solution of 30% ethylene glycol, 30% sucrose in PB until use.
In Situ Hybridization.
Antisense oligonucleotides matching the TrkB (5′ TGC GAC TGC GTC AGC TCG GTG GGC GGG TTC CCT CTG CCA TCA GCA CTG C 3′) and BDNF exon IX (5′ GGG TTA CAC GAA GGA AGG CTG CAG GGG CAT AGA CAA AAG GCA CTG GAA CT 3′) sequences were obtained from Integrated DNA Technologies. Control sense sequences were also generated and used as a control to confirm a low level of nonspecific interactions. Floating sections containing dorsal hippocampus were mounted on Fisher Superfrost Plus slides, washed in 0.05 M PB, and dried in a desiccator. The oligos were labeled with 33P an in situ hybridization conducted as described, starting after the paraformaldehyde step (48). Air-dried slides were exposed to Kodak MR autoradiography films for 1 week (TrkB) or 2 weeks (BDNF). Films were then developed and images taken on a light box by using a CoolSnap camera. Optical density was measured from the CA1 (TrkB) and CA3 (BDNF) pyramidal cell layers and normalized to background density in stratum radiatum.
Immunocytochemistry and Densitometry.
Immunocytochemistry was performed on sections through the dorsal hippocampal formation level 29 of Paxinos and Watson (49), as described (13, 28) (SI Methods). Monoclonal mouse anti-PSD-95 (1:20,000) was purchased from Sigma. Polyclonal rabbit anti-phosphothreonine 308 Akt (1:1500) was purchased from Cell Signaling Technology.
Data Analysis.
Statistical tests were run on Prism GraphPad software (version 5.0a for Mac). Genotype differences in nonmnemonic parameters were analyzed by using two-tailed t tests. For OP and OR tests, the amount of time spent exploring the novel object or location during the recognition trial was expressed as a fraction of the time spent with both objects, termed “novel object fraction.” A fraction of 0.5 is consistent with chance performance, and recognition was defined as a fraction significantly greater than 0.5 by using a one-sample t test. Effects of genotype and estrous cycle on nonmnemonic behaviors in the OP test were examined by using two-way repeated-measures ANOVA with genotype and cycle stage as the independent measures. In situ hybridization and immunocytochemical data were analyzed by using two-way ANOVA with cycle stage and genotype as the independent measures, and post hoc comparisons with Bonferroni tests.
Supplementary Material
Acknowledgments
Support was provided by National Institutes of Health Grants NS07080 (to B.S.M.), NS052819, MH079513, and IMRO Rising Star Award (to F.S.L.), MH082528 (to J.L.S.), and DA08259 and HL18974 (to T.A.M.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0915105107/DCSupplemental.
References
- 1.Protopopescu X, et al. Hippocampal structural changes across the menstrual cycle. Hippocampus. 2008;18:985–988. doi: 10.1002/hipo.20468. [DOI] [PubMed] [Google Scholar]
- 2.Eberling JL, Wu C, Tong-Turnbeaugh R, Jagust WJ. Estrogen- and tamoxifen-associated effects on brain structure and function. Neuroimage. 2004;21:364–371. doi: 10.1016/j.neuroimage.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338:209–216. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- 4.Sherwin BB. Estrogen and cognitive aging in women. Neuroscience. 2006;138:1021–1026. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 5.Sherwin BB, Tulandi T. “Add-back” estrogen reverses cognitive deficits induced by a gonadotropin-releasing hormone agonist in women with leiomyomata uteri. J Clin Endocrinol Metab. 1996;81:2545–2549. doi: 10.1210/jcem.81.7.8675575. [DOI] [PubMed] [Google Scholar]
- 6.Yonkers KA, O’Brien PM, Eriksson E. Premenstrual syndrome. Lancet. 2008;371:1200–1210. doi: 10.1016/S0140-6736(08)60527-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou J, Zhang H, Cohen RS, Pandey SC. Effects of estrogen treatment on expression of brain-derived neurotrophic factor and cAMP response element-binding protein expression and phosphorylation in rat amygdaloid and hippocampal structures. Neuroendocrinology. 2005;81:294–310. doi: 10.1159/000088448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jezierski MK, Sohrabji F. Neurotrophin expression in the reproductively senescent forebrain is refractory to estrogen stimulation. Neurobiol Aging. 2001;22:309–319. doi: 10.1016/s0197-4580(00)00230-x. [DOI] [PubMed] [Google Scholar]
- 10.Gibbs RB. Levels of trkA and BDNF mRNA, but not NGF mRNA, fluctuate across the estrous cycle and increase in response to acute hormone replacement. Brain Res. 1998;810:294. doi: 10.1016/s0006-8993(98)00945-7. [DOI] [PubMed] [Google Scholar]
- 11.Scharfman HE, et al. Changes in hippocampal function of ovariectomized rats after sequential low doses of estradiol to simulate the preovulatory estrogen surge. Eur J Neurosci. 2007;26:2595–2612. doi: 10.1111/j.1460-9568.2007.05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spencer JL, et al. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spencer JL, Waters EM, Milner TA, McEwen BS. Estrous cycle regulates activation of hippocampal Akt, LIM kinase, and neurotrophin receptors in C57BL/6 mice. Neuroscience. 2008;155:1106–1119. doi: 10.1016/j.neuroscience.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato K, Akaishi T, Matsuki N, Ohno Y, Nakazawa K. beta-Estradiol induces synaptogenesis in the hippocampus by enhancing brain-derived neurotrophic factor release from dentate gyrus granule cells. Brain Res. 2007;1150:108–120. doi: 10.1016/j.brainres.2007.02.093. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu E, Hashimoto K, Iyo M. Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: the possibility to explain ethnic mental traits. Am J Med Genet B Neuropsychiatr Genet. 2004;126:122–123. doi: 10.1002/ajmg.b.20118. [DOI] [PubMed] [Google Scholar]
- 16.Pivac N, et al. Ethnic differences in brain-derived neurotrophic factor Val66Met polymorphism in Croatian and Korean healthy participants. Croat Med J. 2009;50:43–48. doi: 10.3325/cmj.2009.50.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gratacòs M, et al. Brain-derived neurotrophic factor Val66Met and psychiatric disorders: meta-analysis of case-control studies confirm association to substance-related disorders, eating disorders, and schizophrenia. Biol Psychiatry. 2007;61:911–922. doi: 10.1016/j.biopsych.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 18.Egan MF, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 19.Chen ZY, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen ZY, et al. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 2004;24:4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Good MA, Barnes P, Staal V, McGregor A, Honey RC. Context- but not familiarity-dependent forms of object recognition are impaired following excitotoxic hippocampal lesions in rats. Behav Neurosci. 2007;121:218–223. doi: 10.1037/0735-7044.121.1.218. [DOI] [PubMed] [Google Scholar]
- 22.Luine VN. The prefrontal cortex, gonadal hormones and memory. Horm Behav. 2006;51:181–182. doi: 10.1016/j.yhbeh.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev. 2007;31:673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brake WG, et al. Novel target sites for estrogen action in the dorsal hippocampus: an examination of synaptic proteins. Endocrinology. 2001;142:1284–1289. doi: 10.1210/endo.142.3.8036. [DOI] [PubMed] [Google Scholar]
- 26.Li C, et al. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci USA. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang UE, Hellweg R, Sander T, Gallinat J. The Met allele of the BDNF Val66Met polymorphism is associated with increased BDNF serum concentrations. Mol Psychiatry. 2009;14:120–122. doi: 10.1038/mp.2008.80. [DOI] [PubMed] [Google Scholar]
- 28.Znamensky V, Akama KT, McEwen BS, Milner TA. Estrogen levels regulate the subcellular distribution of phosphorylated Akt in hippocampal CA1 dendrites. J Neurosci. 2003;23:2340–2347. doi: 10.1523/JNEUROSCI.23-06-02340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frick KM, Berger-Sweeney J. Spatial reference memory and neocortical neurochemistry vary with the estrous cycle in C57BL/6 mice. Behav Neurosci. 2001;115:229–237. doi: 10.1037/0735-7044.115.1.229. [DOI] [PubMed] [Google Scholar]
- 31.Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- 32.Frick KM. Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm Behav. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frick KM, Stillner ET, Berger-Sweeney J. Mice are not little rats: species differences in a one-day water maze task. Neuroreport. 2000;11:3461–3465. doi: 10.1097/00001756-200011090-00013. [DOI] [PubMed] [Google Scholar]
- 34.Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. Shifts in preferred learning strategy across the estrous cycle in female rats. Horm Behav. 2004;45:330–338. doi: 10.1016/j.yhbeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Harburger LL, Bennett JC, Frick KM. Effects of estrogen and progesterone on spatial memory consolidation in aged females. Neurobiol Aging. 2007;28:602–610. doi: 10.1016/j.neurobiolaging.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 36.El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- 37.Ehrlich I, Klein M, Rumpel S, Malinow R. PSD-95 is required for activity-driven synapse stabilization. Proc Natl Acad Sci USA. 2007;104:4176–4181. doi: 10.1073/pnas.0609307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang PC, Yang CH, Huang CC, Hsu KS. Phosphatidylinositol 3-kinase activation is required for stress protocol-induced modification of hippocampal synaptic plasticity. J Biol Chem. 2008;283:2631–2643. doi: 10.1074/jbc.M706954200. [DOI] [PubMed] [Google Scholar]
- 39.Sanna PP, et al. Phosphatidylinositol 3-kinase is required for the expression but not for the induction or the maintenance of long-term potentiation in the hippocampal CA1 region. J Neurosci. 2002;22:3359–3365. doi: 10.1523/JNEUROSCI.22-09-03359.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizuno M, et al. Phosphatidylinositol 3-kinase: a molecule mediating BDNF-dependent spatial memory formation. Mol Psychiatry. 2003;8:217–224. doi: 10.1038/sj.mp.4001215. [DOI] [PubMed] [Google Scholar]
- 41.Stein V, House DR, Bredt DS, Nicoll RA. Postsynaptic density-95 mimics and occludes hippocampal long-term potentiation and enhances long-term depression. J Neurosci. 2003;23:5503–5506. doi: 10.1523/JNEUROSCI.23-13-05503.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg ME. BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci. 2004;24:7366–7377. doi: 10.1523/JNEUROSCI.1739-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 44.Jiang X, et al. BDNF variation and mood disorders: a novel functional promoter polymorphism and Val66Met are associated with anxiety but have opposing effects. Neuropsychopharmacology. 2005;30:1353–1361. doi: 10.1038/sj.npp.1300703. [DOI] [PubMed] [Google Scholar]
- 45.Gatt JM, et al. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. (Translated from Eng) Mol Psychiatry. 2009 doi: 10.1038/mp.2008.143. (in Eng) [DOI] [PubMed] [Google Scholar]
- 46.Hariri AR, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turner CD, Bagnara JT. General endocrinology. 5th Ed. Philadelphia: Saunders; 1971. pp x 659 pp. [Google Scholar]
- 48.Hunter RG, Vicentic A, Rogge G, Kuhar MJ. The effects of cocaine on CART expression in the rat nucleus accumbens: a possible role for corticosterone. Eur J Pharmacol. 2005;517:45–50. doi: 10.1016/j.ejphar.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 49.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th Ed. San Diego: Academic; 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.